Abstract
Calicotome villosa is a spontaneous Mediterranean legume that can be a good candidate as pioneer plants to limit regression of vegetation cover and loss of biodiversity in Tunisian arid soils. In order to grow legumes in such soils, pairing rhizobia and nodule associated bacteria (NAB) might provide numerous advantages. In this work, cultivable biodiversity of rhizobial symbionts and NAB in nodules of C. villosa plants growing in five arid regions of south Tunisia was characterized. Phylogenetic analysis using 16S rDNA gene, dnak, recA and nodD sequences separated nodule-forming bacteria in six clades associated to genera Ensifer, Neorhizobium, Phyllobacterium and Rhizobium. Among NAB, the strain Variovorax sp. CT7.15 was selected due to its capacity to solubilise phosphate and, more interestingly, its high level of aminocyclopropane-1-carboxylate deaminase (ACC deaminase) activity. C. villosa plants were inoculated with representative rhizobia of each phylogenetic group and co-inoculated with the same rhizobia and strain CT7.15. Compared with single rhizobia inoculation, co-inoculation significantly improved plant growth and nodulation, ameliorated plant physiological state and increased nitrogen content in the plants, independently of the rhizobia used. These results support the benefits of pairing rhizobia and selected NAB to promote legume growth in arid or degraded soils.
Keywords: arid soils, ACC deaminase, legumes, nodule associated bacteria, rhizobia
1. Introduction
Soils in Tunisia’s arid regions are characterized by low nutrient content, which is often a limiting factor in plant production. Hence, these areas suffer from rapid regression of vegetation cover, loss of biodiversity and a significant decline in biological activity [1]. Therefore, to limit the massive genetic erosion of species, it is necessary to promote the cultivation of spontaneous plants, especially those used for the restoration of nitrogen soil imbalance. Calicotome belongs to the Genisteae tribe of the subfamily Faboideae; this genus includes four species: Calicotome spinosa, Calicotome villosa, Calicotome infesta and Calicotome intermedia [2]. Calicotome villosa is a spontaneous Mediterranean legume (a 30–150 cm spiny shrub displaying yellow flowers during the spring) that is quite common in the north of Africa and Spain [3]. Calicotome can be pioneer plants growing in barren soils due to a deep and wide root system and their capacity, as legume, to fix atmospheric nitrogen in symbiosis with rhizobia. Rhizobia are Gram-negative soil bacteria classified within α- and β- proteobacteria subclasses that induce the development of root nodules in legumes. Inside the nodule, rhizobia reduce atmospheric N into ammonia to be assimilated by the plant in exchange for plant-derived organic acids [4]. Calicotome spp. nodule-forming bacteria are frequently slow-growing rhizobia affiliated to Bradyrhizobium [5,6]. Contrary to these results, fast-growing rhizobia were identified among isolates from root nodules of C. villosa, which were affiliated to Rhizobium genus [7,8].
An increasing number of studies on rhizobial diversity using standard cultivation methods have reported the presence of the so-called nonrhizobial endophytes (NRE) [9] or nodule associated bacteria (NAB) [10] in nodules of different legume species [11]. Besides rhizobia, other rhizospheric bacteria colonize the interior tissues of plants and contribute in favourable ways to the plant development. These bacteria belong to different genera including Acinetobacter, Agrobacterium, Arthrobacter, Bacillus, Bosea, Enterobacter, Micromonospora, Mycobacterium, Paenibacillus, Pseudomonas, Stenotrophomonas and Variovorax, among others [11,12]. Even though those bacteria do not induce nodule formation, they might be able to colonize the nodules formed by rhizobia strains, and this association may be beneficial for both partners [11,13,14]. These nodule endophytes can behave as plant-growth-promoting bacteria (PGPBs), enhancing plant growth by a variety of direct and indirect mechanisms [15]. Direct mechanisms include facilitation of nutrient acquisition, such as atmospheric nitrogen fixation; production of siderophores for iron uptake and phosphate solubilization; production of phytohormones, like auxins, abscisic acid or cytokinins; and the presence of 1-aminocyclopropane-1-carboxylate deaminase (ACC deaminase) activity, among others [15,16]. Another direct mechanism to increase legume nodulation can be the creation of additional infection sites [17]. PGPB inhabiting nodules and legume rhizosphere involved in plant growth through facilitating plant nodulation could be named as rhizobia helper bacteria (RHB) [17,18].
The aim of this work was to examine the cultivable biodiversity of nodule-inducing rhizobia and NAB in nodules of C. villosa plants growing in arid regions of south Tunisia and also to select NAB that could facilitate plant nodulation and growth, in order to establish rhizobia–NAB couples with potential use in the revegetation of these arid soils.
2. Materials and Methods
2.1. Localization of the Plants and Characteristics of the Soils
Calicotome villosa nodules were collected from wild plants growing in five arid zones of Tunisia: Gabès, Matmata, Zarate, Médenine and Tataouine (Figure S1, Table 1). pH of the soil was determined using a portable meter and a calibrated electrode system (CrisonpH/mVp-506, Hach Lange SLU, Barcelona, Spain). The conductivity of the soil water was determined using a conductivity meter (Crison-522, Hach Lange SLU, Barcelona, Spain) after dilution with distilled water (1:1). Lastly, three 0.5 g dry subsamples of each soil were digested with 6 mL HNO3 (3:1, v/v), 0.5 mL HF and 1 mL H2O2 at 130 °C for 5 h, and ion concentrations were measured by inductively coupled plasma (ICP) spectroscopy (ARL-Fison3410, Thermo Scientific, Waltham MA, USA).
Table 1.
Origins of Calicotome villosa nodules isolates, pH and conductivity of soils and ecological characteristics, C and P content of their sampling sites.
| Coordinates | Ecosystem and Soil Characteristics | pH | Conductivity (µS/cm2) |
Corg % |
P2O5 % |
|
|---|---|---|---|---|---|---|
| S1—Gabes | 33°46′ N, 10°5′ E | Oued bed, sandy gravel | 8.2 ± 0.3 | 19 ± 0.5 | 0.16 ± 0.01 | <0.005 |
| S2—Matmata | 33°29′ N, 10°4′ E | Oued bed, sandy gravel | 9.1 ± 0.4 | 48 ± 0.4 | 0.34 ± 0.02 | <0.005 |
| S3—Zarate | 33°41′ N, 10°21′ E | Sandy (protected area) | 8.3 ± 0.2 | 20 ± 0.6 | 0.11 ± 0.01 | <0.005 |
| S4—Medenine | 33°14′ N, 10°19′ E | Sandy gravel | 9.3 ± 0.2 | 32 ± 0.3 | 0.16 ± 0.03 | <0.005 |
| S5—Tataouine | 33°11′ N, 10°22′ E | Sandy gravel | 8.4 ± 0.3 | 29 ± 0.4 | 0.36 ± 0.02 | <0.005 |
2.2. Isolation and Bacterial Growth
Bacterial strains were isolated from nodules of wild C. villosa plants growing in arid soils described in the previous section. Root nodules were dissected from roots and rinsed thoroughly in water. Surfaces were first disinfected for 1 min with 95% ethanol followed by sodium hypochlorite (5% (v/v)) for 3 min and then extensively rinsed several times with sterile distilled water. Nodules were then crushed on sterile plates and streaked onto yeast-extract mannitol agar (YMA). Plates were incubated at 28 °C for 3–5 days [19]. The purity of the culture was validated by picking and restreaking individual colonies on fresh plates. Bacteria with different colony morphology, among those isolated from nodules of plants growing in the same location, were selected for further identification. To assess the efficiency of the disinfection procedure, for each isolation experiment two sterile and noncrushed nodules were rolled over YMA agar and incubated under the same conditions as the samples. Bacteria were also grown in YMA plates at increasing temperatures ranging from 25 to 40 °C, pH from 4 to 9 and NaCl concentrations from 1% to 2.5% for 3–5 days.
