COVID-19, where Co stands for corona, VI stands for virus, and D denotes disease, in the recent past referred to as 2019 novel coronavirus or 2019-nCoV, has impacted numerous lives and businesses, and has led to a surreal emergency state within world communities. COVID-19 and the future emergence of dangerous viruses will have strong and as yet possibly unanticipated consequences and impact on the present and future use of cellular therapies. In this commentary, we offer a dispassionate assessment of where we believe COVID-19, as well as future emerging viruses, might compromise successful cell transplantation (Fig. 1). These therapies include hematopoietic cell transplantation (HCT) using umbilical cord blood (CB), bone marrow (BM), and mobilized peripheral blood, which contain hematopoietic stem (HSC) and progenitor (HPC) cells, as well as various cellular populations involved in the emerging fields of reparative and regenerative medicine. Such cell populations include HSC, HPC, mesenchymal stem/stromal cells (MSC), and immune cells such as lymphocytes used in chimeric antigen receptor (CAR) T-cell therapies, as well as pluripotent stem cell–based therapies.
FIG. 1.
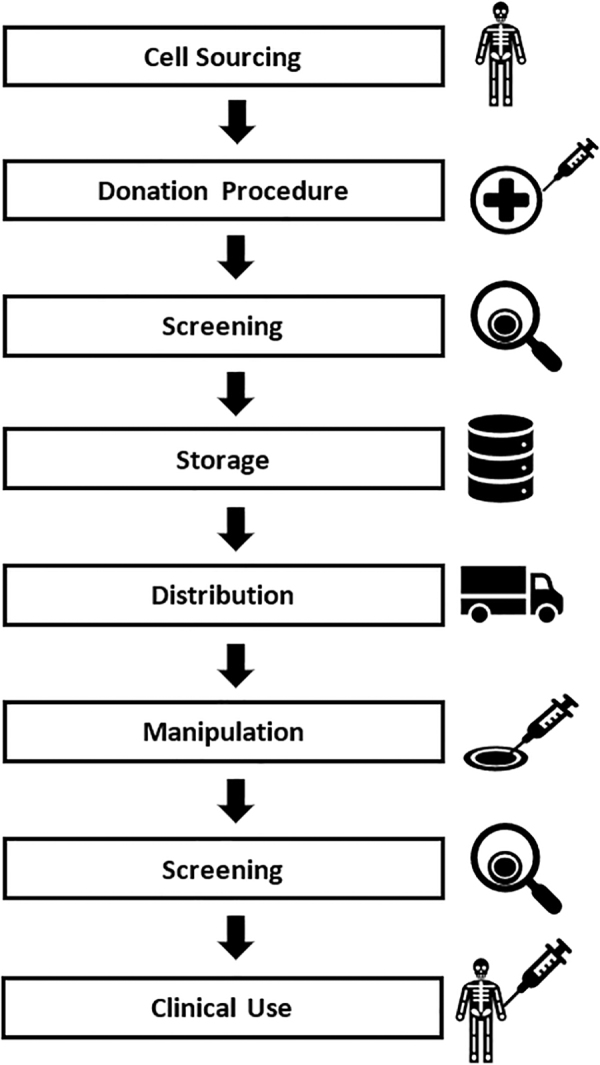
Potential stages of impact for emerging viruses on cellular therapies: anticipating risk points for viral infection during cellular therapy.
Concerns with COVID-19 and other-to-emerge viruses reflect the cells themselves, their collections, storage as cryopreserved cells, their in vitro modifications that include addition of new genes, and/or their expansion ex vivo, and the donors and recipients of these manipulated cells. With the outbreak of COVID-19 and the attendant concerns, fewer persons are currently donating cells for cellular therapies. The pool of available donors is already woefully inadequate. The current situation both literally and psychologically shrinks further the uninfected number of potential donors. This includes CB for HCT, those cells that have been donated since the known start of this highly infectious virus, and likely even CB stored prior to the start of the COVID-19 pandemic. All will have to be checked for the presence of COVID-19, and in the future for other emerging dangerous viruses.
