Abstract
Background: Lack of awareness about the life-limiting nature of renal failure is a significant barrier to palliative care for older adults with end-stage renal disease.
Objective: To train nephrologists to use the best case/worst case (BC/WC) communication tool to improve shared decision making about dialysis initiation for older patients with limited life expectancy.
Design: This is a pre-/postinterventional pilot study.
Setting/Subjects: There were 16 nephrologists and 30 patients of age 70 years and older with estimated glomerular filtration rate (eGFR) <20 mL/min per 1.73 m2 in outpatient nephrology clinics, in Madison, WI.
Measurements: Performance of tool elements, content of communication about dialysis, shared decision making, acceptability of the intervention, decisions to pursue dialysis, and palliative care referrals were measured.
Results: Fifteen of 16 nephrologists achieved competence performing the BC/WC tool with standardized patients, executing at least 14 of 19 items. Nine nephrologists met with 30 patients who consented to audio record their clinic visit. Before training, clinic visits focused on laboratory results and preparation for dialysis. After training, nephrologists noted that declining kidney function was “bad news,” presented dialysis and “no dialysis” as treatment options, and elicited patient preferences. Observer-measured shared decision-making (OPTION 5) scores improved from a median of 20/100 (interquartile range [IQR] 15–35) before training to 58/100 (IQR 55–65). Patients whose nephrologist used the BC/WC tool were less likely to make a decision to initiate dialysis and were more likely to be referred to palliative care.
Conclusions: Nephrologists can learn to use the BC/WC tool with older patients to improve shared decision making about dialysis, which may increase access to palliative care.
Keywords: best case/worst case, dialysis, doctor–patient communication, ESRD, palliative care, shared decision making
Introduction
Each year, >200,000 adults of age 65 years and older receive dialysis for end-stage renal disease (ESRD).1 Although dialysis provides clear benefits for some, older patients with multiple comorbidities face high mortality and treatment burden, including frequent inpatient admissions, chronic pain, fatigue, cognitive dysfunction, and depression.2 Guidelines recommend shared decision making to fully inform patients about dialysis,3 yet nephrologists and their patients face multiple barriers to making a shared decision,4 including failure to perceive dialysis as a treatment choice.5,6
Older adults with life-limiting ESRD would benefit from palliative care to cultivate prognostic awareness,7 clarify goals, and alleviate symptoms whether they choose to initiate dialysis or not. In comparison with patients with other serious illnesses, patients with ESRD are less likely to receive palliative care,8 have higher rates of ICU admissions, and death in the ICU, fewer documented advance directives and do-not-resuscitate orders and shorter hospice stays.8–10 Barriers to palliative care for patients with ESRD include an “all-or-nothing” approach to care with dialysis as the default,11 limited consideration of patients' goals and values regarding dialysis,12 and the perception by both patients and nephrologists that undergoing dialysis is not a choice.13,14
Nephrologists at the University of Wisconsin (UW) in Madison, WI, approached our research team with concerns about end-of-life care for their patients. They were troubled by the revolving door between the hospital and dialysis unit when patients experienced multiple late-stage events, for example, hemodynamic instability and ischemia, which made it difficult to support life outside the hospital. Although they had started a palliative care program for patients with ESRD, it was underutilized. Nephrologists articulated two specific problems: (1) these patients had limited, if any, understanding of their prognosis and (2) many older adults, for whom dialysis offered limited survival advantage, were not apprised of conservative management. They asked us to develop an intervention nephrologists could use to improve shared decision making, provide upstream information about prognosis, and increase utilization of palliative care.
We previously developed a communication tool called best case/worst case (BC/WC) that employs scenario planning and a graphic aid to illustrate options, express prognostic uncertainty, and to describe a range of outcomes within the context of the patient's underlying health state.15 We tested this tool with surgeons and frail older patients considering difficult surgical decisions and found robust improvements in shared decision making.16 We believed this tool could be adapted for outpatient nephrology clinics to improve decisions about dialysis.17 The objective of this pilot study was to adapt the BC/WC tool, train nephrologists to use it in outpatient clinics, and evaluate its impact on shared decision making and access to palliative care.
