Abstract
Objective
The objective of this study is to examine the magnitude and pattern of small-area geographic variation in rates of preventable hospitalisations for ambulatory care-sensitive conditions (ACSC) across Canada (excluding Québec).
Design and setting
A cross-sectional study conducted in Canada (excluding Québec) using data from the 2006 Canadian Census Health and Environment Cohort (CanCHEC) linked prospectively to hospitalisation records from the Discharge Abstract Database (DAD) for the three fiscal years: 2006–2007, 2007–2008 and 2008–2009.
Primary outcome measure
Preventable hospitalisations (ACSC).
Participants
The 2006 CanCHEC represents a population of 22 562 120 individuals in Canada (excluding Québec). Of this number, 2 940 150 (13.03%) individuals were estimated to be hospitalised at least once during the 2006–2009 fiscal years.
Methods
Age-standardised annualised ACSC hospitalisation rates per 100 000 population were computed for each of the 190 Census Divisions. To assess the magnitude of Census Division-level geographic variation in rates of preventable hospitalisations, the global Moran’s I statistic was computed. ‘Hot spot’ analysis was used to identify the pattern of geographic variation.
Results
Of all the hospitalisation events reported in Canada during the 2006–2009 fiscal years, 337 995 (7.10%) events were ACSC-related hospitalisations. The Moran’s I statistic (Moran’s I=0.355) suggests non-randomness in the spatial distribution of preventable hospitalisations. The findings from the ‘hot spot’ analysis indicate a cluster of Census Divisions located in predominantly rural and remote parts of Ontario, Manitoba and Saskatchewan and in eastern and northern parts of Nunavut with significantly higher than average rates of preventable hospitalisation.
Conclusion
The knowledge generated on the small-area geographic variation in preventable hospitalisations can inform regional, provincial and national decision makers on planning, allocation of resources and monitoring performance of health service providers.
Keywords: epidemiology, statistics & research methods, quality in health care
Strengths and limitations of this study.
This study examines the geographic variation in rates of ambulatory care-sensitive condition (ACSC)-related hospitalisation using the 2006 Canadian Census Health and Environment Cohort (CanCHEC) linked prospectively to hospitalisation records from the 2006–2009 Discharge Abstract Database (DAD).
We determined the magnitude of Census Division-level variation (ie, spatial autocorrelation) in rates of ACSC-related hospitalisations by computing the global Moran’s I statistic, which assesses the degree to which rates are similar or dissimilar across geographic areas.
We identified the location of clusters of Census Divisions with significantly lower (ie, ‘cold spots’) or higher (ie, ‘hot spots’) ACSC hospitalisation rates using the local indicator of spatial association.
Geographic areas were defined using the boundaries of Census Divisions, and although results may differ depending on the definition of geographic units, the methodological approach adopted in this study is generalisable to other geographic units.
Limitations of this study include the absence of hospitalisation records in the DAD from Québec and the lower coverage rates for residents of the territories, young adults, individuals of lower socioeconomic status and rural residents.
INTRODUCTION
Hospitalisations due to ambulatory care-sensitive conditions (ACSC) are an important indicator of access and quality of primary care services,1–4 and therefore an important focus of health service research in Canada1 5 6 and internationally.7–11 Though not all hospitalisations are preventable, with appropriate screening, monitoring, management and follow-up in primary care settings, many ACSC-related hospitalisations can be avoided.4 12
The Canadian Institute of Health Information (CIHI) compiles provincial and health region-level aggregated data on preventable hospitalisations related to the following ACSC: chronic obstructive pulmonary disease (COPD), asthma, heart failure and pulmonary oedema, hypertension, angina, diabetes and grand mal status and other epileptic convulsions.4 The most recent, age-standardised Canadian estimates from CIHI indicate that in 2017–2018, 327 per 100 000 population had an ACSC-related hospitalisation, a decrease from 349 per 100 000 population in 2010.13 However, these national figures obscure substantial geographic variation in these rates, which has persisted since 2001 when such data became available.14 In 2017–2018, British Columbia had the lowest hospitalisation rate for ACSC (294 per 100 000 population) and Nunavut had the highest rate (751 per 100 000 population), a rate approximately 2.5 times greater than in British Columbia.13 There is also some evidence of substantial variation in these rates within provinces.6 15–18 Within Ontario, for example, in 2017–2018, there was an almost threefold difference in preventable hospitalisation rates between the Central Local Health Integration Network (LHIN; 195 per 100 000 population) and the North-West LHIN (575 per 100 000 population, respectively).13
International research suggests that more pronounced differences in the rates of preventable hospitalisations may be found across smaller geographic areas (ie, small-area variation), including administrative units responsible for the local delivery of primary care services.8 10 19 20 However, there is only limited Canadian research examining small-area variation in preventable hospitalisations. A 2008 report commissioned by the CIHI found substantial differences in age-standardised ACSC-related hospitalisation rates across 15 Census Metropolitan Areas (CMA), with Regina CMA having the highest rate of 518 per 100 000 population and Ottawa-Gatineau CMA reporting the lowest rate of 181 per 100 000 population.16 The scope of this study, however, was restricted to a very limited number of large urban areas.
