Abstract
Folate and alcohol are dietary factors affecting the risk of cancer development in humans. The interaction between folate status and alcohol consumption in carcinogenesis involves multiple mechanisms. Alcoholism is typically associated with folate deficiency due to reduced dietary folate intake. Heavy alcohol consumption also decreases folate absorption, enhances urinary folate excretion and inhibits enzymes pivotal for one-carbon metabolism. While folate metabolism is involved in several key biochemical pathways, aberrant DNA methylation, due to the deficiency of methyl donors, is considered as a common downstream target of the folate-mediated effects of ethanol. The negative effects of low intakes of nutrients that provide dietary methyl groups, with high intakes of alcohol are additive in general. For example, low methionine, low-folate diets coupled with alcohol consumption could increase the risk for colorectal cancer in men. To counteract the negative effects of alcohol consumption, increased intake of nutrients, such as folate, providing dietary methyl groups is generally recommended. Here mechanisms involving dietary folate and folate metabolism in cancer disease, as well as links between these mechanisms and alcohol effects, are discussed. These mechanisms include direct effects on folate pathways and indirect mediation by oxidative stress, hypoxia, and microRNAs.
Keywords: folate metabolism, alcohol consumption, tumorigenesis, oxidative stress, microRNAs, folate enzymes
1. Introduction
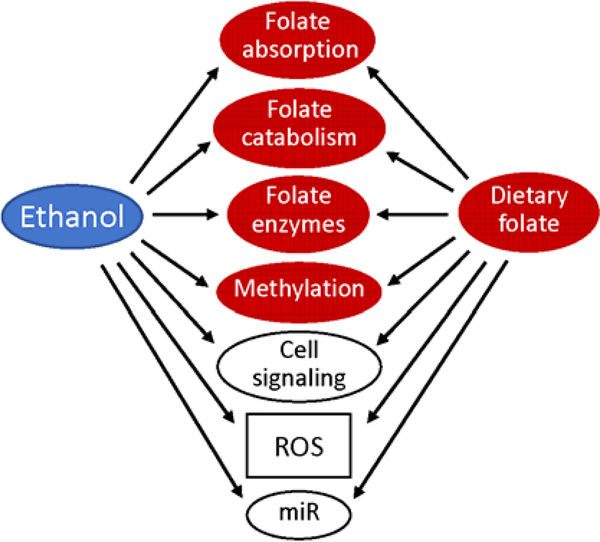
Folate and alcohol are dietary factors affecting the risk of cancer development in humans [1–4]. This conclusion is primarily based on numerous epidemiological studies; precise molecular mechanisms underlying the link between alcohol consumption or folate metabolism and cancer initiation and progression remain largely unknown. The assessment of the combined effect of these two dietary components is obviously more intricate and is a challenging task at molecular, cellular, organism and population levels [5]. The problem is exacerbated by the fact that effects of both folate intake and alcohol consumption are cancer type-specific and can be also modified by other dietary components as well as the personal genetic and epigenetic landscape [6–9]. Thus, though alcohol consumption has been investigated as a potential risk factor for numerous cancers, epidemiological studies have linked it more strongly to the increased risk of breast cancer, cancers of digestive tract and upper respiratory tract [10, 11]. Even with regard to these cancer types, the relationship between alcohol and cancer is not simple. For example, the study of 2812 breast cancer cases from the French E3N-EPIC cohort concluded that there were no association between high alcohol consumption and increased risk of breast cancer among premenopausal women but found a positive linear correlation among post-menopausal women [12]. Of note, this study also indicated that low folate intake increased alcohol-associated breast cancer risk [12]. Some reports also imply that moderate alcohol consumption could be associated with decreased cancer risk especially in the context of specific diets like Mediterranean diet [13–15]. Nevertheless, the prevalent view in the literature is that alcohol consumption is associated with the increased risk of several cancers while folate supplementation can reduce this risk [11, 16, 17]. Accordingly, this review considers potential mechanisms underlying such effects (schematically depicted in Fig. 1).
Fig. 1.
Ethanol suppresses key biological processes directly relevant to folate metabolism (red shapes); this effect can be alleviated by dietary folate. Several pathways outside of folate metabolism are affected by both ethanol and folate (open shapes).
2. Role of folate in tumorigenesis and malignancy progression
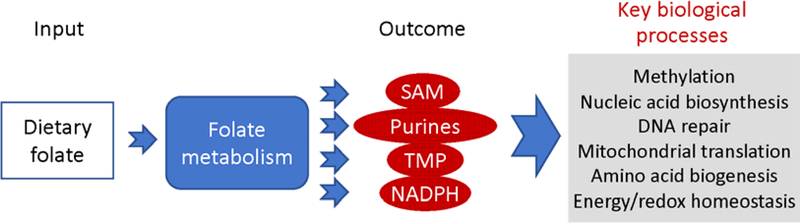
Several mechanisms for ethanol’s effect on carcinogenesis have been proposed, including the induction of oxidative stress, acetaldehyde-associated mutagenesis, perturbation of estrogen metabolism, and via folate metabolism (reviewed in [5, 18, 19]). Folate is an important dietary component because humans cannot synthesize it [20]. In the cell, folate functions as a coenzyme in numerous reactions of one-carbon transfer, which are required for the de novo purine and TMP biosynthesis, NADPH generation, and for metabolism of several amino acids, including re-methylation of homocysteine to methionine [21–23]. The latter reaction is linked to the biosynthesis of S-adenosylmethionine, the universal methyl donor involved in more than 100 methylation reactions in the cell [24]. Importantly, it has been recently reported that 5-methyltetrahydrofolate, the coenzyme remethylating homocysteine, can directly methylate mitochondrial tRNA, an important step in mitochondrial protein translation [25]. Another reaction important for the mitochondrial protein biosynthesis is the formylation of Met-tRNA by 10-formyltetrahydrofolate [21]. At a more general level, folate metabolism regulates such key biological processes as nucleic acid biosynthesis, mitochondrial protein biosynthesis, methylation of DNA, RNA, proteins and small molecules, DNA repair, and amino acid biogenesis (the role of folate in the cell is schematically depicted in Fig. 2) [21, 23, 26]. Accordingly, dietary folate deficiency or insufficient folate intake have been associated with several diseases including neural tube defects, cardiovascular diseases and cancer [23, 27, 28].
Fig. 2.
Role of folate in the cell. Folate taken up from the diet (Input) functions as a coenzyme in reactions of one-carbon transfer (Folate metabolism). These reactions are important for the biosynthesis of several essential molecules (Outcome), which are required for key biological processes.
The link between folate metabolism and the malignant transformation, as well as tumor progression, is however, not so simple though. Epidemiological studies provide ample data that dietary folate supplementation inversely correlates with the risk of several cancer types [2, 3, 29, 30]. At the same time, cancer cells critically depend on folate supplementation to support active nucleotide biosynthesis which is linked to the increased demand for nucleic acids during the period of rapid proliferation. Studies of the effect of folate on proliferation in cell culture and animal models provided experimental support for such mechanism [31–34]. The importance of folate for cancer cells provided the basis for cancer treatment using folate antimetabolites [35–38]. These compounds are structural analogs of folate but function as inhibitors of folate metabolizing enzymes. One of the first antifolates methotrexate, had been effectively used as chemotherapeutic since late 1940s while newer such drugs have been developed recently and approved for the treatment of different types of malignant tumors [36, 39]. Overall, folate has opposite effects on tumorigenesis versus the effect on cancer cell proliferation, which partially explains inconsistency of epidemiological data. To add to this complexity, the effect of folate on cancer metastasis is even less clear. For example, in in vivo models of tumorigenesis, folate deficiency suppressed proliferation but enhanced metastatic potential likely through the effect on epithelial-mesenchymal transition [40]. Of note, a promoting effect of folate on metastasis were also observed [33].
3. Folate transport and ethanol-induced folate deficiency
Folate was one of the factors intensively investigated with regard to the effect of alcohol consumption (reviewed in [41–43]). Studies from the early 1960s demonstrated that folate deficiency is common among alcoholics and that the positive hematopoietic response to the folate intake in these patients could be completely suppressed by excessive alcohol amounts [44, 45]. Recent studies in animals have confirmed these findings. Thus, rats subjected to chronic ethanol ingestion had decreased levels of folate in serum and red blood cells [46, 47]. Among other mechanisms, folate malabsorption could be one of the main causes of ethanol effect. Folate cannot pass through the cellular membrane on its own. Transport is carried out by three transporters, reduce folate carrier (RFC) [48, 49], proton-coupled folate transporter (PCFT) [50] and folate receptor alpha (FRα or FOLR1) [51]. PCFT and RFC are folate transporters responsible for folate uptake by enterocytes [52] so the effect of ethanol on intestinal folate absorption proceeds through regulation of these proteins [53]. Several mechanisms of such effect have been demonstrated. Ethanol decreases the expression of PCFT and RFC [54–57] most likely through the regulation of gene methylation [58]. Of note, methylation of CpG sites in genes encoding all three folate transporters has been demonstrated [59]. Chronic alcoholism can also affect the kinetics of folate absorption, which could be associated with altered lipid composition and mis-localization of transporters within specific lipid domains in the plasma membrane [46, 60]. Renal excretion is also one of the major factors contributing to ethanol-induced folate deficiency, due to reduced re-uptake of folate by kidneys caused by decreased expression of RFC and FRa [61, 62]. All three folate transporters are abundantly expressed in cancer cells [63, 64]. The effect of ethanol on the folate uptake by tumors is not clear though it has been reported that lower expression of FRα in pancreatic ductal adenocarcinoma was associated with alcohol consumption [65]. Interestingly, in this study, high FRα expression in surgically removed tumors was significantly associated with favorable prognosis. The mechanism of such effect is not clear, and in many cases the opposite phenomenon, a beneficial effect of the FRα overexpression on cancer development was reported [64].