Methods
Study design
From June 2017 to April 2018, we performed a pre-/poststudy to assess the feasibility and tolerability of an intervention to train nephrologists to use the BC/WC tool with patients with ESRD. We evaluated the effect of the tool on shared decision making, dialysis decisions, and access to palliative care. We also administered surveys to assess the utility of these measures for use in a future efficacy study. The UW Institutional Review Board approved this study (ID2016-0671) and the National Palliative Care Research Center provided funding to support nephrologist training, patient enrollment, and data collection. All enrolled nephrologists and patients provided written informed consent for permission to audio record clinic visits, undergo interviews, and allow the study team to perform chart review. Each nephrologist received $150 for attending the two-hour training session and patients and family members each received $40 for study participation.
Intervention
We met biweekly for 10 months with nephrologists, palliative care clinicians, and educators to adapt the BC/WC tool for use in decision making about dialysis. We identified several key differences between surgical and dialysis decisions: practice setting (inpatient vs. outpatient), acuity of illness (urgent vs. chronic progressive), treatment type (one-time intervention vs. long-term life-sustaining therapy), and the trajectory of illness (imminent rapid decline vs. gradual functional decline over time). We recognized that it would be difficult for nephrologists to abruptly discuss prognostic information with patients who are familiar with their diagnosis and are living with the slow progression of chronic disease. This is in distinct contrast to patients who have an inciting event such as acute surgery or cancer where the novel diagnosis creates space for a conversation about prognosis. We combined these new elements with the initial BC/WC components: presenting a choice between two treatment options (life with dialysis and life without dialysis); using stories to illustrate the best, worst, and most likely scenarios for both treatment options; encouraging patient deliberation; and making a goal-concordant treatment recommendation.
We developed a two-hour training program for nephrologists to learn to use the BC/WC communication tool. Based on our previously successful model for training surgeons18,19 using adult learning theory,20,21 this program includes a 10-minute introduction, expert demonstration, and individual preparation and practice with standardized patients with one-on-one expert feedback, that is, coaching. The training is focused on teaching nephrologists to translate their clinical knowledge into the BC/WC format using scenario planning22,23 to describe how patients might experience treatment and their overall health trajectory with and without dialysis. Nephrologists also learn how to generate a graphic aid. To evaluate fidelity using the tool, we developed a 19-item checklist to account for novel elements important for this setting (Supplementary Fig. S1). To enhance fidelity, two months after training we invited all trained nephrologists to participate in one-on-one in-person coaching sessions to reinforce essential tool elements.
Participants
We asked all practicing nephrologists (attending MDs, fellows, and advanced practice providers [APPs]) at UW to participate in the training program. We invited those who regularly care for older patients with chronic kidney disease (CKD) and those who expressed interest in learning the tool even though their practice involved other areas of nephrology, for example, transplant.
We approached patients of study-enrolled nephrologists in outpatient clinics who were of age 70 years and older with an eGFR of <20 mL/min per 1.73 m2 to participate in this study. We excluded patients who lacked decision-making capacity, did not speak English, or were currently on dialysis.
Data collection
We audio-recorded clinic visits between nephrologists and study-enrolled patients before and after training. We reviewed patient charts for up to six months after enrollment, recording initiation of dialysis, emergency room and hospital admissions, surgical procedures, palliative care consultations, and death. We also administered a modified version of the Practitioner Opinion Survey24 to all trained nephrologists three and six months after training to gather feedback on clinician use of the BC/WC tool. To patients, we administered the Quality of Communication (QOC) questionnaire, the Shared Decision Making (SDM Q-9) survey, selected questions from the Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey, and the Kidney Disease Quality of Life (KDQOL-36) survey three and six months after study enrollment.
