Abstract
Background
We sought to determine the prognostic role of indoleamine 2,3-dioxygenase 1 (IDO1) by evaluating IDO1 expression in circulating tumour cells (CTCs) at baseline and after completion of chemoradiotherapy in patients with locally advanced (LA) head and neck squamous cell carcinoma (HNSCC) treated with curative intent.
Methods
In a prospective cohort of 113 patients with LA HNSCC, we evaluated expression of IDO1 in the EpCAM+ CTC fraction at baseline and after cisplatin chemoradiation. The prognostic value of combined programmed cell death ligand-1 (PDL-1) and IDO1 expression was assessed.
Results
IDO1 was significantly overexpressed at baseline compared with the post-treatment counterparts (p=0.007). IDO1 messenger RNA (mRNA) expression at baseline was associated with better survival in terms of progression-free survival (PFS) (HR=0.19, p=0.017). Post-treatment IDO1 mRNA levels were correlated with unfavourable prognosis in terms of overall survival (OS) (HR=3.27, p=0.008). Patients with combined decreased expression levels of PDL-1 and IDO1 after treatment exhibited superior PFS (p=0.043) and OS (p=0.021).
Conclusions
Our results strongly suggest that IDO1 mRNA expression is an independent prognostic factor for clinical outcome. Our study provides useful information for future trials combining chemoradiation with immune checkpoint inhibitors and IDO1 inhibitors.
Keywords: head and neck cancer, cell death, CTCs, IDO1
Key questions.
What is already known about this subject?
Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme that participates in the catabolism of the essential amino acid L-tryptophan, causing its depletion, and contributes to immune suppression and tolerance in the tumour microenvironment.
What does this study add?
In patients with locally advanced head and neck cancer treated with chemoradiation, IDO1, an enzyme that catalyses tryptophan metabolism, is a surrogate biomarker of ‘inflamed’ good prognosis phenotype at baseline. On the contrary, persistent IDO1 overexpression at the end of treatment may antagonise induction of immunogenic cell death by chemoradiation.
How might this impact on clinical practice?
Our study provides useful information for future trials combining chemoradiation with immune checkpoint inhibitors and IDO1 inhibitors.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a malignancy with well-known contributing factors, such as tobacco and alcohol consumption; in addition, human papilloma virus is implicated in the pathogenesis of an increasing proportion of oropharyngeal cancers.1 2 Despite advances in multimodality treatment, the 5-year progression-free survival (PFS) rates of patients with locally advanced (LA) disease do not exceed 40%–50%, and survival rates in the recurrent or metastatic setting remain poor.3 The discovery of novel therapeutic agents aimed at minimising toxicity associated with chemotherapy and radiation and improving patient outcomes.
Analysis of tumour microenvironment in patients with a variety of solid tumours has revealed that cancer evolution and treatment response are both influenced by the interplay between malignant cells and cells of the immune system. More specifically, it has been demonstrated that detection of CD8+ T cells is an indicator of an effective antitumour immune response4 5 and correlates with the upregulation of immune inhibitory mechanisms mediating immune suppression. Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme that participates in the catabolism of the essential amino acid L-tryptophan, causing its depletion, and contributes to immune suppression and tolerance in the tumour microenvironment.6 These IDO1-inducing signals may be constitutively present in the inflammatory microenvironment of the tumour and may be stimulated by the dying cells and release of tumour antigens that is triggered by chemotherapy. However, it remains largely unknown to what degree IDO1 is produced after chemotherapy.7 Several studies suggest that IDO1 expression determines the choice between immunogenic and tolerogenic cell death in response to chemotherapy.
On the other hand, detection of circulating tumour cells (CTCs) is used for real-time monitoring of tumour status8 and has been shown to correlate with prognosis in several cancers.9 10 In addition, molecular characterisation of CTCs potentially provides valuable information for the development of novel drugs.
Based on these considerations, we sought to prospectively determine IDO1 messenger RNA (mRNA) expression in CTCs at baseline and after completion of cisplatin chemoradiation therapy (CRT) in a cohort of patients with LA HNSCC treated with curative intent. To achieve this, we first developed a highly sensitive, specific and reproducible real-time quantitative real-time reverse transcription PCR (RT-qPCR) assay for the quantification of IDO1 mRNA expression in CTCs. We demonstrate for the first time that high IDO1 mRNA expression at baseline is associated with favourable overall survival (OS), whereas high IDO1 mRNA expression at the end of treatment is associated with shorter OS.
