Abstract
In this work, the antibacterial activity of deflazacort and several of its synthetic precursors was tested against a panel of bacterial pathogens responsible for most drug-resistant infections including Staphylococcus aureus, Enterococcus spp., Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. The derivative of deflazacort, PYED-1 (pregnadiene-11-hydroxy-16α,17α-epoxy-3,20-dione-1) showed the best antibacterial activity in a dose-dependent way. We focused on the action of PYED-1 against S. aureus cells. PYED-1 exhibited an additive antimicrobial effect with gentamicin and oxacillin against the methicillin-resistant S. aureus isolate 00717. In addition to its antimicrobial effect, PYED-1 was found to repress the expression of several virulence factors of S. aureus, including toxins encoded by the hla (alpha-haemolysin), hlb (beta-haemolysin), lukE-D (leucotoxins E-D), and sea (staphylococcal enterotoxin A) genes, and cell surface factors (fnbB (fibronectin-binding protein B) and capC (capsule biosynthesis protein C)). The expression levels of autolysin isaA (immunodominant staphylococcal antigen) were also increased.
Keywords: antimicrobial activity, Staphylococcus aureus, steroids, deflazacort (DFZ), multidrug pathogens, anti-virulence agent, quantitative real-time PCR, checkerboard assay
1. Introduction
Hospital-acquired infections represent a major problem in high or upper-middle income countries, with an incidence rate of 5% in the United States and 7.1% in Europe [1]. The therapeutic options for infections caused by multidrug-resistant (MDR) pathogens are often extremely limited. Despite intensive searches for new antimicrobial agents, there are, to date, a few active candidates [2], and new antibacterial substances acting through non-conventional mechanisms are needed [3].
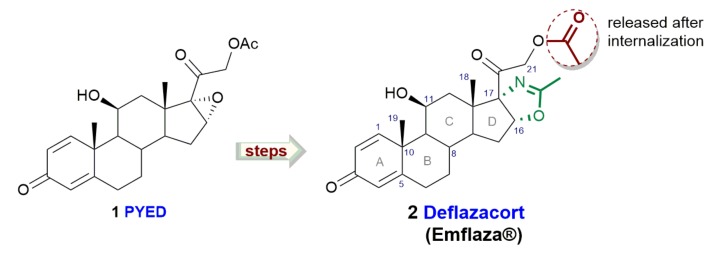
Steroids, a family of naturally-occurring secondary metabolites characterized by a four-fused ring structure, have exhibited a wide range of biological activities and have been shown to function as antitumoral, antiviral, antibacterial, and antioxidant agents [4]. The heterocyclic corticosteroid deflazacort (DFZ, Figure 1), an oxazoline-derivative of prednisolone, has been recently approved for the treatment of Duchenne dystrophy [5] showing high efficacy and good tolerability.
Figure 1.
Deflazacort and its synthetic precursor PYED-1 (pregnadiene-11-hydroxy-16α,17α-epoxy-3,20-dione-1).
In the frame of a study devoted to the development of a novel and convenient synthetic strategy for the preparation of DFZ, we recently found that a DFZ synthetic precursor called PYED-1 (pregnadiene-11-hydroxy-16α,17α-epoxy-3,20-dione-1), exhibited a good antibacterial activity against Staphylococcus aureus ATCC 29213 and Acinetobacter baumannii ATCC 17978 without showing cytotoxicity [6]. In addition, we demonstrated that PYED-1 has a weak effect against Stenotrophomonas maltophilia clinical isolates but at sub-inhibitory concentrations it inhibits biofilm formation of the reference S. maltophilia K279a strain [7].
Here, an improved protocol for the synthesis of PYED-1 has been reported, along with the evaluation of the in vitro antimicrobial activity of PYED-1 and some steroidal synthetic precursors and analogues of DFZ against a panel of bacterial pathogens responsible for most drug-resistant infections, including Staphylococcus aureus, Enterococcus spp., Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. We focused our attention on the activity of PYED-1 against S. aureus. This microorganism causes a variety of infections that range from topical to life-threatening infections and represents a major problem in both the hospital and community settings [8,9]. S. aureus has rapidly-acquired resistance to many antibiotic drug classes, causing infections that are difficult to eradicate [10]. Additive antimicrobial activity with conventional antibiotics against S. aureus cells by checkerboard microdilution method was assessed. Finally, real-time PCR was performed to evaluate the effect of PYED-1 on S. aureus genes involved in the virulence factor production.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals and solvents were purchased with the highest degree of purity (Sigma-Aldrich, Alfa Aesar, VWR) and used without further purification. The reactions were monitored by TLC (precoated silica gel plate F254, Merck) and the products were detected by exposure to ultraviolet radiation, iodine vapor, and chromic mixture. The purity of the compounds was determined by CHNS analysis and was ≥95% in all cases. NMR spectra were recorded on NMR spectrometers operating at 400 MHz (Bruker DRX, Bruker AVANCE) using CDCl3 solutions. Coupling constant values (J) were reported in Hz. Chemical synthesis and structural characterization of compounds 3 to 7 has been realized as previously reported [6].