Analysis
We used qualitative content analysis and OPTION-525 scoring, a well-validated instrument for observer-measured shared decision making, to analyze transcripts of the audio-recorded visits between nephrologists and patients. Six coders individually read and scored each transcript using OPTION-5. We met as a group to reconcile differences in these scores until consensus was achieved. We used an inductive coding strategy, that is, without predetermined codes, and a process called constant comparison to generate a coding taxonomy for qualitative analysis.26 We used an iterative process of consensus building and triangulation with investigators from different backgrounds as a springboard for deeper exploration of the themes and constructs identified in the data. We focused our analysis on the content of communication about dialysis decision making and confirmed consistency between the data and constructs by cross-referencing the coded data once higher level analysis was completed.27 We utilized NVivo 11 (QSR International) to catalogue these data.
We used descriptive statistics to summarize clinical, demographic, and survey data. We used Stata 14 (StataCorp LLC) for all statistical analyses.
Results
Participant characteristics
We recruited 25 nephrologists to undergo BC/WC training. Four nephrologists did not respond to the invitation, one declined, and four nephrologists left the institution before they could undergo training. A total of 16 nephrologists were enrolled including 2 APPs who practice nephrology exclusively. Nine of the trained nephrologists routinely saw patients who met our inclusion criteria and would benefit from the BC/WC intervention.
Fifty-two patients met study criteria. Twelve declined and 10 were not approached per nephrologist request. We enrolled 13 patients before training nephrologists to use the BC/WC tool and 17 patients after training nephrologists to use the BC/WC tool. Study patients had an average eGFR of 16 mL/min per 1.73 m2 and had multiple comorbidities, most commonly heart disease, with >90% of patients having two or more comorbid conditions (Table 1). One patient in the intervention group, who was enrolled with an eGFR of <20, was found on subsequent chart review to have an eGFR of 24. This patient was included in analysis when the patient's nephrologist endorsed this strategy given the patient's overall prognosis.
Table 1.
Patient Characteristics
| Pre (N = 13) | Post (N = 17) | |
|---|---|---|
| Female, n (%) | 5 (39) | 11 (65) |
| Age, median (range) | 78 (71–89) | 76 (71–96) |
| eGFR, mL/min per 1.73 m2, mean (range) | 16 (12–18) | 16 (10–24) |
| Comorbidities | % afflicted | % afflicted |
| Coronary artery disease | 54 | 24 |
| Diabetes | 39 | 59 |
| Heart failure | 39 | 24 |
| Cancer | 39 | 30 |
| Peripheral vascular disease | 15 | 12 |
| Pulmonary hypertension | 8 | 6 |
| COPD | 8 | 0 |
| Cerebrovascular disease | 0 | 6 |
| Has at least two comorbidities | 92 | 94 |
| Has at least three comorbidities | 62 | 59 |
COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
Intervention outcomes
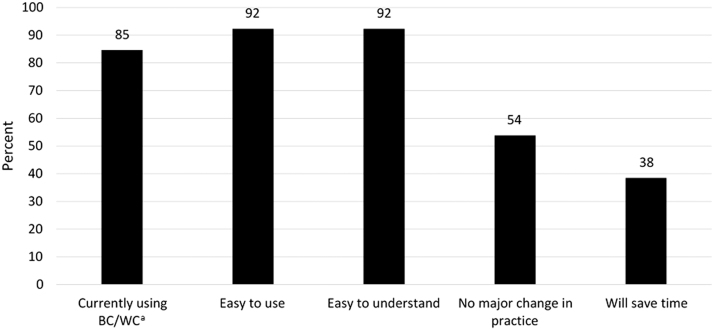
Sixteen nephrologists completed the two-hour training session. Fifteen (8 staff MDs, 4 nephrology fellows, and 3 APPs) achieved competence by performing at least 14 of 19 items in the check-list adherence criteria (median 17, IQR 16–17) with a standardized patient. Elements most frequently omitted were writing “What is important to you now?” on the graphic aid and describing a long-term outcome for the worst case scenario. Eighty-five percent of learners reported continued use of the BC/WC tool three to six months after training. Ninety-two percent believed the tool was easy to use and easy for patients to understand, yet close to half felt that using the tool would require a major change in their practice, and less than half believed it would save time (Fig. 1).
