Abstract
Sport-related concussion (SRC) has become a major health problem, affecting millions of athletes each year. Despite the increasing occurrence and prevalence of SRC, its underlying mechanism and recovery course have yet to be fully elucidated. The National Collegiate Athletic Association–Department of Defense Grand Alliance: Concussion Assessment, Research and Education (CARE) Consortium is a large-scale, multisite study of the natural history of concussion across multiple sports. The Advanced Research Core (ARC) of CARE is focused on the advanced biomarker assessment of a reduced subject cohort. This paper reports findings from two ARC sites to evaluate cerebral blood flow (CBF) changes in acute SRC, as measured using advanced arterial spin labeling (ASL) magnetic resonance imaging (MRI). We compared relative CBF maps assessed in 24 concussed contact sport athletes obtained at 24–48 h after injury to those of a control group of 24 matched contact sport players. Significantly less CBF was detected in several brain regions in concussed athletes, while clinical assessments also indicated clinical symptom and performance impairments in SRC patients. Correlations were found between decreased CBF in acute SRC and clinical assessments, including Balance Error Scoring System total score and Immediate Post-Concussion Assessment and Cognitive Test memory composite and impulse control composite scores, as well as days from injury to asymptomatic. Although using different ASL MRI sequences, our preliminary results from two sites are consistent with previous reports and suggest that advanced ASL MRI methods might be useful for detecting acute neurobiological changes in acute SRC.
Keywords: Cerebral blood flow, Concussion, Traumatic brain injury, Arterial spin labeling, Contact sport, MRI
Introduction
Sport-related concussion (SRC) is recognized as a major health problem, affecting millions of people each year during athletic participation (Centers for Disease and Prevention 2007). A concussion typically occurs following transmission of direct or indirect impulsive forces to the head, which result in a rapid onset of short-lived neurological impairments, and presents clinically with cognitive, physical, and behavioral signs and symptoms (Jordan 2013; McCrory et al. 2013). Despite the increasing occurrence and prevalence of SRC, important clinical issues, such as the time course of neuro-physiological recovery and the relationship between underlying neural recovery, return to play, and risk for developing long-term impairments, have yet to be fully addressed (Manley and Maas 2013; McCrea et al. 2003, 2010, 2013; McCrory et al. 2013; Slobounov et al. 2012; McCrea and Guskiewicz 2014).
The National Collegiate Athletic Association (NCAA)–Department of Defense (DoD) Grand Alliance: Concussion Assessment, Research and Education (CARE) Consortium is a large-scale, multisite study of the natural history of concussion in both sexes across multiple sports (http://www.careconsortium.net/). As stated on the Care ultimately enhance the safety and health of our student-athletes, service members, youth sports participants, and the broader public.” The CARE project includes a Clinical Science Core (CSC) and an Advanced Research Core (ARC). While the CSC is focused on expansive longitudinal neuropsychological testing of subjects, the ARC is focused on advanced biomarker assessment of a reduced subject cohort. Magnetic resonance imaging (MRI) is one of the key components of the CARE ARC biomarker assessment project.
Cerebral blood flow (CBF) is closely coupled with brain functioning. Reduced CBF during the acute and subacute phases of mild traumatic brain injury (mTBI) has been detected using various imaging methods such as single-photon emission computed tomography (Audenaert et al. 2003; Gowda et al. 2006) and perfusion computed tomography (Metting et al. 2014), which have several disadvantages including financial cost, ionizing radiation, and limited repetition of acquisitions. Arterial spin labeling (ASL) is an advanced MRI technique capable of measuring CBF noninvasively by using magnetically labeled arterial blood water as an endogenous contrast tracer, without radiation exposure, and is hence very suitable for longitudinal studies of CBF in healthy and diseased individuals, or as a surrogate marker of brain function and metabolism (Detre et al. 2009; Wang et al. 2003a, 2011). As the first part of currently still ongoing CARE ARC project, the aim of this work was to evaluate CBF changes in acute SRC using ASL perfusion MRI.
