Abstract
Background: Retropharyngeal lymph node metastases (RPMs) from differentiated thyroid carcinoma (DTC) are rare. Treatment includes surgical resection, radioactive iodine (RAI) therapy, or external beam radiation therapy (EBRT). The objective of this study was to describe our experience in the management of DTC-associated RPMs.
Methods: Patients diagnosed with a DTC-associated RPM from 1999 to 2018 were identified at our institution, using key search terms in imaging and histology reports. Patient and tumor characteristics were recorded, and patients were grouped according to RPM management: observation, nonsurgical treatment, or surgical resection. The estimated rates of local RPM control, disease-specific survival (DSS), and distant metastasis-free probability (DMFP) were calculated by using the Kaplan-Meier method.
Results: Of the 65 patients identified, 53 (82%) had an RPM as a manifestation of recurrent disease. Twenty-five patients (38%) underwent observation, 13 (20%) received nonsurgical treatment (RAI, EBRT, and/or systemic therapy), and 27 (42%) underwent surgical resection. In the observation cohort, all patients had a stable RPM, which in the majority (80%) of cases remained <1.5 cm during the period of observation (median 28 months). Of the 13 patients in the nonsurgical treatment cohort, 3 received RAI therapy, 7 received EBRT, and 3 received systemic therapy only.
In the surgical cohort, the median RPM maximum diameter was 2.0 cm (range 0.8–4.2 cm). The size of the RPM was predictive of surgical resection versus observation (p < 0.001). A transcervical approach was employed in 19 patients, and a transoral approach was used in 8 patients. The 5-year rate of local RPM control was 92%. For the whole cohort, the 5- and 10-year DMFP were 72% and 62%, respectively; the 5- and 10-year DSS were 93% and 81%, respectively.
Conclusions: DTC-associated RPMs manifest as recurrent disease in the majority of patients. Select patients with a small-volume and nonprogressive RPM may be suitable for observation, whereas surgery is likely warranted in large or progressing RPMs. In general, the presence of an RPM from DTC appears to be associated with aggressive disease.
Keywords: retropharyngeal lymph node metastasis, thyroid carcinoma, management, surgery, transoral robotic surgery
Introduction
The retropharyngeal space is an inverted-pyramid-shaped virtual space bordered by the visceral fascia and superior constrictor muscles anteriorly, the prevertebral fascia posteriorly, the carotid sheath laterally, the skull base superiorly, and the greater cornu of the hyoid inferiorly. Its contents are limited to fat and retropharyngeal lymph nodes (1). Rouvière (2) classified retropharyngeal lymph nodes into a medial chain and lateral chain, in which lymph nodes can be identified anywhere between the base of the skull cephalad and the greater cornu of the hyoid bone caudad.
Regional lymph node metastases are present in an estimated 30–80% of patients at initial presentation of differentiated thyroid carcinoma (DTC) (3–6). The first-echelon nodes are commonly Level VI, followed by Level II–V and Level VII lymph nodes. Small case series and individual case reports have estimated the incidence of retropharyngeal lymph node metastasis (RPM) in DTC patients to be 0.43–5% (7–11). DTC-associated RPMs are rarely found at initial presentation and more commonly manifest in the context of recurrent or persistent disease (9). Treatment may involve surgical resection, radioactive iodine (RAI) therapy, external beam radiation therapy (EBRT), systemic therapy, or observation. The aim of RPM management is to maximize curative intent while minimizing associated morbidity. The optimal management strategy for a DTC-associated RPM is based on the size, location, and progression of the RPM as well as on the overall disease burden and patient characteristics. Retropharyngeal disease from DTC is a rare event. However, with increasing prevalence of DTC and widespread use of imaging and thyroglobulin measurements, the rates of RPM detection are likely to increase. The aim of our study was to discuss the considerations in treatment and prognosis for DTC-associated RPM.
Materials and Methods
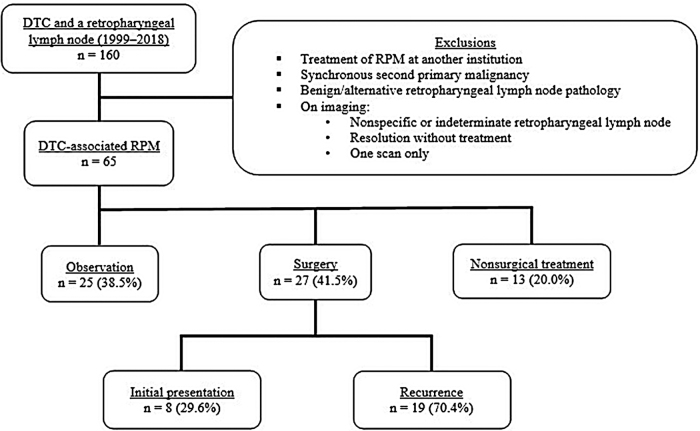
After institutional review board approval, DTC patients were identified as having a retropharyngeal lymph node, using the search term “retropharyngeal” in imaging and histopathology reports, from January 1999 to June 2018. Patients with a synchronous second malignancy or treatment of RPM at another institution were excluded. Patients were also excluded if, on imaging, the retropharyngeal lymph node was (i) present on one scan only, (ii) deemed nonspecific or indeterminate, or (iii) resolved without treatment (Fig. 1). Patients were classified into three cohorts based on management: (i) observation, (ii) nonsurgical treatment (RAI therapy, EBRT, or systemic therapy), or (iii) surgical resection. Patients may have received their initial thyroid surgery at another institution and subsequently referred to our institution for management of the RPM.