To address this gap, the objective of this study was to examine the magnitude and pattern of geographic variation in preventable hospitalisations in Canada (excluding Québec) across small geographic areas, defined by the boundaries of Census Divisions (CDs). CDs are standard census geographic units that generally correspond to municipalities, as determined by provincial and territorial legislation, or neighbouring municipalities amalgamated for the purposes of regional planning and managing some of the common services.21 CDs vary in their areas and population sizes and, in 2006, there were 190 CDs in Canada (excluding Québec). A reference map of CDs can be found on the Statistics Canada website.22 We hypothesised that the overall magnitude of geographic variation and the distribution of preventable hospitalisations across CDs in Canada is not random but rather exemplifies spatial dependence where CDs with lower and higher than average ACSC-related hospitalisation rates are clustered together. The presence of small-area geographic differences in rates of potentially preventable hospitalisations may suggest the presence of substantial inequalities in access to appropriate primary care across CDs.6 16 17 Thus, identifying CDs with disproportionately high rates of ACSC-related hospitalisations can support decision makers in planning, allocation of resources and monitoring performance of health service providers as well as lead to an improvement in primary care quality to reduce the burden of preventable hospitalisations.23 24 Moreover, methodological approaches and findings from this baseline study can lend to further examination of whether or not clusters of CDs with lower or higher rates of preventable hospitalisations are emerging, stable or declining.
Methods
Data
To assess the magnitude and pattern of geographic variation in rates of ACSC-related hospitalisation, we conducted a cross-sectional study using the 2006 Canadian Census Health and Environment Cohort (CanCHEC) linked prospectively to hospitalisation records from the 2006–2009 Discharge Abstract Database (DAD). The 2006 CanCHEC consists of about 20% of the non-institutional respondents to the 2006 Census of Canada who were given long-form census questionnaire, totalling over 4.6 million individuals. The cohort reliably captures characteristics of the entire Canadian population, residing in large metropolitan regions or small remote settlements25 as it is representative of approximately 95%–97% of the provincial populations and 93%–94% of the territorial populations.25 26
The individual-level records for the members of the 2006 CanCHEC were recently linked by Statistics Canada to the DAD records for three fiscal years: 2006–2007, 2007–2008 and 2008-2009.27 The DAD is a census of hospital discharges for all provinces and territories excluding Québec, which does not report hospitalisation data to the DAD, and includes administrative and clinical data for approximately three million hospital discharges per year.28 The DAD provides information on the main diagnoses, date of admission and treatment information. Each hospital record consists of up to 25 diagnoses and 20 intervention codes based on the International Classification of Disease 10th Revision, Canadian Modification codes (ICD-10-CA) and volume 4 of the Canadian Classification of Health Interventions.29 30
The record linkage of the 2006 CanCHEC and 2006–2009 DAD involved the hierarchical deterministic exact method31 and was based on personal identifiers common to both data sources (ie, date of birth, sex and postal code). A validation study conducted by Statistics Canada indicated that linkage rates approached 100%, with weighted coverage rates exceeding 80% (ie, the weighted CanCHEC represents over 80% of hospitalisations during the 2006–2009 period), and that the linked files are suitable for health-related research as the data are broadly representative of the population of all provinces and territories, excluding Québec.27 Methodological details on the 2006 CanCHEC, data linkage and findings from the linkage validation study are available elsewhere.27
Rates of preventable hospitalisations
Following the CIHI’s previously established definition of ACSC, validated for use in Canada,4 we used the first three characters of each ‘most responsible’ diagnosis to identify ACSC-related hospitalisation events. Selected ACSC include grand mal status and other epileptic convulsions, COPD, asthma, diabetes, heart failure and pulmonary oedema, hypertension and angina (excluding cases with cardiac procedures). For each CD, we computed age-standardised annualised ACSC hospitalisation rate per 100 000 population. Specifically, hospitalisation records over three fiscal years (ie, 2006–2007, 2007–2008 and 2008–2009) were pooled to produce a stable estimate of ACSC-related hospitalisation rate in each CD and to detect differences between these geographic areas. Sampling weights were used in line with the 2006 census design. The estimated population-level counts of hospitalisation events in each CD were rounded to a base of 5 as required by Statistic Canada’s confidentiality procedures. The rate for each CD was computed by dividing the estimated annualised and rounded count of ACSC-related hospitalisations in that CD by the total population of that CD and then expressed as per 100 000 population. Finally, direct standardisation was carried out using the entire 2006 Census Canada as the reference population and four age groups (ie, 0–19, 20–39, 40–59, ≥60 years).