2-((4aS,4bR,5S,6aS,6bS,7aR)-4b-bromo-5-hydroxy-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7a,8,8a,8b,9,10-dodecahydro-6bH-naphtho [2’,1’:4,5]indeno[1-b]oxiren-6b-yl)-2-oxoethyl acetate (compound 10). Phthalic anhydride (0.13 g, 0.86 mmol) was added in one portion to a stirred solution of compound 9 (provided by Symbiotec Pharmalab PVT) in dichloromethane (DCM) 2.0 mL at room temperature. After few minutes, phthalic anhydride was completely dissolved to give a yellow clear solution. At the same temperature 50% aqueous H2O2 was then added dropwise in 2h and the mixture was then heated to reflux temperature, until formation of a white precipitate was observed. The reaction mixture was stirred at the in-refluxing DCM for 24 h. Water and solid sodium bicarbonate (NaHCO3) were added to the cooled reaction mixture until pH 7 was reached, and then the mixture extracted with dichloromethane (CH2Cl2). The organic layer was washed with brine while the aqueous phase was again extracted with chloroform/methanol (CHCl3/MeOH, 9/1). The resulting organic layers were washed with brine. All the organic phases were combined, dried with sodium sulfate anhydrous (Na2SO4) and concentrated under reduced pressure to give a yellow solid. Ethyl acetate was added to the crude residue. The resulting precipitate was decanted, and the orange mother liquors removed. This operation was repeated until a white powder was obtained. All the mother liquors previously obtained were combined and concentrated. The resulting precipitate was decanted, separated from the mother liquors and repeatedly washed with ethyl acetate until to obtain epoxide derivative 10 as a white powder (85 mg, 82%yield). 1H NMR (400 MHz): δ 1.41 (s, 3H, H-18), 1.48-1.55 (m, 1H, H-14), 1.70 (s, 3H, H-19), 1.71-1.82 (m, 2H, H-7), 1.94-2.05 (m, 3H, H-12a, H-15), 2.15 (s, 3H, H-23), 2.16–2.25 (m, 1H, H-8), 2.36–2.49 (m, 2H, H-12b, H-6a), 2.55–2.68 (m, 1H, H-6b), 3.85 (s, 1H, H-16), 4.59 (d, J = 13.4, 1H, H-21a), 4.67 (d, J = 13.4, 1H, H-21b), 4.76 (bs, 1H, H-11), 6.07 (bs, 1H, H-4), 6.32 (d, J = 10.1, 1H, H-2), 7.21 (d, J = 10.1, 1H, H-1). 13C NMR (100 MHz): 18.3, 20.4, 24.9, 27.0, 28.3, 30.4, 33.5, 37.3, 39.3, 42.1, 50.2, 61.1, 65.7, 70.5, 75.9, 85.3, 125.1, 129.3, 152.3, 165.5, 170.4, 186.2, and 198.9. Anal. calcd for C23H27BrO6: C, 57.63; H, 5.68; Br, and 16.67. Found: C, 57.75; H, 5.66; and Br, 16.62.
2-((4aR,5S,6aS,6bS,7aR)-5-hydroxy-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7a,8,8a,8b,9,10-dodecahydro-6bH-naphtho[2’,1’:4,5]indeno[1,2-b]oxiren-6b-yl)-2-oxoethyl acetate (PYED-1) was prepared from compound 10 by treatment with tributyltin hydride (Bu3SnH) and azobisisobutyronitrile (AIBN) according to the procedure previously reported [6].
2.2. Biological Screening
2.2.1. Bacterial Strains and Growth Conditions
Bacterial clinical isolates belong to a collection previously established at the Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II. In accordance with European regulations, each isolate was associated with a unique ID and subsequently anonymized. Epidemiological features of strains were in accordance to previous publications [11,12,13,14]. No ethical approval was required for the study because there was no access to patient data. Antimicrobial susceptibility to conventional antibiotics of all isolates was determined using the VITEK 2 system (BioMérieux) and/or the Phoenix microbiology system (BD Diagnostics) and the results were interpreted in accordance with the European Committee on Antimicrobial Susceptibility Testing [15] guidelines. The minimal inhibitory concentration (MIC) of colistin of A. baumannii isolates was determined by broth microdilution assay according to Clinical Laboratory Standards Institute guidelines [16]. The MIC of oxacillin was determined by broth microdilution method in cation-adjusted Mueller–Hinton broth (CA-MHB) supplemented with 2% NaCl. The plates were incubated at 35 °C and the MIC was determined after 24 and 48 h of incubation. The presence of the staphylococcal cassette chromosome mec (SCCmec) in methicillin-resistant S. aureus (MRSA) isolates was assessed using the Xpert MRSA kit (Cepheid, Sunnyvale, CA). The kit contains primers and probes for the detection of the spa, mecA, and SCCmec sequences inserted into the SA chromosomal attB site. The Xpert MRSA assay was performed using a GeneXpert Dx system and the analysis was performed as per the manufacturer’s protocol.
2.2.2. Antimicrobial Activity of Steroidal Compounds
MIC values of steroidal compounds against planktonic bacteria were examined by a broth microdilution method previously described [17]. Briefly, compounds were dissolved in dimethyl sulfoxide (DMSO) to the concentration of 50 mg/mL. Two-fold serial dilutions ranging from 2 μg/mL to 1000 mg/mL of the compounds were prepared in triplicate and placed into a polystyrene 96-well plate. Bacterial cell suspensions were prepared at 0.5 McFarland standard using BD PhoenixSpec™ nephelometer and were subsequently diluted in cation-adjusted Mueller–Hinton broth (CA-MHB) to final culture density of approximately 5 × 106 colony forming unit (CFU)/mL. One hundred microliter of bacteria (5 x 105 CFU) were then added to the microtiter plates containing 100 μL of serial dilutions of steroidal compounds. Only CA-MHB was added in negative control wells. Wells with no compounds were used on each plate as positive growth control. Plates were incubated at 37 °C for 18–24 h under shaking (300 rpm). To evaluate microbial growth, the optical density at 595 nm was measured by using a microplate reader (Bio-Rad Laboratories S.r.l.). The effects of DMSO concentrations, ranging from 0.1% to 1%, on bacteria growth kinetics were separately tested. To calculate the minimum bactericidal concentration (MBC), bacterial suspensions from MIC assay microtiter wells were diluted in PBS and spot-plated on TSA plates to count colonies after incubation at 37 °C for 18 h. The MBC was determined as the lowest concentration of substance, which produced ≥99.9% killing (≥3log10) after 24 h of incubation as compared to the colony count of the starting inoculum. All tests were performed in triplicate and repeated three times.
2.2.3. Time Kill Assay
The killing kinetics of PYED-1 at 1 x, 2 x, and 4 x MIC were determined against S. aureus ATCC 29213 strain, as previously described [18]. Tubes containing CA-MHB with different concentrations of PYED-1 were inoculated with S. aureus ATCC 29213 to a density of approximately 5 x 106 CFU/mL and incubated at 37 °C under shaking (300 rpm). The tube without PYED-1 was a growth control. Viable bacterial counts were performed after 0, 1, 2, 3, 4, 5, 7, and 24 h incubation by plating serial 10-fold dilutions of broth cultures onto TSA plates.
2.2.4. Checkerboard Assay
A checkerboard method in 96-well microtiter plates containing CA-MHB was used to test the effects of the interactions of PYED-1 with either oxacillin or gentamicin against methicillin-resistant S. aureus (MRSA). In brief, PYED-1 and each tested antibiotic were serially diluted in microtiter plate wells along the y and x axes. Checkerboard plates with a concentration of 106 CFU/mL of the tested bacteria were incubated overnight at 37 °C. To evaluate microbial growth, the optical density at 595 nm was measured by using a microplate reader (Bio-Rad Laboratories S.r.l.). The effect of the interactions of PYED-1 with each of the tested antibiotics was quantified by calculating the fractional inhibitory concentration (FIC) index as follows: FIC index = FIC of PYED-1 + FIC of antibiotic, where FIC of PYED-1 (or antibiotic) is the ratio of MIC of PYED-1 (or antibiotic) in combination and MIC of PYED-1 (or antibiotic) alone. The following intervals of FIC index were used to interpret the experimental outcomes: ≤0.5, synergistic; >0.5 to ≤1.0, additive; >1.0 to ≤2.0, indifferent; and >2.0, antagonistic effects [19]. All experiments were repeated three times.