Materials and methods
Study design
In a single-institution study, 113 patients with LA HNSCC participated in this analysis. Written informed consent was obtained from all patients.
For this population of patients, our group has previously published results regarding expression of immunogenic cell death (ICD) biomarkers.11 Inclusion criteria have been previously described11; patients with newly diagnosed, histologically confirmed squamous cell carcinoma of the oral cavity, oropharynx, larynx or hypopharynx were included. Patients had tumours not amenable to surgical treatment or wished to preserve their larynx. Exclusion criteria have been previously described.11 Determination of disease stage was done using the TNM classification by performing a CT scan of the head and neck, thorax and abdomen. All patients underwent cisplatin chemoradiation, and registration was done before the initiation of treatment. All patients received high-dose cisplatin (100 mg/m2 every 21 days) in combination with radiotherapy. Eighty-five per cent of patients received >200 mg/m2 cisplatin. All patients received 66 Gy in 30 daily fractions over 6 weeks to the primary tumour site and involved nodes. Sample collection occurred at two timepoints: at baseline and at the end of CRT (a week after treatment was stopped). All patients were subjected to standard follow-up, which was CT of the head and neck and evaluation by an ear, nose and throat physician every 3 months for the first 2 years and every 6 months after the period of 2 years. The first assessment of treatment response was done 12 weeks post-CRT.
We evaluated the expression of IDO1 in the EpCAM+ CTC fraction at baseline and after cisplatin-based CRT.
Isolation of EpCAM(+) CTCs
For the isolation of EpCAM+ CTCs from peripheral blood (30 mL), we followed our previously described protocols.12 13
RNA extraction
The miRNeasy micro kit (QIAGEN, Germany) was used for the isolation of total RNA from the EpCAM(+) CTC fraction, according to the manufacturer’s instructions. cDNA synthesis cDNA synthesis was performed using the SuperScript First-Strand Synthesis System for RT-PCR (Life Technologies, USA) according to manufacturer’s protocol, using 7 µL of isolated total RNA as starting template.
RT-qPCR assay for the quantification of IDO mRNA
Primer and probe design
We designed in silico the primers and hydrolysis probes (TaqMan) for IDO1 and β2-microglobulin (B2M, used as a reference gene14) using Primer Premier V.5.0 software (Premier Biosoft, California, USA). Our primers and probes were carefully designed to completely avoid primer–dimer formation, false priming sites, formation of hairpin structures and hybridisation to genomic DNA, while amplifying specifically only the genes of interest. The sequences of primers and probes are available on request.
qPCR
Expressional analysis of genes was conducted using the TaqMan chemistry, in a Rotor Gene Q Real-Time PCR machine (Qiagen), after thorough optimisation of the methodology (data not shown).
Regarding IDO1, each cDNA was amplified in a 10 µL reaction containing 2.5 µL of the PCR synthesis buffer (5×), 1.6 µL MgCl2 (25 mM), 0.2 µL deoxyribonucleotide triphosphates (dNTPs) (10 mM), 0.1 µL Taq DNA polymerase (5 U/µL), 1 µL of forward and reverse primers (2.5 μΜ), 0.83 µL TaqMan probe (3 µM) and H2O to the final volume. The 10 µL reaction mixture for B2M contained 1 µL of PCR synthesis buffer (5×), 1.2 µL MgCl2 (25 mM), 0.15 µL dNTPs (10 mM), 0.3 µL Bovine Serum Albumin Solution (BSA) (10 µg/µL), 0.1 µL Taq DNA polymerase 5 U/µL, 0.25 µL of forward and reverse primers (10 μΜ), 0.83 µL TaqMan probe (3 µM) and H2O to the final volume. The thermal cycling protocol for IDO1 consisted of an initial 2 min polymerase activation step at 95°C and 50 cycles of 95°C for 10 s for template denaturation, 60°C for 20 s for primer annealing and 72°C for 20 s for extension. Finally, the cycling conditions for B2M were 95°C for 2 min, 45 cycles of 95°C for 10 s, annealing at 58°C for 20 s and extension at 72°C for 20 s.