Methods
Participants
This study was approved by the Medical College of Wisconsin (MCW) Institutional Review Board (IRB). The participating ARC sites deferred to MCW via a reliance agreement, so the MCW IRB is the central IRB of record. All subjects provided written informed consent before participating. Participants were recruited from an ongoing prospective CARE ARC study. A total 24 concussed athletes with useful ASL data are included in this report: 14 from one ARC study site (University of North Carolina at Chapel Hill; UNC) and 10 from another study site (University of Wisconsin-Madison; UW). Additional noninjured contact sport players (N = 24), referred to as the contact control (CC) group, were selected from the same study sites to closely match injured athletes based on age, gender, sport, and estimated premorbid level of intelligence. (See Wechsler Test of Adult Reading [WTAR] measure listed below.) Table 1 summarizes the sample characteristics and degree of matching between the groups used in these analyses.
Table 1.
Sample characteristics and group matching
| SRC (N = 24) N (%) or M (SD) |
CC (N = 24) N (%) or M (SD) |
p | |
|---|---|---|---|
| Demographics | |||
| Age | 18.96 (1.20) | 19.33 (1.52) | 0.348 |
| Male | 19 (79.2%) | 19 (79.2%) | 1.000 |
| BMI | 27.26 (4.87) | 27.24 (3.77) | 0.988 |
| Prior concussions | 9 (37.5%) | 11 (45.8%) | 0.558 |
| WTAR standard score | 110.92 (12.27) | 111.09 (10.30) | 0.959 |
| Sport | 0.980 | ||
| Football (male) | 12 (50.0%) | 11 (45.8%) | |
| Ice hockey | 3 (12.5%) | 4 (16.7%) | |
| Lacrosse | 3 (12.5%) | 3 (12.5%) | |
| Soccer | 6 (25.0%) | 6 (25.0%) |
SRC sport-related concussion, CC contact control, BMI body mass index, WTAR Wechsler Test of Adult Reading (estimate of premorbid verbal intellectual ability)
Baseline and post-injury clinical assessments
All participants completed a 90-min, preseason baseline clinical assessment protocol. Post-injury testing for concussed players occurred 24–48 h after injury. Follow-up assessments for the CC group were performed as soon as possible after identification. Baseline clinical assessments were individually proctored by paid research assistants and included a demographics and health history interview, WTAR, Sport Concussion Assessment Tool–3 (SCAT3) symptom checklist (McCrory et al. 2013), Standardized Assessment of Concussion (SAC) (McCrea et al. 1997), Balance Error Scoring System (BESS) (Riemann and Guskiewicz 2000), Immediate Post-Concussion and Cognitive Testing (ImPACT) computerized neurocognitive test (CNT) battery (Iverson et al. 2006), and other self-report and computerized measures. ImPACT produces four clinical composite scores: Verbal Memory (VERM), Visual Memory (VISM), Visual–Motor Speed (VMS), and Reaction Time (RT), as well as supplementary Impulse Control (IMPC) and Cognitive Efficiency (COGE) scores that can aid in interpreting performance data. Follow-up assessments were similar to baseline assessments but were used to obtain injury/recovery (rather than health history) information and did not repeat the WTAR.
Clinical data analysis
Sample demographics and patient history variables were compared between the SRC and CC groups using chi-squared statistics (nominal variables) and independent samples t-tests (continuous variables). Degrees of freedom for t-tests were corrected when Levene’s test indicated heteroscedasticity between groups. In order to mirror neuroimaging analyses, statistical analyses of the clinical assessment variables reported below emphasized SRC versus CC group comparisons for the primary clinical assessment measures at the 24–48-h assessment. ImPACT composite scores were aggregated into Memory and Speed composite scores (Schatz and Maerlender 2013). In particular, the four primary composite scores were each z-scored using the sample’s baseline performance on each measure and then combined into a Memory (mean of VERM and VISM composite z-scores) and Speed (mean of VMS and reverse RT composite z-scores) composite at each time point. Preliminary analyses revealed small practice effects (improvements) from baseline to 24–48 h in the CC group on the BESS examination. Consequently, to report more accurate estimates of the acute effects of SRC on clinical variables, analysis of covariance (ANCOVA) models were utilized to compare the 24–48-h clinical assessment performance between groups (SRC, CC), using baseline performance as a covariate. The overall significance status of the between-group comparisons was unchanged when covarying for baseline performance. Statistical Package for Social Sciences (SPSS, version 24.0) was used to assess differences in demographic and clinical measures between groups. For continuous variables, ANCOVAs were employed with correction for multiple comparisons, while χ2 tests were used to examine group differences in categorical variables. Partial eta-squared (ηp2 effect size was also estimated using SPSS.