FIG. 1.
Exclusion criteria and patient cohorts. DTC, differentiated thyroid carcinoma; RPM, retropharyngeal lymph node metastasis.
Assessment of the retropharyngeal lymph node
A retropharyngeal lymph node was defined as a lymph node located within the boundaries of the retropharyngeal space. A retropharyngeal lymph node was considered to be an RPM if imaging was indicative of DTC metastasis with keywords such as “consistent with,” “suspicious for,” or “probably” on an imaging report intended to connote a diagnostic certainty level of >75%. RPMs were identified on cross-sectional imaging by using computed tomography (CT), magnetic resonance imaging (MRI), RAI whole-body scan, or positron emission tomography (PET) and confirmed on histopathology when available. In the observation and nonsurgical cohort, the diagnosis of an RPM was based on imaging characteristics that were suspicious for metastases and did not have histological confirmation as the majority of these patients had metastatic disease elsewhere. Of the 160 patients identified, 65 met the inclusion criteria.
Management and outcomes
Patient and primary tumor characteristics were recorded as well as details of RPM management and oncological outcomes. Recurrences were categorized as locoregional or distant and confirmed by cyto-histopathology or detected on imaging. Locoregional recurrence was defined as DTC in regional cervical lymph nodes or the thyroid bed. Distant metastasis-free probability (DMFP) was calculated from the date of initial surgery to the date of first structural recurrence at a distant site. Disease-specific survival (DSS) was calculated from the date of initial surgery to the date of last appointment with the institution's disease management team or date of death with evidence of locoregional or distant disease at final follow-up. Local RPM control, defined as absence of recurrent or persistent disease within the retropharyngeal space after surgical resection, was calculated from the date of RPM surgery.
Statistical analyses
Statistical analyses were carried out by using SPSS version 25.0 software (IBM). Survival outcomes were calculated by using the Kaplan-Meier method. The difference in median maximum RPM diameter on initial imaging between the surgical cohort and observation cohort was assessed by using the Mann-Whitney U test. A p-value <0.05 was considered significant.
Results
Patient, primary tumor, and RPM characteristics
Patient characteristics are summarized in Table 1. The median age at initial surgery was 42 years (range 16–82 years); 59% were female. Forty-five patients (69%) had their initial surgery at another institution. Using the American Joint Committee on Cancer (AJCC) eighth edition pathological TNM staging system (12), 39% of the patients had advanced T classification (T3 or T4). A central and/or lateral neck dissection was performed in 51 patients (79%); 39 patients (60%) had pathological N1b disease. The median maximum diameter of a metastatic regional lymph node at initial surgery was 2.9 cm (range 0.4–5.2 cm). Fifty-five patients had papillary thyroid carcinoma, seven patients had poorly DTC, and three patients had Hürthle cell carcinoma. Excluding the patients with poorly DTC, 28 patients (43%) were deemed at high risk for recurrence according to the American Thyroid Association (ATA) risk stratification system (13). Of the 65 patients, 72% were stage I, 12% were stage II, 5% were stage III, 5% were stage IV, and 6% did not have sufficient information to determine staging. The RPM was a manifestation of recurrent disease in 53 patients (82%) (Table 2). The RPM was ipsilateral to the primary tumor in 52 patients (80%). The median maximum RPM diameter on initial imaging was 1.3 cm (range 0.4–4.2 cm). CT was the imaging modality used for RPM detection in 68% of patients.
Table 1.