Normalisation of qPCR data in clinical samples
qPCR data for IDO1 expression were normalised in respect to B2M expression in the same cDNAs, using the 2–ΔΔCq approach.15 CTCs isolated through positive immune-magnetic enrichment are partly contaminated, since ‘contamination’ of peripheral blood mononuclear cells in the EpCAM(+) CTC fraction could affect IDO assay specificity, we assessed this contamination by analysing peripheral blood samples from 20 healthy individuals in exactly the same way as patients. We estimated a cut-off based on IDO1 normalised expression in respect to B2M expression in this control group. Using this approach, we defined a sample as IDO1 overexpressed based on the fold change of each target gene expression in the EpCAM(+) CTC fraction in respect to the corresponding EpCAM(+) fraction in the group of 20 healthy individuals.
Statistical analysis
The Wilcoxon statistical test was performed to analyse the differential expression of IDO1 before and after treatment. The non-parametric Mann-Whitney U, Jonckheere-Terpstra and Kruskal-Wallis tests were used appropriately in order to assess the associations of IDO1 expression levels with patients’ clinicopathological parameters. Independent associations between baseline IDO1 levels and tumour characteristics were assessed by linear regression. The association between IDO1 levels and response to treatment was evaluated by logistic regression.
Our survival analysis included the construction of Kaplan-Meier survival curves, and the long-rank statistical test was performed for the assessment of any differences in the Kaplan-Meier curves and the estimation of the p value. The prognostic value of IDO1 expression for PFS and OS was evaluated by univariate and multivariate bootstrap Cox proportional hazards regression models, where the 95% CI was calculated using the bias corrected and accelerated approach, and the resulting p values were evaluated by the test for trend approach. PFS was defined as the time from registration to the date of tumour progression or death from other causes or censored at the time of the last contact. OS was defined as the time from registration to the study to death from any cause or censored at the time of the last contact. Statistical analyses were performed using the SPSS Statistics V.22.0 software. Two-tailed tests were used and p values of <0.05 were considered statistically significant.
Results
Patient population
We collected samples from 113 patients with LA HNSCC at two timepoints: at baseline and after completion of CRT. Baseline patient characteristics have been described in our previously published paper.11
IDO1 levels at baseline and post-treatment
In our cohort, 73 patients had evaluable specimens for CTC gene expression analysis. Forty patients were not included in the analysis because of low-quality RNA. Wilcoxon signed-rank test analysis revealed a significant overexpression of IDO1 at baseline compared with the post-treatment counterpart (p=0.007, online supplementary figure S1).
esmoopen-2019-000646supp001.pdf (54.5KB, pdf)
Association of IDO1 levels with tumour characteristics
According to our statistical analysis, IDO1 at baseline exhibited increased expression levels in male patients (p=0.030, online supplementary table S1). Moreover, a significant IDO1 upregulation was observed in heavy and ex-heavy smokers not only at baseline (p<0.001, online supplementary table S1) but also postchemoradiation (p=0.029, online supplementary table S2).
esmoopen-2019-000646supp002.pdf (155.2KB, pdf)
esmoopen-2019-000646supp003.pdf (179.7KB, pdf)
Association with clinical outcome
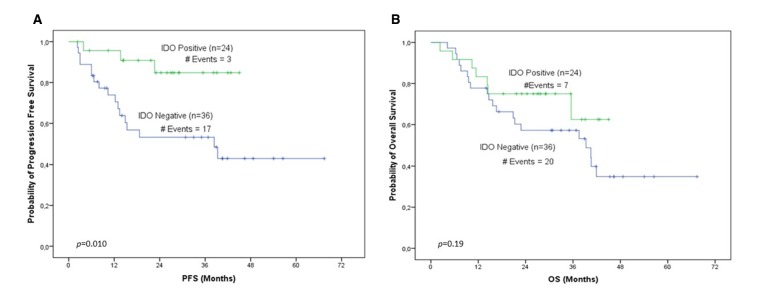
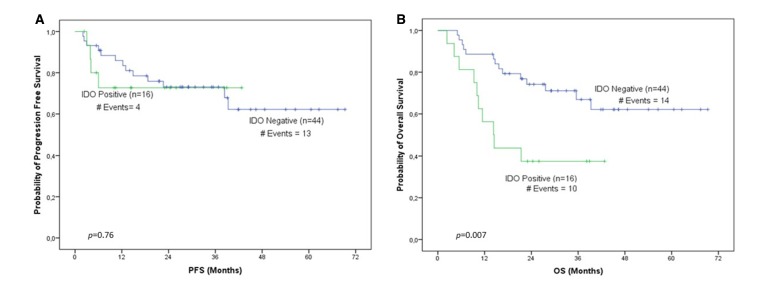