FIG. 1.
Practitioner opinion survey (N = 13). Percentage of respondents who answered “agree” and “strongly agree” to selected questions on the practitioner opinion survey. aBC/WC = best case/worst case.
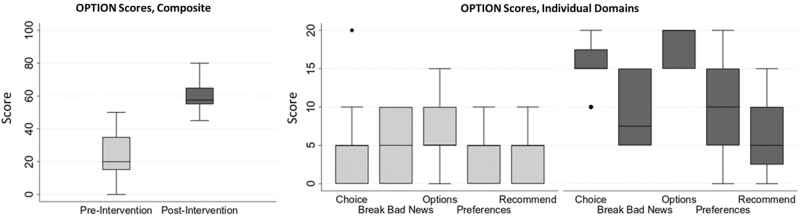
Before training, OPTION 5 scores were low, median 20/100 (IQR 15–35). After training, OPTION 5 scores increased considerably, median 58/100 (IQR 55–65), with improvement in two domains: choice presentation and description of options (Fig. 2). Nephrologists' checklist adherence scores when using the tool with actual patients in clinic were slightly lower (median 13, IQR 11–15). Items omitted included writing “What is important to you now” on the graphic aid, making a treatment recommendation and description of the most likely outcome.
FIG. 2.
Change in observer-measured shared decision making during audio-recorded clinic visits after nephrologists attended BC/WC training.
Content of communication about dialysis was distinctly different. Before training, nephrologists discussed laboratory results, the need for future clinic visits, and preparation for dialysis (Table 2). For example, one nephrologist discussed the importance and logistics of monthly laboratory draws to monitor renal function, whereas another discussed vascular access, “…and the next visit I'll probably send you to get a surgery for a fistula. ‘Cause that takes another couple months to be ready to use for dialysis.” Discussions about dialysis focused on the mode of dialysis, the procedure for vascular access, and when to start dialysis.
Table 2.
Content of Communication Used by Nephrologists in Clinic Visits with Patients with eGFR <20 before and after Best Case/Worst Case Training
| Topic | Representative quotes |
|---|---|
| Before BC/WC training | |
| Discussing dialysis mode | “One of them is similar to the machine we have in center here. Where ya hook up, and you get this fistula, uh put in your arm as a connection to blood vessels—it's big surgery to do that…The other one…is a machine that you run overnight, k and you have a tube that's in the belly.” |
| Preparation for dialysis | “Three months I'm gonna have you come back and see me, I think we should go forward with planning for the surgery. Just to get the catheter placed whether or not we use it…at this point…I'd say, we should start training. So you can learn, how to do it.” |
| Describing access procedures | “So, let's say…We place a peritoneal dialysis catheter, you start feeling better…We leave it in there, we might even just bury it under the skin. And then if we needed, it can be taken back out and used whenever. So I don't think there's really a downside to getting that thing placed…” |
| Access logistics | “And when I get those results back we'll talk about them and I'll show you where I think we have a big enough vein to work with to put something in your arm, to create something in your arm—and I'll go over it more in detail when I know what we have to work with and that'll help me decide what we need to do. So I can't explain what we're gonna do yet until I get these vein studies back.” |
| After BC/WC training | |
| Breaking bad news | “So your kidney function is such that you know we kinda know where we're goin’ with this. You know this isn't gonna get better to the point that dialysis isn't in your future. Something is gonna change eventually ‘cause you can't stay at this low level forever.” |
| Presenting options | “Because that's what we're trying to talk about today is, dialysis versus no dialysis…they come into the center, which usually means about 3 to 4 hours on the machine…there is kind of a rollercoaster where people do feel good and feel bad and they kinda go back and forth…” “We like to mention that there's palliative care…Palliative means, palliate, makes you feel better. Relieve symptoms…we like people to know that there's something to support that part of it. I can support dialysis with a machine. But people need more support than just a machine, whether they choose to go on dialysis or not.” |
| Scenario planning | “But in the best case scenario, people who go on dialysis…they do okay, initially, but there's a change in their day to day life. There's a change in what they do, and that's a really important part of this.” “When we look at things like the worst case, that someone starts dialysis…[it] can put them into situations where they're now going into the hospital pretty frequently, and they decline pretty fast.” |
| Eliciting goals and values | “I think one of the things that we need to kind of figure out when we talk about things working for you, is what does working for you look like? In other words, what things do you like to do, what do you enjoy doing, what's important to you?” |
BC/WC, best case/worst case.