Patient and Primary Tumor Characteristics
| Characteristic | No. of patients (%) |
|||
|---|---|---|---|---|
| Whole cohort (n = 65) | Observation (n = 25) | Nonsurgical (n = 13) | Surgery (n = 27) | |
| Median age | 42 (16–82) years | 38 (16–76) years | 58 (20–82) years | 50 (25–67) years |
| <55 years | 48 (73.8) | 22 (88.0) | 6 (46.2) | 20 (74.1) |
| ≥55 years | 17 (26.2) | 3 (12.0) | 7 (53.8) | 7 (25.9) |
| Sex | ||||
| Female | 38 (58.5) | 17 (68.0) | 6 (46.2) | 15 (55.6) |
| Male | 27 (41.5) | 8 (32.0) | 7 (53.8) | 12 (44.4) |
| Pathological T stage | ||||
| T1 | 16 (24.6) | 7 (18.0) | 2 (15.4) | 7 (25.9) |
| T2 | 15 (23.1) | 6 (24.0) | 2 (15.4) | 7 (25.9) |
| T3 | 12 (18.5) | 4 (16.0) | 3 (23.1) | 5 (18.5) |
| T4 | 13 (20.0) | 6 (24.0) | 4 (30.8) | 3 (11.1) |
| Unknown | 9 (13.8) | 2 (8.0) | 2 (15.4) | 5 (18.5) |
| Pathological N stage | ||||
| N0 | 5 (7.7) | 3 (12.0) | 0 | 2 (7.4) |
| N1a | 17 (26.2) | 8 (32.0) | 1 (7.7) | 8 (29.6) |
| N1b | 39 (60.0) | 13 (52.0) | 10 (76.9) | 16 (59.3) |
| Unknown | 4 (6.2) | 1 (4.0) | 2 (15.4) | 1 (3.7) |
| M Stage | ||||
| M0 | 61 (93.8) | 24 (96.0) | 11 (84.6) | 26 (96.3) |
| M1 | 1 (1.5) | 0 | 0 | 1 (3.7) |
| Unknown | 3 (4.6) | 1 (4.0) | 2 (15.4) | 0 |
| Extrathyroidal extension | ||||
| Macroscopic | 16 (24.6) | 6 (24.0) | 5 (38.5) | 5 (18.5) |
| Microscopic | 29 (44.6) | 12 (48.0) | 6 (46.2) | 11 (40.7) |
| Not identified | 8 (12.3) | 3 (12.0) | 0 | 5 (18.5) |
| Unknown | 12 (18.5) | 4 (16.0) | 2 (15.4) | 6 (22.2) |
| Initial histology | ||||
| Papillary | 55 (84.6) | 23 (92.0) | 8 (61.5) | 24 (88.9) |
| Poorly differentiated | 7 (10.8) | 1 (4.0) | 4 (30.8) | 2 (7.4) |
| Hürthle cell | 3 (4.6) | 1 (4.0) | 1 (7.7) | 1 (3.7) |
| Neck dissection at initial surgery | ||||
| None | 13 (20.0) | 7 (28.0) | 0 | 6 (22.2) |
| Central | 10 (15.4) | 4 (16.0) | 1 (7.7) | 5 (18.5) |
| Lateral | 15 (23.1) | 6 (24.0) | 4 (30.8) | 5 (18.5) |
| Central and lateral | 26 (40.0) | 8 (32.0) | 7 (53.8) | 11 (40.7) |
| Unknown | 1 (1.5) | 0 | 1 (7.7) | 0 |
| Median maximum LN diameter | 2.9 (0.4–5.2) cm | 2.5 (0.4–5.2) cm | 2.6 (2.3–3.6) cm | 3.4 (0.7–5.0) cm |
| ATA risk | ||||
| Low | 0 | 0 | 0 | 0 |
| Intermediate | 22 (33.8) | 12 (48.0) | 5 (38.5) | 5 (18.5) |
| High | 28 (43.1) | 10 (40.0) | 3 (23.1) | 15 (55.6) |
| Unknown | 8 (12.3) | 2 (8.0) | 1 (7.7) | 5 (18.5) |
| Not applicable | 7 (10.8) | 1 (4.0) | 4 (30.8) | 2 (7.4) |
| AJCC stage | ||||
| I | 47 (72.3) | 21 (84.0) | 5 (38.5) | 21 (77.8) |
| II | 8 (12.3) | 2 (8.0) | 3 (23.1) | 3 (11.1) |
| III | 3 (4.6) | 1 (4.0) | 1 (7.7) | 1 (3.7) |
| IV | 3 (4.6) | 0 (0.0) | 2 (15.4) | 1 (3.7) |
| Unknown | 4 (6.2) | 1 (4.0) | 2 (15.4) | 1 (3.7) |
Table 2.