Median follow-up was 27.16 months (range 2.3–69.3), during which 24 patients progressed and 34 died. In our cohort, IDO1 levels were evaluated for association with PFS and OS. According to Kaplan-Meier analysis for IDO1 expression levels at baseline, patients stratified as IDO1 positive unequivocally demonstrated (p=0.010) longer PFS intervals compared with IDO1-negative ones (figure 1A). Furthermore, the cumulative probability of 3-year PFS for IDO1-positive patients was 0.85, whereas the corresponding probability for IDO1-negative ones was 0.55. Bootstrap univariate Cox proportional hazard regression analysis corroborated the favourable prognostic value of IDO1 at baseline, since IDO1 overexpression was associated with better survival in terms of PFS (HR=0.23, p=0.018; table 1), and patients categorised as IDO1 positive were 4.35 times less likely to relapse compared with IDO1-negative ones. Interestingly, post-treatment IDO1 mRNA levels seem to constitute a significant marker of unfavourable prognosis in terms of OS since both Kaplan-Meier analysis (p=0.007, figure 2B) and bootstrap univariate Cox proportional hazard regression analysis (HR=2.92, p=0.011; table 1) demonstrated that patients categorised as IDO1 positive are characterised by inferior OS intervals compared with those belonging to the IDO1-negative group. An also interesting finding was that post-treatment IDO1 expression levels retained their significant prognostic value as a continuous variable (HR=2.45, p=0.019; table 1).
Figure 1.
IDO1 expression levels before treatment. Shown are the Kaplan-Meier (A) PFS curve (p=0.010) and the (B) OS curve (p=0.19) for IDO1 expression levels before treatment. IDO1, indoleamine 2,3-dioxygenase 1; OS, overall survival; PFS, progression-free survival.
Table 1.
Univariate Cox proportional hazard regression analysis showing the correlation of IDO levels before and after treatment with PFS and OS
| Univariate analysis (N=60) | |||||||
| PFS | OS | ||||||
| Variable | HR* | 95% CI† | P value | Variable | HR* | 95% CI† | P value |
| Log IDO1 (before treatment) | 0.66 | 0.23 to 1.07 | 0.26 | Log IDO1 (before treatment) | 1.35 | 0.51 to 3.31 | 0.39 |
| IDO1 (before treatment) | IDO1 (before treatment) | ||||||
| Negative | 1.00 | Negative | 1.00 | ||||
| Positive | 0.23 | 0.02 to 0.50 | 0.018 | Positive | 0.57 | 0.19 to 1.36 | 0.21 |
| Log IDO (after treatment) | 1.19 | 0.49 to 2.89 | 0.60 | Log IDO (after treatment) | 2.45 | 1.17 to 7.60 | 0.019 |
| IDO (after treatment) | IDO (after treatment) | ||||||
| Negative | 1.00 | Negative | 1.00 | ||||
| Positive | 1.2 | 0.25 to 3.23 | 0.75 | Positive | 2.92 | 1.10 to 7.70 | 0.011 |
Bold values indicate statistical significance.
*HR estimated from Cox proportional hazard regression model.
†CI of the estimated HR. Results are based on 1000 bootstrap samples and obtained after the bias corrected and accelerated approach.
OS, overall survival; PFS, progression-free survival.
Figure 2.
IDO1 expression levels after treatment. Shown are the Kaplan-Meier (A) PFS curve (p=0.76) and the (B) OS curve (p=0.007) for IDO1 expression levels after treatment. IDO1, indoleamine 2,3-dioxygenase 1; OS, overall survival; PFS, progression-free survival.
A multivariate Cox regression analysis, adjusted for important and established prognostic parameters, such as smoking status, alcohol consumption, TNM stage and gender, revealed that IDO1 expression levels before treatment correlate with favourable prognosis for patients with HNSCC (HR=0.19, p=0.017) (table 2). Next, we evaluated the independence of IDO1 expression levels after treatment in predicting the probability of OS in patients with HNSCC by developing the same Cox multivariate proportional hazard regression model. According to this model, IDO1 expression levels after treatment are associated with unfavourable prognosis in terms of OS for those patients (HR=3.27, p=0.008) (table 2).