4. Folate degradation
Reduced folates are unstable in vitro and rapidly undergo oxidative degradation but they are protected from degradation in the cell through binding to numerous folate enzymes [66]. Despite of such protection, in vivo folate catabolism is an active process [67–70]. The degradation of folate can be non-enzymatic but is also catalyzed by ferritin [71]. As alcohol consumption induces folate deficiency, the question has been asked of whether ethanol contributes to enhanced folate catabolism [72]. In fact, it has been shown that in vitro ethanol metabolism can induce folate degradation [73]. Major enzymatic pathways of ethanol metabolism via catalase, alcohol dehydrogenase or CYP450 lead to the formation of acetaldehyde, which is further metabolized to acetate by several aldehyde dehydrogenases [74, 75]. The oxidation of acetaldehyde can also occur in the reaction catalyzed the ubiquitous enzyme xanthine oxidase, which produces superoxide radicals [73]. Superoxide radicals in turn cause cleavage of folates with 5-methyltetrahydrofolate being much more susceptible to this degradation than folic acid [73].
While it is not clear whether this mechanism takes place in vivo, it has been recently shown that spontaneous folate decomposition produces formaldehyde, a cytotoxic metabolite which can damage DNA [76]. Formaldehyde is converted to formic acid by a pathway including as an intermediate step the catalysis by the alcohol dehydrogenase 5 (ADH5) enzyme [77]. Interestingly, it has been shown that ethanol exposure of zebrafish embryos reduces ADH5 mRNA [78]. Of note, the final step of formaldehyde detoxification in the proposed mechanism, which is the clearance of formic acid, requires tetrahydrofolate [76]. In fact, this folate-dependent pathway is the main mechanism of formate clearance and methanol detoxification in humans, and it requires two folate metabolizing enzymes, MTHFD1 and ALDH1L1 [79, 80]. Another recent report underscored the enhanced folate degradation associated with the accumulation of specific reduced folate, THF, in the cytosol [81]. This study also highlighted the role of folate metabolizing enzyme ALDH1L1 in the prevention of THF degradation. The binding of THF was proposed as a likely mechanism for such protection. In agreement with the mechanism of folate protection by ALDH1L1, up-regulation of the ALDH1L1 gene prevented folate degradation and alleviated the oxidative stress induced by ethanol exposure in zebrafish embryos [82, 83]. Interestingly, ALDH1L1 is one of the most down-regulated proteins in several cancers and it has been suggested as putative tumor suppressor (reviewed in [84]).
5. Effect of ethanol on folate metabolizing enzymes
Reactions constituting folate metabolism are carried out by about two dozen of specific enzymes [21]. The functions of many of these enzymes have been linked to tumorigenesis and malignancy progression [20, 84–86]. Several of these enzymes are well-known targets of ethanol [42]. Thus, ethanol has been shown to produce inhibitory effect on the activities of MTHFR and MTR in an animal model [87]. This mechanism can contribute to carcinogenesis by affecting the liver S-adenosylmethionine pool thus altering methylation capacity of the cell [88]. Ethanol also decreases thymidylate synthase mRNA levels in regenerating liver after partial hepatectomy [89], which could inhibit DNA biosynthesis and diminish the DNA repair capability. Two folate enzymes involved in the metabolism of 10-formyltetrahydrofolate, ALDH1L1 and ALDH1L2, were also investigated as targets of ethanol and in response mechanisms to alcohol consumption (reviewed recently in [42]). An OMICS-type study has also reported that prenatal ethanol exposure of mouse embryos leads to the decreased DHFR expression [90]. Though the mechanism of this effect is not clear, it could have a far-reaching effect on carcinogenesis since DHFR is a key enzyme incorporating dietary folic acid into the reduced folate pool [91, 92]. FPGS, the enzymes conjugating folate to glutamate, was downregulated in the intestine and kidney of rats fed ethanol for 3 month [58]. This effect was likely the result of the FPGS gene hypermethylation, observed in this study. The FPGS-catalyzed reaction is crucial for folate retention inside the cell and the loss of the enzyme in mice is embryonically lethal [93]. Therefore, the decrease of FPGS activity in response to alcohol consumption could be a contributor to folate-mediated ethanol toxicity and a factor playing a role in carcinogenesis.
Folate-metabolizing enzymes themselves could also mediate effects of ethanol on the cell. In support of this notion, studies indicated that single nucleotide polymorphisms in enzymes of folate pathways could modify cancer risk associated with alcohol consumption [94–99]. Several studies demonstrated, in a more direct way, that enzymes of folate metabolism are involved in cellular response to the ethanol exposure. For example, in a zebrafish model, exposure of embryos to ethanol led to the up-regulation of ALDH1L1 (10-formyltetrahydrofolate dehydrogenase), which alleviated ethanol-induced oxidative stress [83]. In another study, mice with mild MTHFR deficiency (heterozygous disruption of Mthfr mimicking the Mthfr 677C→T SNP in humans [100]) had lower capacity to repair DNA and displayed more neuronal damage in the brain in response to the ethanol feeding [101]. Paradoxically, the MTHFR 677TT genotype has been shown to play a protective role against alcohol dependence [102]. Furthermore, subjects with the MTHFR 677TT genotype constituted a subgroup of alcoholic patients with a decreased risk for developing hepatic toxicity [102]. Overall, the mechanistic studies on the interaction between ethanol and folate enzyme are limited.
6. Molecular mechanisms underlying effects of ethanol on folate homeostasis
The interaction between alcohol consumption and dietary folate intake is relevant not only to cancer but also to liver diseases and disorders of embryonic development. Indeed, studies in micropigs have shown that folate deficiency enhances perturbations in hepatic methionine metabolism, decreases S-adenosylmethionine and glutathione, and increases DNA damage and lipid oxidation while promoting alcoholic liver injury [103, 104]. As well, both alcohol consumption and dietary folate deficiency have a teratogenic effect. Dietary folate deficiency has long been known as a cause of neural tube defects (NTDs) with most common such defect being spina bifida [105]. For the reason of the NTDs prevention, in 1996 the FDA mandated the supplementation of grain foods in the US with the synthetic form of the vitamin, folic acid. Prenatal exposure to maternal consumption of the ethanol is a common cause of developmental abnormalities, known as fetal alcohol spectrum disorder (FASD), associated with neurological, behavioral, and cognitive deficits [106–108]. In general, there are many common mechanisms regulating embryonic development and tumorigenesis [109–112]. For example, FASD are linked to an impaired immune system which consequently leads to an elevated risk of cancer and other diseases [113]. It has been recently shown that the transcriptional repressor Snai2, which is involved in the induction of epithelial-mesenchymal transition in cancer and development, is deregulated in response to ethanol thus causing apoptosis in avian neural crest progenitors [114]. Of note, folate metabolism is also involved in the regulation of proliferation, apoptosis and epithelial-mesenchymal transition [33, 81, 115–117]. Furthermore, DNA methylation, which is intrinsically linked to folate metabolism and plays roles in the regulation of embryonic development and in tumorigenesis, is also deregulated by alcohol [88, 118–123]. Thus, analysis of cellular responses to ethanol and folate in developmental processes can provide clues for mechanistic links between these nutrients and the malignant transformation. Several examples of such links are discussed below.
Exposure to ethanol produces a pleiotropic effect on the cell with alcohol consumption causing genetic abnormalities, epigenetic dysregulation, induction of cell signaling, and metabolic abnormalities, global events activating whole arrays of downstream cellular responses [5]. In support of such a wide-spread effect, OMICs studies have shown that exposure to ethanol causes dramatic alterations in the overall gene expression [124–128]. Interestingly, in one of these studies, a significant number of affected targets were ribosomal genes [124]. Inactivating or deleterious mutations of some of these genes cause Diamond-Blackfan anemia, which are conditions characterized by macrocytic anemia and cancer predisposition, and representative of a class of disorders known as ribosomopathies [129]. Importantly, it has been recently reported that ribosomal proteins are commonly deleted in human cancers and that this phenomenon is often associated with p53 mutations [130]. One of the ribosomal proteins involved in the response to ethanol was rps3a [90, 124]. Curiously, this protein physically interacts with mitochondrial folate metabolizing enzymes, MTHFD2 [131]. While this enzyme is a resident of mitochondria, it can translocate to the nucleus which is likely a mechanism for the regulation of cellular proliferation [132]. In fact, numerous reports linked MTHFD2 expression to enhanced cellular proliferation and highlighted upregulation of the enzyme as a cancer trait [132–138]. These findings raise the question of whether MTHFD2 could be a mediator of the effect of alcohol consumption on malignant transformation or progression of initiated cells.