Characteristics of the Retropharyngeal Lymph Node Metastases
| Characteristic | No. of patients (%) |
|||
|---|---|---|---|---|
| Whole cohort (n = 65) | Observation (n = 25) | Nonsurgical (n = 13) | Surgery (n = 27) | |
| Time of RPM diagnosis | ||||
| Initial presentation | 12 (18.5) | 3 (12.0) | 1 (7.7) | 8 (29.6) |
| Recurrent disease | 53 (81.5) | 22 (88.0) | 12 (92.3) | 19 (70.4) |
| RPM location | ||||
| Ipsilateral | 52 (80.0) | 18 (72.0) | 10 (76.9) | 24 (88.9) |
| Contralateral | 7 (10.8) | 4 (16.0) | 2 (15.4) | 1 (3.7) |
| Bilateral | 2 (3.1) | 1 (4.0) | 1 (7.7) | 0 |
| Unknown | 4 (6.2) | 2 (8.0) | 0 | 2 (7.4) |
| Median maximum RPM diameter | 1.3 (0.4–4.2) cm | 1.1 (0.4–4.1) cm | 1.2 (0.7–2.4) cm | 2.0 (0.8–4.2) cm |
| Concurrent disease at RPM diagnosisa | ||||
| None | 20 (30.8) | 8 (32.0) | 2 (15.4) | 10 (37.0) |
| Locoregional | 40 (61.5) | 15 (60.0) | 10 (76.9) | 15 (55.6) |
| Distant | 15 (23.1) | 4 (16.0) | 6 (46.2) | 5 (18.5) |
| Previous or concomitant MRND | ||||
| Yes | 60 (92.3) | 21 (84.0) | 12 (92.3) | 27 (100) |
| No | 5 (7.7) | 4 (16.0) | 1 (7.7) | 0 |
| Imaging for RPM detection | ||||
| CT | 44 (67.7) | 19 (76.0) | 9 (69.2) | 16 (59.3) |
| MRI | 15 (23.1) | 4 (16.0) | 1 (7.7) | 10 (37.0) |
| PET | 3 (4.6) | 2 (8.0) | 0 | 1 (3.7) |
| 131I RAI | 3 (4.6) | 0 | 3 (23.1) | 0 |
| Median follow-up from RPM diagnosis | 35 (2–228) months | 28 (2–182) months | 32 (4–102) months | 56 (5–228) months |
Some patients had both locoregional and distant concurrent disease.
CT, computed tomography; MRI, magnetic resonance imaging; MRND, modified radical neck dissection; PET, positron emission tomography; RAI, radioactive iodine; RPM, retropharyngeal lymph node metastasis.
Outcomes for the whole cohort
The median follow-up was 78 months (range 8–487 months) from the initial thyroid surgery and 35 months (range 2–228 months) from the time of RPM diagnosis (Table 2). Of the 65 patients, 25 underwent observation, 13 received nonsurgical treatment, and 27 had surgical resection of the RPM. At the time of RPM diagnosis, concurrent distant disease was present in 16% of the patients in the observation cohort, 46% of the patients in the nonsurgical treatment cohort, and 19% of the patients in the surgery cohort. Twenty-four patients had distant disease on final follow-up. The 5- and 10-year rates of DMFP were 72% and 62%, respectively. The 5- and 10-year rates of DSS were 93% and 81%, respectively. The cause of death was distant metastatic disease in the majority (90%) of cases. No patient had uncontrolled RPM disease or died as a result of extensive disease within the retropharyngeal space.
Outcomes for the observation cohort
Twenty-five patients received no treatment for the RPM. The median duration of observation was 28 months (range 2–182 months) (Table 2). The RPM was a manifestation of recurrent disease in 88% of patients. At the time of RPM diagnosis, 15 patients (60%) had concurrent locoregional disease and 4 patients (16%) had concurrent distant disease. The median maximum RPM diameter was 1.1 cm on initial imaging (Table 2). Twenty patients (80%) had small-volume nonprogressive disease, which remained <1.5 cm during the period of observation. In the remaining five patients, the RPM was >1.5 cm on initial imaging but showed no progression during the observation period. All 25 patients had evidence of structural disease at final follow-up: 19 patients (76%) had locoregional disease, 6 patients (24%) had distant disease, and there were no disease-specific deaths.
Outcomes for the nonsurgical treatment cohort
At the time of RPM diagnosis, concurrent locoregional disease was present in 10 of the 13 patients in the nonsurgical treatment cohort, and concurrent distant disease was present in 6 patients. This heterogeneous group included three patients whose RPM was incidentally found on an RAI whole-body posttherapy scan. For all three patients, subsequent cross-sectional imaging showed complete resolution of disease, and no evidence of disease was found at final follow-up.
Three patients received EBRT to the RPM. In all three patients, the RPM had been progressing in size and was >2 cm before treatment. Four other patients received EBRT to the thyroid bed or lateral neck for recurrent locoregional disease. In these seven patients, EBRT achieved stability, a reduction in the size, or complete resolution of the RPM. The median maximum RPM diameter before EBRT was 2.0 cm (range 0.8–2.9 cm), and the median maximum RPM diameter after EBRT was 1.0 cm (0.0–2.5 cm).