After training, nephrologists continued to discuss laboratory values and dietary changes, but the content of the conversation about dialysis changed. Nephrologists alerted patients about the need to make a decision about dialysis and broke bad news. For example, “…So, what we really wanna do today…[is discuss] the bigger picture, and the bigger picture meaning that the kidneys are something that are, well, really not holding out and, not gonna last forever.” They presented dialysis and no dialysis as two viable treatment options and described the short- and long-term experience of each. Nephrologists also included narrative about life without dialysis, “You kind of do the things that we're doing now, and we would continue that way. Doesn't mean we're not doing things, we're just saying, we're just gonna react, or treat the things that are…affecting your symptoms or so…hopefully keeping you out of the hospital more.”
Nephrologists also used BC/WC to convey prognostic uncertainty. For example, “…we've watched your kidney function over time, and it has gotten slowly worse…best case scenario would be that it stays at 15 forever. And you do n't have any symptoms and things don't change. That would be wonderful. The worst case would be that the symptoms show up really fast and people who don't have kidney function…would pass away from either the toxins building up or the fluid building up.” Another nephrologist described the most likely scenario for life with dialysis, discussing increased fatigue while on dialysis due to the patient's underlying heart failure. After describing treatment options with BC/WC, nephrologists elicited patients' goals and values, and three nephrologists specifically introduced patients to palliative care and recommended referral during the clinic visit, “Um, we have…someone trained with exposure to nephrology…specifically, is trained in palliative care. And when people choose not to do dialysis, that's where we kind of guide them.”
Patient outcomes
Patients and family members who were exposed to BC/WC appreciated the clarity of treatment choice and honest information about prognosis for both treatment options (Table 3). They valued how the BC/WC tool allowed them to deliberate about treatment choice, to anticipate what life with dialysis might be like, and prepare for the future. Some were startled to suddenly receive prognostic information, whereas others expressed frustration that this information had not been shared earlier.
Table 3.
Patient and Family Member Attitudes Toward the Best Case/Worst Case Communication Tool
| Clarifies the treatment choice | “I mean to see it on paper you know the pros on one side and the cons on the other side that—that helps a lot. And he had it all diagrammed out so…having all this information, having it right there on paper for me, not on the chart on the wall that I can't take home.” “Oh yeah and it, it gave you the two options you, I mean you had it there on paper…I don't know if it changed my thinking other than it just made it very clear. That you [have options] X and Y.” |
| Promotes discussion of difficult prognoses | “So she was coming up with some stuff that we'd never heard before…it was good to know—no, I appreciate her telling me that.” “Well, I mean…the life expectancy. He says maximum five years and more likely two or three years after I go on dialysis. Um…I'm not sure I needed to know that…” |
| Facilitates dialogue and provides a framework for deliberation | “I think it's all of those questions. You know: how old am I? You know: what is valuable to me? I think there are so many things that go into that decision.” “It just makes it, it was easier to sit down to discuss it with him when I saw it on paper.” |
| Promotes understanding of expectations and preparation for adverse events | “So I'm glad he brought that up. So I guess the needles and that…and the palliative care um, and being nauseous were the things I took away from him chatting with me about dialysis, or being end of life or whatever you call it.” “I think [the nephrologist]…tells it like it is. And I think that's what I want. I don't want anybody to sugarcoat anything for me…it's better than being in limbo and not knowing a lot of things.” |
Four patients whose nephrologist used the BC/WC tool expressed desire for dialysis compared with eight patients who were not exposed to the BC/WC tool. Of the 17 patients exposed to the tool, 6 received palliative care referrals and 2 ultimately attended an appointment with a palliative care specialist (Table 4). Survey completion rate ranged from 97% to 85%. QOC measurements had good variability among respondents due to the end-of-life component of the instrument, whereas SDM Q-9 and CAHPS scores demonstrated high ceiling effects (Supplementary Fig. S2). SF-12 physical composite scores declined over time, whereas the other domains of the KDQOL-36 remained stable.