2.3. Genetic Diversity by BOX-PCR and Identification of Cultivable Bacteria
Genomic DNA was extracted using the G-spin genomic DNA extraction kit (INtRon Biotechnology, Inc., Gyeongii-do, South Korea), following the manufacturer’s instructions. Genetic diversity in bacteria isolated from nodules collected in each region was studied by BOX-PCR using BOX A1R primer (5′-CTA CGG CAA GGC GAC GCT GAC G-3′). PCR steps were programmed as described in Mesa et al. [20] using 40 ng of genomic DNAs as templates. PCR products were electrophoresed through a 1.5% agarose gel at 75 V for 2 h. Gel was visualized under UV radiation and photographed. The gel was photographed, and the fingerprints were analysed with CLIQS 1D Pro Software (TotalLab Ltd., Newcastle-Upon-Tyne, UK). Dendrograms were reconstructed using the BOX-PCR profile. The similarities were determined by calculating Pearson’s product moment correlation coefficient [21].
Bacteria representative of each genetic profile were identified by PCR amplification and sequencing of the 16S rDNA following the conditions described in Rivas et al. [22]. EzTaxon server was used to determine homologies with DNA sequences from described bacterial type strains [23]. Partial 16S rDNA sequences were deposited in GenBank (Table 2).
Table 2.
Closest species to the representative strains isolated from Calicotome villosa based on the 16S rRNA partial sequence using EzTaxon server [23].
| Strain | Accession No. | Related Species | Sequenced Fragment (bp) | Identity (%) |
|---|---|---|---|---|
| CE36 | MH327917 | Rhizobium pakistanense | 928 | 98.99 |
| CE5.11 | MH327918 | Phyllobacterium ifriqiyense | 1029 | 99.70 |
| CE5.13 | MH327919 | Rhizobium sullae | 957 | 99.34 |
| CG2.6 | MH327920 | Rhizobium radiobacter | 1303 | 99.54 |
| CG2.8 | MH327921 | Rhizobium radiobacter | 1336 | 98.39 |
| CT4.4 | MH327922 | Rhizobium esperanzae | 1381 | 98.22 |
| CZ2.7 | MH327923 | Rhizobium esperanzae | 1360 | 97.41 |
| CZ4.12 | MH327924 | Neorhizobium alkalisoli | 1300 | 99.77 |
| CZ4.17 | MH327925 | Neorhizobium alkalisoli | 925 | 99.67 |
| CZ7.21 | MH327926 | Rhizobium pakistanense | 1017 | 98.80 |
| CM61 | MH327927 | Ensifer meliloti | 1020 | 99.51 |
| CT17 | MH327928 | Phyllobacterium catacumbae | 1432 | 95.36 |
| CTEM17 | MH327929 | Phyllobacterium ifriqiyense | 1382 | 96.44 |
| CT17.15 | MK121778 | Variovorax paradoxus | 1328 | 96.61 |
| CTEM29 | MK121779 | Brevundimonas sp. | 1100 | 97.36 |
| CM4.10 | MK121780 | Sphingomonas aquatilis | 1302 | 97.93 |
| CZ3.18 | MK121781 | Bacillus cereus | 889 | 97.64 |
| CT7.13 | MK659946 | Pseudomonas sp. | 979 | 96.78 |
| CM8.1 | MK656260 | Ensifer meliloti | 1049 | 90.14 |
| CE5.20 | MK660379 | Sphingomonas sp. | 1229 | 97.15 |
| CT29 | MK590375 | Inquilinus sp. | 1347 | 97.99 |
Housekeeping genes recA (recombinase A) and dnak (chaperone protein DnaK) were amplified using primer couples recA6F/recA555R and dnak1466F/dnak1777R following the method described by Mertens et al. [24]. PCR amplification of symbiotic nodD gene was performed following the conditions described in [22,25].
PCR products were purified with ExoSAP-IT PCR Product Cleanup (Thermo Fisher, Waltham, MA, USA), and sequencing was performed at StabVida (Lisbon, Portugal). For phylogenetic analysis, sequences were assembled and then aligned using ClustalW program [26], with relevant sequences obtained from the GenBank database. The phylogenetic trees with 1000 bootstrap replications were constructed using MEGA 6.0 software [27]. Selected gene sequences obtained in this work were deposited in the GenBank database, and their accession numbers are shown in the phylogenetic trees.
2.4. Determination of PGP Properties and Enzymatic Activities
Bacterial cultures were plated on nitrogen-free broth (NFB) medium as preliminary test for nitrogen fixation [28]. Strains able to grow in NFB medium were tested for acetylene reduction following the protocol described in [29]. Positive phosphate solubilization was resolved on NBRIP (National Botanical Research Institute’s phosphate growth medium) plates when bacterial growth caused the appearance of surrounding transparent halos [30]. Plates were incubated 3 days at 28 °C. The quantification of IAA (indole-3-acetic acid) was evaluated by a colorimetric method as detailed in Mesa et al. [20]. Quantitative measurement of ACC deaminase activity of bacterial strains was carried out using the method described by Penrose and Glick [31]. The reaction was determined by comparing the absorbance at 540 nm of the sample to a standard curve of α-ketobutyrate. Then, total protein concentration of toluenized cells was estimated using bovine serum albumin (BSA) to create the protein calibration curve [32]. Finally, the enzyme activity was calculated based on the µmoles of released α-ketobutyrate per mg of protein per hour.
Lipase and protease activities were performed growing the strains in Tween80 and casein agar plates, respectively, and the presence of halos around bacteria growth indicates positive enzymatic activities [33]. Chitinase activity was observed according to Mesa et al. [20]. Pectinase and cellulase activities were examined as described in Elbeltagy et al. [34]. DNAse activity was studied in DNA agar plates and revealed with 1 N HCl. Concerning amylase activity; bacteria were grown in starch agar (Scharlab SL, Barcelona, Spain) plates. Plates were revealed by covering plate surface with 10 mL of lugol.
2.5. Plant Inoculation and Growth
Seeds of Calicotome villosa were first treated with pure sulfuric acid for 1 min, rinsed abundantly six times with sterile distilled water, and then allowed to germinate on 1% agar plates at 28 °C for approximately 72 h. Germinated seeds were transferred to sterilized plastic pots containing sterile soil from Gabès region. Soil was sterilized at 121 °C, 1 atm for 20 min, three times (mixing vigorously the soil after each sterilization cycle). Seeds were irrigated with nitrogen-free buffered nodulation medium (BNM) [35] and inoculated with 108 bacterial cells per mL of the desired bacterial inoculum. For each strain, three replicates were considered. Noninoculated plants were included as negative controls. Plants were rinsed with BNM approximately once a week for 5 months and nodulation was observed. Cross-inoculation assays using Medicago sativa, Lens culinaris and Lotus edulis were developed using the same protocol except for seeds germination. In that case, seeds were treated with 5% sodium hypochlorite and rinsed six times with sterile distilled water.
For co-inoculation experiments, germinated seeds of C. villosa were inoculated with the selected isolates by immersion in 108 cells per mL solution (resuspended in BNM), transferred into plastic pots filled with sterilized soil from Gabès and then inoculated with the remaining bacteria. The experimental design consisted of two replicates (three plants per replicate) of each of the four following treatments: (i) noninoculated control plants; (ii) plants inoculated with the ACC-deaminase-producing strain Variovorax sp. CT7.15; (iii) plants inoculated separately with selected nodule-inducing rhizobial strains: Phyllobacterium sp. CTEM17, Rhizobium sp. CZ2.7, Rhizobium pakistanense CE36, Neorhizobium sp. CZ4.17 and Ensifer sp. CM61; and (iv) plants co-inoculated with the selected rhizobia and the strain Variovorax sp. CT7.15. Pots were placed in the greenhouse (18/8 h light/dark at 25/23 °C) and rinsed with BNM solution once a week for 5 months. Plants were then collected and data regarding growth parameters, i.e., plant height, shoot and root dry weight and number of nodules, were recorded.