The remaining three patients received systemic therapy for incurable, progressive, PET-avid, RAI-refractory locoregional and distant disease. One patient was treated with targeted therapy in the form of an angiogenesis inhibitor followed by a kinase inhibitor for progressive and extensive regional nodal disease and lung metastasis. The second patient had extensive regional and mediastinal recurrent disease with anaplastic transformation, which was treated with paclitaxel and an MEK inhibitor. The third patient had poorly DTC at initial presentation and distant disease at the time of RPM diagnosis, which was treated with an angiogenesis inhibitor followed by a kinase inhibitor. Two patients underwent MSK-IMPACT genetic testing, which identified a BRAFV600E mutation in each. Two of the three patients died of the disease by final follow-up.
Outcomes for the surgical cohort
Twenty-seven (42%) patients underwent surgical resection of the RPM. Fourteen patients (52%) had the histological subtype tall cell variant or tall cell features confirmed on histopathology at initial surgery or on subsequent locoregional recurrences. At the time of RPM diagnosis, concurrent locoregional disease was present in 15 patients (56%) and concurrent distant disease was present in 5 (19%). The median maximum RPM diameter on initial imaging was 2.0 cm (range 0.8–4.2 cm), significantly larger than that in the observation cohort (p = 0.001).
Nineteen patients (70%) underwent a transcervical approach and eight (30%) underwent a transoral approach. A transoral approach was first performed for a DTC-associated RPM at our institution in 2013. It was reserved for patients with a supero-medially located RPM whereas a transcervical approach was adopted in the majority of cases (68%) when a concurrent neck dissection was required. Of the eight patients who underwent a transoral approach, six had robotically assisted surgery. Postoperatively, dysphagia was the most commonly reported complication, occurring in about 25% of the patients in each subgroup. One patient had a hypoglossal nerve injury following the transcervical approach.
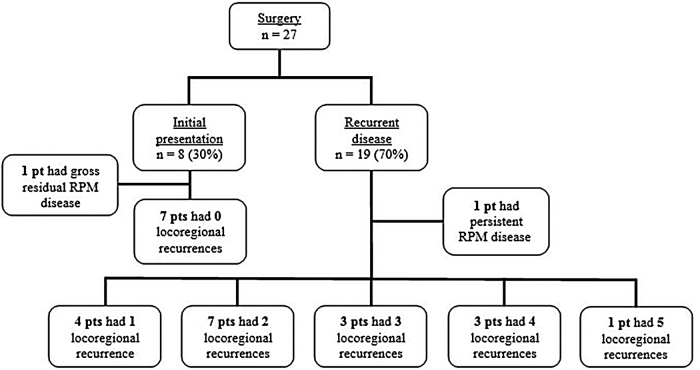
The estimated five-year rate of local RPM control was 92%. The RPM was identified at initial presentation in 8 (30%) of the 27 patients. One of these patients had poorly DTC with M1 disease at initial presentation; this patient had gross residual disease in one of three RPMs after initial surgery and underwent postoperative chemoradiotherapy. There were no other locoregional recurrences in these eight patients by final follow-up (Fig. 2), but one patient developed distant disease to the lungs at 49 months (Table 3). In contrast, 19 patients developed a DTC-associated RPM as a manifestation of recurrent disease, of whom 14 had ≥2 locoregional recurrences (Fig. 2). One patient with recurrent disease had a persistent RPM on postoperative imaging and was managed with RAI therapy. Of the 19 patients presenting with recurrent disease, 8 developed distant disease by final follow-up and 3 patients subsequently died of the disease (Table 3).
FIG. 2.
Locoregional recurrence in the surgical cohort. pt, patient.
Table 3.
Responses to Therapy in the Surgical Cohort at Final Follow-Up
| Response to therapya | No. of patients (n = 27) |
|
|---|---|---|
| RPM at initial presentation (n = 8) | RPM at disease recurrence (n = 19) | |
| Excellent | 2 | 2 |
| Indeterminate | 3 | 6 |
| Biochemical incomplete | 1 | 1 |
| Structural incomplete | 2 | 10 |
| Locoregional disease | 0 | 2 |
| Distant disease | 2 | 8 |
American Thyroid Association classification.