2.2.5. RNA
Total RNA was isolated from S. aureus ATCC 29213 cells grown overnight in CA-MHB were diluted to an OD600 of 0.05 and grown at 37 °C at 200 rpm to an OD600 of 0.3–0.4. The culture was subsequently split into two tubes, treated with either PYED-1 at the concentration of 4 μg/mL or 0.008% DMSO, and incubated at 37 °C at 200 rpm for a further 3 h. Two volumes of RNAprotect Bacteria Reagent was added to the cell suspensions and incubated for 5 min at room temperature. Next, the cell suspensions were centrifuged at 5000× g for 10 min and the supernatant was decanted. RNA was purified according to the previously-reported method [20] with minor modifications. Bacterial cells were resuspended in 200 μL of 20 mg/mL proteinase K and 200 μL TE buffer (30 mM Tris HCl, 1 mM EDTA, pH 8.0) containing 20 mg/mL lysozyme and 12.5 μg/mL lysostaphin and incubated at 37 °C for 1 h. The resulting protoplasts were used for RNA isolation with the RNeasy Mini Kit, following recommendation of the manufacturer (Qiagen, Germany). Residual DNA were removed with DNase Max Kit (Qiagen). RNA was quantified using a Nano-drop instrument (Thermo Fisher).
2.2.6. RT-PCR
Total RNA was reverse transcribed into cDNA using QuantiTect Reverse Transcription Kit (Qiagen), according to the manufacturer’s protocol. The RT-PCR was performed as previously described [13], using a SYBR Green master mix (Applied Biosystems). The primer pairs used in PCR experiments are reported in Table 1.
Table 1.
Gene target list and oligonucleotide sequences.
| Gene | Primer Sequence |
|---|---|
| agrA Fw | TGCGAAGACGATCCAAAAC |
| agrA Rv | TTTAGCTTGCTCAAGCACCTC |
| aur Fw | GATGGTCGCACATTCACAAG |
| aur Rv | CGCCTGACTGGTCCTTATATTC |
| capC Fw | CATCCAGAGCGGAATAAAGC |
| capC Rv | CGGAAATACCCGCTAATGAC |
| clfB Fw | TTATGGTGGTGGAAGTGCTG |
| clfB Rv | TGGACTTGGTTCTGGATCTG |
| fnbB Fw | GAACATGGTCAAGCACAAGG |
| fnbB Rv | ACGCCATAATTACCGTGACC |
| hla Fw | TCTTGGAACCCGGTATATGG |
| hla Rv | AGCGAAGTCTGGTGAAAACC |
| hlb Fw | GTGCCAAAGCCGAATCTAAG |
| hlb Rv | ATCAGCGCGTTTATATTGTCC |
| isaA Fw | TCCGACAAACACTGTTGACC |
| isaA Rv | AATCCCCAAGCACCTAAACC |
| lytM Fw | ACGGTGTCGACTATGCAATG |
| lytM Rv | ATTGCCGCCACCATAGTTAC |
| lukD Fw | GTACTTAAGGCAGCCGGAAAC |
| lukD Rv | CGCCCCAATAAAACTGTGAG |
| lukE Fw | ATGGGGTGTTAAAGCAAACG |
| lukE Rv | TCTCTTGCTGAACCTGTTGG |
| rpoB Fw | ACAACCACTTGGCGGTAAAG |
| rpoB Rv | ATGCTTCAAGTGCCCATACC |
| saeR Fw | CCAAGGGAACTCGTTTTACG |
| saeR Rv | ACGCATAGGGACTTCGTGAC |
| sea Fw | ATTGCCCTAACGTGGACAAC |
| sea Rv | TGCTCCCTGCAATTCAGAC |
| sigB Fw | TGATCGCGAACGAGAAATC |
| sigB Rv | ATTGCCGTTCTCTGAAGTCG |
| spa Fw | AGATGACCCAAGCCAAAGTG |
| spa Rv | CTTTCGGTGCTTGAGATTCATT |
The rpoB gene was used as the housekeeping control to normalize the expressions of genes of interest. RNA samples not treated with reverse transcriptase were routinely included as no template controls. Changes in transcript levels were determined using the 2−ΔΔCT method [21]. RNA expression levels were determined by using three independent cultures and all analyses were performed in triplicate.
2.2.7. Statistical Analysis
Each experiment was performed in triplicate and repeated at least three time on different days. Arithmetic means and standard deviations were used to statistically analyze continuous variables. Student’s t test was used to determine statistical differences between two means.
3. Results
3.1. Chemistry
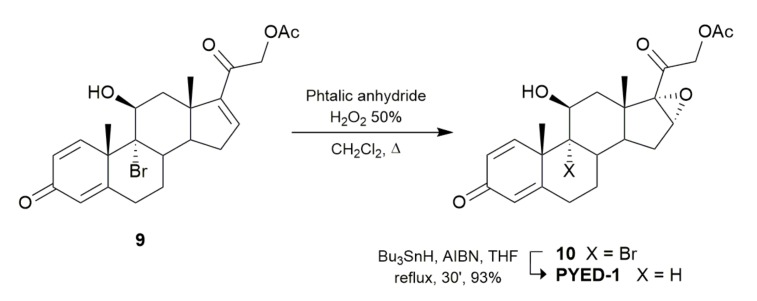
To deeply study the biological potential of PYED-1, an improved protocol for its preparation was first designed (Figure 2).
Figure 2.
Synthetic transformations from brominated compound 9 to epoxide PYED-1.
As previously described [6], C16–C17 double bond oxidation of 9-bromotriene acetate (compound 9) with meta-chloroperbenzoic acid (mCPBA) led to the corresponding PYED-1, albeit with uncomplete conversion (47% yield). With the aim to improve the reaction yield, the use of a different organic peroxy acid, i.e., monoperphthalic acid, was investigated [22]. As shown in Figure 2, treatment of compound 9 with phthalic anhydride in the presence of 50% of aqueous H2O2 in refluxing DCM gave, after 24 h, the corresponding brominated epoxide (compound 10) in better yields (82%). Debromination of this latter was then accomplished by treatment with Bu3SnH and AIBN in refluxing tetrahydrofuran (THF) affording the pure PYED-1 in 93% yield.