Table 4.
Patient Decisions About Dialysis during Audio-Recorded Clinic Visits and Outcomes during Six Months of Follow-Up
| Pre (N = 13) | Post (N = 17) | |
|---|---|---|
| No decision about dialysis | 3 | 8 |
| Desires no dialysis | 2 | 3 |
| Desires some form of dialysis | 8 | 4 |
| Initiated dialysis | 1 | 1 |
| Died | 1 | 0 |
| Palliative care referrals | 2 | 6 |
| Palliative care appointment scheduled | 1 | 3 |
| Attended palliative care appointment | 1 | 2 |
Discussion
Nephrologists can successfully learn to use the BC/WC communication tool to discuss dialysis treatment options with older patients with ESRD. Observer-measured shared decision making improved and the content of communication shifted from management of renal disease and dialysis access to discussion about prognosis and treatment options. In this small pilot study, more patients deferred decision making about dialysis and received a referral to palliative care after exposure to the BC/WC tool. These findings have important implications for nephrologists, patients, and palliative care for patients with ESRD.
For nephrologists, use of a framework to present dialysis as one of two treatment options, rather than a foregone response to renal failure, can alter the content of their communication with older patients with ESRD. Structured elements facilitate translation of information about treatment and prognosis, enabling nephrologists to describe the burdens of dialysis, the overall health trajectory of end-stage disease, and work with patients to consider whether life with dialysis will meet patients' goals.15 In using scenario planning, nephrologists encourage patients to visualize a previously unimaginable reality and prepare for major shifts in a way that simple prognostication, for example, 25% median survival at five years, cannot.22,23 Although we considered presenting a choice between dialysis and “conservative management,” this framing led our nephrologists to believe that patients should choose dialysis or palliative care. To be clear that palliative care can support all patients with ESRD, we labeled conservative management “no dialysis” or “life without dialysis” to avoid this misperception. Centers with more established concurrent palliative care programs may not have this issue.
Although nephrologists noted BC/WC was easy to use, easy for patients to understand, and reported continued use of the tool six months after training, they are pressured to prepare patients for dialysis as renal function declines. These clinical demands leave little time to discuss the life-limiting nature of ESRD or to consider life without dialysis. Although enthusiasm for the intervention was similar to our previous experience with surgeons,28 nephrologists had more challenges incorporating this tool within their busy clinic schedule and saw this as a major change in their clinical practice. This is likely due to different practice patterns whereby nephrologists focus on disease management, whereas surgeons routinely discuss surgical intervention and informed consent. Our study design considered the BC/WC tool as a one-time intervention. However, the graphic aid and the conversation can be used over time to continue the conversation about dialysis initiation with other clinicians and in other settings (Supplementary Fig. S3). Some of our study participants reported using the tool this way, which could both improve the efficacy of the intervention and reduce concerns associated with implementing a new tool in a busy clinic.
For patients, the BC/WC tool supports shared decision making by revealing options and providing stories they can use to reflect on preferences. The graphic aid can be referred to later as they consider options. Nonetheless, patients had mixed reactions to hearing about the life-limiting nature of their disease, ranging from frustration that this was previously undisclosed to irritation that prognosis was suddenly now revealed. BC/WC is designed to bridge the gap between prognostic understanding and treatment choice but requires empathy about the difficult nature of this conversation. Some patients may benefit from inviting engagement into this discussion, for example, “I am sorry to tell you your kidney disease has gotten worse…would you like to talk about what that means?”29
Use of the BC/WC tool may be more challenging at institutions without an established relationship between nephrology and palliative care. At UW, patients have access to outpatient palliative care specifically designed for patients with renal disease. This resource augments the care nephrologists provide, enables continued discussion of treatment preferences so that dialysis decisions are not delayed indefinitely, and facilitates documentation of care goals and specialty end-of-life care when needed. Nephrologists at our institution are invested in using the BC/WC tool, in part, due to a department-wide focus on reducing high rates of intensive treatments at the end of life and low utilization of palliative care.