Patient and public involvement
No patients involved.
Statistical analysis
To assess the magnitude of CD-level variation (ie, spatial autocorrelation) in rates of preventable hospitalisations, we computed the global Moran’s I statistic, which assesses the degree to which rates are similar or dissimilar across geographic areas.32 This was computed using the formula:
where, zi and zj for areas i and j are the deviations from the mean, wij is the matrix of row-standardised weights, and
Moran’s I values range from −1 to +1, which represent extreme negative and positive spatial correlations, respectively (ie, CDs with either low or high preventable hospitalisation rates are geographically clustered together) and 0 indicates spatial randomness (no spatial correlation between CDs). To test the null hypothesis of no spatial correlation, a Monte Carlo simulation was used with 1000 random permutations to produce the rank of observed Moran’s I in relation to the simulated values, with p<0.05 indicating statistical significance.
To determine the location of clusters of CDs with significantly lower (ie, ‘cold spots’) or higher (ie, ‘hot spots’) ACSC hospitalisation rates, we assessed whether preventable hospitalisation rate in each CD is closer to the rates of its neighbours or to the national average. This was achieved using the local indicator of spatial association (LISA)32 33 using the formula:
The statistical significance of LISA estimates was tested using a Monte Carlo simulation which compares the actual observed LISA values for each CD with the distribution of repeatedly randomised values. A LISA significance map was produced to identify clusters of CDs with significantly higher or lower rates of ACSC hospitalisations compared with their neighbours. All analyses were conducted using R.34 Patients were not involved in this study.
Results
The 2006 CanCHEC represents a population of 22 562 120 individuals in Canada, except Québec. Of this number, 2 940 150 (13.03%) individuals were estimated to be hospitalised at least once during the 2006–2009 fiscal years. In total, the weighted number of hospitalisation events reported by all members of this population was 4 762 195. Out of that number, 337 995 (7.10%) events were ACSC-related hospitalisations. The most common ACSC diagnosis was COPD (33.23%), followed by heart failure and pulmonary oedema (26.10%), angina (16.01%), diabetes (9.47%), asthma (7.15%), grand mal (5.05%) and hypertension (2.99%).