3.2. Antimicrobial Activity of a Panel of Steroid Derivatives
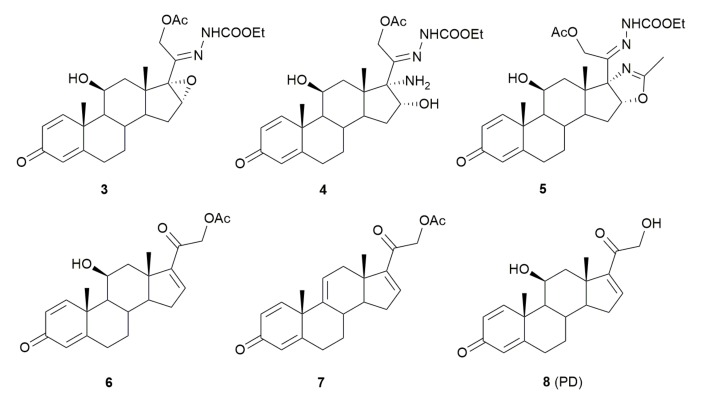
PYED-1 was shown to be effective against both S. aureus and A. baumannii [6]. Here, we have chosen to extend the antimicrobial activity study of DFZ and its precursors (Figure 3) against a panel of bacterial pathogens including S. aureus, Enterococcus spp., A. baumannii, P. aeruginosa, K. pneumoniae, E. coli, and Enterobacter spp.
Figure 3.
Deflazacort (DFZ) glucocorticoid precursors and analogues.
The MIC of all steroid derivatives was determined by broth microdilution assay. The antibacterial activity of steroid derivatives against selected bacteria varied significantly, MIC values ranging from 4 to 750 μg/mL (Table 2).
Table 2.
Minimum inhibitory concentration (MIC) (μg/mL) and minimum bactericidal concentration (MBC) (μg/mL) values of PD, DFZ and its precursors against a panel of Gram-negative and Gram-positive pathogens.
| Bacteria | Compound | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PYED-1 | 2 (DFZ) | 3 | 4 | 5 | 6 | 7 | 8 (PD) | |||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
|
Staphylococcus aureus ATCC 29213 |
16 | 16 | >1000 | >1000 | 375 | >1000 | >1000 | >1000 | 750 | >1000 | 256 | 512 | 1000 | 1000 | >1000 | >1000 |
|
Enterococcus faecalis ATCC 29212 |
4 | 16 | >1000 | >1000 | 187 | >1000 | >1000 | >1000 | 375 | >1000 | 128 | 256 | 1000 | 1000 | >1000 | >1000 |
|
Acinetobacter baumannii ATCC 17978 |
16 | 16 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 512 | 512 | 1000 | 1000 | >1000 | >1000 |
|
Pseudomonas aeruginosa ATCC 27859 |
128 | 1000 | >1000 | >1000 | 750 | >1000 | >1000 | >1000 | >1000 | >1000 | 1000 | 1000 | 1000 | 1000 | >1000 | >1000 |
|
Escherichia coli ATCC 25922 |
128 | 128 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 1000 | 1000 | 1000 | 1000 | >1000 | >1000 |
|
Klebsiella pneumoniae ATCC 700603 |
>1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
|
Enterobacter aerogenes ATCC 13048 |
>1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
PYED-1 proved to be the most effective growth inhibitor, with 4 and 16 μg/mL MIC values against Enterococcus faecalis and S. aureus respectively, instead shown a 16 μg/mL MIC values against A. baumannii and 128 μg/mL against both E. coli and Pseudomonas aeruginosa. No antimicrobial activity was found for DFZ and compound 4. The intermediates 3 and 5 showed only a weak antimicrobial activity against E. faecalis and S. aureus, and only the compound 3 showed a weak antimicrobial activity against P. aeruginosa. All compounds did not exert any appreciable inhibition of the other Gram-negative bacteria tested, K. pneumoniae and E. aerogenes, up to the concentration of 1000 mg/mL. To examine whether the inhibition of bacterial growth could be related to the DMSO used to dissolve the compounds tested, the bacterial growth was measured in the presence of increasing concentrations of DMSO. No growth differences were observed in the presence of any of the DMSO concentrations used (data not shown).
With the aim to identify which functionalities could be responsible for the antimicrobial activity of PYED-1 we evaluated the biological potential of compounds 6, 7, and 8, which share common residues with PYED-1. Compound 6, in which the epoxy function at C16–C17 positions was replaced by a double bond (Figure 3), exhibited antibacterial activity against S. aureus, E. faecalis, A. baumannii, P. aeruginosa, and E. coli strains, but at much higher concentration than PYED-1 (Table 2). In contrast, both compound 7, in which the hydroxyl function at C10 position is absent, and compound 8 (PD) in which the acetyl group at 21 hydroxyl function is missing, functioned only at concentration of 1 mg/mL, or more (Table 2). Data directly imply that the epoxy function on C16–C17 positions of the steroidal scaffold, the C10 hydroxyl group, and the acetyl residue are all crucial for the antimicrobial activity exhibited by PYED-1.
To test whether PYED-1 was also active against clinical strains, we selected for each tested species 8 to 11 clinical isolates exhibiting different antibiotic resistance profiles. For all species, the results obtained with clinical isolates were comparable to those obtained using PYED-1 against the reference ATCC strain (Table 3).
Table 3.