Table 2.
Multivariate Cox regression analysis showing the correlation of IDO expression before and after treatment with PFS and OS
| Multivariate analysis (N=60) | |||||||
| PFS | OS | ||||||
| Variable | HR* | 95% CI† | P value | Variable | HR* | 95% CI† | P value |
| IDO1 (before treatment) | IDO1 (after treatment) | ||||||
| Negative | 1.00 | Negative | 1.00 | ||||
| Positive | 0.19 | 0.03 to 0.46 | 0.017 | Positive | 3.27 | 1.03 to 2.05 | 0.008 |
| Smoke | Smoke | ||||||
| Light/never | 1.00 | Light/Never | 1.00 | ||||
| Heavy | 0.60 | 0.09 to 3.35 | 0.34 | Heavy | 0.66 | 0.17 to 1.91 | 0.38 |
| Ethanol | EtOH | ||||||
| Social/no | 1.00 | Social/no | 1.00 | ||||
| Heavy | 3.26 | 0.58 to 3.78 | 0.071 | Heavy | 2.14 | 0.57 to 8.67 | 0.16 |
| TNM stage‡ | TNM stage | ||||||
| I–III | 1.00 | I–III | 1.00 | ||||
| IV | 2.33 | 0.30 to 9.45 | 0.15 | IV | 2.12 | 0.28 to 1.42 | 0.18 |
| Sex | Sex | ||||||
| Male | 1.00 | Male | 1.00 | ||||
| Female | 0.80 | 0.27 to 2.41 | 0.80 | Female | 0.77 | 0.23 to 1.63 | 0.62 |
Bold values indicate statistical significance.
*HR estimated from Cox proportional hazard regression model.
†CI of the estimated HR. Results are based on 1000 bootstrap samples and obtained after the bias corrected and accelerated approach.
‡TNM staging system.
OS, overall survival; PFS, progression-free survival.
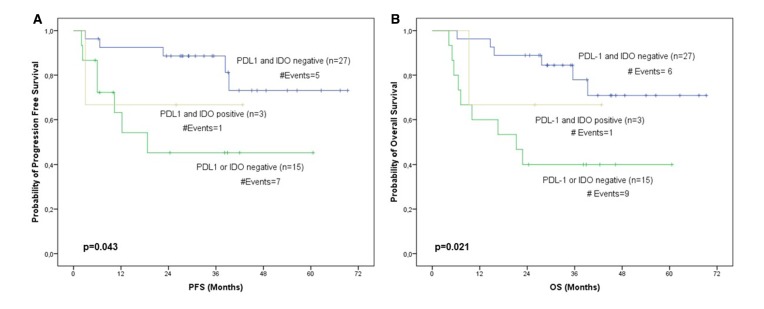
Moreover, given that we have previously assessed PDL-1 expression levels in CTCs after treatment at the same patient cohort,16 we evaluated the prognostic value of IDO1 and PDL-1 expression levels after categorisation of patients into PDL-1 and IDO1 negative, PDL-1 or IDO1 negative and PD-L1 and IDO1 positive. Therefore, according to Kaplan-Meier analysis, we found that patients with decreased expression levels of PDL-1 and IDO1 after treatment exhibited superior PFS (p=0.043) and OS (p=0.021) compared with the patients belonging to the other groups (figure 3).
Figure 3.
PDL-1 and IDO1 expression levels after treatment. Shown are the Kaplan-Meier (A) PFS curve (p=0.043) and the (B) OS curve (p=0.021) for PDL-1 and IDO1 expression levels after treatment. IDO1, indoleamine 2,3-dioxygenase 1; OS, overall survival; PFS, progression-free survival.
Discussion
The classical form of cell death induced by chemotherapy is apoptosis, which triggers production of immunosuppressive transforming growth factor-β by the macrophages that phagocytose the debris, leading to immune suppression and tolerance.17 With the majority of chemotherapy agents, a complex state of a combination of these events is observed, with much immunosuppressive apoptosis occurring side-by-side with more immunogenic forms of cell death. Thus, the main question remains which signal dominates on the local immune system. In vivo studies in apoptotic cells have revealed a potent regulatory role for IDO1 in controlling the choice between tolerance and immunity to dying cell.18 In this study, we demonstrate that in a cohort of patients with LA HNSCC, IDO1 is significantly overexpressed at baseline compared with post-treatment counterparts. Most importantly, we show a significant association between IDO1 expression and clinical outcome. More specifically, IDO1-positive patients at baseline were found to have significantly longer PFS and OS compared with IDO1-negative counterparts, whereas IDO1-positive patients post-treatment were characterised by inferior OS; indeed, both baseline and post-treatment IDO1 expression levels remained a strong and independent prognostic factor in multivariate analysis.