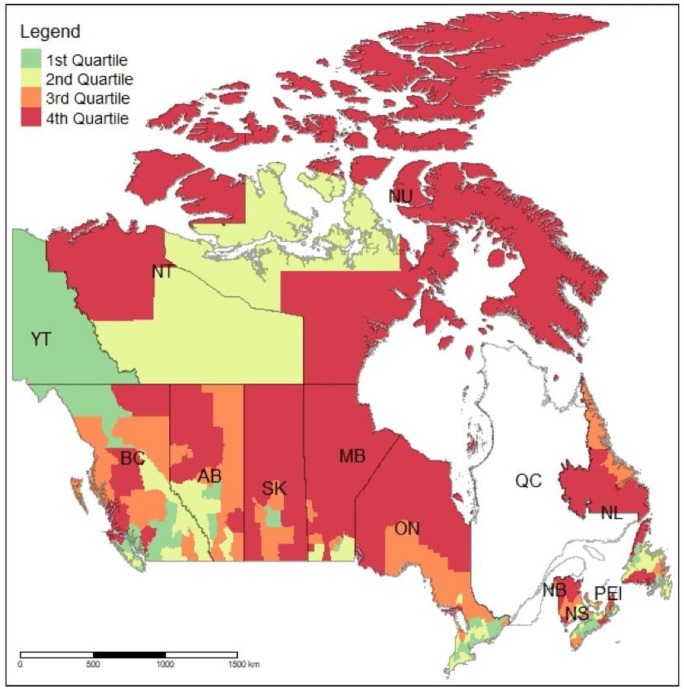
The overall annualised rate of preventable hospitalisation for the 2006 CanCHEC for the 2006–2009 fiscal years was 499 per 100 000 population (table 1). The rates of ACSC-related hospitalisations varied across provinces and territories from the lowest of 436 per 100 000 population in British Columbia to the highest of 1264 per 100 000 population in Nunavut. As hypothesised, the between CD variation in these rates was even more pronounced than the variation across provinces, with the rates ranging from the lowest of 266 per 100 000 population in the White Horse Plains area near the city of Winnipeg in Manitobato the highest of 2131 per 100 000 population in Manitoulin, in central Ontario. The median rate across all CDs was 693 per 100 000 population with the IQR equal to 351 (ie, from 564 and 915 per 100 000 population). Similarly, a substantial level of variation can also be observed between CDs within each province. Figure 1 displays the rates of preventable hospitalisations for all CDs in Canada (excluding Québec) and suggests that there is a substantial level of variation in these rates across Canada and within each province and territory. In general, the rates appear to be highest in CDs located in the northern parts of Newfoundland and Labrador, New Brunswick, Ontario, Manitoba, Saskatchewan and Alberta. They are also relatively high in the interior of British Columbia and in some parts of Nunavut and the Northwest Territories.
Table 1.
Ambulatory care-sensitive condition hospitalisation rates across provinces and Census Divisions, Canada 2006–2009
| ACSC rates between CDs within each province | |||||||
| Province | Population* | ACSC events† | ACSC rates‡ | #CDs | Lowest | Median | Highest |
| Newfoundland and Labrador | 474 405 | 9265 | 651 | 11 | 513 | 513 | 1054 |
| Prince Edward Island | 125 800 | 2720 | 721 | 3 | 672 | 779 | 780 |
| Nova Scotia | 853 525 | 13 335 | 521 | 18 | 368 | 643 | 1353 |
| New Brunswick | 696 650 | 17 465 | 836 | 15 | 566 | 846 | 1094 |
| Ontario | 11 428 170 | 154 715 | 451 | 49 | 296 | 581 | 2131 |
| Manitoba | 1 082 900 | 19 595 | 603 | 23 | 266 | 827 | 2112 |
| Saskatchewan | 907 630 | 23 375 | 858 | 18 | 518 | 1063 | 1576 |
| Alberta | 3 088 730 | 45 325 | 489 | 19 | 373 | 708 | 1401 |
| British Columbia | 3 810 320 | 49 850 | 436 | 28 | 331 | 616 | 1244 |
| Yukon | 28 770 | 460 | 533 | 1 | 533 | 533 | 533 |
| Northwest Territories | 38 440 | 875 | 759 | 2 | 667 | 876 | 1085 |
| Nunavut | 26 775 | 1015 | 1264 | 3 | 564 | 1215 | 1540 |
| Canada | 22 562 120 | 337 995 | 499 | 190 | 266 | 693 | 2131 |
*2006 population size rounded to a base of 10.
†Estimated population-level counts of ACSC hospitalisation events rounded to a base of 5.
‡Standardised and annualised ACSC hospitalisation rates per 100 000 population.
ACSC, ambulatory care-sensitive condition; CD, Census Division.
Figure 1.
Age-standardised annualised hospitalisation rates (quartiles) for ambulatory care-sensitive conditions per 100 000 population: Canada (Census Divisions).
The Moran’s I statistic, computed to assess the overall magnitude of variation in preventable hospitalisation rates between CDs was 0.3550 (expected value=−0.0053; variance=0.0026) suggesting that the overall spatial distribution of preventable hospitalisations between CDs is non-random. The results from the Monte Carlo simulation of Moran’s I indicated that the null hypothesis of no spatial correlation can be rejected (the rank of the observed Moran’s I=1000; the pseudo p value=0.001).