MIC (μg/mL) and MBC (μg/mL) values of PYED-1 on a panel of Gram-negative and Gram-positive multidrug pathogens.
| Bacteria | MIC | MBC | Bacteria | MIC | MBC |
|---|---|---|---|---|---|
| S. aureus ATCC 29213 | 16 | 16 | A. baumannii ATCC 17978 | 16 | 16 |
| S. aureus 00717 | 16 | 16 | A. baumannii AYE | 16 | 16 |
| S. aureus 00142 | 16 | 16 | A. baumannii 60794 | 32 | 32 |
| S. aureus 90356 | 16 | 32 | A. baumannii 30031 | 32 | 32 |
| S. aureus 90159 | 32 | 32 | A. baumannii 30032 | 16 | 16 |
| S. aureus 90319 | 16 | 16 | A. baumannii 90407 | 32 | 64 |
| S. aureus 00709 | 32 | 32 | A. baumannii 70120 | 32 | 64 |
| S. aureus 90623 | 16 | 16 | A. baumannii 62258 | 16 | 16 |
| S. aureus 90246 | 32 | 32 | A. baumannii 3909 | 32 | 32 |
| S. aureus 61035 | 16 | 16 | A. baumannii 3990 | 32 | 16 |
| S. aureus 61486 | 8 | 16 | A. baumannii 4190 | 16 | 32 |
| S. aureus 64428 | 16 | 16 | A. baumannii NM3 | 16 | 32 |
| E. faecalis ATCC 29212 | 4 | 16 | E. coli ATCC 25922 | 128 | 128 |
| E. faecalis 90709 | 8 | 16 | E. coli 90117 | 128 | 128 |
| E. faecalis 30818 | 8 | 16 | E. coli 01283 | 128 | 512 |
| E. faecium 90725 | 8 | 16 | E. coli 01029 | 256 | 1000 |
| E. faecium 90122 | 8 | 16 | E. coli 66570 | 128 | 512 |
| E. faecalis 90842 | 8 | 16 | E. coli 91947 | 256 | 1000 |
| E. faecalis 90837 | 8 | 16 | E. coli 90050 | 256 | 1000 |
| E. faecium 62179 | 8 | 32 | E. coli 65964 | 128 | 256 |
| E. faecium 63131 | 8 | 16 | E. coli 65026 | 128 | 512 |
| P. aeruginosa ATCC 27859 | 128 | 1000 | |||
| P. aeruginosa 01018 | 128 | 1000 | |||
| P. aeruginosa 01493 | 128 | 1000 | |||
| P. aeruginosa 00865 | 64 | 1000 | |||
| P. aeruginosa 91631 | 256 | >1000 | |||
| P. aeruginosa RP73 | 64 | 1000 | |||
| P. aeruginosa 66148 | 128 | 1000 | |||
| P. aeruginosa 66149 | 128 | 1000 | |||
| P. aeruginosa 00445 | 256 | >1000 | |||
| P. aeruginosa 00173 | 256 | >1000 |
3.3. Activity of PYED-1 against S. aureus
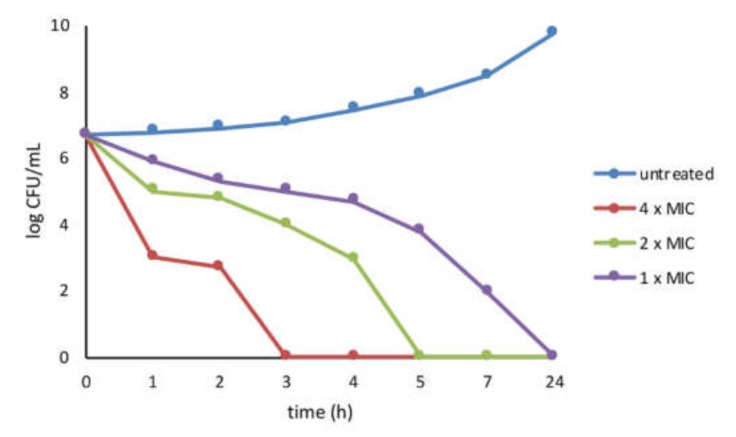
We focused our attention to the inhibitory effect of PYED-1 against S. aureus. The inhibition of bacterial growth by PYED-1 was characterized by means of time–kill assays for the S. aureus ATCC 29213. S. aureus cells treated with PYED-1 at 4 x MIC (64 μg/mL) and 2 x MIC (32 μg/mL) were killed at 3 and 5 h, respectively. After 7 h exposure to 1 x MIC (16 μg/mL), the amount of S. aureus cells dropped to 2 × 10 CFU/mL. After 24 h, there was a total growth inhibition (Figure 4).
Figure 4.
Killing kinetics for S. aureus following treatment with the PYED-1. Growth kinetics were monitored following exposure to PYED-1 at 1 x MIC, 2 x MIC, and 4 x MIC.
Antibiotic combination therapy is frequently used as a possible method of outmaneuvering recalcitrant bacterial pathogens, to improve the antibiotic efficiency as well as to reduce antibiotic doses used [23]. Synergy experiments using combinations of PYED-1 with conventional antibiotics were conducted in order to potentiate or restore the antibacterial activity of currently available antibiotics against S. aureus, a pathogen that is difficult to treat due to the rising number of drug- resistant strains. PYED-1 antibacterial activity was tested in combination with gentamicin and oxacillin against MRSA 00717, an isolate resistant to the selected antibiotics. The MIC value of PYED-1 was 16 μg/mL while the MIC value of gentamicin and oxacillin was 256 μg/mL and 16 μg/mL, respectively. In the checkerboard dilution test, PYED-1 markedly lowered the MICs of gentamicin (from 256 to 16 μg/mL) and oxacillin (from 16 to 1 μg/mL) against the MRSA 00717 isolate. The combination of PYED-1 with either gentamicin or oxacillin had an additive effect, as the FIC values were 0.5625 and 0.5156, respectively (Table 4).
Table 4.
Additive effects of the compound PYED-1 with antibiotics against S. aureus.
| Bacterial Strain | Combination | MICa (μg/mL) | MICc (μg/mL) | FIC |
|---|---|---|---|---|
| S. aureus 00717 | PYED-1/gentamicin | 16/256 | 8/16 | 0.5625 |
| PYED-1/oxacillin | 16/128 | 8/2 | 0.5156 |
MICa, MIC of one sample alone; MICc, MIC of samples in combination; FIC, fractional inhibitory concentration.
3.4. Transcriptional Changes Induced by PYED-1 in S. aureus
To investigate the anti-virulence activity of PYED-1 the expression levels of known S. aureus virulence and regulatory genes [24] were investigated by qRT-PCR (Table 5).
Table 5.