2.6. Photosynthetic Pigments
At the end of the experimental period, photosynthetic pigments in fully developed green leaves from each treatment were extracted using 0.05 g of fresh material in 10 mL of 80% aqueous acetone. After filtering, 1 mL of the suspension was diluted with a further 2 mL of acetone, and chlorophyll a (Chl a) and chlorophyll b (Chl b) contents were determined with a HitachiU-2001 spectrophotometer (Hitachi Ltd., Tokyo, Japan) using three wavelengths (663.2, 646.8 and 470.0 nm). Concentrations of pigments (µg × g of fresh weight−1) were obtained following the method described by Lichtenthaler [36].
2.7. Statistical Analysis
Statistical analysis was performed using the software Statistica v. 6 (Statsoft Inc., Tulsa, OK, USA). Data were first tested for normality with the Kolmogorov–Smirnov test and for homogeneity of variance with the Brown–Forsythe test. Comparisons between means in different inoculation and co-inoculation treatments at the end of the experiment were made by using generalizer linear models (GLM). Significant test results were followed by Fisher tests (LSD) for identification of important contrasts.
3. Results and Discussion
3.1. Biodiversity of Cultivable Bacteria Inhabiting C. villosa Nodules in Tunisian Arid Soils
Since limited information was available on bacteria nodulating or NAB in C. villosa, the first objective of this work was therefore to identify and characterize the nodule cultivable bacteria associated with this legume in arid soils of Tunisia, particularly in five regions with similar chemical properties, low organic material and phosphate content (Table 1).
Based on place where nodules were collected and colony morphology, 43 cultivable bacterial strains were originally isolated. Nodulation tests using C. villosa plants were performed to evaluate the ability of the isolates to renodulate de host plant. Twenty-seven isolates were able to nodulate C. villosa plants under controlled conditions, 12 failed to nodulate and 4 induced small nonfixing white lumps. Isolates able to renodulate C. villosa plants were classified as fast growing (able to grow in 3–5 days at 28 °C).
BOX-PCR fingerprinting of the isolates allowed grouping by genetic profiles (Figure S2). Here, 16S rRNA of 21 strains were sequenced and compared with type strains using Eztaxon server [23] (Table 2). The selection of these strains was performed as follows: among strains with the same BOX-PCR profile only one of them was sequenced (i.e., CZ61 and CZ7.21 or CE5.11 and CE4.6 showed the same profile in Figure S2, and only CZ7.21 and CE5.11 were sequenced). In the same way, strains with undefined profile (i.e., CE36 in Figure S2) were also sequenced. Our results showed the existence of a wide biodiversity in C. villosa nodules, with isolates belonging to 10 different genera.
Strains were also characterized based on some phenotypic parameters, such as growth at different NaCl concentrations, increasing pH and temperature, as well as antibiotic tolerance Although isolates showed a wide diversity in their characteristics, most of them were able to grow at pH ranging from 5 to 9 and temperatures ranging from 25 to 35 °C. In general, isolates showed a low tolerance to NaCl, and only strain CM61 related to E. meliloti grew at 1% NaCl.
3.2. Rhizobia Isolated from C. villosa Nodules in Tunisian Arid Soils
Concerning rhizobia, strains related to Phyllobacterium sp. were isolated from nodules of C. villosa plants growing in Gabès, Médenine and Tataouine regions and Rhizobium sp. in Zarate, Médenine and Tataouine. In Matmata region, nodules were elicited by Ensifer sp. strains. Neorhizobium sp. strains were isolated from nodules in Zarate, in addition to strains related to Rhizobium pakistanense, which were also isolated in Médenine. These strains nodulated C. villosa in greenhouse conditions. Finally, strains that induced small white lumps in C. villosa roots were related to Rhizobium radiobacter and isolated from plants growing in Gabès soils.
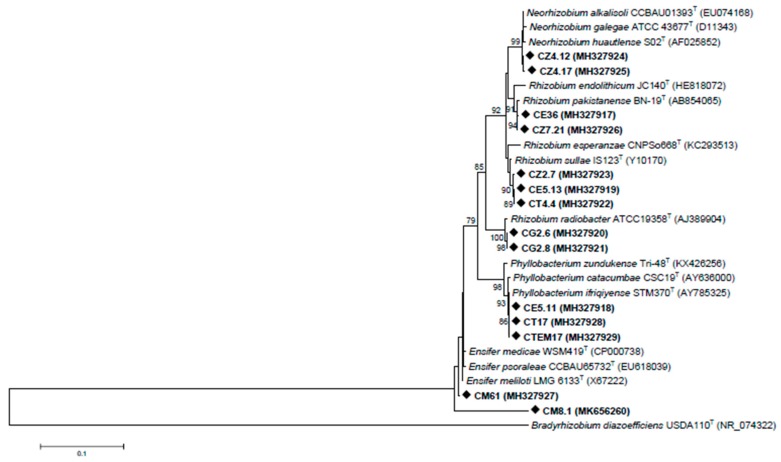
The phylogenetic relationship between the sequenced strains relative to type strains based on 16S rRNA gene grouped rhizobia into six clades (Figure 1). Strains CZ4.12 and CZ4.17 were closely related to Neorhizobium alkalisoli species. Isolates CE36 and CZ7.21 were closely related to Rhizobium pakistanense. Three strains, CT4.4, CE 5.13 and CZ2.7, were related to Rhizobium sullae and Rhizobium esperanzae. CM61 was related to Ensifer meliloti, and CM8.1, also identified as Ensifer sp., was not clearly associated with any species. CE5.11, CT17 and CTEM17 were identified as Phyllobacterium sp. and related to Phyllobacterium ifriqiyense and Phyllobacterium catacumbae. Finally, CG2.6 and CG2.8 were identified as Rhizobium radiobacter.
Figure 1.
Neighbour-joining phylogenetic tree based on the 16S rRNA gene of representative nodule-inducing strains isolated from nodules of Calicotome villosa. Bootstrap confidence levels were derived from 1000 replications, and those greater than 75% are indicated at the internodes. Scale bar = 0.1 nucleotide divergence.
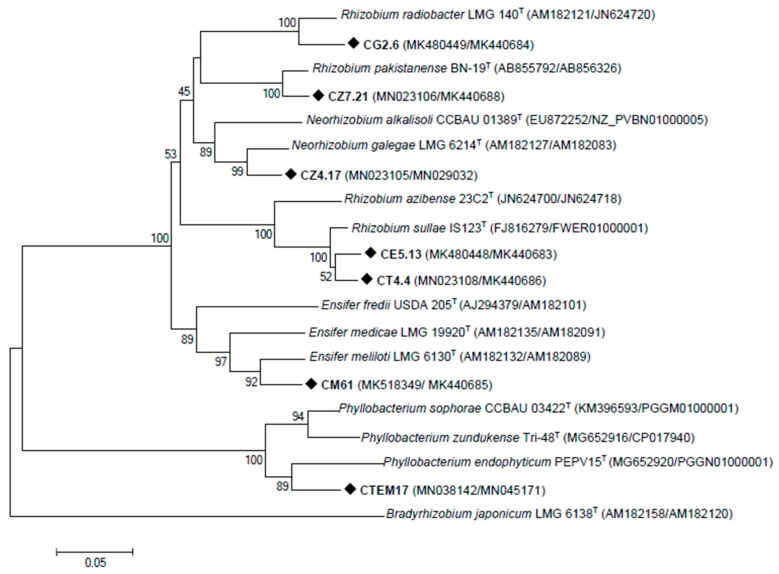
The housekeeping genes are considered as robust markers for assessing the evolutionary genetics of rhizobia [37]. Thus, to confirm the phylogenetic position of the strains isolated in this study, recA and dnak genes of one strain of each clade were sequenced. Strain CT4.4, positioned between R. sullae and R. esperanzae, was also included to clarify its position. Consistent with the 16S rRNA tree, the concatenated phylogenetic tree based on recA and dnak sequences grouped C. villosa nodulating isolates in six different clades (Figure 2). CE5.13 and CT4.4 were closely related to Rhizobium sullae. CG2.6 and CZ7.21 had concatenated sequences that were identical to R. radiobacter and R. pakistanense, respectively, with a high bootstrap support (100%). With lower bootstrap support, strain CM61 was clustered with Ensifer meliloti LMG 6133T. The other strains were distributed between two clusters: strain CZ4.17 aligned with Neorhizobium galegae CCBAU 01393T and closely related with N. alkalisoli, and CTEM17 affiliated with Phyllobacterium cluster with high identity values.