7. microRNA link between alcohol consumption and dietary folate
Alcohol consumption was also investigated with regard to the role of microRNAs (miRNAs) in the teratogenic, liver damaging and carcinogenic effects of ethanol (reviewed in [139–142]). miRNAs, a diverse class of highly conserved small non-coding RNAs that regulate gene expression, play important role in malignant tumor initiation and in metastasis [143–145]. A recent analysis of RNA-Seq paired-end dataset derived from alcohol-exposed neural fold-stage chick crania suggested that miRNAs significantly contribute to gene expression in response to ethanol [127]. Certain dietary components, including folate, can impact tumorigenesis and cancer progression by modulating tissue levels of miRNAs [146]. A growing body of evidence links folate status to the regulation of a large number of miRNAs but also identifies a reverse effect - the regulation of folate metabolizing enzymes by miRNAs (reviewed in [147, 148]). One of the early reports demonstrated that folate deficiency leads to a pronounced but reversible global increase in miRNA expression in human lymphoblastoid cells [149]. In another study, deregulation of miR-122, −23 and −130 was observed in hepatocellular carcinomas developed in rats kept on the folate- and methyl-deficient diet [150]. It has been further shown that miR-122 is significantly downregulated in human primary hepatocellular carcinomas [150]. Interestingly, in a recent study miR-122 protected from ethanol-induced liver disease [151]. Thus, the regulation of miR-122 can be a point of crosstalk between ethanol and folate. Cross-reference of miRNA responding to both the folate status [147] and alcohol consumption or in vitro ethanol exposure identified several potential links including miR-21 [152–156], miR-222 [149, 157, 158] and miR- 34a [159–161] all of which were implemented in cancer disease [162–164].
While numerous reports link folate and ethanol to the regulation of microRNAs, it is not clear yet how the cross-talk between these dietary components regulates progression of known pathologies. One study that linked ethanol-induced birth defects to the up-regulation of miR-10a/miR-10b and associated down-regulation of transcription factor Hoxa1 also demonstrated that folic acid prevented ethanol-induced miR-10a elevation and reduced developmental abnormalities [165]. Of note, miR-10a could also have a role in carcinogenesis [166]. For example, levels of miR-10a/b were significantly increased in peripheral blood mononuclear cells derived from patients with acute myeloid leukemia compared with cells derived from healthy donors [167]. Furthermore, miR-10a/b expression promoted proliferation and inhibited differentiation of HL-60 cells [167]. miR-10 was also suggested as an oncogene involved in breast cancer initiation and progression, with one of the downstream mechanisms being modulation of HOXA1 gene expression [168]. Another mechanism of miR-10b in tumor promotion is associated with Rho GTPase up-regulation leading to Rho-kinase-associated cytoskeleton activation and enhanced tumor cell invasion [169, 170]. In this regard, the role of folate in metastasis promotion through the activation of Rho GTPase-dependent cytoskeleton rearrangement has been reported [33, 171].
8. Folate, ethanol and oxidative stress
Folate can alleviate oxidative stress [172, 173] while ethanol is a known inducer of such stress [174–176]. Ethanol metabolism generates reactive oxygen species and depletes the antioxidant molecule glutathione (GSH) which leads to oxidative stress and lipid and protein damage and then to growth retardation and neurotoxicity [177, 178]. The relationship between alcohol consumption and folate intake is a two-way street: ethanol can decrease folate-dependent antioxidative capacity while folate can alleviate ethanol-induced oxidative stress [177, 179]. In support of this notion, it has been reported that folic acid protects offspring against oxidative stress in the case of ethanol feeding to pregnant rats [180]. The effect of folate in this study was observed in liver and pancreas and was attributed to either the direct quenching of reactive oxygen species or scavenging capacity toward acetaldehyde. These findings are especially important taking into consideration that prenatal folate supplementation can affect the cancer risk later in life [181–186]. While in fact the scavenging behavior of folate in vitro has been reported [187], it is unclear whether the direct reduction of reactive oxygen species by folate takes place in vivo [172]. Instead, the antioxidant effect of folate in vivo is primarily associated with the ability to lower Hcy and thus to alleviate hyperhomocysteinemia-induced effects [188, 189]. It has been shown that folic acid supplementation attenuates xanthine oxidase activity, restores SOD activity and effectively antagonizes oxidative stress in the kidneys of hyperhomocysteinemic rats [190]. In in vitro experiments in this study, incubation of tubular cells with 5-methyltetrahydrofolate abolished Hcy-induced NADPH oxidase activation and reduced the intracellular level of superoxide anion, and also reduced the mRNA levels of NOX4 and p22phox [190]. Of note, the study indicated that the folate effect on oxidative stress can either be associated with Hcy re-methylation or proceed through Hcy-independent mechanisms.
Alcohol consumption has been linked to the induction of hypoxia [191, 192], a state leading to increased generation of reactive oxygen species [193]. Several reports have also addressed the effect of folate on hypoxia. Thus, folic acid has been shown to protect cultured endothelial cells from hypoxia by decreasing both ROS levels and apoptosis linked to the ERK1/2 and NOX4 pathways [194]. The protective mechanism of folate in hypoxia is primarily associated with the induction of nitric oxide production by endothelial NO synthase (eNOS) [195]. This mechanism is linked to the upregulation of DHFR in response to folate administration, which enhances BH4 recycling thus promoting eNOS recoupling [196, 197]. Since eNOS uncoupling is linked to cardiopulmonary disorders [198], the eNOS-related mechanism of folate was mainly investigated in endothelial cells as a potential therapeutic approach to prevent cardiovascular disease [195]. However, solid malignant tumors typically grow under hypoxic conditions and to survive and proliferate in a hypoxic environment, cancer cells undergo genetic and adaptive changes that contribute to the malignant phenotype and to aggressive tumor behavior [199–203]. Interestingly, it has been shown that ubiquitously expressed folate enzymes are downregulated under severe hypoxia [204], which suggests folate metabolism as one of the components of such adaptive response. In support of the role of folate pathways in this response, the suppression of MTHFD2 folate enzyme disturbs NADPH and redox homeostasis and accelerates cell death under hypoxia-induced oxidative stress [205]. Furthermore, it has been recently shown that folic acid supplementation represses hypoxia- induced inflammatory response in promyelomonocytic cells via the elimination of ROS and inhibition of the JAK2/STAT3/NF-κB pathway [206].
9. Summary
Mountains of literature link alcohol consumption and folate intake to the risk of cancer development. While alcohol consumption has positive correlation with the risk of several types of cancer, numerous studies support the idea that increased dietary folate has inverse correlation with tumorigenesis. However, precise molecular mechanisms underlying the effects of ethanol and folate in this respect are diverse and not completely understood. Several of these mechanisms involve the effect of ethanol on folate metabolism and it is likely that ethanol-induced folate deficiency contributes to tumorigenesis. The obvious conclusion here would be that increased folate intake could alleviate effects of alcohol, and numerous studies support such a connection. Ethanol produces folate-dependent as well as folate independent effects, and there are also some cellular nodes which are targeted by both ethanol and folate independently. Thus, the overall response to the combined effect of ethanol and folate will create an intricate circuit, the outcomes of with will further depend on other dietary components, genetic and epigenetic modalities, the sites of interaction, and numerous other factors. It should be also emphasized that the effect of both these dietary constituents is likely different in tumor initiation versus tumor progression and metastasis [19, 20, 207–209]. The role of ethanol and folate in tumor progression and metastasis, however, is much less investigated and awaits future studies.