Discussion
Presentation
Given the propensity of RPMs to present as part of recurrent disease in DTC, theories regarding the lymphatic spread to the retropharyngeal space have been widely postulated. In a recent systematic review of 92 patients, Hartl et al. (14) reported that 74% of patients with DTC-associated RPM had a previous or concomitant lateral nodal metastasis, and 49% of the patients had previously undergone a lateral neck dissection (14). All 25 RPM patients in the study by Wang et al. (15) and all 12 patients in the study by Togashi et al. (10) underwent a neck dissection before or at the time of RPM diagnosis. In our own cohort, 86% of patients initially presented with pN1 disease, 60% with pN1b disease, and the median maximum diameter of a metastatic regional lymph node was 2.9 cm (range 0.4–5.2 cm). Further, 92% of patients had had a previous or concomitant lateral neck dissection. Therefore, it has been hypothesized that DTC-associated RPM may be a manifestation of collateral lymphatic drainage pathways as a result of extensive nodal disease at presentation or previous neck dissections (2,7,10,16,17). Further, Rouvière studied the human lymphatic drainage pathways in 30 cadavers and reported that “in about one-fifth of cases, the thyroid lymph-capillary network is connected directly by a lymphatic trunk with retropharyngeal nodes” (2). The location of the primary thyroid tumor may impact on the route of lymphatic spread and subsequent involvement of lymph nodes in the central compartment, lateral compartment, or, more rarely, retropharyngeal space.
DTC-associated RPM most commonly presents as part of recurrent or persistent disease. In our cohort, 82% of patients had an RPM associated with recurrent disease. This finding is consistent with other studies in the literature, with estimates ranging between 42% and 91% (9,10,15). A smaller percentage of RPMs are identified during the initial investigation of DTC at presentation and even more rarely, patients may present with symptoms of an oropharyngeal mass, which is subsequently diagnosed as a DTC metastasis (15,18). The retropharyngeal space is rarely evaluated by ultrasound, and, therefore, RPMs are usually diagnosed with cross-sectional imaging; PET-CT, CT, or MRI scans are commonly used. Cross-sectional imaging should be considered in patients with lateral neck metastases or an increasing serum thyroglobulin measurement and no evidence of structural recurrence on neck ultrasound.
Management
The prognostic significance of DTC-associated RPM remains largely unknown and the oncological benefit of surgical intervention has not been conclusively demonstrated. Therefore, the decision to treat an RPM needs to be balanced with the risks and morbidity associated with its treatment. In our case series, no patient had an uncontrolled DTC-associated RPM or died as a result of extensive disease within the retropharyngeal space. Therefore, an observational approach was considered in select patients with small-volume RPMs. Indeed, 25 patients in our series were observed after a diagnosis of an RPM (median follow-up 28 months). In this cohort, the RPMs were small; the median maximum diameter on initial imaging was 1.1 cm. In addition, they remained small during the observation period. Given the small-volume and nonprogressive nature of the RPM, observation was deemed appropriate for these patients. Select patients with an RPM may be suitable for nonsurgical treatment such as with RAI, EBRT, or systemic therapy. In our study, the majority of patients selected for nonsurgical treatment of the RPM had concurrent locoregional or distant metastases (77% and 46%, respectively). The principle objectives of nonsurgical treatment are to minimize symptoms and prevent progression of locoregional disease.
Surgical resection has generally been considered the most effective treatment for DTC-associated RPM (8,15) and is discussed in the majority of case series in the literature (8–10,14,15,18–20). The RPMs create a surgical challenge given their location, surrounding anatomy, and proximity to vital neurovascular structures. Minimizing surgical risk and morbidity as well as avoiding unwarranted treatment is essential given the long-term favorable prognosis of DTC. Therefore, at our institution, surgery was reserved for a large or increasing RPM in the majority of patients; the median maximum diameter of the RPM in the surgical cohort was 2.0 cm. A transcervical approach to the retropharyngeal space without mandibulotomy was described by Martin in 1957 and Ballantyne in 1964 (21,22). The transcervical approach provides wide exposure to the retropharyngeal space; however, it is associated with injury to the lower cranial nerves and cervical sympathetic chain. Other more extensive approaches, such as transparotid, infratemporal, or transcervical with mandibulotomy/mandibulectomy, may be required to access the retropharyngeal space but are usually reserved for more aggressive RPM disease secondary to head and neck squamous cell carcinoma. At our institution, the transcervical approach was largely reserved for an infero-laterally located RPM, patients requiring a concurrent neck dissection, or an RPM diagnosed before the introduction of transoral robotic surgery (TORS).
In recent years, many centers have described their experience with the transoral approach (19,20,23). A transoral approach is mainly used in cases of an isolated metastasis amenable to transoral access based on preoperative imaging (20). Shellenberger et al. reported on three patients and Andrew et al. on six patients who underwent RPM surgery by ultrasound-guided transoral resection, all of which were <2.5 cm (20,23). In addition, the use of TORS to the retropharyngeal space has been recently described in the literature (19,24,25). Out of the 27 surgical patients in our cohort, 8 patients underwent transoral surgery, 6 of whom were with a robotically assisted approach. In our series, a transoral approach was reserved for patients with a supero-medially located RPM. The transoral approach is considered to be less invasive and is subsequently generally associated with a shorter inpatient stay and fewer postoperative complications. However, it may give limited access to the internal carotid artery or jugular vein in the rare but serious event of a major hemorrhage.