Figure 2.
Neighbour-joining tree based on concatenated recA and dnaK gene sequences of representative nodule-inducing strains isolated from nodules of Calicotome villosa. The significance of each branch is indicated by the bootstrap value calculated for 1000 replicates (only values higher than 75% are indicated). Scale bar = 0.02 nucleotide divergence.
Considering these results and previous works [7,38,39], Rhizobium and Ensifer seem to be the main microsymbionts for indigenous legumes in Tunisia. Concerning Rhizobium, our isolates were closely related to R. sullae, which was known to nodulate Hedysarum coronarium L. [40], and to R. pakistanense, recovered from groundnut nodules in Pakistan [41]. Ensifer strains were related to E. meliloti, a common microsymbiont of most wild legumes in Tunisian arid soils [39,42,43]. Isolates belonging to Phyllobacterium have been previously described to nodulate wild legumes in Tunisia [7,38,43]. Phyllobacterium strains isolated in this work were closely related to Phyllobacterium ifriqiyense STM370. The first isolation of this reference strain was reported in [44], from nodules of Astragalus algerianus and Lathyrus numidicus in the infra-arid zone of southern Tunisia. On the other hand, the presence of Agrobacterium tumefaciens (formerly Rhizobium radiobacter) in nodules of legumes growing in Tunisia was previously reported [39,43,45,46].
None of our isolates was affiliated to Bradyrhizobium, unlike in previous studies on C. spinosa in the northeast of Algeria [6] and C. spinosa and C. infesta in different areas of Sicily [5]. Several authors have demonstrated that Bradyrhizobium predominates among rhizobia nodulating Genisteae [47,48,49,50]. However, in some other studies, Genistoid plants, such as Genista saharae, Retama raetam or Retama sphaerocarpa, were nodulated by Sinorhizobium (formerly Ensifer), Rhizobium, Mesorhizobium and Phyllobacterium [39,51,52].
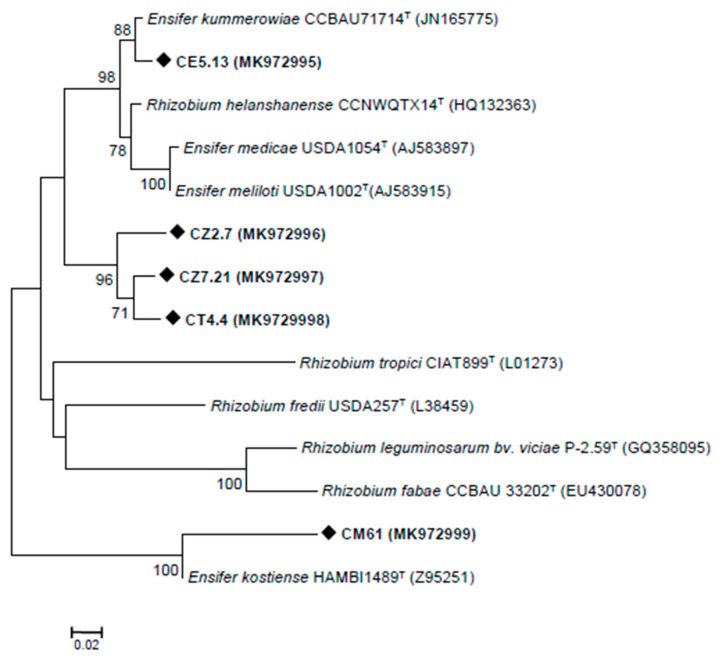
The bacterial nodulation (nod) genes, which encode the production of Nod factor determine the Rhizobium-legume symbiosis [53]. The NodD (LysR-type regulator), which acts as a transcriptional activator for the nod operon, plays an important role in the control of rhizobial host specificity [54]. In order to determine the nature of the symbiotic interaction and the diversity of C. villosa isolates, the symbiotic nodD genes were analysed (Figure 3). Strain CE5.13 nodD sequence displayed a high similarity to that of Ensifer kummerowiae CCBAU71714T. This type strain, isolated from Kummerowia stipulacea, nodulated its host plant and Medicago sativa, but not several other legumes assayed [55]. On the other hand, the strain CM61 shared a 100% nodD sequence similarity with Ensifer kostiense HAMBI1489T, which nodulated Acacia senegal and Prosopis chilensis [56]. Strains CT4.4, CZ2.7 and CZ7.21, grouped closely together and formed a separate linage (supported by high bootstrap values (97%)) from all other strains, that was closest to E. medicae, E. meliloti and E. kummerowiae. We could not amplify nodD gene in any of the Neorhizobium sp., R. radiobacter or Phyllobacterium sp. strains.
Figure 3.
Molecular phylogenetic analysis using the neighbour-joining method of C. villosa root nodule isolates for the nodD gene. Significant bootstraps (70%) are indicated as percentages (1000 replications). Scale bar = 0.02 nucleotide divergence.
Amplification of nodA gene, more commonly used for symbiotic characterization of the strains, was also attempted using different primer couples as described by Tounsi-Hammami et al. [57]. Unfortunately, nodA gene could not be amplified after several attempts. Accordingly, Tounsi-Hammani et al. [57] were able to amplify nodA gene only in 1 out of 14 selected rhizobia able to nodulate Lupinus albus in Tunisian calcareous soils.
The symbiotic genes of rhizobia are reported to have an evolutionary history different from those of the housekeeping genes [58], an argument that is supported by the nodD, 16S and housekeeping gene phylogenies obtained in this study. This may be explained by the fact that the symbiotic genes that codify nodulation are plasmid borne in fast-growing rhizobia, so they can be transferred among species in the rhizosphere.
Finally, a cross-inoculation experiment to test the ability of these autochthonous rhizobia to nodulate representative grain legumes was developed (Table S1). R. sullae and E. meliloti strains nodulated M. sativa, L. culinaris and L. edulis. N. galegea and R. pakistanense strains only nodulated M. sativa, and Phyllobacterium strains did not nodulate any of the legumes assayed.
3.3. NAB Isolated from C. villosa Nodules in Tunisian Arid Soils
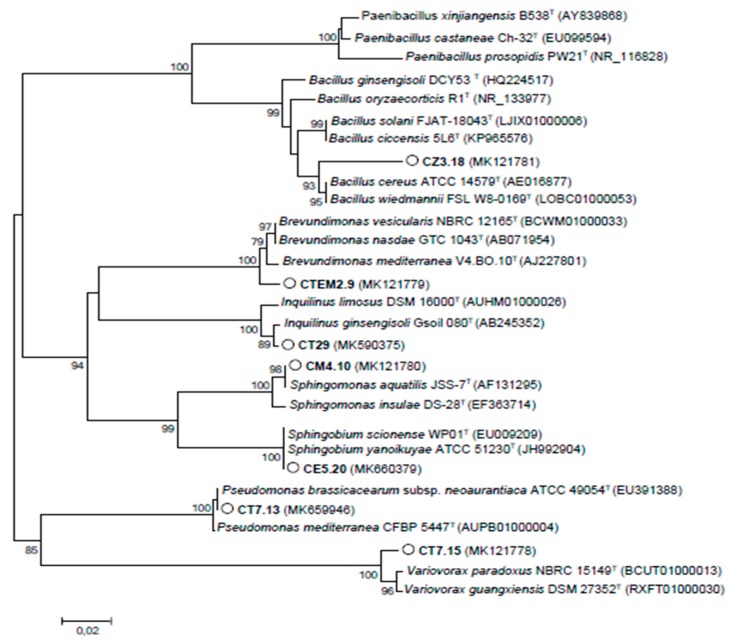
The presence of nodule associated bacteria (or NAB) could be preferentially selected by legumes as these bacteria could provide benefits to counteract nutritional deficiencies, drought and other stresses. Among NAB isolated, Brevundimonas sp. and Sphingomonas sp. strains accompanied Phyllobacterium sp. in nodules collected in Gàbes. Nodules induced by Ensifer sp. in Matmata contained Sphingomonas sp. and Pseudomonas sp. strains. Bacillus sp. was found inhabiting nodules collected in Zarate. Finally, NAB isolated from nodules of Tataouine belonged to genera Inquilinus, Pseudomonas and Variovorax. Based on 16S rRNA gene sequences, a phylogenetic tree of cultivable NAB was constructed (Figure 4).