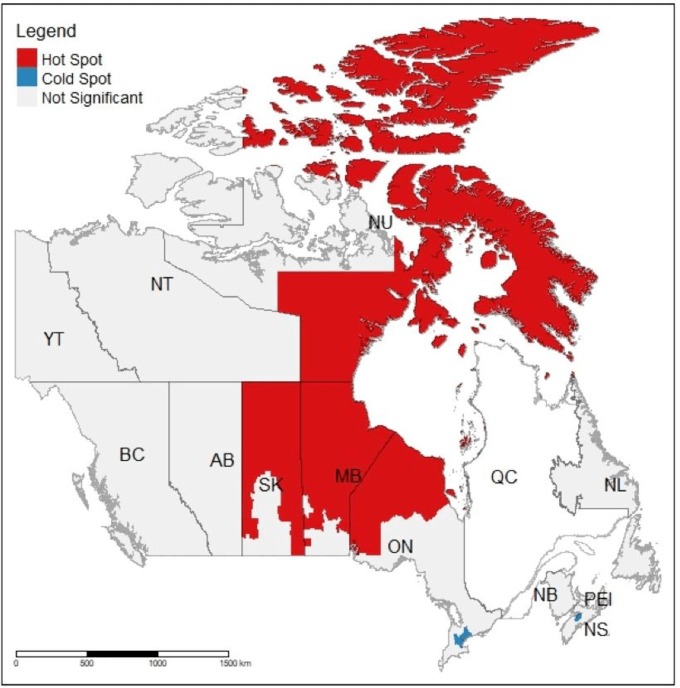
Figure 2 presents the findings from the LISA analysis and depicts the pattern of clustering of CDs with significantly higher (‘hot spots’) and lower (‘cold spots’) rates of preventable hospitalisations. It indicates that a relatively large cluster of CDs with higher than average hospitalisation rates was located in northern parts of Ontario, Manitoba and Saskatchewan and across eastern and northern parts of Nunavut (‘hot spots’). In addition, two clusters of CDs with lower than average rates (‘cold spots’) were found, one in the Greater Toronto Area and one in central Nova Scotia, around the town of Windsor.
Figure 2.
Hot spots and cold spots in preventable hospitalisations: Canada (Census Divisions). This map identifies clusters of census divisions with significantly higher (hot spots) or lower (cold spots) rates of hospitalisations for ambulatory care-sensitive conditions compared with their neighbours.
Discussion
This study addresses an important gap in the literature by providing information on the magnitude and pattern of geographic variation in preventable hospitalisations in Canada. Specifically, our study contributes to the literature by: (1) providing a quantitative assessment of the magnitude of spatial variation in preventable hospitalisations across small geographic areas (ie, CDs); (2) identifying geographic areas with significantly lower or higher concentrations of these events(ie, ‘cold spots’ and ‘hot spots’, respectively); and (3) demonstrating how spatial analysis can be applied to future studies of geographic variation in preventable hospitalisations that may involve data on newer census cohorts linked to more recent hospitalisation records, when these data become available.
Overall, the results of the spatial analysis provide support for the hypothesis that there is a statistically significant and substantial level of spatial variation in preventable hospitalisations across Canada and clustering of CDs with significantly lower and higher rates, which is a novel finding as the previous studies did not conduct any formal statistical assessment of the magnitude or patterns of geographic variations. The presence of a large cluster of CDs with higher than average ACSC-related hospitalisation rates, stretching from northern parts of Ontario, Manitoba and Saskatchewan and across eastern and northern parts of Nunavut, indicates that CDs with significantly higher rates of preventable hospitalisations are more likely to cluster in northern, predominantly rural and remote regions of Canada. In a CIHI report, rural areas in Canada were found to have approximately 60% higher rates of preventable hospitalisations compared with urban areas,14 potentially due to poor access to primary care in these locations.35 36 In contrast, two ‘cold spots’, characterised by lower than average rates of preventable hospitalisations, were found in predominately urban areas (ie, in the Greater Toronto Area and in the urban area of Nova Scotia). This pattern is likely related to differences in primary healthcare in rural compared with urban areas, as barriers related to accessibility (eg, availability) or quality of primary care services are well-known factors related to geographic variation in preventable hospitalisations.7 23 24
It is important to acknowledge that, in addition to the availability of primary healthcare services, the magnitude and pattern of geographic variation in preventable hospitalisations may also be related to differences in sociodemographics, health behaviours and/or health status characteristics of the individuals residing in each CD (ie, compositional effect) or to other area-level factors. Berlin et al8 argue that although an effective primary care system should aid in the prevention of ACSC-related hospitalisations, these events are also dependent on individual-level factors such as propensity to seek care, severity of the disease, compliance issues, financial constraints or accessibility issues. Research by Falster and colleagues20 suggests that as much as 36.9% of geographic variation in preventable hospitalisations in Australia was a result of individual-level sociodemographic and health characteristics. The geographic variation in preventable hospitalisations may, in particular, be driven by the well-known social gradient in health, as described by Marmot.37 In a 2008 study involving 15 CMA areas, for instance, hospitalisations for ACSC were highest among those with low socioeconomic status (SES) and showed a steep gradient between low SES, average SES and high SES.16 Thus, further examination of the determinants of geographic variation, with a focus on individual-level factors, would help ascertain why residents of some CDs are more (or less) likely to be hospitalised for an ACSC, compared with residents in other areas of Canada.