Prognosis
Several studies have shown a potential important association between DTC-associated RPM and distant disease. In a study by Moritani, 10 out of 22 patients with DTC-associated RPM had distant disease at the time of RPM diagnosis and 6 patients subsequently died of distant disease (9). Togashi et al. reported on 12 patients, of whom 5 developed distant disease during the study period (10). Wang et al. reported on 25 patients, 9 developed distant metastases and 6 subsequently died of disease during follow-up (15). In our cohort, we also observed that many patients had concurrent distant metastases or progressed to distant disease; the 5- and 10-year rates of DMFP were 71.7% and 61.9%, respectively. This highlights RPM as a potential marker for aggressive biology.
In the surgical cohort of 27 patients, 19 had RPM as part of recurrent disease and 8 at initial presentation. Of the eight patients who had an RPM resection at the time of initial surgery, none developed a subsequent locoregional recurrence. Conversely, those who presented as part of recurrent disease were associated with multiple locoregional recurrences and progression to distant disease during the follow-up period; 8 of the 19 patients had distant disease at final follow-up. Patients presenting with locoregional recurrence are inherently associated with a poorer prognosis and cannot be compared with those with an RPM at initial presentation. However, it may be worth considering the timing of RPM diagnosis when deciding DTC-associated RPM management. Further studies are required to determine the optimum treatment of a DTC-associated RPM, taking into account the extent of RPM disease and concurrent burden of DTC.
Limitations
Similar to all retrospective studies, these data have limitations. Selection bias from individual physician and patient preferences can never be fully accounted for. In addition, as DTC-associated RPM is a rare event, this study is subject to the limitations associated with small sample size. Limited conclusions can be drawn, given the quality and retrospective nature of the data, regarding the indications and survival benefit of RPM surgery. Further, complications are likely to be underestimated in this study as data were collected retrospectively.
RPM is largely diagnosed using cross-sectional imaging using CT or MRI scans. Given that the majority of patients with thyroid cancer are not evaluated with cross-sectional imaging, the true incidence and impact of RPM in DTC is likely to be underestimated. Further, in this study a retropharyngeal lymph node did not have to be confirmed on histopathology to be identified as an RPM. Therefore, it is possible that a small number of patients in the observational and nonsurgical treatment cohort found to have imaging characteristics suspicious for an RPM might not, in fact, have an RPM. It should also be noted that patients in the observation group are likely to have had, by the nature of selection bias, more quiescent disease compared with patients in the surgical cohort. If further imaging showed evidence of a progressing/enlarging RPM, surgical resection may have been recommended. Data regarding the location of the primary thyroid nodule within the thyroid gland were not available for this cohort, and therefore limited conclusions can be drawn on the route of lymphatic spread direct from the superior pole of the thyroid gland to the retropharyngeal space.
In conclusion, select DTC patients with a small-volume and nonprogressive RPM may be suitable for observation. Surgical resection may be considered for a large, progressing RPM, taking into account the location of the RPM in the retropharyngeal space and the overall extent of DTC tumor burden. In general, the presence of an RPM from DTC appears to be associated with aggressive disease.
Author Disclosure Statement
None of the authors had financial or personal relationships that could potentially influence this work.