At present, we are not aware of the extent to which IDO1 is modified following chemotherapy treatment. In some tumours, IDO1 is constitutively expressed by the tumour cells themselves. This may serve as an immune-escape mechanism or may confer some non-immune survival advantage on the tumours.19 Several studies in hepatocellular carcinoma,20 pancreatic cancer,21 and endometrial22 and ovarian cancers23 have shown that high IDO1 expression is correlated with dismal prognosis. Furthermore, high IDO1 expression has been shown to significantly correlate with poor survival in tumours of the larynx, oral cavity and nasopharynx.24–26 Nevertheless, all these studies have evaluated IDO1 in pretreatment biopsies. This is useful for identifying which tumours constitutively express or stimulate the production of IDO1 as part of their underlying biology, but it gives no information about how much reactive IDO1 may have been induced in response to cell death and inflammation; this would have required treatment biopsies. Using a novel approach, we estimated for the first time IDO1 mRNA expression on CTCs derived for patients with HNSCC undergoing CRT, both at baseline and post-treatment, allowing real-time monitoring of IDO1 expression status.
In our study, 36% of patients had IDO1 overexpression post-treatment compared with 58% of patients at baseline. Interestingly, IDO1-positive patients at baseline were shown to have a significant improvement in PFS and OS compared with IDO1-negative patients. Upregulation of IDO1 is observed in tumours with T cell-inflamed phenotype, which is characterised by the presence of activated cytotoxic CD8+ T cells both within the tumour and the peritumoural stroma. Indeed, there is a growing body of evidence suggesting that these tumours show high expression of IDO1 and PDL-1, which is stimulated by interferon-γ produced by CD8+ T cells in vivo.27 These defined immune-system inhibitory pathways blunt the function of T cells and eventually allow tumour outgrowth. In this situation, upregulation of IDO may be a surrogate for a more robust spontaneous antitumour immune response and thus may be associated with a more favourable prognosis.19 Therefore, in our study, high IDO1 expression at baseline might reflect the presence of a T cell-inflamed phenotype, which is associated with good prognosis and potentially response to immunotherapy28 29 and chemotherapy.30 31 Of note, in a retrospective study by Saloura et al, 33%–47% of 558 HNSCC tumours demonstrated a T cell-inflamed phenotype similar to melanoma based on a gene expression signature.32
On the contrary, we demonstrated that patients characterised as IDO1 positive post-treatment show a statistically significant inferior OS compared with their IDO1-negative counterparts. In the multivariate analysis, post-treatment IDO1 expression levels retained their prognostic significance. Similarly, patients with combined decreased expression of PDL-1 and IDO1 had a statistically significant improvement in OS. Our patients were treated with radical chemoradiotherapy; accumulating evidence suggests that a decisive contribution to the long-term successful elimination of cancer by radiation and several chemotherapy drugs is made by notifying the immune system for the presence of dying cancer cells.33 However, dying tumour cells represent a rich source of antigens that are potentially immunogenic, but which cannot become actually immunogenic unless the relevant inhibitory pathways in the tumour, such as IDO1, are blocked. Indeed, IDO1 and its related downstream pathways may help create an undesirable tolerogenic milieu, which precludes the immune system from responding to antigens released from dying tumour cells.7 Thus, high expression of IDO1 post-treatment might prevent the induction of ICD, block the efficacy of chemoradiotherapy and result in decreased OS.