This pilot study has several limitations: notably it was not designed to make inferences about efficacy of the BC/WC tool. As such, future studies are required to determine whether this intervention improves QOC and access to palliative care or reduces intensive treatment at the end of life. Although we found the intervention was well tolerated by clinicians and we have streamlined BC/WC training, it may be taxing for busy nephrologists with many clinical demands to invest the time to learn a new way to communicate with patients. Finally, although we developed this tool in the outpatient setting because it is an easier venue for implementation and future efficacy testing, there is nothing about the setting that restricts use of the tool. Clinicians may find this tool more useful in the acute setting, but we were unable to evaluate inpatient use due to limitations in our study design.
Conclusion
Nephrologists can learn to use the BC/WC tool with older patients to improve shared decision making about dialysis and continue to use the tool months after training. Patients with ESRD value the forecasting elements of the tool that may facilitate access to palliative care when the tool is used to discuss dialysis in clinic.
Supplementary Material
Acknowledgments
The authors thank Dr. Jonathan Jaffrey, senior vice president and chief population health officer at UW Health and the UW Department of Nephrology, for his support of this project.
This study was presented at the 2018 Annual Assembly of Hospice and Palliative Care in Boston, MA, and the third annual American College of Surgeons Symposium for Palliative Care in 2018.
Authors' Contributions
Research idea and study design were done by R.A.J., M.W., K.S., A.Z., T.C.C., S.K.J., and M.L.S.; data acquisition was carried out by J.L.T., D.A.F., and C.J.Z.; data analysis/interpretation was done by C.J.Z., A.Z., J.L.T., D.A.F., N.D.B., and M.L.S; statistical analysis was carried out by C.J.Z., M.L.S.; and supervision or mentorship was performed by M.L.S. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding Information
M.L.S. is supported by a National Palliative Care Research Center (NPCRC), Pilot/Exploratory Project Award. C.J.Z. is supported by an NIH 2T32HL110853-06 Training Grant. T.C.C. is supported by the Ellen and Peter O. Johnson Chair in Palliative Care. These funding sources had no role in study design, data collection, analysis, or interpretation, article preparation, or the decision to submit this report for publication.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. 2017 USRDS Annual Data Report Reference Tables Volume 2-ESRD: Treatment Modalities. United States Renal Data System; https://www.usrds.org/reference.aspx 2017. (Last accessed October30, 2017) [Google Scholar]
- 2. Jassal SV, Watson D: Dialysis in late life: Benefit or burden. Clin J Am Soc Nephrol 2009;4:2008–2012 [DOI] [PubMed] [Google Scholar]
- 3. Galla JH: Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians Association and the American Society of Nephrology. J Am Soc Nephrol 2000;11:1340–1342 [DOI] [PubMed] [Google Scholar]
- 4. Wong SP, Hebert PL, Laundry RJ, et al. : Decisions about renal replacement therapy in patients with advanced kidney disease in the US Department of Veterans Affairs, 2000–2011. Clin J Am Soc Nephrol 2016;11:1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ladin K, Lin N, Hahn E, et al. : Engagement in decision-making and patient satisfaction: A qualitative study of older patients' perceptions of dialysis initiation and modality decisions. Nephrol Dial Transplant 2017;32:1394–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ladin K, Pandya R, Perrone RD, et al. : Characterizing Approaches to Dialysis Decision Making with Older Adults: A Qualitative Study of Nephrologists. Clin J Am Soc Nephrol 2018;13:1188–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson VA, Jacobsen J, Greer JA, et al. : The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: A communication guide. J Palliat Med 2013;16:894–900 [DOI] [PubMed] [Google Scholar]