RT-PCR analysis of virulence factors gene expression in S. aureus ATCC 29,213 in the presence of PYED-1.
| Gene | Description | Fold Changea ± SD | p Value |
|---|---|---|---|
| capC | Capsule biosynthesis protein C | –2.95 ± 0.13 | 0.0153 |
| fnbB | Fibronectin-binding protein B | –2.11 ± 0.04 | 0.0139 |
| clfB | Clumping factor B | +1.44 ± 0.25 | 0.1988 |
| spa | Surface protein A | –1.51 ± 0.02 | 0.0213 |
| lytM | Peptidoglycan hydrolase | +1.39 ± 0.32 | 0.2177 |
| aur | Aureolysin, zinc metalloproteinase | –1.38 ± 0.22 | 0.0765 |
| isaA | Immunodominant staphylococcal antigen | +4.18 ± 1.24 | 0.0022 |
| hla | Alpha-haemolysin | –10.17 ± 0.02 | 0.0002 |
| hlb | Beta-haemolysin | –21.17 ± 0.01 | 0.0132 |
| lukD | Pore-forming leukocidin | –10.96 ± 0.01 | <0.0001 |
| lukE | Pore-forming leukocidin | –13.52 ± 0.005 | <0.0001 |
| sea | Staphylococcal enterotoxin A | –2.46 ± 0.02 | 0.0115 |
| agrA | Accessory gene regulator protein A | –2.29 ± 0.1 | 0.0146 |
| sigB | RNA polymerase sigma factor B | –2.11 ± 0.09 | 0.0006 |
| saeR | Response regulator SaeR | –8.66 ± 0.03 | <0.0001 |
a—indicates reduction and + indicates increase.
RNA was extracted from exponential S. aureus cells (5 × 108 CFU/mL) untreated and treated at sub-MIC concentration (4 μg/mL) of PYED-1 for 3 h. No growth differences between treated and untreated cells were observed. The expression of spa (surface protein A) and clfB (clumping factor B) genes was not affected, but significant downregulation of fnbB (fibronectin-binding protein B) and capC (capsule biosynthesis protein C) genes was observed. As shown in Table 5, the levels of toxin genes hla (alpha-haemolysin), hlb (beta-haemolysin) lukE-D (leucotoxins E-D), and sea (staphylococcal enterotoxin A) were notably reduced. Downregulation of the transcript levels of agrA (accessory gene regulator protein A), saeR (response regulator SaeR), and sigB (RNA polymerase sigma factor B) genes after PYED-1 treatment by a factor of 2.29, 8.66, and 2.11, respectively, was also noted. Significant upregulation of isaA (immunodominant staphylococcal antigen) gene was observed. The expression of lytM (peptidoglycan hydrolase) and aur (aureolysin) genes was not affected (Table 5).
4. Discussion
PYED-1 is a promising novel antimicrobial agent [6]. Here we extended our initial studies on the antimicrobial activity of this compound, by testing its efficacy against a panel of Gram-negative and Gram-positive pathogens, including S. aureus, Enterococcus spp., A. baumannii, P. aeruginosa, K. pneumoniae, E. coli, and Enterobacter spp. PYED-1 exerted a good inhibitory activity against E. faecalis, S. aureus, and A. baumannii, low to moderate activity against E. coli and P. aeruginosa, while it did not exert any appreciable inhibition against K. pneumoniae and E. aerogenes ( Table 2 and Table 3). A weak effect was also observed in a previous report against S. maltophilia [7].
According to the European Centre for Disease Prevention and Control, S. aureus is one of the most difficult-to-treat pathogens due to the increasing number of drug-resistant strains [10]. The time-kill curve analysis against S. aureus demonstrated a concentration-dependent rapid bactericidal activity of PYED-1 (Figure 4). In line with this, we have previously shown that PYED-1 exhibited bactericidal activity against S. maltophilia, and that the permeabilization of the bacterial membrane might contribute to the effect [7]. Our study demonstrated additivity of PYED-1/gentamicin and PYED-1/oxacillin combinations by checkerboard assay. Data also revealed that PYED-1 has the potential to restore the effectiveness of oxacillin against MRSA strains. This result is an important finding because restoring activity to conventional antibiotics using combinations would enable the clinical use of conventional antibiotics for the treatment of S. aureus infections.
Many studies suggested that drugs targeting virulence factors (e.g., enterotoxins, hemolysins, and adhesins) represent an alternative approach to treat infections caused by MDR bacteria [25]. Intriguingly, we observed that PYED-1 impinges on the expression levels of several virulence factors and regulatory genes involved in S. aureus virulence (Table 5). The pathogenicity of S. aureus depends on the production of numerous extracellular virulence factors. S. aureus produces a series of hemolysins including α- and β-hemolysins. Alpha-hemolysin is the most studied pore-forming toxin and it plays a significant role in skin and soft tissue infections in animal models of staphylococcal infection [26]. Highly virulent S. aureus strains can produce up to five different bicomponent leukotoxins, by which S. aureus targets and kills neutrophils. The pore-forming leukocidin LukE-D is a critical virulence factor involved in S. aureus bloodstream infection [27].
The regulation of virulence factors induced by an antibiotic may result in either worsening or mitigation of the infection. For diseases caused by S. aureus, the regulation of secretion of toxins, and the selection of antibiotic according to its ability to affect these properties could be very important for the outcome of S. aureus diseases. At suboptimal concentrations, linezolid and clindamycin significantly inhibits the production of α-hemolysin and staphylococcal enterotoxins (A and B). Investigation of the expression of several toxins of S. aureus in the presence of PYED-1 showed protein A [28,29]. In contrast, β-lactams and glycopeptides induce the expression of α-hemolysin, enterotoxins and toxic shock syndrome toxin-1 [30]. We found that the levels of hla, hlb lukD, lukE, and sea genes were notably reduced (Table 5). The combination of PYED-1, which downregulates toxins expression, with β-lactams antibiotics, is able to induce expression of α-hemolysin and enterotoxins at subinhibitory concentrations and may therefore be highly beneficial for the treatment of S. aureus infections.
In S. aureus, the expression of several virulence genes is regulated by two-component systems, such Agr operon and SaeRS, the DNA-binding protein SarA and the alternative sigma factor SigB. These regulators coordinately control hla expression [31,32]. SaeRS also activates the expression of the leukotoxins LukE-D [33]. We observed that treatment with PYED-1 resulted in reduced expression of agrA, saeR, and sigB genes (Table 5). This nicely explains the downregulation of the transcript levels of controlled hla, hlb, lukD, lukE, and sea genes. These results are in accord with literature data. Duan et al. [34] showed subinhibitory concentration of resveratrol decreased the expression of hla and inhibited the regulation of saeRS. Subinhibitory concentrations of thymol significantly inhibited the transcription of hla, sea, and seb in S. aureus and acted partly through the agr locus [35].