Figure 4.
Neighbour-joining phylogenetic tree based on the 16S rRNA gene of endophytic strains isolated from nodules of Calicotome villosa. Bootstrap confidence levels were derived from 1000 replications, and those greater than 75% are indicated at the internodes. Scale bar = 0.1 nucleotide divergence.
Zakhia et al. [8] reported the presence of Bacillus, Inquilinus and Sphingomonas in nodules of spontaneous C. villosa plants growing in Tunisia. In the same work, Pseudomonas strains were found in nodules of other spontaneous legumes growing in the same region. Variovorax has been isolated from nodules of Acacia growing in southeastern Australia [59] and nodules of Crotalaria growing in Ethiopia [60].
3.4. PGP Properties of the Isolates
Assessing the presence of PGP properties is an important task, enabling the selection of the most efficient bacterial inoculants. In that way, the abilities to solubilize phosphate and to produce IAA and the presence of ACC deaminase activity were assayed in all the isolates. Most of the nodule-forming rhizobacteria (29 out of 31) were able to solubilize phosphate, while only the Variovorax sp. strains among the nonrhizobia did it. Soil phosphate is frequently found in insoluble form in soil, so bacteria with phosphate-solubilising activity may provide an available form to the plant. Concerning IAA production, a hormone directly involved in plant root elongation, only the strain R. radiobacter CG2.6 was able to produce low hormone levels (0.84 mgL−1). Strains Brevundimonas sp. CTEM2.9 and Variovorax sp. CT7.5 and CT7.15 showed ACC deaminase activity. Particularly, Variovorax sp. CT7.15 showed a high level of activity, up to 27.26 µmol α-ketobutyrate mg of protein−1h−1. The phytohormone ethylene (C2H4) is an important modulator of plant growth and developmental processes [61,62]. For many plants, C2H4 stimulates germination and breaks the seed dormancy, but, during germination, a high concentration of ethylene inhibits root elongation [63]. Bacteria having ACC deaminase activity can regulate ethylene levels in the plant, thereby limiting the damage to the plant [31].
Nitrogen fixation was also studied in NAB isolated from C. villosa nodules. Only strains of Variovorax sp., both CT7.5 and CT7.15, grew in nitrogen-free medium. Nevertheless, they could not reduce acetylene, indicating that they did not fix atmospheric nitrogen.
Based on these results, Variovorax sp. CT7.15 was selected to study its capability to improve C. villosa growth and nodulation in co-inoculation with nodule-inducing rhizobia.
The presence of enzymatic activities related with organic matter degradation in Variovorax sp. CT7.15 was also studied. This strain showed pectinase, amylase and lipase activities; and was negative for cellulase, chitinase, DNAse and protease.
3.5. Co-Inoculation of C. villosa with Rhizobia and Variovorax sp. CT7.15 Strain Improves Plant Nodulation and Growth
The need for more research on pairing rhizobia and NAB in order to grow legumes on arid or eroded soils has been recently pointed out [11]. In this context, surface disinfected C. villosa seeds were inoculated with selected rhizobia or co-inoculated with the same rhizobia and the strain Variovorax sp. CT7.15. One nodule-forming rhizobia of each phylogenetic clade, among those that induced the formation a greater number of nodules in re-inoculation experiments (data not shown), was selected.
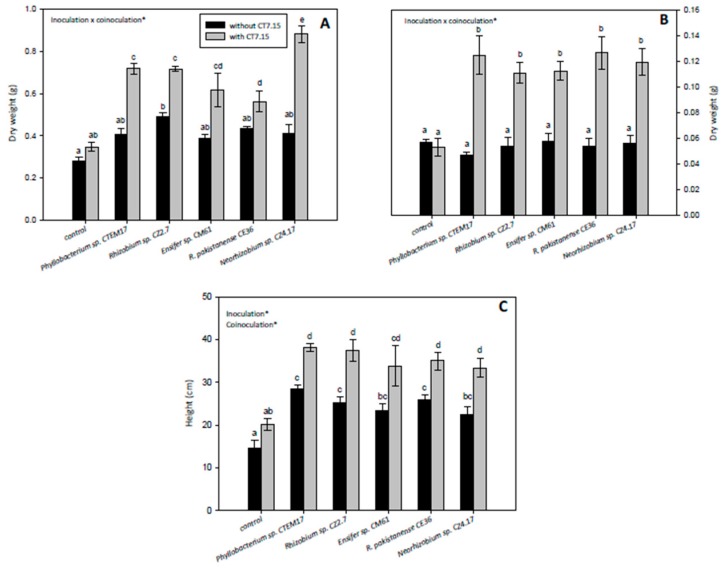
As expected, inoculation with rhizobia increased plant growth (Figure 5). Inoculation improved both plant height and dry weight, particularly due to an increase in shoot weight, independently of the rhizobia (Figure 5A,C). Nevertheless, significant differences in aerial part dry weight were only found in plants inoculated with Rhizobium sp. CZ2.7 (Figure 5A). On the other hand, plants co-inoculated with rhizobia and Variovorax sp. CT7.15 showed significantly higher shoot dry weight (Figure 5A), root dry weight (Figure 5B) and plant height than those inoculated only with rhizobia (Figure 5C). Compared to single inoculation, the increases in shoot biomass ranged from 30% (R. pakistanense) to 100% (Neorhizobium sp.) (Figure 5A), and increases in plant height were around 30% (Figure 5C). Concerning root dry weights, co-inoculated plants duplicated the root weight of plants inoculated only with rhizobia (Figure 5B). Increase in root biomass could be very helpful in the absorption of water and nutrients in arid soils.
Figure 5.
Aerial part dry weight (A), root dry weight (B) and plant height (C) of Calicotome villosa in response to inoculation with different rhizobia (Phyllobacterium sp. CTEM17, Rhizobium sp. CZ2.7, Ensifer sp. CM61, R. pakistanense CE36 and Neorhizobium sp. CZ4.17) in absence and presence of strain CT7.15 as co-inoculant for 5 months. Values represent mean ± SE, n = 6. Different letters indicate means that are significantly different from each other (GLM, inoculation × co-inoculation; LSD test, p < 0.05). Inoculation, co-inoculation or inoculation × co-inoculation in the corner of the panels indicate main or interaction significant effects (∗ p < 0.001).
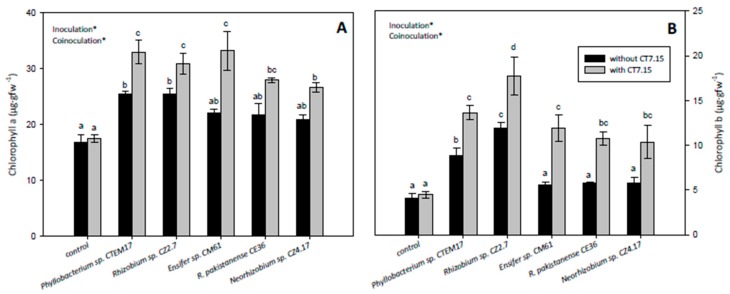
The effect of plant inoculation either with rhizobia or co-inoculation with CT7.15 on chlorophyll concentrations was also recorded (Figure 6). Inoculation with rhizobia increased chlorophyll a and b concentrations compared to control plants, with their values being significantly higher in plants inoculated with Phyllobacterium sp. and Rhizobium sp. Plants co-inoculated with rhizobia and strain CT7.15 showed higher concentrations of both chlorophylls than plants inoculated only with rhizobia, with significant differences independent of the rhizobia (Figure 6A,B). These results indicated that co-inoculation with the Variovorax strain CT7.15 ameliorates the physiological state of the C. villosa plants.