Acknowledgments
The authors thank Dr. David Horita for carefully reading the manuscript and thoughtful comments. S.A.K. is supported by the National Institutes of Health grant R01 DK117854.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Boffetta P, Hashibe M, Alcohol and cancer, Lancet Oncol, 7 (2006) 149–156. [DOI] [PubMed] [Google Scholar]
- [2].Chen P, Li C, Li X, Li J, Chu R, Wang H, Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis, Br J Cancer, 110 (2014) 2327–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kennedy DA, Stern SJ, Moretti M, Matok I, Sarkar M, Nickel C, Koren G, Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis, Cancer Epidemiol, 35 (2011) 2–10. [DOI] [PubMed] [Google Scholar]
- [4].Stevens VL, McCullough ML, Sun J, Jacobs EJ, Campbell PT, Gapstur SM, High levels of folate from supplements and fortification are not associated with increased risk of colorectal cancer, Gastroenterology, 141 (2011) 98–105, 105 e101. [DOI] [PubMed] [Google Scholar]
- [5].Rossi M, Jahanzaib Anwar M, Usman A, Keshavarzian A, Bishehsari F, Colorectal Cancer and Alcohol Consumption-Populations to Molecules, Cancers (Basel), 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wien TN, Pike E, Wisloff T, Staff A, Smeland S, Klemp M, Cancer risk with folic acid supplements: a systematic review and meta-analysis, BMJ Open, 2 (2012) e000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heinen MM, van den Brandt PA, Schouten LJ, Goldbohm RA, Schouten HC, Verhage BA, Dietary one-carbon nutrient intake and risk of lymphoid and myeloid neoplasms: results of the Netherlands cohort study, Cancer Epidemiol Biomarkers Prev, 23 (2014) 2153–2164. [DOI] [PubMed] [Google Scholar]
- [8].Nishihara R, Wang M, Qian ZR, Baba Y, Yamauchi M, Mima K, Sukawa Y, Kim SA, Inamura K, Zhang X, Wu K, Giovannucci EL, Chan AT, Fuchs CS, Ogino S, Schernhammer ES, Alcohol, one-carbon nutrient intake, and risk of colorectal cancer according to tumor methylation level of IGF2 differentially methylated region, Am J Clin Nutr, 100 (2014) 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mason JB, Tang SY, Folate status and colorectal cancer risk: A 2016 update, Mol Aspects Med, 53 (2017) 73–79. [DOI] [PubMed] [Google Scholar]
- [10].Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, van den Brandt PA, Brinton L, Buring JE, Eliassen AH, Falk R, Gapstur SM, Giles GG, Goodman G, Hoffman-Bolton J, Horn-Ross PL, Inoue M, Kolonel LN, Krogh V, Lof M, Maas P, Miller AB, Neuhouser ML, Park Y, Robien K, Rohan TE, Scarmo S, Schouten LJ, Sieri S, Stevens VL, Tsugane S, Visvanathan K, Wilkens LR, Wolk A, Weiderpass E, Willett WC, Zeleniuch- Jacquotte A, Zhang SM, Zhang X, Ziegler RG, Smith-Warner SA, Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies, Int J Epidemiol, 45 (2016) 916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zakhari S, Hoek JB, Alcohol and breast cancer: reconciling epidemiological and molecular data, Adv Exp Med Biol, 815 (2015) 7–39. [DOI] [PubMed] [Google Scholar]
- [12].Fagherazzi G, Vilier A, Boutron-Ruault MC, Mesrine S, Clavel-Chapelon F, Alcohol consumption and breast cancer risk subtypes in the E3N-EPIC cohort, Eur J Cancer Prev, 24 (2015) 209–214. [DOI] [PubMed] [Google Scholar]
- [13].Klarich DS, Brasser SM, Hong MY, Moderate Alcohol Consumption and Colorectal Cancer Risk, Alcohol Clin Exp Res, 39 (2015) 1280–1291. [DOI] [PubMed] [Google Scholar]
- [14].Giacosa A, Barale R, Bavaresco L, Gatenby P, Gerbi V, Janssens J, Johnston B, Kas K, La Vecchia C, Mainguet P, Morazzoni P, Negri E, Pelucchi C, Pezzotti M, Rondanelli M, Cancer prevention in Europe: the Mediterranean diet as a protective choice, Eur J Cancer Prev, 22 (2013) 90–95. [DOI] [PubMed] [Google Scholar]
- [15].Schwingshackl L, Hoffmann G, Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies, Int J Cancer, 135 (2014) 1884–1897. [DOI] [PubMed] [Google Scholar]
- [16].Peng Q, Chen H, Huo JR, Alcohol consumption and corresponding factors: A novel perspective on the risk factors of esophageal cancer, Oncol Lett, 11 (2016) 3231–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Galeone C, Edefonti V, Parpinel M, Leoncini E, Matsuo K, Talamini R, Olshan AF, Zevallos JP, Winn DM, Jayaprakash V, Moysich K, Zhang ZF, Morgenstern H, Levi F, Bosetti C, Kelsey K, McClean M, Schantz S, Yu GP, Boffetta P, Lee YC, Hashibe M, La Vecchia C, Boccia S, Folate intake and the risk of oral cavity and pharyngeal cancer: a pooled analysis within the International Head and Neck Cancer Epidemiology Consortium, Int J Cancer, 136 (2015) 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dumitrescu RG, Shields PG, The etiology of alcohol-induced breast cancer, Alcohol, 35 (2005) 213–225. [DOI] [PubMed] [Google Scholar]
- [19].Ratna A, Mandrekar P, Alcohol and Cancer: Mechanisms and Therapies, Biomolecules, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Strickland KC, Krupenko NI, Krupenko SA, Molecular mechanisms underlying the potentially adverse effects of folate, Clin Chem Lab Med, 51 (2013) 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tibbetts AS, Appling DR, Compartmentalization of Mammalian folate-mediated one-carbon metabolism, Annu Rev Nutr, 30 (2010) 57–81. [DOI] [PubMed] [Google Scholar]
- [22].Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD, Quantitative flux analysis reveals folate-dependent NADPH production, Nature, 510 (2014) 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ducker GS, Rabinowitz JD, One-Carbon Metabolism in Health and Disease, Cell Metab, 25 (2017) 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Glynn SA, Albanes D, Folate and cancer: a review of the literature, Nutr Cancer, 22 (1994) 101–119. [DOI] [PubMed] [Google Scholar]
- [25].Morscher RJ, Ducker GS, Li SH, Mayer JA, Gitai Z, Sperl W, Rabinowitz JD, Mitochondrial translation requires folate-dependent tRNA methylation, Nature, 554 (2018) 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP, Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease, J Neurosci, 22 (2002) 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stover PJ, Physiology of folate and vitamin B12 in health and disease, Nutr Rev, 62 (2004) S3–12; discussion S13. [DOI] [PubMed] [Google Scholar]
- [28].Stover PJ, One-carbon metabolism-genome interactions in folate-associated pathologies, J Nutr, 139 (2009) 2402–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tio M, Andrici J, Cox MR, Eslick GD, Folate intake and the risk of upper gastrointestinal cancers: a systematic review and meta-analysis, J Gastroenterol Hepatol, 29 (2014) 250–258. [DOI] [PubMed] [Google Scholar]
- [30].Giovannucci E, Epidemiologic studies of folate and colorectal neoplasia: a review, J Nutr, 132 (2002) 2350S–2355S. [DOI] [PubMed] [Google Scholar]
- [31].Rodriguez JM, Miranda D, Bunout D, Ronco AM, de la Maza MP, Hirsch S, Folates Induce Colorectal Carcinoma HT29 Cell Line Proliferation Through Notch1 Signaling, Nutr Cancer, 67 (2015) 706–711. [DOI] [PubMed] [Google Scholar]
- [32].Tomaszewski JJ, Cummings JL, Parwani AV, Dhir R, Mason JB, Nelson JB, Bacich DJ, O’Keefe DS, Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate, Prostate, 71 (2011) 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Oleinik NV, Helke KL, Kistner-Griffin E, Krupenko NI, Krupenko SA, Rho GTPases RhoA and Rac1 mediate effects of dietary folate on metastatic potential of A549 cancer cells through the control of cofilin phosphorylation, J Biol Chem, 289 (2014) 26383–26394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ashkavand Z, O’Flanagan C, Hennig M, Du X, Hursting SD, Krupenko SA, Metabolic Reprogramming by Folate Restriction Leads to a Less Aggressive Cancer Phenotype, Mol Cancer Res, 15 (2017) 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chattopadhyay S, Moran RG, Goldman ID, Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications, Mol Cancer Ther, 6 (2007) 404–417. [DOI] [PubMed] [Google Scholar]
- [36].Goldman ID, Chattopadhyay S, Zhao R, Moran R, The antifolates: evolution, new agents in the clinic, and how targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs, Curr Opin Investig Drugs, 11 (2010) 1409–1423. [PubMed] [Google Scholar]
- [37].Visentin M, Zhao R, Goldman ID, The antifolates, Hematol Oncol Clin North Am, 26 (2012) 629–648, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Longley DB, Harkin DP, Johnston PG, 5-fluorouracil: mechanisms of action and clinical strategies, Nat Rev Cancer, 3 (2003) 330–338. [DOI] [PubMed] [Google Scholar]
- [39].Gonen N, Assaraf YG, Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance, Drug Resist Updat, 15 (2012) 183–210. [DOI] [PubMed] [Google Scholar]
- [40].deficient tumor microenvironment promotes epithelial-to-mesenchymal transition and cancer stem-like phenotypes, Oncotarget, 7 (2016) 33246–33256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Medici V, Halsted CH, Folate, alcohol, and liver disease, Mol Nutr Food Res, 57 (2013) 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Krupenko SA, Krupenko NI, ALDH1L1 and ALDH1L2 Folate Regulatory Enzymes in Cancer, Adv Exp Med Biol, 1032 (2018) 127–143. [DOI] [PubMed] [Google Scholar]