Many antibiotics can affect the expression of S. aureus surface proteins. Tigecycline treatment downregulated the expression of the cap genes, which mediate the synthesis of the capsule polysaccharide [36]. Oxacillin, moxifloxacin, and linezolid led to the development of a hyper-adhesive phenotype due to an increase in fnbA/B transcription [37]. The presence of PYED-1 also reduced the level of other virulence-associated genes in S. aureus, such as fnbB and capC genes in planktonic cells (Table 5), influencing the adherence properties of S. aureus. Since microbial adherence is the initial step of S. aureus pathogenicity, the ability of PYED-1 to affect this property may be an important factor for attenuation of bacterial infections.
Autolysins have been implicated in many cellular functions [38]. IsaA is a lytic transglycosylase involved in cellular elongation, septation, recycling of muropeptides, and pore formation [39,40]. The expression levels of the autolysin gene isaA were notably upregulated after PYED-1 treatment (Table 5). Overexpression of isaA could lead to a reduction in biofilm formation, a major virulence factor involved in S. aureus pathogenicity. These results are in accord to Payne et al. [41] that showed tannic acid inhibits the formation of biofilms by increasing the extracellular level of IsaA.
In light of the results obtained with S. aureus, further research on the anti-virulence activity of PYED-1 against other multidrug pathogens is merited.
5. Conclusions
The results of the present study revealed that PYED-1, alone or in combination with antibiotics, exerts a strong antimicrobial activity against S. aureus growth. PYED-1 has also the potential to restore the effectiveness of oxacillin against MRSA strains. Moreover, the finding that PYED-1 affects the expression levels of S. aureus virulence genes make this drug a promising candidate for the treatment of S. aureus infectious diseases.
Acknowledgments
We gratefully acknowledge Pierpaolo Di Nocera for comments and critical reading of the manuscript.
Author Contributions
Conceptualization, E.D.G. and A.G.; methodology, A.V., A.E., E.A., V.D.I., and D.D.; validation, A.V. and E.D.G; formal analysis, A.V. and E.D.G.; investigation, A.V., A.E., E.A., V.D.I., and D.D.; resources, A.E., E.A., V.D.I., and D.D.; data curation, A.V. and E.D.G.; writing—original draft preparation, A.G. and E.D.G.; visualization, A.G. and E.D.G.; supervision, A.G. and E.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Boev C., Kiss E. Hospital-Acquired Infections. Crit. Care Nurs. Clin. North Am. 2017;29:51–65. doi: 10.1016/j.cnc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Tommasi R., Brown D.G., Walkup G.K., Manchester J.I., Miller A.A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 2015;14:529–542. doi: 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder M., Brooks B.D., Brooks A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes. 2017;8:39. doi: 10.3390/genes8010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ke S. Recent Progress of Novel Steroid Derivatives and Their Potential Biological Properties. Mini-Rev. Med. Chem. 2018;18:745–775. doi: 10.2174/1389557517666171003103245. [DOI] [PubMed] [Google Scholar]
- 5.Shieh P.B., McIntosh J., Jin F., Souza M., Elfring G., Narayanan S., Trifillis P., Peltz S.W., McDonald C., Darras B., et al. Deflazacort versus prednisone/prednisolone for maintaining motor function and delaying loss of ambulation: A post HOC analysis from the ACT DMD trial. Muscle Nerve. 2018;58:639–645. doi: 10.1002/mus.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito A., De Gregorio E., De Fenza M., D’Alonzo D., Satawani A., Guaragna A. Expeditious synthesis and preliminary antimicrobial activity of deflazacort and its precursors. RSC Adv. 2019;9:21519–21524. doi: 10.1039/C9RA03673C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito A., Vollaro A., Esposito E.P., D’Alonzo D., Guaragna A., Zarrilli R., De Gregorio E. Antibacterial and Antivirulence Activity of Glucocorticoid PYED-1 against Stenotrophomonas maltophilia. Antibiot. 2020;9:105. doi: 10.3390/antibiotics9030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David M.Z., Daum R.S. Treatment of Staphylococcus aureus Infections. Curr. Top. Microbiol. Immunol. 2017;409:325–383. doi: 10.1007/82_2017_42. [DOI] [PubMed] [Google Scholar]
- 9.Turner N.A., Sharma-Kuinkel B.K., Maskarinec S.A., Eichenberger E.M., Shah P.P., Carugati M., Holland T.L., Fowler V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Genet. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vestergaard M., Frees D., Ingmer H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019;7:747–765. doi: 10.1128/microbiolspec.GPP3-0057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Gregorio E., Roscetto E., Iula V.D., Martinucci M., Zarrilli R., Di Nocera P.P., Catania M.R. Development of a real-time PCR assay for the rapid detection of Acinetobacter baumannii from whole blood samples. New Microbiol. 2015;38:251–257. [PubMed] [Google Scholar]
- 12.Giannouli M., Cuccurullo S., Crivaro V., Di Popolo A., Bernardo M., Tomasone F., Amato G., Brisse S., Triassi M., Utili R., et al. Molecular Epidemiology of Multidrug-Resistant Acinetobacter baumannii in a Tertiary Care Hospital in Naples, Italy, Shows the Emergence of a Novel Epidemic Clone. J. Clin. Microbiol. 2010;48:1223–1230. doi: 10.1128/JCM.02263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinucci M., Roscetto E., Iula V.D., Votsi A., Catania M.R., De Gregorio E. Accurate identification of members of the Burkholderia cepacia complex in cystic fibrosis sputum. Lett. Appl. Microbiol. 2016;62:221–229. doi: 10.1111/lam.12537. [DOI] [PubMed] [Google Scholar]
- 14.Sahl J.W., Del Franco M., Pournaras S., Colman R.E., Karah N., Dijkshoorn L., Zarrilli R. Phylogenetic and genomic diversity in isolates from the globally distributed Acinetobacter baumannii ST25 lineage. Sci. Rep. 2015;5:15188. doi: 10.1038/srep15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing (EUCAST) Clinical Breakpoints. [(accessed on 15 September 2012)]; Available online: http://www.eucast.org/clinical_breakpoints/
- 16.CLSI (2015) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical Laboratory Standards Institute; Wayne, IN, USA: 2015. Approved Standard—Tenth edition. CLSI document M07-A10. [Google Scholar]