Figure 6.
Physiological parameters. Chlorophyll a (A) and chlorophyll b (B) in randomly selected leaves of Calicotome villosa in response to inoculation with different rhizobia (Phyllobacterium sp. CTEM17, Rhizobium sp. CZ2.7, Ensifer sp. CM61, R. pakistanense CE36 and Neorhizobium sp. CZ4.17) in absence and presence of strain CT7.15 as co-inoculant for 5 months. Values represent mean ± SE, n = 6. Different letters indicate means that are significantly different from each other (GLM, inoculation × co-inoculation; LSD test, p < 0.05). Inoculation, co-inoculation or inoculation × co-inoculation in the corner of the panels indicate main or interaction significant effects (∗ p < 0.001).
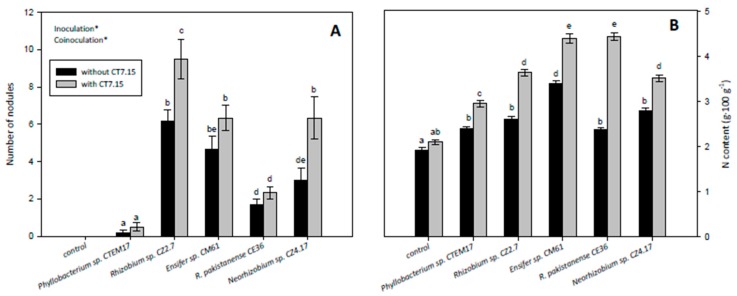
Finally, nodulation and plant N content were also evaluated in C. villosa plants inoculated with rhizobia and co-inoculated with the Variovorax sp. strain (Figure 7). C. villosa plants co-inoculated with rhizobia and the ACC-deaminase-producing bacterium showed higher number of nodules than plants inoculated only with rhizobia (Figure 7A). Differences in nodule number were significant in plants inoculated with Rhizobium sp. CZ2.7 and Neorhizobium sp. CZ4.17 strains (Figure 7A). This effect on nodulation rate might be explained by the ACC-deaminase activity of CT7.15, which decreases levels of ethylene, a well-known negative regulator of nodulation [64,65].
Figure 7.
Number of nodules (A) in Calicotome villosa and nitrogen content (B) in randomly selected leaves of Calicotome villosa in response to inoculation with different rhizobia (Phyllobacterium sp. CTEM17, Rhizobium sp. CZ2.7, Ensifer sp. CM61, R. pakistanense CE36 and Neorhizobium sp. CZ4.17) in absence and presence of strain CT7.15 as co-inoculant for 5 months. Values represent mean ± SE, n = 6. Different letters indicate means that are significantly different from each other (GLM, inoculation × co-inoculation; LSD test, p < 0.05). Inoculation, co-inoculation or inoculation × co-inoculation in the corner of the panels indicate main or interaction significant effects (∗ p < 0.001).
As expected, plants inoculated with rhizobia showed higher values of N than control plants, with statistically significant differences (Figure 7B). On the other hand, co-inoculated plants showed higher values in N content than plants inoculated only with rhizobia, with significant differences independent of the rhizobia (Figure 7B). Although plants inoculated with CT7.15 strain showed higher values of N than control plant, differences were not significant (Figure 7B). Increase in N content in co-inoculated plants might be related not only with the increase in nodule number but also with the ACC deaminase activity of CT7.15, since this enzyme converts ACC (precursor of ethylene) into ammonia and α-ketobutyrate, providing the plant a nitrogen source [66].
4. Conclusions
In conclusion, co-inoculation of C. villosa plants with autochthonous nodule-inducing rhizobia and the strain Variovorax sp. CT7.15 promoted plant growth and nodulation and improved plant physiological state in arid soils, increasing the effects of single rhizobia inoculation. These results support the benefits of pairing rhizobia and selected NAB to grow legumes in arid or degraded soils.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/4/541/s1. Figure S1, (A) Map of Tunisia showing the location of sampling sites. (B,C) Nodules of Calicotome villosa wild type plants collected for this work. Figure S2, Box-PCR patterns of the bacteria isolates from Calicotome villosa compared using dendrogram. Scales at the top of the dendrograms represent similarity. Table S1, Cross-nodulation of representative rhizobia isolated from C. villosa nodules with some grain legumes.
Author Contributions
Conceptualization, A.F., M.M. and I.D.R.-L.; methodology, K.B., S.N.-T. and E.M.-N.; software, S.N.-T.; validation, E.P., A.F. and K.B.; formal analysis, K.B., S.N.-T. and E.M.-N.; investigation, K.B. and S.N.-T.; resources, M.Á.C., E.P. and S.R.-G.; data curation, E.M.-N. and S.R.-G.; writing—Original draft preparation, I.D.R.-L., K.B. and S.N.-T.; writing—Review and editing, I.D.R.-L. and K.B.; visualization, M.M. and I.D.R.-L; supervision, M.M. and M.Á.C.; project administration, M.Á.C. and E.P.; funding acquisition, S.R.-G., M.M. and I.D.R.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by grants from the Ministry for the Higher Education and Scientific Research (Tunisia) and University of Gabès, and projects from Ministerio de Economía y Competitividad (MINECO Project CGL2016-75550-R cofounded by FEDER) and Junta de Andalucía (Project 2017/391).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Tarhouni M., Ouled-Belgacem A., Henchi B., Neffati M. A preliminary overview of the effects of seasonal drought and animal pressure around watering points on plant species using adaptative strategy analyses in the Tunisian arid zone. Ecol. Mediterr. 2006;32:39–48. [Google Scholar]
- 2.Quezel P., Santa S. Nouvelle Flore de L’Algérie et des Régions Désertiques Méridionales. Editions du Centre National de la Recherche Scientifique; Paris, France: 1962. pp. 475–476. [Google Scholar]
- 3.Greuter W., Burdet H.M., Long G. Med.-Checklist 4: A Critical Inventory of Vascular Plants of the Circum Mediterranean Countries: Dicotyledones (Lauraceae Rhamnaceae) Genève Conservatoire et Jardin Botanique de la Ville de Genève; Geneva, Switzerland: 1989. [Google Scholar]
- 4.Poole P., Ramachandran V., Terpolilli V. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018;16:291–303. doi: 10.1038/nrmicro.2017.171. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale M., Lanza A., Bonni M.L., Marsala S., Puglia A.M., Quatrini P. Diversity of rhizobia nodulating wild shrubs of Sicily and some neighboring islands. Arch. Microbiol. 2008;190:461–470. doi: 10.1007/s00203-008-0394-2. [DOI] [PubMed] [Google Scholar]
- 6.Salmi A., Boulila F., Bourebaba Y., Le Roux C., Belhadi D., De Lajudie P. Phylogenetic diversity of Bradyrhizobium strains nodulating Calicotome spinosa in the Northeast of Algeria. Syst. Appl. Microbiol. 2018;41:452–459. doi: 10.1016/j.syapm.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Zakhia F., Jeder H., Domergue O., Willems A., Cleyet-Marel J.-C., Gillis M., Dreyfus B., de Lajudie P. Characterisation of wild legume nodulating bacteria (LNB) in the infra-arid zone of Tunisia. Syst. Appl. Microbiol. 2004;27:380–395. doi: 10.1078/0723-2020-00273. [DOI] [PubMed] [Google Scholar]
- 8.Zakhia F., Jeder H., Willems A., Gillis M., Dreyfus B., de Lajudie P. Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya. Microb. Ecol. 2006;51:375–393. doi: 10.1007/s00248-006-9025-0. [DOI] [PubMed] [Google Scholar]
- 9.De Meyer S.E., De Beuf K., Vekeman B. A large diversity of non-rhizobial endophytes found in legume root nodules in Flanders (Belgium) Soil Biol. Biochem. 2015;83:1–11. doi: 10.1016/j.soilbio.2015.01.002. [DOI] [Google Scholar]