A relevant question is how to harness the immunogenic potential of chemotherapy or chemoradiotherapy to increase its anticancer efficacy. First, IDO1 is emerging as a mechanism that influences the crucial choice of whether dying cells will eventually become tolerogenic or immunogenic. Therefore, if the tolerogenic IDO1 pathway can be blocked, then conventional chemotherapy may be more spontaneously immunogenic. Indeed, preclinical mouse models show that IDO1 inhibitor drugs are synergistic with a variety of chemotherapeutic agents in several tumour models,34 35 and clinical trials are currently evaluating potential combinations of IDO1 inhibitors and chemotherapy agents. In addition, early data demonstrated antitumour activity in bladder cancer with an IDO1 small molecule inhibitor combined with anti-programmed cell death protein 1 (PD-1) therapy.36 Immune checkpoints modulate signalling and either inhibit or stimulate T-cell response. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) and PD-1 are distinct examples of coinhibitory molecules.37 Although intriguing phase II clinical trial data of IDO1/PD-1 doublets drove rapid clinical development of IDO1 inhibitors, producing tantalising clinical efficacy data, results of the phase III KEYNOTE-252/ECHO-301 clinical trial that evaluated the combination of IDO1 inhibitor epacadostat and anti-PD-1 antibody pembrolizumab in unresectable melanoma were disappointing.38
Of note, using CellSearch system in CTCs expressing PDL-1 in the same cohort of patients,16 we have previously shown that the percentage of CTCs expressing a molecular marker is not directly correlated with the total number of CTCs, since CTCs are highly heterogeneous, and therefore, it is not expected that they are all overexpressing IDO-1. Thus, since our RT-qPCR assay does not enumerate CTCs, our findings are directly related to IDO1 overexpression rather that the total number of CTCs. Therefore, IDO1 expression levels at the end of treatment do not reflect disease burden (minimal residual disease).
A major limitation of our study is that it is a single-institution cohort and our results need to be validated in large cohorts with longer follow-up.
In conclusion, IDO1 is one of the regulatory mechanisms that trigger immune suppression and tolerance in the tumour microenvironment. Our study strongly suggests that IDO1 mRNA expression is an independent prognostic factor for clinical outcome; high IDO1 mRNA expression is associated with favourable outcomes at baseline and poor survival after treatment. Despite disappointing results in a phase III trial, interests in therapeutic application of IDO1 inhibition are constantly increasing, particularly since the clinical implementation of immunotherapeutic approaches.
Acknowledgments
We thank the patients and their families.
Footnotes
Contributors: Design and conception: AP, PE, AK-S and GKo. Acquisition of data and analysis: PE, AK-S, AS, GKo, EK, EGi, PM, EGa, EM, GD, IK, EV, MA, MG, GKa, EL and AP. Manuscript draft: PE, A-KS and AP. Final review and approval: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Medical Ethical Committee of Attikon University hospital (Athens, Greece) and complies with the principles laid down in the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. meta-analysis of chemotherapy on head and neck cancer. Lancet 2000;355:949–55. [PubMed] [Google Scholar]
- 2.Pignon J-P, le Maître A, Maillard E, et al. Meta-Analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 3.Baxi S, Fury M, Ganly I, et al. Ten years of progress in head and neck cancers. J Natl Compr Canc Netw 2012;10:806–10. 10.6004/jnccn.2012.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 2014;232:199–209. 10.1002/path.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother 2006;29:233–40. 10.1097/01.cji.0000199193.29048.56 [DOI] [PubMed] [Google Scholar]
- 6.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J 2010;16:354–9. 10.1097/PPO.0b013e3181eb3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson TS, Mcgaha T, Munn DH. Chemo-Immunotherapy: role of indoleamine 2,3-dioxygenase in defining immunogenic versus tolerogenic cell death in the tumor microenvironment. Adv Exp Med Biol 2017;1036:91–104. 10.1007/978-3-319-67577-0_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun T, Zou K, Yuan Z, et al. Clinicopathological and prognostic significance of circulating tumor cells in patients with head and neck cancer: a meta-analysis. Onco Targets Ther 2017;10:3907–16. 10.2147/OTT.S136530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xenidis N, Perraki M, Kafousi M, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol 2006;24:3756–62. 10.1200/JCO.2005.04.5948 [DOI] [PubMed] [Google Scholar]
- 10.Tinhofer I, Konschak R, Stromberger C, et al. Detection of circulating tumor cells for prediction of recurrence after adjuvant chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol 2014;25:2042–7. 10.1093/annonc/mdu271 [DOI] [PubMed] [Google Scholar]