Limitations
One limitation of any analysis involving the DAD is the absence of hospitalisation records from Québec. As these records are currently not shared with the CIHI or Statistics Canada, we were not able to directly address this limitation. Second, the validation study of the 2006 CanCHEC-DAD linked files indicated that coverage rates were slightly lower for residents of the territories, young adults, individuals of lower SES and rural residents27; however, sampling weights used to extrapolate the observed counts to the whole population accounted for some of this under-representation. Furthermore, the boundaries of CDs were used in this study to define geographic areas. Although the Modifiable Areal Unit Problem indicates that the results may differ depending on the definition of geographic units,38–40 the methodological approach adopted in this study is generalisable to other geographic units. The results of this study are also based on the assumption that during the 2006–2009 time period, members of the 2006 CanCHEC did not move from CDs that they reported as their home addresses in the 2006 Census; however, residential mobility within the boundaries of each CD would not affect the results. Lastly, estimates generated from the recently released 2006 CanCHEC-DAD linked files may not reflect the current rates of preventable hospitalisation in Canada.
Additionally, the CIHI definition of ACSC may not capture all preventable hospitalisations and not all ACSC hospitalisations may be preventable.4 12 However, at present, these are the best national data that are available for conducting analysis on small-area geographic variation in preventable hospitalisations. Moreover, for surveillance purposes, findings from the current study can be used as a baseline estimate to be compared with the results of future assessments of geographic variation in preventable hospitalisation involving linked files from newer census cohorts, when these data become available.
Conclusions
The knowledge on the magnitude and pattern of small-area geographic variation in preventable hospitalisations can inform regional, provincial and national decision makers on planning resources and monitoring performance of health service providers. As preventable hospitalisations are an important indicator of access and quality of primary care services, identifying of clusters of CDs with disproportionately high rates of ACSC-related hospitalisations can lead to an improvement in primary care quality in these areas to reduce the burden of preventable hospitalisations. Ultimately, this can lead to the reduction of substantial inequalities in the rates of preventable hospitalisations across Canada.
The current study provides valuable insight into small-area geographic variation in preventable hospitalisations in Canada. We found that the pattern of ‘hot spots’ in ACSC-related hospitalisations does not follow provincial boundaries, which is a novel observation in the Canadian context and suggests the need to focus on intraprovincial comparisons. As suggested by the existing literature, there may be a wide range of inter-related factors that can potentially contribute to this variation. Although it is often assumed that small-area geographic variation in preventable hospitalisations is related to characteristics of the health care system, this variation may also be related to individual-level and area-level socioeconomic factors rooted in the local contexts,10 20 23 or to health-related behaviour associated with low SES.
Ultimately, people interact with the healthcare system in the geographic areas in which they reside, and future research should assess the nature of these interactions and how they may contribute to the observed geographic variation in ACSC-related hospitalisations.
Supplementary Material
Footnotes
Twitter: @anderskk3, @aclark82
Contributors: PW: planned and undertook statistical analysis and interpretation. PW and AM: drafted and finalised the manuscript. SA, KKA, AFC, MC, SJF, JG, MH, SH, SK, KN, RP, SSa, SSS, SSt and AT: reviewed the manuscript. All authors contributed to the design of the study; reviewed, commented on and approved the final manuscript.
Funding: This research was funded by the Schulich School of Medicine & Dentistry through the Collaborative Research Seed Grant and by the Children’s Health Foundation.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Ethical approval for this study was not required as the study uses anonymous and confidential secondary data from Statistics Canada. Consent from respondents was obtained at the time of data collection. Data were provided by Statistics Canada through the Research Data Centres programme and accessed under the Statistics Act of Canada. The analyses and the interpretation are the authors’ alone.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. Not Applicable.