- [43].Zakhari S, Hoek JB, Epidemiology of Moderate Alcohol Consumption and Breast Cancer: Association or Causation?, Cancers (Basel), 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Herbert V, Zalusky R, Davidson CS, Correlation of folate deficiency with alcoholism and associated macrocytosis, anemia, and liver disease, Ann Intern Med, 58 (1963) 977–988. [DOI] [PubMed] [Google Scholar]
- [45].Sullivan LW, Herbert V, Suppression Hematopoiesis by Ethanol, J Clin Invest, 43 (1964) 2048–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hamid A, Kaur J, Mahmood A, Evaluation of the kinetic properties of the folate transport system in intestinal absorptive epithelium during experimental ethanol ingestion, Mol Cell Biochem, 304 (2007) 265–271. [DOI] [PubMed] [Google Scholar]
- [47].Hamid A, Wani NA, Rana S, Vaiphei K, Mahmood A, Kaur J, Down-regulation of reduced folate carrier may result in folate malabsorption across intestinal brush border membrane during experimental alcoholism, FEBS J, 274 (2007) 6317–6328. [DOI] [PubMed] [Google Scholar]
- [48].Moscow JA, Gong M, He R, Sgagias MK, Dixon KH, Anzick SL, Meltzer PS, Cowan KH, Isolation of a gene encoding a human reduced folate carrier (RFC1) and analysis of its expression in transport-deficient, methotrexate-resistant human breast cancer cells, Cancer Res, 55 (1995) 3790–3794. [PubMed] [Google Scholar]
- [49].Gelineau-van Waes J, Heller S, Bauer LK, Wilberding J, Maddox JR, Aleman F, Rosenquist TH, Finnell RH, Embryonic development in the reduced folate carrier knockout mouse is modulated by maternal folate supplementation, Birth Defects Res A Clin Mol Teratol, 82 (2008) 494–507. [DOI] [PubMed] [Google Scholar]
- [50].Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID, Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption, Cell, 127 (2006) 917–928. [DOI] [PubMed] [Google Scholar]
- [51].Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, Finnell RH, Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development, Nat Genet, 23 (1999) 228–232. [DOI] [PubMed] [Google Scholar]
- [52].Visentin M, Diop-Bove N, Zhao R, Goldman ID, The intestinal absorption of folates, Annu Rev Physiol, 76 (2014) 251–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hamid A, Wani NA, Kaur J, New perspectives on folate transport in relation to alcoholism-induced folate malabsorption--association with epigenome stability and cancer development, FEBS J, 276 (2009) 2175–2191. [DOI] [PubMed] [Google Scholar]
- [54].Wani NA, Hamid A, Khanduja KL, Kaur J, Folate malabsorption is associated with down-regulation of folate transporter expression and function at colon basolateral membrane in rats, Br J Nutr, 107 (2012) 800–808. [DOI] [PubMed] [Google Scholar]
- [55].Said HM, Mee L, Sekar VT, Ashokkumar B, Pandol SJ, Mechanism and regulation of folate uptake by pancreatic acinar cells: effect of chronic alcohol consumption, Am J Physiol Gastrointest Liver Physiol, 298 (2010) G985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wani NA, Kaur J, Reduced levels of folate transporters (PCFT and RFC) in membrane lipid rafts result in colonic folate malabsorption in chronic alcoholism, J Cell Physiol, 226 (2011) 579–587. [DOI] [PubMed] [Google Scholar]
- [57].Wani NA, Nada R, Kaur J, Biochemical and molecular mechanisms of folate transport in rat pancreas; interference with ethanol ingestion, PLoS One, 6 (2011) e28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wani NA, Hamid A, Kaur J, Alcohol-associated folate disturbances result in altered methylation of folate-regulating genes, Mol Cell Biochem, 363 (2012) 157–166. [DOI] [PubMed] [Google Scholar]
- [59].Farkas SA, Befekadu R, Hahn-Stromberg V, Nilsson TK, DNA methylation and expression of the folate transporter genes in colorectal cancer, Tumour Biol, 36 (2015) 5581–5590. [DOI] [PubMed] [Google Scholar]
- [60].Wani NA, Thakur S, Najar RA, Nada R, Khanduja KL, Kaur J, Mechanistic insights of intestinal absorption and renal conservation of folate in chronic alcoholism, Alcohol, 47 (2013) 121–130. [DOI] [PubMed] [Google Scholar]
- [61].Hamid A, Kaur J, Decreased expression of transporters reduces folate uptake across renal absorptive surfaces in experimental alcoholism, J Membr Biol, 220 (2007) 69–77. [DOI] [PubMed] [Google Scholar]
- [62].Romanoff RL, Ross DM, McMartin KE, Acute ethanol exposure inhibits renal folate transport, but repeated exposure upregulates folate transport proteins in rats and human cells, J Nutr, 137 (2007) 1260–1265. [DOI] [PubMed] [Google Scholar]
- [63].Matherly LH, Hou Z, Gangjee A, The promise and challenges of exploiting the proton-coupled folate transporter for selective therapeutic targeting of cancer, Cancer Chemother Pharmacol, 81 (2018) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cheung A, Bax HJ, Josephs DH, Ilieva KM, Pellizzari G, Opzoomer J, Bloomfield J, Fittall M, Grigoriadis A, Figini M, Canevari S, Spicer JF, Tutt AN, Karagiannis SN, Targeting folate receptor alpha for cancer treatment, Oncotarget, 7 (2016) 52553–52574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cai L, Michelakos T, Ferrone CR, Zhang L, Deshpande V, Shen Q, DeLeo A, Yamada T, Zhang G, Ferrone S, Wang X, Expression status of folate receptor alpha is a predictor of survival in pancreatic ductal adenocarcinoma, Oncotarget, 8 (2017) 37646–37656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Suh JR, Herbig AK, Stover PJ, New perspectives on folate catabolism, Annu Rev Nutr, 21 (2001) 255–282. [DOI] [PubMed] [Google Scholar]
- [67].Caudill MA, Gregory JF, Hutson AD, Bailey LB, Folate catabolism in pregnant and nonpregnant women with controlled folate intakes, J Nutr, 128 (1998) 204–208. [DOI] [PubMed] [Google Scholar]
- [68].Geoghegan FL, McPartlin JM, Weir DG, Scott JM, para-acetamidobenzoylglutamate is a suitable indicator of folate catabolism in rats, J Nutr, 125 (1995) 2563–2570. [DOI] [PubMed] [Google Scholar]
- [69].Higgins JR, Quinlivan EP, McPartlin J, Scott JM, Weir DG, Darling MR, The relationship between increased folate catabolism and the increased requirement for folate in pregnancy, BJOG, 107 (2000) 1149–1154. [DOI] [PubMed] [Google Scholar]
- [70].McNulty H, McPartlin JM, Weir DG, Scott JM, Folate catabolism is related to growth rate in weanling rats, J Nutr, 125 (1995) 99–103. [DOI] [PubMed] [Google Scholar]
- [71].Suh JR, Oppenheim EW, Girgis S, Stover PJ, Purification and properties of a folate-catabolizing enzyme, J Biol Chem, 275 (2000) 35646–35655. [DOI] [PubMed] [Google Scholar]
- [72].Kelly D, Reed B, Weir D, Scott J, Effect of acute and chronic alcohol ingestion on the rate of folate catabolism and hepatic enzyme induction in mice, Clin Sci (Lond), 60 (1981) 221–224. [DOI] [PubMed] [Google Scholar]
- [73].Shaw S, Jayatilleke E, Herbert V, Colman N, Cleavage of folates during ethanol metabolism. Role of acetaldehyde/xanthine oxidase-generated superoxide, Biochem J, 257 (1989) 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Peter Guengerich F, Avadhani NG, Roles of Cytochrome P450 in Metabolism of Ethanol and Carcinogens, Adv Exp Med Biol, 1032 (2018) 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Heit C, Dong H, Chen Y, Shah YM, Thompson DC, Vasiliou V, Transgenic mouse models for alcohol metabolism, toxicity, and cancer, Adv Exp Med Biol, 815 (2015) 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Burgos-Barragan G, Wit N, Meiser J, Dingler FA, Pietzke M, Mulderrig L, Pontel LB, Rosado IV, Brewer TF, Cordell RL, Monks PS, Chang CJ, Vazquez A, Patel KJ, Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism, Nature, 548 (2017) 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu L, Swenberg JA, Crossan GP, Patel KJ, Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen, Mol Cell, 60 (2015) 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hu Y, Khan IA, Dasmahapatra AK, Disruption of circulation by ethanol promotes fetal alcohol spectrum disorder (FASD) in medaka (Oryzias latipes) embryogenesis, Comp Biochem Physiol C Toxicol Pharmacol, 148 (2008) 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Krupenko SA, FDH: an aldehyde dehydrogenase fusion enzyme in folate metabolism, Chem. Biol. Interact, 178 (2009) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Strickland KC, Holmes RS, Oleinik NV, Krupenko NI, Krupenko SA, Phylogeny and evolution of aldehyde dehydrogenase-homologous folate enzymes, Chem. Biol. Interact, 191 (2011) 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zheng Y, Lin TY, Lee G, Paddock MN, Momb J, Cheng Z, Li Q, Fei DL, Stein BD, Ramsamooj S, Zhang G, Blenis J, Cantley LC, Mitochondrial One-Carbon Pathway Supports Cytosolic Folate Integrity in Cancer Cells, Cell, 175 (2018) 1546–1560 e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chang WN, Lee GH, Kao TT, Lin CY, Hsiao TH, Tsai JN, Chen BH, Chen YH, Wu HR, Tsai HJ, Fu TF, Knocking down 10-Formyltetrahydrofolate dehydrogenase increased oxidative stress and impeded zebrafish embryogenesis by obstructing morphogenetic movement, Biochim Biophys Acta, 1840 (2014) 2340–2350. [DOI] [PubMed] [Google Scholar]