- 10.Rajendran G., Patel M.H., Joshi S.J. Isolation and characterization of nodule-associated Exiguobacterium sp. from the root nodules of fenugreek (Trigonella foenu-graecum) and their possible role in plant growth promotion. Int. J. Microbiol. 2012;2012:693982. doi: 10.1155/2012/693982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Hidalgo P., Hirsch A.M. The nodule microbiome: N2-Fixing rhizobia do not live alone. Phytobiomes. 2017;1:52. doi: 10.1094/PBIOMES-12-16-0019-RVW. [DOI] [Google Scholar]
- 12.Velázquez E., Martínez-Hidalgo P., Carro L., Alonso P., Peix A., Trujillo M.E., Martínez-Molina E. Nodular endophytes: An untapped diversity. In: González B.R.M., González-López J., editors. Beneficial Plant—Microbial Interactions: Ecology and Applications. CRC Press; Boca Raton, FL, USA: 2013. pp. 214–236. [Google Scholar]
- 13.Pandya M., Kumar G.N., Rajkumar S. Invasion of rhizobial infection thread by non-rhizobia for colonization of Vigna radiata root nodules. FEMS Microbiol. Lett. 2013;348:58–65. doi: 10.1111/1574-6968.12245. [DOI] [PubMed] [Google Scholar]
- 14.Zgadzaj R., James E.K., Kelly S., Kawaharada Y., de Jonge N., Jensen D.B., Madse L.H., Radutoiu S. A legume genetic framework controls infection of nodules by symbiotic and endophytic bacteria. PLoS Genet. 2015;11:e1005280. doi: 10.1371/journal.pgen.1005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olanrewaju O.S., Glick B.R., Babalola O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33:197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamalero E., Glick B.R. Bacterial modulation of plant ethylene levels. Plant. Physiol. 2015;169:13. doi: 10.1104/pp.15.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehboob I., Naveed M., Zahir Z.A., Sessitsch A. Potential of rhizosphere bacteria for improving Rhizobium-legume symbiosis. In: Arora N.K., editor. Plant Microbe Symbiosis: Fundamentals and Advances. Springer; New Delhi, India: 2013. [Google Scholar]
- 18.Martínez-Hidalgo P., Galindo-Villardón P., Trujillo M.E., Igual J.M., Martínez-Molina E. Micromonospora from nitrogen-fixing nodules of alfalfa (Medicago sativa L.). A new promising plant probiotic bacteria. Sci. Rep. 2014;4:6389. doi: 10.1038/srep06389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent J.M. A manual for the practical study of root nodule bacteria. IBM Handb. 1970;45:440. [Google Scholar]
- 20.Mesa J., Mateos-Naranjo E., Caviedes M.A., Redondo-Gomez S., Pajuelo E., Rodriguez-Llorente I.D. Scouting contaminated estuaries: Heavy metal resistant and plant growth promoting rhizobacteria in the native metal rhizoaccumulator Spartina maritima. Mar. Pollut. Bull. 2015;90:150–159. doi: 10.1016/j.marpolbul.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Jobson J. Applied Multivariate Data Analysis. Volume 1. Springer Verlag; New York, NY, USA: 1991. p. 621. [Google Scholar]
- 22.Rivas R., Velazquez E., Willems A., Vizcaino N., Subba-Rao N.S., Mateos P.F., Gillis M., Martinez-Molina E. A new species of Devosia that forms a unique nitrogen-fixing root-nodule symbiosis with the aquatic legume Neptunia natans (L.f.) Druce. Appl. Environ. Microbiol. 2002;68:5217–5222. doi: 10.1128/AEM.68.11.5217-5222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun J., Lee J.-H., Jung Y., Kim M., Kim S., Kim B.K., Lim Y.W. EzTaxon: A web based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2007;57:2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- 24.Martens M., Delaere M., Coopman R., de Vos P., Gillis M., Willems A. Multilocus sequence analysis of Ensifer and related taxa. Int. J. Syst. Evol. Microbiol. 2007;57:489–503. doi: 10.1099/ijs.0.64344-0. [DOI] [PubMed] [Google Scholar]
- 25.Haukka K., Lindström K., Young J.P. Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Appl. Environ. Microbiol. 1998;64:419–426. doi: 10.1128/AEM.64.2.419-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chenna R., Sugawara H., Koike T., Lopez R., Gibson T.J., Higgins D.G., Thompson J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Döebereiner J. Isolation and identification of aerobic nitrogen-fixing bacteria from soil and plants. In: Alef K., Nannipieri P., editors. Methods in Applied Soil Microbiology and Biochemistry. Academic Press; London, UK: 1995. pp. 134–141. [Google Scholar]
- 29.Buendía-Clavería A.M., Chamber M., Ruiz-Sainz J.E. A comparative study of the physiological characteristics, plasmid content and symbiotic properties of different Rhizobium fredii strains. Syst. Appl. Microbiol. 1989;12:203–209. [Google Scholar]
- 30.Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 31.Penrose D.M., Glick B.R. Methods for isolating and characterizing ACC deaminase containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 32.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dyebinding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Harley J.P., Prescott L.M. Laboratory Exercises in Microbiology. 5th ed. The McGraw-Hill; New York, NY, USA: 2002. p. 466. [Google Scholar]
- 34.Elbeltagy A., Nishioka K., Suzuki H., Sato T., Sato Y.I., Morisaki H., Mitsui H., Minamisawa K. Isolation and characterization of endophytic bacteria from wild and traditionally cultivated rice varieties. Soil Sci. Plant. Nutr. 2000;46:617–629. doi: 10.1080/00380768.2000.10409127. [DOI] [Google Scholar]
- 35.Ehrhardt D.W., Atkinson E.M., Long S.R. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenthaler H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- 37.Zhang Y.M., Tian C.F., Sui X.H., Chen W.F., Chen W.X. Robust markers reflecting phylogeny and taxonomy of rhizobia. PLoS ONE. 2012;7:e44936. doi: 10.1371/journal.pone.0044936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Romdhane S., Nasr H., Samba-Mbaye R., Neyra M., Ghorbal M. Diversity of Acacia tortilis rhizobia revealed by PCR/RFLP on crushed root nodules in Tunisia. Ann. Microbiol. 2005;55:249–258. [Google Scholar]
- 39.Mahdhi M., De Lajudie P., Mars M. Phylogenetic and symbiotic characterization of rhizobial bacteria nodulating Argyrolobium uniflorum in Tunisian arid soils. Can. J. Microbiol. 2008;54:209–217. doi: 10.1139/W07-131. [DOI] [PubMed] [Google Scholar]
- 40.Squartini A., Struffi P., Döring H., Selenska-Pobell S., Tola E., Giacomini A., Vendramin E., Velázquez E., Mateos P.F., Martinez-Molina E., et al. Rhizobium sullae sp. nov. (formerly ‘Rhizobium hedysari’), the root-nodule microsymbiont of Hedysarum coronarium L. Int. J. Syst. Evol. Microbiol. 2002;52:1267–1276. doi: 10.1099/00207713-52-4-1267. [DOI] [PubMed] [Google Scholar]
- 41.Khalid R., Zhang Y.J., Ali S., Sui X.H., Zhang X.X., Amara U., Chen W., Hayat R. Rhizobium pakistanensis sp. nov.; isolated from groundnut (Arachis hypogaea) nodules grown in rainfed Pothwar, Pakistan. Antonie Van Leeuwenhoek. 2014;107:281–290. doi: 10.1007/s10482-014-0326-x. [DOI] [PubMed] [Google Scholar]