- 17.Pane K., Cafaro V., Avitabile A., Torres M., Vollaro A., De Gregorio E., Catania M.R., Di Maro A., Bosso A., Gallo G., et al. Identification of Novel Cryptic Multifunctional Antimicrobial Peptides from the Human Stomach Enabled by a Computational–Experimental Platform. ACS Synth. Boil. 2018;7:2105–2115. doi: 10.1021/acssynbio.8b00084. [DOI] [PubMed] [Google Scholar]
- 18.Ferrazzano G.F., Scioscia E., Sateriale D., Pastore G., Colicchio R., Pagliuca C., Cantile T., Alcidi B., Coda M., Ingenito A., et al. In Vitro Antibacterial Activity of Pomegranate Juice and Peel Extracts on Cariogenic Bacteria. BioMed Res. Int. 2017;2017:1–7. doi: 10.1155/2017/2152749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moellering R.C. Antimicrobial Combinations. Rinsho Yakuri/Japanese J. Clin. Pharmacol. Ther. 1993;24:293–300. doi: 10.3999/jscpt.24.293. [DOI] [Google Scholar]
- 20.De Gregorio E., Esposito E.P., Zarrilli R., Di Nocera P.P. Contact-Dependent Growth Inhibition Proteins in Acinetobacter baylyi ADP1. Curr. Microbiol. 2018;75:1434–1440. doi: 10.1007/s00284-018-1540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Swern D. Organic Reactions. Wiley Online Library; Hoboken, NJ, USA: 2011. Epoxidation and Hydroxylation of Ethylenic Compounds with Organic Peracids; pp. 378–434. [Google Scholar]
- 23.Tängdén T. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Upsala J. Med. Sci. 2014;119:149–153. doi: 10.3109/03009734.2014.899279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenul C., Horswill A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2018;6:669–686. doi: 10.1128/microbiolspec.GPP3-0031-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L., Yi T., Shen Z., Teng Z., Wang J. Aloe-emodin Attenuates Staphylococcus aureus Pathogenicity by Interfering With the Oligomerization of α-Toxin. Front. Microbiol. 2019;9:157. doi: 10.3389/fcimb.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kebaier C., Chamberland R.R., Allen I.C., Gao X., Broglie P.M., Hall J.D., Jania C., Doerschuk C.M., Tilley S.L., Duncan J. Staphylococcus aureus α-Hemolysin Mediates Virulence in a Murine Model of Severe Pneumonia Through Activation of the NLRP3 Inflammasome. J. Infect. Dis. 2012;205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonzo F., Torres V.J. Bacterial Survival Amidst an Immune Onslaught: The Contribution of the Staphylococcus aureus Leukotoxins. PLoS Pathog. 2013;9:1003143. doi: 10.1371/journal.ppat.1003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbert S., Barry P., Novick R.P. Subinhibitory Clindamycin Differentially Inhibits Transcription of Exoprotein Genes in Staphylococcus aureus. Infect. Immun. 2001;69:2996–3003. doi: 10.1128/IAI.69.5.2996-3003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardo K., Pakulat N., Fleer S., Schnaith A., Utermöhlen O., Krut O., Müller S., Krönke M. Subinhibitory Concentrations of Linezolid Reduce Staphylococcus aureus Virulence Factor Expression. Antimicrob. Agents Chemother. 2004;48:546–555. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens D.L., Ma Y., Salmi D.B., McIndoo E., Wallace R.J., Bryant A.E. Impact of Antibiotics on Expression of Virulence? Associated Exotoxin Genes in Methicillin? Sensitive and Methicillin? Resistant Staphylococcus aureus. J. Infect. Dis. 2007;195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y.Q., Willard J., Yeaman M.R., Cheung A.L., Bayer A.S. Regulation of Staphylococcus aureusα-Toxin Gene(hla)Expression byagr, sarA, andsaeIn Vitro and in Experimental Infective Endocarditis. J. Infect. Dis. 2006;194:1267–1275. doi: 10.1086/508210. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Yeo W., Bae T. The SaeRS Two-Component System of Staphylococcus aureus. Genes. 2016;7:81. doi: 10.3390/genes7100081. [DOI] [Google Scholar]
- 33.Nygaard T.K., Pallister K.B., Ruzevich P., Griffith S., Vuong C., Voyich J.M. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 2010;201:241–254. doi: 10.1086/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan J., Li M., Hao Z., Shen X., Liu L., Jin Y., Wang S., Guo Y., Yang L., Wang L., et al. Subinhibitory concentrations of resveratrol reduce alpha-hemolysin production in Staphylococcus aureus isolates by downregulating saeRS. Emerg. Microbes Infect. 2018;7:136. doi: 10.1038/s41426-018-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu J., Wang D., Xiang H., Feng H., Jiang Y., Xia L., Dong J., Lu J., Yu L., Deng X. Subinhibitory Concentrations of Thymol Reduce Enterotoxins A and B and α-Hemolysin Production in Staphylococcus aureus Isolates. PLoS ONE. 2010;5:e9736. doi: 10.1371/journal.pone.0009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith K., Gould K.A., Ramage G., Gemmell C.G., Hinds J., Lang S. Influence of Tigecycline on Expression of Virulence Factors in Biofilm-Associated Cells of Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009;54:380–387. doi: 10.1128/AAC.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasigade J.-P., Moulay A., Lhoste Y., Tristan A., Bes M., Vandenesch F., Etienne J., Lina G., Laurent F., Dumitrescu O. Impact of sub-inhibitory antibiotics on fibronectin-mediated host cell adhesion and invasion by Staphylococcus aureus. BMC Microbiol. 2011;11:263. doi: 10.1186/1471-2180-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith T., Blackman S.A., Foster S.J. Autolysins of Bacillus subtilis: Multiple enzymes with multiple functions. Microbiology. 2000;146:249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- 39.Stapleton M.R., Horsburgh M.J., Hayhurst E.J., Wright L., Jonsson I.-M., Tarkowski A., Kokai-Kun J.F., Mond J.J., Foster S.J. Characterization of IsaA and SceD, Two Putative Lytic Transglycosylases of Staphylococcus aureus. J. Bacteriol. 2007;189:7316–7325. doi: 10.1128/JB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheurwater E., Reid C.W., Clarke A. Lytic transglycosylases: Bacterial space-making autolysins. Int. J. Biochem. Cell Boil. 2008;40:586–591. doi: 10.1016/j.biocel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Payne D.E., Martin N.R., Parzych K.R., Rickard A.H., Underwood A., Boles B.R. Tannic Acid Inhibits Staphylococcus aureus Surface Colonization in an IsaA-Dependent Manner. Infect. Immun. 2012;81:496–504. doi: 10.1128/IAI.00877-12. [DOI] [PMC free article] [PubMed] [Google Scholar]