- [83].Hsiao TH, Lin CJ, Chung YS, Lee GH, Kao TT, Chang WN, Chen BH, Hung JJ, Fu TF, Ethanol-induced upregulation of 10-formyltetrahydrofolate dehydrogenase helps relieve ethanol-induced oxidative stress, Mol Cell Biol, 34 (2014) 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Krupenko SA, Krupenko NI, Loss of ALDH1L1 folate enzyme confers a selective metabolic advantage for tumor progression, Chem Biol Interact, 302 (2019) 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK, Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation, Science, 336 (2012) 1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gupta R, Yang Q, Dogra SK, Wajapeyee N, Serine hydroxymethyl transferase 1 stimulates pro-oncogenic cytokine expression through sialic acid to promote ovarian cancer tumor growth and progression, Oncogene, 36 (2017) 4014–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Villanueva JA, Halsted CH, Hepatic transmethylation reactions in micropigs with alcoholic liver disease, Hepatology, 39 (2004) 1303–1310. [DOI] [PubMed] [Google Scholar]
- [88].Varela-Rey M, Woodhoo A, Martinez-Chantar ML, Mato JM, Lu SC, Alcohol, DNA methylation, and cancer, Alcohol Res, 35 (2013) 25–35. [PMC free article] [PubMed] [Google Scholar]
- [89].Yoshida Y, Komatsu M, Ozeki A, Nango R, Tsukamoto I, Ethanol represses thymidylate synthase and thymidine kinase at mRNA level in regenerating rat liver after partial hepatectomy, Biochim Biophys Acta, 1336 (1997) 180–186. [DOI] [PubMed] [Google Scholar]
- [90].Feltes BC, de Faria Poloni J, Nunes IJ, Bonatto D, Fetal alcohol syndrome, chemo-biology and OMICS: ethanol effects on vitamin metabolism during neurodevelopment as measured by systems biology analysis, OMICS, 18 (2014) 344–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bailey SW, Ayling JE, The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake, Proc Natl Acad Sci U S A, 106 (2009) 15424–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bertino JR, Cancer research: from folate antagonism to molecular targets, Best Pract Res Clin Haematol, 22 (2009) 577–582. [DOI] [PubMed] [Google Scholar]
- [93].Raz S, Stark M, Assaraf YG, Folylpoly-gamma-glutamate synthetase: A key determinant of folate homeostasis and antifolate resistance in cancer, Drug Resist Updat, 28 (2016) 43–64. [DOI] [PubMed] [Google Scholar]
- [94].Suzuki T, Matsuo K, Sawaki A, Mizuno N, Hiraki A, Kawase T, Watanabe M, Nakamura T, Yamao K, Tajima K, Tanaka H, Alcohol drinking and one-carbon metabolism-related gene polymorphisms on pancreatic cancer risk, Cancer Epidemiol Biomarkers Prev, 17 (2008) 2742–2747. [DOI] [PubMed] [Google Scholar]
- [95].Sangrajrang S, Sato Y, Sakamoto H, Ohnami S, Khuhaprema T, Yoshida T, Genetic polymorphisms in folate and alcohol metabolism and breast cancer risk: a case-control study in Thai women, Breast Cancer Res Treat, 123 (2010) 885–893. [DOI] [PubMed] [Google Scholar]
- [96].Svensson T, Yamaji T, Budhathoki S, Hidaka A, Iwasaki M, Sawada N, Inoue M, Sasazuki S, Shimazu T, Tsugane S, Alcohol consumption, genetic variants in the alcohol- and folate metabolic pathways and colorectal cancer risk: the JPHC Study, Sci Rep, 6 (2016) 36607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cheng TY, Makar KW, Neuhouser ML, Miller JW, Song X, Brown EC, Beresford SA, Zheng Y, Poole EM, Galbraith RL, Duggan DJ, Habermann N, Bailey LB, Maneval DR, Caudill MA, Toriola AT, Green R, Ulrich CM, Folate-mediated one-carbon metabolism genes and interactions with nutritional factors on colorectal cancer risk: Women’s Health Initiative Observational Study, Cancer, 121 (2015) 3684–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Suzuki T, Matsuo K, Hasegawa Y, Hiraki A, Wakai K, Hirose K, Saito T, Sato S, Ueda R, Tajima K, One-carbon metabolism-related gene polymorphisms and risk of head and neck squamous cell carcinoma: case-control study, Cancer Sci, 98 (2007) 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Suzuki T, Matsuo K, Hiraki A, Saito T, Sato S, Yatabe Y, Mitsudomi T, Hida T, Ueda R, Tajima K, Impact of one-carbon metabolism-related gene polymorphisms on risk of lung cancer in Japan: a case control study, Carcinogenesis, 28 (2007) 1718–1725. [DOI] [PubMed] [Google Scholar]
- [100].Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. , A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase, Nat Genet, 10 (1995) 111–113. [DOI] [PubMed] [Google Scholar]
- [101].Fowler AK, Hewetson A, Agrawal RG, Dagda M, Dagda R, Moaddel R, Balbo S, Sanghvi M, Chen Y, Hogue RJ, Bergeson SE, Henderson GI, Kruman II, Alcohol-induced one-carbon metabolism impairment promotes dysfunction of DNA base excision repair in adult brain, J Biol Chem, 287 (2012) 43533–43542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Saffroy R, Benyamina A, Pham P, Marill C, Karila L, Reffas M, Debuire B, Reynaud M, Lemoine A, Protective effect against alcohol dependence of the thermolabile variant of MTHFR, Drug Alcohol Depend, 96 (2008) 30–36. [DOI] [PubMed] [Google Scholar]
- [103].Halsted CH, Villanueva JA, Devlin AM, James SJ, Interactions of ethanol and folate deficiency in development of alcoholic liver disease in the micropig, Trans Am Clin Climatol Assoc, 113 (2002) 151–162; discussion 162–153. [PMC free article] [PubMed] [Google Scholar]
- [104].Halsted CH, Villanueva JA, Devlin AM, Niemela O, Parkkila S, Garrow TA, Wallock LM, Shigenaga MK, Melnyk S, James SJ, Folate deficiency disturbs hepatic methionine metabolism and promotes liver injury in the ethanol-fed micropig, Proc Natl Acad Sci U S A, 99 (2002) 10072–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Blom HJ, Shaw GM, den Heijer M, Finnell RH, Neural tube defects and folate: case far from closed, Nat Rev Neurosci, 7 (2006) 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA, Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders, Pediatrics, 138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, Conry JL, LeBlanc N, Loock CA, Lutke J, Mallon BF, McFarlane AA, Temple VK, Rosales T, Canada N Fetal Alcohol Spectrum Disorder Research, Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan, CMAJ, 188 (2016) 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Riley EP, Infante MA, Warren KR, Fetal alcohol spectrum disorders: an overview, Neuropsychol Rev, 21 (2011) 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ma Y, Zhang P, Wang F, Yang J, Yang Z, Qin H, The relationship between early embryo development and tumourigenesis, J Cell Mol Med, 14 (2010) 2697–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Guo X, Wang XF, Signaling cross-talk between TGF-beta/BMP and other pathways, Cell Res, 19 (2009) 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Maguire LH, Thomas AR, Goldstein AM, Tumors of the neural crest: Common themes in development and cancer, Dev Dyn, 244 (2015) 311–322. [DOI] [PubMed] [Google Scholar]
- [112].Hadjimichael C, Chanoumidou K, Papadopoulou N, Arampatzi P, Papamatheakis J, Kretsovali A, Common stemness regulators of embryonic and cancer stem cells, World J Stem Cells, 7 (2015) 1150–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mead EA, Sarkar DK, Fetal alcohol spectrum disorders and their transmission through genetic and epigenetic mechanisms, Front Genet, 5 (2014) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Flentke GR, Baulch JW, Berres ME, Garic A, Smith SM, Alcohol-mediated calcium signals dysregulate pro-survival Snai2/PUMA/Bcl2 networks to promote p53-mediated apoptosis in avian neural crest progenitors, Birth Defects Res, 111 (2019) 686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hoeferlin LA, Oleinik NV, Krupenko NI, Krupenko SA, Activation of p21-Dependent G1/G2 Arrest in the Absence of DNA Damage as an Antiapoptotic Response to Metabolic Stress, Genes Cancer, 2 (2011) 889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hoeferlin LA, Fekry B, Ogretmen B, Krupenko SA, Krupenko NI, Folate stress induces apoptosis via p53-dependent de novo ceramide synthesis and up-regulation of ceramide synthase 6, J Biol Chem, 288 (2013) 12880–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fekry B, Jeffries KA, Esmaeilniakooshkghazi A, Szulc ZM, Knagge KJ, Kirchner DR, Horita DA, Krupenko SA, Krupenko NI, C16-ceramide is a natural regulatory ligand of p53 in cellular stress response, Nat Commun, 9 (2018) 4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Robertson KD, Wolffe AP, DNA methylation in health and disease, Nat Rev Genet, 1 (2000) 11–19. [DOI] [PubMed] [Google Scholar]
- [119].Smith ZD, Meissner A, DNA methylation: roles in mammalian development, Nat Rev Genet, 14 (2013) 204–220. [DOI] [PubMed] [Google Scholar]
- [120].Okano M, Bell DW, Haber DA, Li E, DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development, Cell, 99 (1999) 247–257. [DOI] [PubMed] [Google Scholar]
- [121].Baylin SB, Herman JG, DNA hypermethylation in tumorigenesis: epigenetics joins genetics, Trends Genet, 16 (2000) 168–174. [DOI] [PubMed] [Google Scholar]