Imaging protocol
Data were collected at two ARC sites: UNC and UW. ASL data from UNC was acquired on a Siemens 3 T Trio system (Erlangen, Germany) with a 32-channel head coil. The commercially available two-dimensional pulsed ASL (2D PASL) was applied using parameters TI1 = 700 ms (time between the inversion pulse and the beginning of periodic saturation pulses), TI1s = 1600 ms (time between the inversion pulse and the end of periodic saturation pulses), and TI2 = 1800 ms (time between the inversion pulse and acquisition of the proximal image), which were chosen so as to minimize intravascular signal intensity at 3 T (Donahue et al. 2006; Wang et al. 2002). Interleaved label and control images were acquired using a gradient-echo single-shot echo-planar imaging readout, with acquisition parameter repetition time/echo time (TR/TE) = 3204/13 ms, field of view (FOV) = 224 mm, and matrix = 64 × 64. The imaging region consisted of 36 contiguous ascending axial slices of 4.5 mm thickness. Each perfusion measurement consisted of 109 dynamics (54 control and label image pairs) plus one M0 image (the equilibrium brain tissue magnetization used to normalize the difference perfusion map) with a scan time of approximately 5 min. To minimize the head motion artifact, Siemens online 3D Prospective Acquisition Correction was applied during the ASL scan.
ASL data from UW were collected on General Electric Healthcare (GE) Discovery MR750 whole body 3 T MRI scanner (Waukesha, WI) with a Nova Medical, Inc., 32-channel head coil (Wilmington, MA). The GE three-dimensional (3D) pseudo continuous ASL (3D-pCASL) sequence was used with parameters TR/TE = 4632/10.5 ms, FOV = 240 mm, matrix = 128 × 128, post-labeling duration (PLD) = 1525 ms, labeling duration = 1450 ms, spiral readout of 8 arms and 512 samples, number of excitations = 3, slice thickness = 4 mm, total = 36 slices. A background suppression pulse was applied to maximize the sensitivity to blood perfusion (Alsop et al. 2015), scan time = 4:29 min.
In addition, high-resolution anatomical images were collected for coregistration with the functional images. These included a T1-weighted magnetization-prepared rapid acquisition with gradient echo with the following parameters: TR/TE = 7.6/3.0 ms, FA = 8°, FOV = 256 mm, 1 mm isotropic resolution, and TI = 900 ms. A T2-weighted CUBE image was also acquired with TR/TE = 2500/63.6 ms, FA = 90°, FOV = 256 mm, and 1 mm isotropic resolution. To detect incidental findings including cerebral vascular abnormalities, a high-resolution (isotropic voxel size of 1 mm3) T2-weighted image and 3D fluid attenuated inversion recovery image was also acquired.
Image processing
Individual T1 anatomical and ASL image quality was checked for potential distortion and artifact. In addition, individual T1, T2 and FLAIRE were screened by licensed neuroradiologists for intracranial lesions or other potential incidental findings.
The T1 anatomical image was skull-stripped and transformed to Montreal Neurological Institute (MNI) space using a nonlinear registration. In addition, individual gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) probability maps were estimated using the SPM12 segmentation function (http://www.fil.ion.ucl.ac.uk/spm/).
All ASL image processing was performed using previously published methods (Wang et al. 2011, 2012). All PASL images were first realigned to the M0 image with the same spatial resolution. The label images were then pairwise subtracted from the time-matched control images to produce a perfusion weighted time series, and then quantitative CBF map in units of ml/100 g/min for each PASL scan was generated using one compartment model (Wang et al. 2003b). Similarly, a CBF map from the pCASL data was also generated online using the one compartment model. All individual CBF maps were normalized to MNI space in SPM12, resampled to 2 mm3 voxels, and smoothed with a full width at half maximum kernel of 6 × 6 × 6 mm. After that, a regression algorithm developed by Asllani et al. to correct for partial volume effects in ASL data (Asllani et al. 2008, 2009). The algorithm is based on a model that represents the voxel intensity as a weighted sum of pure tissue contribution, where the weighting coefficients are the tissue’s fractional volume in the voxel (Asllani et al. 2008). Using this algorithm, CBF for GM and WM were estimated independently based on individual GM, WM, and CSF probability maps (Asllani et al. 2008, 2009). Since there is a known difference between GM and WM perfusion and our current protocols are not optimized for the detection of WM CBF (Wu et al. 2013), this work focuses on our GM CBF findings. Furthermore, relative CBF (rCBF) was calculated by normalizing CBF with mean GM CBF in order to reduce data noise caused by intersubject variations in global CBF (Aslan and Lu 2010).