Funding Information
This research was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1. Chong VF, Fan YF. 2000. Radiology of the retropharyngeal space. Clin Radiol 55:740–748 [DOI] [PubMed] [Google Scholar]
- 2. Rouvière H 1938 Anatomie des lymphatiques de l'homme [Anatomy of the Human Lymphatic System]. Edward Brothers, Inc., New York, NY, pp 16–65
- 3. Smith VA, Sessions RB, Lentsch EJ. 2012. Cervical lymph node metastasis and papillary thyroid carcinoma: does the compartment involved affect survival? Experience from the SEER database. J Surg Oncol 106:357–362 [DOI] [PubMed] [Google Scholar]
- 4. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. 2008. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 144:1070–1077 [DOI] [PubMed] [Google Scholar]
- 5. Pereira JA, Jimeno J, Miquel J, Iglesias M, Munne A, Sancho JJ, Sitges-Serra A. 2005. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 138:1095–1100 [DOI] [PubMed] [Google Scholar]
- 6. Thompson AM, Turner RM, Hayen A, Aniss A, Jalaty S, Learoyd DL, Sidhu S, Delbridge L, Yeh MW, Clifton-Bligh R, Sywak M. 2014. A preoperative nomogram for the prediction of ipsilateral central compartment lymph node metastases in papillary thyroid cancer. Thyroid 24:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCormack KR, Sheline GE. 1970. Retropharyngeal spread of carcinoma of the thyroid. Cancer 26:1366–1369 [DOI] [PubMed] [Google Scholar]
- 8. Desuter G, Lonneux M, Plouin-Gaudon I, Jamar F, Coche E, Weynand B, Rahier J, Gregoire V, Andry G, Hamoir M. 2004. Parapharyngeal metastases from thyroid cancer. Eur J Surg Oncol 30:80–84 [DOI] [PubMed] [Google Scholar]
- 9. Moritani S. 2016. Parapharyngeal metastasis of papillary thyroid carcinoma. World J Surg 40:350–355 [DOI] [PubMed] [Google Scholar]
- 10. Togashi T, Sugitani I, Toda K, Kawabata K, Takahashi S. 2014. Surgical management of retropharyngeal nodes metastases from papillary thyroid carcinoma. World J Surg 38:2831–2837 [DOI] [PubMed] [Google Scholar]
- 11. Qiu ZL, Xu YH, Song HJ, Luo QY. 2011. Localization and indentificationof parapharyngeal metastases from differentiated thyroid carcinoma by (131)I-SPECT/CT. Head Neck 33:171–177 [DOI] [PubMed] [Google Scholar]
- 12. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP 2017 AJCC Cancer Staging Manual. 8th ed. New York [DOI] [PubMed]
- 13. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartl DM, Leboulleux S, Velayoudom-Cephise FL, Mirghani H, Deandreis D, Schlumberger M. 2015. Management of retropharyngeal node metastases from thyroid carcinoma. World J Surg 39:1274–1281 [DOI] [PubMed] [Google Scholar]
- 15. Wang XL, Xu ZG, Wu YH, Liu SY, Yu Y. 2012. Surgical management of parapharyngeal lymph node metastasis of thyroid carcinoma: a retrospective study of 25 patients. Chin Med J 125:3635–3639 [PubMed] [Google Scholar]
- 16. Goyal N, Pakdaman M, Kamani D, Caragacianu D, Goldenberg D, Randolph GW. 2017. Mapping the distribution of nodal metastases in papillary thyroid carcinoma: where exactly are the nodes? Laryngoscope 127:1959–1964 [DOI] [PubMed] [Google Scholar]
- 17. Otsuki N, Nishikawa T, Iwae S, Saito M, Mohri M, Nibu K. 2007. Retropharyngeal node metastasis from papillary thyroid carcinoma. Head Neck 29:508–511 [DOI] [PubMed] [Google Scholar]
- 18. Lombardi D, Nicolai P, Antonelli AR, Maroldi R, Farina D, Shaha AR. 2004. Parapharyngeal lymph node metastasis: an unusual presentation of papillary thyroid carcinoma. Head Neck 26:190–196 [DOI] [PubMed] [Google Scholar]
- 19. Givi B, Troob SH, Stott W, Cordeiro T, Andersen PE, Gross ND. 2016. Transoral robotic retropharyngeal node dissection. Head Neck 38:E981–E986 [DOI] [PubMed] [Google Scholar]
- 20. Andrews GA, Kwon M, Clayman G, Edeiken B, Kupferman ME. 2011. Technical refinement of ultrasound-guided transoral resection of parapharyngeal/retropharyngeal thyroid carcinoma metastases. Head Neck 33:166–170 [DOI] [PubMed] [Google Scholar]
- 21. Ballantyne AJ. 1964. Significance of retropharyngeal nodes in cancer of the head and neck. Am J Surg 108:500–504 [DOI] [PubMed] [Google Scholar]
- 22. Martin H. 1957. Surgery of the Head and Neck Tumors. Hoeber Harper, New York, NY [Google Scholar]
- 23. Shellenberger T, Fornage B, Ginsberg L, Clayman GL. 2007. Transoral resection of thyroid cancer metastasis to lateral retropharyngeal nodes. Head Neck 29:258–266 [DOI] [PubMed] [Google Scholar]
- 24. Moore MW, Jantharapattana K, Williams MD, Grant DG, Selber JC, Holsinger FC. 2011. Retropharyngeal lymphadenectomy with transoral robotic surgery for papillary thyroid cancer. J Robot Surg 5:221. [DOI] [PubMed] [Google Scholar]
- 25. Byeon HK, Duvvuri U, Kim WS, Park YM, Hong HJ, Koh YW, Choi EC. 2013. Transoral robotic retropharyngeal lymph node dissection with or without lateral oropharyngectomy. J Craniofac Surg 24:1156–1161 [DOI] [PubMed] [Google Scholar]