- 11.Economopoulou P, Koutsodontis G, Strati A, et al. Surrogates of immunologic cell death (ICD) and chemoradiotherapy outcomes in head and neck squamous cell carcinoma (HNSCC). Oral Oncol 2019;94:93–100. 10.1016/j.oraloncology.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 12.Strati A, Markou A, Parisi C, et al. Gene expression profile of circulating tumor cells in breast cancer by RT-qPCR. BMC Cancer 2011;11:422. 10.1186/1471-2407-11-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markou A, Strati A, Malamos N, et al. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem 2011;57:421–30. 10.1373/clinchem.2010.154328 [DOI] [PubMed] [Google Scholar]
- 14.Zavridou M, Mastoraki S, Strati A, et al. Evaluation of preanalytical conditions and implementation of quality control steps for reliable gene expression and DNA methylation analyses in liquid biopsies. Clin Chem 2018;64:1522–33. 10.1373/clinchem.2018.292318 [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 16.Strati A, Koutsodontis G, Papaxoinis G, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol 2017;28:1923–33. 10.1093/annonc/mdx206 [DOI] [PubMed] [Google Scholar]
- 17.Green DR, Ferguson T, Zitvogel L, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol 2009;9:353–63. 10.1038/nri2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravishankar B, Liu H, Shinde R, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A 2012;109:3909–14. 10.1073/pnas.1117736109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol 2016;37:193–207. 10.1016/j.it.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan K, Wang H, Chen M-shan, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol 2008;134:1247–53. 10.1007/s00432-008-0395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 2008;206:849–54. discussion 54-6. 10.1016/j.jamcollsurg.2007.12.014 [DOI] [PubMed] [Google Scholar]
- 22.Ino K, Yoshida N, Kajiyama H, et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer 2006;95:1555–61. 10.1038/sj.bjc.6603477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto A, Nikaido T, Ochiai K, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 2005;11:6030–9. 10.1158/1078-0432.CCR-04-2671 [DOI] [PubMed] [Google Scholar]
- 24.Ben-Haj-Ayed A, Moussa A, Ghedira R, et al. Prognostic value of indoleamine 2,3-dioxygenase activity and expression in nasopharyngeal carcinoma. Immunol Lett 2016;169:23–32. 10.1016/j.imlet.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 25.Ye J, Liu H, Hu Y, et al. Tumoral indoleamine 2,3-dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch 2013;462:73–81. 10.1007/s00428-012-1340-x [DOI] [PubMed] [Google Scholar]
- 26.Seppälä M, Halme E, Tiilikainen L, et al. The expression and prognostic relevance of indoleamine 2,3-dioxygenase in tongue squamous cell carcinoma. Acta Otolaryngol 2016;136:729–35. 10.3109/00016489.2016.1152631 [DOI] [PubMed] [Google Scholar]
- 27.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013;5:200ra116. 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014–22. 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 30.Halama N, Braun M, Kahlert C, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res 2011;17:678–89. 10.1158/1078-0432.CCR-10-2173 [DOI] [PubMed] [Google Scholar]
- 31.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28:105–13. 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 32.Saloura V, Zuo Z, Koeppen H, et al. Correlation of T-cell inflamed phenotype with mesenchymal subtype, expression of PD-L1, and other immune checkpoints in head and neck cancer. J Clin Oncol 2014;32:6009 10.1200/jco.2014.32.15_suppl.6009 [DOI] [Google Scholar]
- 33.Showalter A, Limaye A, Oyer JL, et al. Cytokines in immunogenic cell death: applications for cancer immunotherapy. Cytokine 2017;97:123–32. 10.1016/j.cyto.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou D-Y, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res 2007;67:792–801. 10.1158/0008-5472.CAN-06-2925 [DOI] [PubMed] [Google Scholar]
- 35.Li M, Bolduc AR, Hoda MN, et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer 2014;2:21. 10.1186/2051-1426-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabernero J, Luke JJ, Joshua AM, et al. BMS-986205, an indoleamine 2,3-dioxygenase 1 inhibitor (IDO1i), in combination with nivolumab (NIVO): updated safety across all tumor cohorts and efficacy in pts with advanced bladder cancer (advBC). J Clin Oncol 2018;36:4512 10.1200/JCO.2018.36.15_suppl.4512 [DOI] [Google Scholar]
- 37.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georgina Long V V, Hamid O, Gajewski T, et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study. J Clin Oncol 2018;36:108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000646supp001.pdf (54.5KB, pdf)
esmoopen-2019-000646supp002.pdf (155.2KB, pdf)
esmoopen-2019-000646supp003.pdf (179.7KB, pdf)