- [122].Baylin SB, Jones PA, A decade of exploring the cancer epigenome - biological and translational implications, Nat Rev Cancer, 11 (2011) 726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jones PA, Baylin SB, The epigenomics of cancer, Cell, 128 (2007) 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Berres ME, Garic A, Flentke GR, Smith SM, Transcriptome Profiling Identifies Ribosome Biogenesis as a Target of Alcohol Teratogenicity and Vulnerability during Early Embryogenesis, PLoS One, 12 (2017) e0169351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Masuo Y, Imai T, Shibato J, Hirano M, Jones OA, Maguire ML, Satoh K, Kikuchi S, Rakwal R, Omic analyses unravels global molecular changes in the brain and liver of a rat model for chronic Sake (Japanese alcoholic beverage) intake, Electrophoresis, 30 (2009) 1259–1275. [DOI] [PubMed] [Google Scholar]
- [126].Zeng HL, Yang Q, Du H, Li H, Shen Y, Liu T, Chen X, Kamal GM, Guan Q, Cheng L, Wang J, Xu F, Proteomics and metabolomics analysis of hepatic mitochondrial metabolism in alcohol-preferring and non-preferring rats, Oncotarget, 8 (2017) 102020–102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Al-Shaer AE, Flentke GR, Berres ME, Garic A, Smith SM, Exon level machine learning analyses elucidate novel candidate miRNA targets in an avian model of fetal alcohol spectrum disorder, PLoS Comput Biol, 15 (2019) e1006937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Yin HQ, Je YT, Kim M, Kim JH, Kong G, Kang KS, Kim HL, Yoon BI, Lee MO, Lee BH, Analysis of hepatic gene expression during fatty liver change due to chronic ethanol administration in mice, Toxicol Appl Pharmacol, 235 (2009) 312–320. [DOI] [PubMed] [Google Scholar]
- [129].Narla A, Ebert BL, Ribosomopathies: human disorders of ribosome dysfunction, Blood, 115 (2010) 3196–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ajore R, Raiser D, McConkey M, Joud M, Boidol B, Mar B, Saksena G, Weinstock DM, Armstrong S, Ellis SR, Ebert BL, Nilsson B, Deletion of ribosomal protein genes is a common vulnerability in human cancer, especially in concert with TP53 mutations, EMBO Mol Med, 9 (2017) 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Koufaris C, Nilsson R, Protein interaction and functional data indicate MTHFD2 involvement in RNA processing and translation, Cancer Metab, 6 (2018) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gustafsson Sheppard N, Jarl L, Mahadessian D, Strittmatter L, Schmidt A, Madhusudan N, Tegner J, Lundberg EK, Asplund A, Jain M, Nilsson R, The folate-coupled enzyme MTHFD2 is a nuclear protein and promotes cell proliferation, Sci Rep, 5 (2015) 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, Huang J, Asplund A, Mootha VK, Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer, Nat Commun, 5 (2014) 3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ben-Sahra I, Hoxhaj G, Ricoult SJ, Asara JM, Manning BD, mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle, Science, 351 (2016) 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Miyo M, Konno M, Colvin H, Nishida N, Koseki J, Kawamoto K, Tsunekuni K, Nishimura J, Hata T, Takemasa I, Mizushima T, Doki Y, Mori M, Ishii H, The importance of mitochondrial folate enzymes in human colorectal cancer, Oncol Rep, (2016). [DOI] [PubMed] [Google Scholar]
- [136].Pikman Y, Puissant A, Alexe G, Furman A, Chen LM, Frumm SM, Ross L, Fenouille N, Bassil CF, Lewis CA, Ramos A, Gould J, Stone RM, DeAngelo DJ, Galinsky I, Clish CB, Kung AL, Hemann MT, Vander Heiden MG, Banerji V, Stegmaier K, Targeting MTHFD2 in acute myeloid leukemia, J Exp Med, 213 (2016) 1285–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Tedeschi PM, Vazquez A, Kerrigan JE, Bertino JR, Mitochondrial Methylenetetrahydrofolate Dehydrogenase (MTHFD2) Overexpression Is Associated with Tumor Cell Proliferation and Is a Novel Target for Drug Development, Mol Cancer Res, 13 (2015) 1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Green NH, Galvan DL, Badal SS, Chang BH, LeBleu VS, Long J, Jonasch E, Danesh FR, MTHFD2 links RNA methylation to metabolic reprogramming in renal cell carcinoma, Oncogene, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Balaraman S, Tingling JD, Tsai PC, Miranda RC, Dysregulation of microRNA expression and function contributes to the etiology of fetal alcohol spectrum disorders, Alcohol Res, 35 (2013) 18–24. [PMC free article] [PubMed] [Google Scholar]
- [140].Torres JL, Novo-Veleiro I, Manzanedo L, Alvela-Suarez L, Macias R, Laso FJ, Marcos M, Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease, World J Gastroenterol, 24 (2018) 4104–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Mandal C, Halder D, Jung KH, Chai YG, Maternal alcohol consumption and altered miRNAs in the developing fetus: Context and future perspectives, J Appl Toxicol, 38 (2018) 100–107. [DOI] [PubMed] [Google Scholar]
- [142].Nagadia R, Pandit P, Coman WB, Cooper-White J, Punyadeera C, miRNAs in head and neck cancer revisited, Cell Oncol (Dordr), 36 (2013) 1–7. [DOI] [PubMed] [Google Scholar]
- [143].Farazi TA, Spitzer JI, Morozov P, Tuschl T, miRNAs in human cancer, J Pathol, 223 (2011) 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Iorio MV, Croce CM, MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review, EMBO Mol Med, 4 (2012) 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Calin GA, Croce CM, MicroRNA signatures in human cancers, Nat Rev Cancer, 6 (2006) 857–866. [DOI] [PubMed] [Google Scholar]
- [146].Shah MS, Davidson LA, Chapkin RS, Mechanistic insights into the role of microRNAs in cancer: influence of nutrient crosstalk, Front Genet, 3 (2012) 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Beckett EL, Veysey M, Lucock M, Folate and microRNA: Bidirectional interactions, Clin Chim Acta, 474 (2017) 60–66. [DOI] [PubMed] [Google Scholar]
- [148].Shookhoff JM, G.l. Gallicano, A new perspective on neural tube defects: folic acid and microRNA misexpression, Genesis, 48 (2010) 282–294. [DOI] [PubMed] [Google Scholar]
- [149].Marsit CJ, Eddy K, Kelsey KT, MicroRNA responses to cellular stress, Cancer Res, 66 (2006) 10843–10848. [DOI] [PubMed] [Google Scholar]
- [150].Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST, Ghoshal K, Downregulation of miR-122 in the rodent and human hepatocellular carcinomas, J Cell Biochem, 99 (2006) 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [151].Satishchandran A, Ambade A, Rao S, Hsueh YC, Iracheta-Vellve A, Tornai D, Lowe P, Gyongyosi B, Li J, Catalano D, Zhong L, Kodys K, Xie J, Bala S, Gao G, Szabo G, MicroRNA 122, Regulated by GRLH2, Protects Livers of Mice and Patients From Ethanol-Induced Liver Disease, Gastroenterology, 154 (2018) 238–252 e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Beckett EL, Martin C, Choi JH, King K, Niblett S, Boyd L, Duesing K, Yates Z, Veysey M, Lucock M, Folate status, folate-related genes and serum miR-21 expression: Implications for miR-21 as a biomarker, BBA Clin, 4 (2015) 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Shah T, Mishra S, More A, Otiv S, Apte K, Joshi K, Combination of vitamin B12 active forms improved fetal growth in Wistar rats through up-regulation of placental miR-16 and miR- 21 levels, Life Sci, 191 (2017) 97–103. [DOI] [PubMed] [Google Scholar]
- [154].Sathyan P, Golden HB, Miranda RC, Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium, J Neurosci, 27 (2007) 8546–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Dippold RP, Vadigepalli R, Gonye GE, Hoek JB, Chronic ethanol feeding enhances miR- 21 induction during liver regeneration while inhibiting proliferation in rats, Am J Physiol Gastrointest Liver Physiol, 303 (2012) G733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Marts LT, Green DE, Mills ST, Murphy T, Sueblinvong V, MiR-21-Mediated Suppression of Smad7 Induces TGFbeta1 and Can Be Inhibited by Activation of Nrf2 in Alcohol- Treated Lung Fibroblasts, Alcohol Clin Exp Res, 41 (2017) 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Baker BC, Mackie FL, Lean SC, Greenwood SL, Heazell AEP, Forbes K, Jones RL, Placental dysfunction is associated with altered microRNA expression in pregnant women with low folate status, Mol Nutr Food Res, 61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Ignacio C, Mooney SM, Middleton FA, Effects of Acute Prenatal Exposure to Ethanol on microRNA Expression are Ameliorated by Social Enrichment, Front Pediatr, 2 (2014) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Li W, Li Z, Zhou D, Zhang X, Yan J, Huang G, Maternal folic acid deficiency stimulates neural cell apoptosis via miR-34a associated with Bcl-2 in the rat foetal brain, Int J Dev Neurosci, 72 (2019) 6–12. [DOI] [PubMed] [Google Scholar]
- [160].Geoffroy A, Kerek R, Pourie G, Helle D, Gueant JL, Daval JL, Bossenmeyer-Pourie C, Late Maternal Folate Supplementation Rescues from Methyl Donor Deficiency-Associated Brain Defects by Restoring Let-7 and miR-34 Pathways, Mol Neurobiol, 54 (2017) 5017–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Meng F, Glaser SS, Francis H, Yang F, Han Y, Stokes A, Staloch D, McCarra J, Liu J, Venter J, Zhao H, Liu X, Francis T, Swendsen S, Liu CG, Tsukamoto H, Alpini G, Epigenetic regulation of miR-34a expression in alcoholic liver injury, Am J Pathol, 181 (2012) 804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hermeking H, The miR-34 family in cancer and apoptosis, Cell Death Differ, 17 (2010) 193–199. [DOI] [PubMed] [Google Scholar]