There is also the concern of how to ship these cellular sources for therapy (e.g., CB and BM) across countries. The National Marrow Donor Program has had to obtain special permission for transport of these precious lifesaving cells, and in these uncertain times, other shippers of cells for therapy may also need to obtain such special permission. So, why the concern for COVID-19 screening, even for many cells stored frozen (e.g., CB for CB banking efforts) in the recent and not so recent past? The cells stored frozen in a cryopreserved state may already have been infected with COVID-19. Cells frozen that are potentially contaminated with other infectious agents are usually not stored with those believed not to be contaminated. Moreover, viruses are very small particles, and if recent cell samples are placed in the same freezer tanks, the virus might be capable of breaching the freezer bag and surviving long enough to infect other stored frozen cells. “Leakage” of the virus from one sample frozen bag to another in liquid nitrogen storage, especially in the gaseous phase of the liquid nitrogen, is not an impossible scenario. Caution indicates screening cells intended for cellular therapy before they are stored and again before they are used clinically. Stored cryopreserved CB units usually have small aliquots of the CB stored for analysis if needed, and these small samples can be tested for COVID-19. This would add to the extensive list of other infectious agents that are currently screened for by CB banking companies. This requires a rigorously reliable test to screen for COVID-19.
Scientific and clinical investigators who are working with cells for cellular therapy, whether involved in research efforts or actual clinical transplantation, must assume that all the human samples they are working with may contain COVID-19, and continue to undertake the required rigorous safety precautions necessary to protect themselves.
There is also the real concern that COVID-19 contamination might change the phenotypic and/or functional characteristics of the cells. This is presently an unknown, but the possibility exists that if the virus does influence the cells of interest, it could make it difficult to obtain consistent and reproducible results—a cornerstone for reliable scientific investigations. COVID-19 influences many organs, not just the lung, and what of cellular therapies using MSC and immune cells, such as CART cells? CART cell therapy is associated with the devastating side effects elicited by the CART cell-induced cytokine storm. While investigators have been dealing with efforts to dampen the life-threatening side effects of the CART cell-induced cytokine storm, how will COVID-19, if present in the CART cells, donors, or recipients of these cells, impact this promising treatment? Viruses are known to induce cytokine release. Will COVID-19 exacerbate the cytokine storm elicited by the CART cells?
This then brings us to the recipients of HCT and other cellular therapies. In the short term, transplants as well as other procedures are necessarily being scaled back because of the risk of exposure to a potentially immunocompromised recipient, the lack of available ventilators for the recipient, and the current inability to assess whether the transplant is infected. If the recipients are infected with COVID-19, even if it only elicits mild effects that allow for recovery, how will the virus in the recipients influence the infused cells? In HCT, especially with CB, would this result in delayed time to engraftment for each donor cell type, lower survival, increased relapse rates, and acute and chronic graft-versus-host disease? Pregnant recipients have the further potential concern, as recent news reports suggest, that COVID-19 can even cross the mother–child placental barrier.
We are currently in the midst of uncertain times regarding the potential impact and influences of COVID-19 and a future of continuing emergence of dangerous viruses on cell products and their use in cellular therapies. It is important that we seriously consider how these viruses will now and in the future affect the cell products, the storage of cryopreserved cells, and their use for various renditions of cellular therapy, including that of reparative and regenerative medicine. Internationally, health organizations and scientific societies are offering guidance on COVID-19 and stem-cell transplants and surveying their members on future patient impact (see Table 1 for some examples). Now, not later, is the time to begin scientific investigations into the potential roles of COVID-19 and other-to-emerge viruses, not just for the potential to infect recipients of the cellular therapies, but also for how they might influence the cell products and their use for recipients in need of these valuable and in some cases lifesaving therapies.
Table 1.
Health Organization and Scientific Society Guidance on Impact of COVID-19 on Stem-Cell Transplants
| American Society for Transplantation and Cellular Therapy | https://www.astct.org/communities/community-home?CommunityKey=d3949d84-3440-45f4-8142-90ea05adb0e5 |
| American Society of Hematology | https://www.hematology.org/COVID-19 |
| American Society of Transplantation | https://www.myast.org/covid-19-information |
| European Society for Blood and Marrow Transplantation | https://www.ebmt.org/covid-19-and-bmt |
| The Lancet | https://www.thelancet.com/coronavirus |
| The Transplantation Society | https://www.tts.org/covid-19 |
| World Health Organization | https://www.who.int/emergencies/diseases/novel-coronavirus-2019 |
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded in part by NIH HL R35 (Outstanding Investigator Award) 139599 to HEB, and by the Center for Urban Responses to Environmental Stressors Grant #P30 ES020957 from the National Institute of Environmental Health Sciences to G.C.P.