- 42.Fterich A., Mahdhi M., Lafuente A., Pajuelo E., Caviedes M.A., Rodriguez-Llorente I.D., Mars M. Taxonomic and symbiotic diversity of bacteria isolated from nodules of Acacia tortilis subsp. raddiana in arid soils of Tunisia. Can. J. Microbiol. 2012;58:738–751. doi: 10.1139/w2012-048. [DOI] [PubMed] [Google Scholar]
- 43.Mahdhi M., Houidheg N., Mahmoudi N., Msaddak A., Rejili M., Mars M. Characterization of rhizobial bacteria nodulating Astragalus corrugatus and Hippocrepis areolata in Tunisian Arid Soils. Polish J. Microbiol. 2016;65:331–339. doi: 10.5604/17331331.1215612. [DOI] [PubMed] [Google Scholar]
- 44.Mantelin S., Fischer-Le Saux M., Zakhia F., Béna G., Bonneau S., Jeder H., de Lajudie P., Cleyet-Marel J.C. Emended description of the genus Phyllobacterium and description of four novel species associated with plant roots: Phyllobacterium bourgognense sp. nov.; Phyllobacterium ifriqiyense sp. nov.; Phyllobacterium leguminum sp. nov. and Phyllobacterium brassicacearum sp. nov. Int. J. Syst. Evol. Microbiol. 2006;56:827–839. doi: 10.1099/ijs.0.63911-0. [DOI] [PubMed] [Google Scholar]
- 45.Mhamdi R., Mrabet M., Laguerre G., Tiwari R., Aouani M.E. Colonization of Phaseolus vulgaris nodules by Agrobacterium-like strains. Can. J. Microbiol. 2005;51:105–111. doi: 10.1139/w04-120. [DOI] [PubMed] [Google Scholar]
- 46.Mrabet M., Mnasri B., Ben Romdhane S., Laguerre G., Aouani M.E., Mhamdi R. Agrobacterium strains isolated from root nodules of common bean specifically reduce nodulation by Rhizobium gallicum. FEMS Microbiol. Ecol. 2006;56:304–309. doi: 10.1111/j.1574-6941.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 47.Kalita M., Stepkowski T., Lotocka B., Malek W. Phylogeny of nodulation genes and symbiotic properties of Genista tinctoria bradyrhizobia. Arch. Microbiol. 2006;186:87–97. doi: 10.1007/s00203-006-0124-6. [DOI] [PubMed] [Google Scholar]
- 48.Msaddak A., Durán D., Rejili M., Mars M., Ruiz Argüeso T., Imperial J., Palacios J., Rey L. Diverse bacteria affiliated with the genera Microvirga, Phyllobacterium and Bradyrhizobium nodulate Lupinus micranthus growing in soils of Northern Tunisia. Appl. Environ. Microb. 2017;83:e02820-16. doi: 10.1128/AEM.02820-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stępkowski T., Banasiewicz J., Granada C., Andrews M., Passaglia L. Phylogeny and phylogeography of rhizobial symbionts nodulating legumes of the tribe Genisteae. Genes. 2018;9:163. doi: 10.3390/genes9030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinuesa P., León-Barrios M., Silva C., Willems A., Jarabo-Lorenzo A., Pérez-Galdona R., Werner D., Martínez-Romero E. Bradyrhizobium canariense sp. nov.; an acid-tolerant endosymbiont that nodulates endemic genistoid legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum bv. genistearum, Bradyrhizobium genospecies alpha and Bradyrhizobium genospecies beta. Int. J. Syst. Evol. Microbiol. 2005;55:569–575. doi: 10.1099/ijs.0.63292-0. [DOI] [PubMed] [Google Scholar]
- 51.Chaïch K., Bekki A., Bouras N., Holtz M.D., Soussou S., Mauré L., Brunel B., de Lajudie P., Cleyet-Marel J.-C. Rhizobial diversity associated with the spontaneous legume Genista saharae in the northeastern Algerian Sahara. Symbiosis. 2016;71:111–120. doi: 10.1007/s13199-016-0414-y. [DOI] [Google Scholar]
- 52.Ruiz-Díez B., Fajardo S., Puertas-Mejía M.A., de Felipe Mdel R., Fernández-Pascual M. Stress tolerance, genetic analysis and symbiotic properties of root-nodulating bacteria isolated from Mediterranean leguminous shrubs in Central Spain. Arch. Microbiol. 2009;191:35–46. doi: 10.1007/s00203-008-0426-y. [DOI] [PubMed] [Google Scholar]
- 53.Laguerre G., Nour S.M., Macheret V., Sanjuan J., Drouin P., Amarger N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology. 2001;147:981–993. doi: 10.1099/00221287-147-4-981. [DOI] [PubMed] [Google Scholar]
- 54.Jacovo E.D., Valentine T.A., Maluk M., Toorop P., Lopez del Egido L., Frachon N., Iannetta P.P.M. Towards a characterisation of the wild legume bitter vetch (Lathyrus linifolius L. (Reichard) Bässler): Heteromorphic seed germination, root nodule structure and N-fixing rhizobial symbionts. Plant Biol. 2019;21:523–532. doi: 10.1111/plb.12902. [DOI] [PubMed] [Google Scholar]
- 55.Wei G.H., Wang E.T., Tan Z.Y., Zhu M.E., Chen W.X. Rhizobium indigoferae sp. nov. and Sinorhizobium kummerowiae sp. nov.; respectively isolated from Indigofera spp. and Kummerowia stipulacea. Int. J. Syst. Evol. Microbiol. 2002;52:2231–2239. doi: 10.1099/00207713-52-6-2231. [DOI] [PubMed] [Google Scholar]
- 56.Nick G., de Lajudie P., Eardly B.D., Suomalainen S., Paulin L., Zhang X., Gillis M., Lindström K. Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov.; isolated from leguminous trees in Sudan and Kenya. Int. J. Syst. Bacteriol. 1999;49:1359–1368. doi: 10.1099/00207713-49-4-1359. [DOI] [PubMed] [Google Scholar]
- 57.Tounsi-Hammami S., Le Roux C., Dhane-Fitouri S., de Lajudie P., Duponnois R., Jeddi F.B. Genetic diversity of rhizobia associated with root nodules of white lupin (Lupinus albus L.) in Tunisian calcareous soils. Syst. Appl. Microbiol. 2019;42:448–456. doi: 10.1016/j.syapm.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Zinga M.K., Jaiswal S.K., Dakora F.D. Presence of diverse rhizobial communities responsible for nodulation of common bean (Phaseolus vulgaris) in South African and Mozambican soils. FEMS Microbiol. Ecol. 2016;93:fiw236. doi: 10.1093/femsec/fiw236. [DOI] [PubMed] [Google Scholar]
- 59.Hoque M.S., Broadhurst L.M., Thrall P.H. Genetic characterization of root-nodule bacteria associated with Acacia salicina and A. stenophylla (Mimosaceae) across south-eastern Australia. Int. J. Syst. Evol. Microbiol. 2011;61:299–309. doi: 10.1099/ijs.0.021014-0. [DOI] [PubMed] [Google Scholar]
- 60.Aserse A.A., Räsänen L.A., Aseffa F., Hailemariam A., Lindström K. Diversity of sporadic symbionts and nonsymbiotic endophytic bacteria isolated from nodules of woody, shrub, and food legumes in Ethiopia. Appl. Microbiol. Biotechnol. 2013;97:10117–10134. doi: 10.1007/s00253-013-5248-4. [DOI] [PubMed] [Google Scholar]
- 61.Glick B.R. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007;26:227–242. doi: 10.1080/07352680701572966. [DOI] [Google Scholar]
- 62.Van de Poel B., Smet D., Van Der Straeten D. Ethylene and hormonal crosstalk in vegetative growth and development. Plant Physiol. 2015;169:61–72. doi: 10.1104/pp.15.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finch-Savage W.E., Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 64.Johnson P.R., Ecker J.R. The ethylene gas signal transduction pathway: A Molecular Perspective. Annu. Rev. Gen. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- 65.Shaharoona B., Arshad M., Zahir Z.A. Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.) Lett. Appl. Microbiol. 2005;42:155–159. doi: 10.1111/j.1472-765X.2005.01827.x. [DOI] [PubMed] [Google Scholar]
- 66.Honma M., Shimomura T. Metabolism of 1-aminocyclopropane- 1-carboxylic acid. Agric. Biol. Chem. 1978;42:1825–1831. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.