- [163].Krichevsky AM, Gabriely G, miR-21: a small multi-faceted RNA, J Cell Mol Med, 13 (2009) 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafre SA, Farace MG, Agami R, Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation, EMBO J, 26 (2007) 3699–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, Wang J, Zhou SF, Li Y, Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation, Hum Reprod, 24 (2009) 562–579. [DOI] [PubMed] [Google Scholar]
- [166].Lund AH, miR-10 in development and cancer, Cell Death Differ, 17 (2010) 209–214. [DOI] [PubMed] [Google Scholar]
- [167].Bi L, Sun L, Jin Z, Zhang S, Shen Z, MicroRNA-10a/b are regulators of myeloid differentiation and acute myeloid leukemia, Oncol Lett, 15 (2018) 5611–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Balatti V, Oghumu S, Bottoni A, Maharry K, Cascione L, Fadda P, Parwani A, Croce C, Iwenofu OH, MicroRNA Profiling of Salivary Duct Carcinoma Versus Her2/Neu Overexpressing Breast Carcinoma Identify miR-10a as a Putative Breast Related Oncogene, Head Neck Pathol, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC, Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up- regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion, J Biol Chem, 285 (2010) 36721–36735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Bourguignon LY, Wong G, Shiina M, Up-regulation of Histone Methyltransferase, DOT1L, by Matrix Hyaluronan Promotes MicroRNA-10 Expression Leading to Tumor Cell Invasion and Chemoresistance in Cancer Stem Cells from Head and Neck Squamous Cell Carcinoma, J Biol Chem, 291 (2016) 10571–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Oleinik NV, Krupenko NI, Krupenko SA, ALDH1L1 inhibits cell motility via dephosphorylation of cofilin by PP1 and PP2A, Oncogene, 29 (2010) 6233–6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Stanger O, Wonisch W, Enzymatic and non-enzymatic antioxidative effects of folic acid and its reduced derivates, Subcell Biochem, 56 (2012) 131–161. [DOI] [PubMed] [Google Scholar]
- [173].Lai KG, Chen CF, Ho CT, Liu JJ, Liu TZ, Chern CL, Novel roles of folic acid as redox regulator: Modulation of reactive oxygen species sinker protein expression and maintenance of mitochondrial redox homeostasis on hepatocellular carcinoma, Tumour Biol, 39 (2017) 1010428317702649. [DOI] [PubMed] [Google Scholar]
- [174].Albano E, Alcohol, oxidative stress and free radical damage, Proc Nutr Soc, 65 (2006) 278–290. [DOI] [PubMed] [Google Scholar]
- [175].Das SK, Vasudevan DM, Alcohol-induced oxidative stress, Life Sci, 81 (2007) 177–187. [DOI] [PubMed] [Google Scholar]
- [176].Zakhari S, Alcohol metabolism and epigenetics changes, Alcohol Res, 35 (2013) 6–16. [PMC free article] [PubMed] [Google Scholar]
- [177].Ojeda L, Nogales F, Murillo L, Carreras O, The role of folic acid and selenium against oxidative damage from ethanol in early life programming: a review, Biochem Cell Biol, 96 (2018) 178–188. [DOI] [PubMed] [Google Scholar]
- [178].Arteel GE, Oxidants and antioxidants in alcohol-induced liver disease, Gastroenterology, 124 (2003) 778–790. [DOI] [PubMed] [Google Scholar]
- [179].Zhang Y, Wang H, Li Y, Peng Y, A review of interventions against fetal alcohol spectrum disorder targeting oxidative stress, Int J Dev Neurosci, 71 (2018) 140–145. [DOI] [PubMed] [Google Scholar]
- [180].Cano MJ, Ayala A, Murillo ML, Carreras O, Protective effect of folic acid against oxidative stress produced in 21-day postpartum rats by maternal-ethanol chronic consumption during pregnancy and lactation period, Free Radic Res, 34 (2001) 1–8. [DOI] [PubMed] [Google Scholar]
- [181].Milne E, Greenop KR, Bower C, Miller M, van Bockxmeer FM, Scott RJ, de Klerk NH, Ashton LJ, Gottardo NG, Armstrong BK, Aus CBTC, Maternal use of folic acid and other supplements and risk of childhood brain tumors, Cancer Epidemiol Biomarkers Prev, 21 (2012) 1933–1941. [DOI] [PubMed] [Google Scholar]
- [182].Chiavarini M, Naldini G, Fabiani R, Maternal Folate Intake and Risk of Childhood Brain and Spinal Cord Tumors: A Systematic Review and Meta-Analysis, Neuroepidemiology, 51 (2018) 82–95. [DOI] [PubMed] [Google Scholar]
- [183].Ly A, Lee H, Chen J, Sie KK, Renlund R, Medline A, Sohn KJ, Croxford R, Thompson LU, Kim YI, Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring, Cancer Res, 71 (2011) 988–997. [DOI] [PubMed] [Google Scholar]
- [184].Sie KK, Medline A, van Weel J, Sohn KJ, Choi SW, Croxford R, Kim YI, Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring, Gut, 60 (2011) 1687–1694. [DOI] [PubMed] [Google Scholar]
- [185].Thompson JR, Gerald PF, Willoughby ML, Armstrong BK, Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case-control study, Lancet, 358 (2001) 1935–1940. [DOI] [PubMed] [Google Scholar]
- [186].Ciappio ED, Mason JB, Crott JW, Maternal one-carbon nutrient intake and cancer risk in offspring, Nutr Rev, 69 (2011) 561–571. [DOI] [PubMed] [Google Scholar]
- [187].Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T, Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity, Free Radic Biol Med, 30 (2001) 1390–1399. [DOI] [PubMed] [Google Scholar]
- [188].Obeid R, Herrmann W, Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia, FEBS Lett, 580 (2006) 2994–3005. [DOI] [PubMed] [Google Scholar]
- [189].Mattson MP, Shea TB, Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders, Trends Neurosci, 26 (2003) 137–146. [DOI] [PubMed] [Google Scholar]
- [190].Hwang SY, Siow YL, Au-Yeung KK, House J, K. O, Folic acid supplementation inhibits NADPH oxidase-mediated superoxide anion production in the kidney, Am J Physiol Renal Physiol, 300 (2011) F189–198. [DOI] [PubMed] [Google Scholar]
- [191].Nath B, Szabo G, Hypoxia and hypoxia inducible factors: diverse roles in liver diseases, Hepatology, 55 (2012) 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].French SW, The role of hypoxia in the pathogenesis of alcoholic liver disease, Hepatol Res, 29 (2004) 69–74. [DOI] [PubMed] [Google Scholar]
- [193].Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT, Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing, Cell Metab, 1 (2005) 401–408. [DOI] [PubMed] [Google Scholar]
- [194].Cheng F, Lan J, Xia W, Tu C, Chen B, Li S, Pan W, Folic Acid Attenuates Vascular Endothelial Cell Injury Caused by Hypoxia via the Inhibition of ERK1/2/NOX4/ROS Pathway, Cell Biochem Biophys, 74 (2016) 205–211. [DOI] [PubMed] [Google Scholar]
- [195].Moens AL, Vrints CJ, Claeys MJ, Timmermans JP, Champion HC, Kass DA, Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease, Am J Physiol Heart Circ Physiol, 294 (2008) H1971–1977. [DOI] [PubMed] [Google Scholar]
- [196].Gao L, Chalupsky K, Stefani E, Cai H, Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity, J Mol Cell Cardiol, 47 (2009) 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Chalupsky K, Kracun D, Kanchev I, Bertram K, Gorlach A, Folic Acid Promotes Recycling of Tetrahydrobiopterin and Protects Against Hypoxia-Induced Pulmonary Hypertension by Recoupling Endothelial Nitric Oxide Synthase, Antioxid Redox Signal, 23 (2015) 1076–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Gielis JF, Lin JY, Wingler K, Van Schil PE, Schmidt HH, Moens AL, Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders, Free Radic Biol Med, 50 (2011) 765–776. [DOI] [PubMed] [Google Scholar]
- [199].Harris AL, Hypoxia--a key regulatory factor in tumour growth, Nat Rev Cancer, 2 (2002) 38–47. [DOI] [PubMed] [Google Scholar]
- [200].Wilson WR, Hay MP, Targeting hypoxia in cancer therapy, Nat Rev Cancer, 11 (2011) 393–410. [DOI] [PubMed] [Google Scholar]
- [201].Semenza GL, Hypoxia-inducible factors in physiology and medicine, Cell, 148 (2012) 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Semenza GL, Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype, EMBO J, 36 (2017) 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Gorrini C, Harris IS, Mak TW, Modulation of oxidative stress as an anticancer strategy, Nat Rev Drug Discov, 12 (2013) 931–947. [DOI] [PubMed] [Google Scholar]
- [204].Raz S, Sheban D, Gonen N, Stark M, Berman B, Assaraf YG, Severe hypoxia induces complete antifolate resistance in carcinoma cells due to cell cycle arrest, Cell Death Dis, 5 (2014) e1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Ju HQ, Lu YX, Chen DL, Zuo ZX, Liu ZX, Wu QN, Mo HY, Wang ZX, Wang DS, Pu HY, Zeng ZL, Li B, Xie D, Huang P, Hung MC, Chiao PJ, Xu RH, Modulation of Redox Homeostasis by Inhibition of MTHFD2 in Colorectal Cancer: Mechanisms and Therapeutic Implications, J Natl Cancer Inst, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ma J, Zhen X, Huang X, Jiang X, Folic acid supplementation repressed hypoxia-induced inflammatory response via ROS and JAK2/STAT3 pathway in human promyelomonocytic cells, Nutr Res, 53 (2018) 40–50. [DOI] [PubMed] [Google Scholar]
- [207].Meadows GG, Zhang H, Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival, Alcohol Res, 37 (2015) 311–322. [PMC free article] [PubMed] [Google Scholar]
- [208].Zhang H, Zhu Z, Zhang F, Meadows GG, Alcohol consumption and antitumor immunity: dynamic changes from activation to accelerated deterioration of the immune system, Adv Exp Med Biol, 815 (2015) 313–331. [DOI] [PubMed] [Google Scholar]
- [209].Mason JB, Unraveling the complex relationship between folate and cancer risk, Biofactors, 37 (2011) 253–260. [DOI] [PubMed] [Google Scholar]