References
- 1.Sanmartin C, Khan S, LHAD Research Team . Hospitalizations for Ambulatory Care Sensitive Conditions (ACSC): The factors that matter : Health research working paper series. Ottawa: Statistics Canada, Health Information and Research Division, 2011. [Google Scholar]
- 2.Magan P, Otero A, Alberquilla A, et al. Geographic variations in avoidable hospitalizations in the elderly, in a health system with universal coverage. BMC Health Serv Res 2008;8:42. 10.1186/1472-6963-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AD, Goldacre MJ, Hicks N, et al. Hospitalization for ambulatory care-sensitive conditions: a method for comparative access and quality studies using routinely collected statistics. Can J Public Health 2001;92:155–9. 10.1007/BF03404951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Institute for Health Information Ambulatory care sensitive conditions, 2017. Available: http://indicatorlibrary.cihi.ca/display/HSPIL/Ambulatory+Care+Sensitive+Conditions
- 5.Cloutier-Fisher D, Penning MJ, Zheng C, et al. The devil is in the details: trends in avoidable hospitalization rates by geography in British Columbia, 1990-2000. BMC Health Serv Res 2006;6:104. 10.1186/1472-6963-6-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez M, Vellanky S, Herring J, et al. Variations in Canadian rates of hospitalization for ambulatory care sensitive conditions. Healthc Q 2008;11:20–2. 10.12927/hcq.2008.20087 [DOI] [PubMed] [Google Scholar]
- 7.Billings J, Anderson GM, Newman LS. Recent findings on preventable hospitalizations. Health Aff 1996;15:239–49. 10.1377/hlthaff.15.3.239 [DOI] [PubMed] [Google Scholar]
- 8.Berlin C, Busato A, Rosemann T, et al. Avoidable hospitalizations in Switzerland: a small area analysis on regional variation, density of physicians, hospital supply and rurality. BMC Health Serv Res 2014;14:289. 10.1186/1472-6963-14-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weeks WB, Ventelou B, Paraponaris A. Rates of admission for ambulatory care sensitive conditions in France in 2009-2010: trends, geographic variation, costs, and an international comparison. Eur J Health Econ 2016;17:453–70. 10.1007/s10198-015-0692-y [DOI] [PubMed] [Google Scholar]
- 10.Ansari Z, Rowe S, Ansari H, et al. Small area analysis of ambulatory care sensitive conditions in Victoria, Australia. Popul Health Manag 2013;16:190–200. 10.1089/pop.2012.0047 [DOI] [PubMed] [Google Scholar]
- 11.Busby J, Purdy S, Hollingworth W. How do population, general practice and hospital factors influence ambulatory care sensitive admissions: a cross sectional study. BMC Fam Pract 2017;18:67. 10.1186/s12875-017-0638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker RL, Chen G, McAlister FA, et al. Hospitalization for uncomplicated hypertension: an ambulatory care sensitive condition. Can J Cardiol 2013;29:1462–9. 10.1016/j.cjca.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Canadian Institute for Health Information Your Health System - Ambulatory Care Sensitive Conditions, 2019. Available: https://yourhealthsystem.cihi.ca/hsp/indepth?lang=en#/indicator/019/2/C5001/
- 14.Canadian Institute for Health Information Health indicators. Ottawa, ON, 2008. https://secure.cihi.ca/free_products/HealthIndicators2008_ENGweb.pdf [Google Scholar]
- 15.Lin G, Allan DE, Penning MJ. Examining distance effects on hospitalizations using GIS: a study of three health regions in British Columbia, Canada. Environ Plan A 2002;34:2037–53. 10.1068/a3528 [DOI] [Google Scholar]
- 16.Canadian Institute for Health Information Reducing gaps in health: a focus on socio-economic status in urban Canada. Ottawa, ON, 2008. [Google Scholar]
- 17.Manitoba Centre for Health Policy and Evaluation. Concept: ambulatory care sensitive (ACS) conditions, 2007. Available: http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1023
- 18.Fransoo R, Martens P, et al. , The Need to Know Team, . The 2013 RHA indicators atlas. Winnepeg, MB, 2013. http://mchp-appserv.cpe.umanitoba.ca/reference//RHA_2013_web_version.pdf [Google Scholar]