- 8. Wachterman MW, Pilver C, Smith D, et al. : Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med 2016;176:1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wachterman MW, Halipern SM, Keating NL, et al. : Hospice length of stay, healthcare utilization, and spending at the end of life among patients with end-stage renal disease. In: Schwarze ML, ed. JAMA Intern Med 2018;178:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurella Tamura M, Montez-Rath ME, Hall YN, et al. : Advance directives and end-of-life care among nursing home residents receiving maintenance dialysis. Clin J Am Soc Nephrol 2017;12:435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong SY, McFarland LV, Liu C, et al. : Care practices for patients with advanced kidney disease who forgo maintenance dialysis. JAMA Intern Med 2019;179:305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong SP, Vig EK, Taylor JS, et al. : Timing of initiation of maintenance dialysis: A qualitative analysis of the electronic medical records of a national cohort of patients from the department of veterans affairs. JAMA Intern Med 2016;176:228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ladin K, Smith AK: Active medical management for patients with advanced kidney disease. JAMA Intern Med 2019;179:313–315 [DOI] [PubMed] [Google Scholar]
- 14. Kaufman SR, Shim JK, Russ AJ: Old age, life extension, and the character of medical choice. J Gerontol B Psychol Sci Soc Sci 2006;61:S175–S184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwarze ML, Taylor LJ: Managing uncertainty—Harnessing the power of scenario planning. N Engl J Med 2017;377:206–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor LJ, Nabozny MJ, Steffens NM, et al. : Best case/worst case: A framework to improve surgeon communication in high-stakes surgical decisions. JAMA Surg 2017;152:531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grubbs V: Time to recast our approach for older patients with ESRD: The best, the worst, and the most likely. Am J Kidney Dis 2018;71:605–607 [DOI] [PubMed] [Google Scholar]
- 18. Kruser JM, Nabozny MJ, Steffens NM, et al. : “Best case/worst case”: Qualitative evaluation of a novel communication tool for difficult in-the-moment surgical decisions. J Am Geriatr Soc 2015;63:1805–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwarze M: Best Case/Worst Case Training Program. https://www.hipxchange.org/BCWC 2016. (Last accessed October16, 2017)
- 20. Ericsson KA, Krampe RT, Teschromer C: The role of deliberate practice in the acquisition of expert performance. Psychol Rev 1993;100:363–406 [Google Scholar]
- 21. Reed S, Shell R, Kassis K, et al. : Applying adult learning practices in medical education. Curr Probl Pediatr Adolesc Health Care 2014;44:170–181 [DOI] [PubMed] [Google Scholar]
- 22. Wack P: Uncharted waters ahead. Harvard Business Review 1985;Sept–Oct:73–89 [Google Scholar]
- 23. Wack P: Shooting the rapids. Harvard Business Review 1985;Nov–Dec:139–150 [Google Scholar]
- 24. O'Connor A, Cranney A: User Manual—Acceptability [document on the Internet]. pp. 1–5. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Acceptability.pdf 1996. (Last accessed July7, 2013)
- 25. Elwyn G, Edwards A, Wensing M, et al. : Shared decision making: Developing the OPTION scale for measuring patient involvement. Qual Saf Health Care 2003;12:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miles MB, Huberman M: Early steps in analysis. In: Qualitative Data Analysis. Thousand Oaks, CA: SAGE Publications, 1994, pp. 50–89 [Google Scholar]
- 27. Carter N, Bryant-Lukosius D, DiCenso A, et al. : The use of triangulation in qualitative research. Oncol Nurs Forum 2014;41:545–547 [DOI] [PubMed] [Google Scholar]
- 28. Kruser JM, Taylor LJ, Campbell TC, et al. : “Best case/worst case”: Training surgeons to use a novel communication tool for high-risk acute surgical problems. J Pain Symptom Manage 2017;53:711–719.e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cortez D, Maynard DW, Campbell TC: Creating space to discuss end-of-life issues in cancer care. Patient Educ Couns 2019;102:216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.