- 19.Arandelovic A, Acampora A, Federico B, et al. The use of preventable hospitalization for monitoring the performance of local health authorities in long-term care. Health Policy 2018;122:309–14. 10.1016/j.healthpol.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 20.Falster MO, Jorm LR, Douglas KA, et al. Sociodemographic and health characteristics, rather than primary care supply, are major drivers of geographic variation in preventable hospitalizations in Australia. Med Care 2015;53:436–45. 10.1097/MLR.0000000000000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistics Canada Census division (CD), 2015. Available: http://www12.statcan.gc.ca/census-recensement/2011/ref/dict/geo008-eng.cfm
- 22.Statistics Canada Census divisions, 2006 reference maps, 2006. Available: http://www12.statcan.gc.ca/census-recensement/2011/geo/map-carte/pdf/030114_0304-0418_2006.pdf
- 23.Ansari Z, Laditka JN, Laditka SB. Access to health care and hospitalization for ambulatory care sensitive conditions. Med Care Res Rev 2006;63:719–41. 10.1177/1077558706293637 [DOI] [PubMed] [Google Scholar]
- 24.Rosano A, Loha CA, Falvo R, et al. The relationship between avoidable hospitalization and accessibility to primary care: a systematic review. Eur J Public Health 2013;23:356–60. 10.1093/eurpub/cks053 [DOI] [PubMed] [Google Scholar]
- 25.Statistics Canada 2006 census of population, 2006. Available: http://www12.statcan.gc.ca/census-recensement/2006/index-eng.cfm
- 26.Statistics Canada Census profile, 2016 census, 2018. Available: http://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E
- 27.Rotermann M, Sanmartin C, Trudeau R, et al. Linking 2006 census and hospital data in Canada. Health Rep 2015;26:10–20 http://www.statcan.gc.ca/pub/82-003-x/2015010/article/14228-eng.pdf [PubMed] [Google Scholar]
- 28.Canadian Institute for Health Information Data quality documentation, discharge Abstract Database—Multi-Year information. Ottawa: CIHI, 2012. https://www.cihi.ca/en/dad_multi-year_en.pdf [Google Scholar]
- 29.Canadian Institute for Health Information International statistical classification of diseases and related health problems, 10th revision. Ottawa: CIHI, 2006. [Google Scholar]
- 30.Canadian Institute for Health Information Canadian classification of health interventions. Ottawa, ON, 2006. http://hcaiinfo.ca/Health_Care_Facility_Provider/documents/appendices/CCI_Vol4_2006%20Alpha%20Index.pdf [Google Scholar]
- 31.Bernier J, Nobrega K. Overview of record linkage. SYMPOSIUM 99: Combining Data from Different Sources. Ottawa, ON: Statistics Canada, 1999. [Google Scholar]
- 32.Moran PAP. A test for the serial independence of residuals. Biometrika 1950;37:178–81. 10.1093/biomet/37.1-2.178 [DOI] [PubMed] [Google Scholar]
- 33.Anselin L. Local indicators of spatial Association-LISA. Geogr Anal 1995;27:93–115. 10.1111/j.1538-4632.1995.tb00338.x [DOI] [Google Scholar]
- 34.R Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.R-project.org [Google Scholar]
- 35.Altmayer CA, Ardal S, Woodward GL, et al. Variation in emergency department visits for conditions that may be treated in alternative primary care settings. CJEM 2005;7:252–6. 10.1017/S1481803500014391 [DOI] [PubMed] [Google Scholar]
- 36.Gilliland JA, Shah TI, Clark A, et al. A geospatial approach to understanding inequalities in accessibility to primary care among vulnerable populations. PLoS One 2019;14:e0210113. 10.1371/journal.pone.0210113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marmot M. Social determinants of health inequalities. Lancet 2005;365:1099–104. 10.1016/S0140-6736(05)74234-3 [DOI] [PubMed] [Google Scholar]
- 38.Gehlke CE, Biehl K. Certain effects of grouping upon the size of the correlation coefficient in census tract material. J Am Stat Assoc 1934;29:185A:169–70. [Google Scholar]
- 39.Flowerdew R, Manley DJ, Sabel CE. Neighbourhood effects on health: does it matter where you draw the boundaries? Soc Sci Med 2008;66:1241–55. 10.1016/j.socscimed.2007.11.042 [DOI] [PubMed] [Google Scholar]
- 40.Openshaw S. The Modifiable Areal Unit Problem - Concepts and Techniques in Modern Geography. Norwich, UK: Geo Books, 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.