Imaging statistical analysis
For voxelwise group comparison on individual CBF maps, the Analysis of Functional NeuroImages (AFNI, http://www.afni.nimh.nih.gov) mixed-effects multilevel analysis tool was applied to perform an ANCOVA that incorporates both the variability across subjects and the precision estimate of each effect of interest from individual subject analyses (Chen et al. 2012). Since two ARC sites included in this study have different ASL protocols, we have included the site as a covariate in all analyses. Although there is no significant difference between groups with regard to the number of previous concussions, the number of previous concussions was not distributed evenly across groups. Therefore, we have also included the previous number of concussions as a covariate for potential confounds. We have conducted the group analyses twice, with and without number of prior concussions as an additional covariate. For all the CBF analyses described above, the height threshold was set at p < 0.05 and the spatial extent threshold was set at cluster volume > 137 voxels, which corresponds to p < 0.05 corrected for familywise error of multiple comparisons according to Monte Carlo simulations (10,000 iterations) implemented in AFNI’s 3dClustSim (Chow et al. 2013; Okonkwo et al. 2014; Cox et al. 2017). In additional analyses, results were also evaluated using a more conservative threshold of corrected p < 0.01 as determined using 3dCluStim by setting the height threshold at p < 0.01 with the minimal cluster size of 67 voxels.
Results
Participant characteristics
This study analyzed a total of 24 SRC patients and 24 CC subjects. Concussed and control groups did not differ significantly with respect to age, sex, body mass index (BMI), or WTAR standard score (Table 1). The study included athletes of four types of contact sports, including football, ice hockey, lacrosse, and soccer. SRC and CC groups matched very well on contact sport type. Although there was no difference between the two groups with regard to overall concussion history (dichotomized), two SRC patients had three previous concussions and three SRC patients had two previous concussions, while only one CC subject had two previous concussion and no CC subject had three previous concussions. Therefore, the number of prior concussions was tested as a potential confounding covariate in group analyses, as stated above.
In the concussed group, no patient reported any loss of consciousness associated with injury, three (5.6%) reported post-traumatic amnesia, and five (10.4%) reported retrograde amnesia.
Clinical measures
Baseline assessments
Both SRC and CC subjects were equivalent in ratings on the SCAT3 symptom checklist and performance on the SAC and BESS at baseline (Table 2). ImPACT data were collected at only one site (UW) and showed no difference between SRC and CC groups at baseline. Within 24–48 h post-injury, concussed patients produced significantly higher SCAT3 symptom ratings (p < 0.001, ηp 2 = 0.321), higher (more impaired) BESS total scores (p < 0.026, ηp 2 = 0.115), and significantly lower ImPACT memory scores (p < 0.006, ηp 2 p = 0.388) in comparison with CC subjects, as evaluated using the ANCOVA models after controlling for baseline performance (Table 3).
Table 2.
Descriptive statistics for clinical assessment variables at baseline (pre-season) and 24–48 h after injury
| SRC | CC | |||
|---|---|---|---|---|
| N | M (SD) | N | M (SD) | |
| SCAT3 symptom severity (BL) | 22 | 5.05 (9.16) | 23 | 2.78 (5.74) |
| SCAT3 symptom severity (48-h) *** | 22 | 19.95 (16.63) | 23 | 3.43 (5.81) |
| SAC total (BL) | 22 | 26.86 (1.94) | 23 | 27.35 (2.10) |
| SAC total (48-h) | 22 | 26.41 (2.06) | 23 | 27.26 (2.38) |
| BESS total (BL) | 23 | 14.43 (5.38) | 21 | 14.52 (3.93) |
| BESS total (48-h) * | 23 | 17.22 (10.82) | 21 | 11.24 (5.85) |
| ImPACT memory composite (BL) | 10 | −0.17 (0.82) | 9 | 0.19 (0.72) |
| ImPACT memory composite (48-h) ** | 10 | −0.66 (0.94) | 9 | 0.53 (0.51) |
| ImPACT speed composite (BL) | 10 | −0.06 (1.18) | 9 | 0.07 (0.64) |
| ImPACT speed composite (48-h) | 10 | −0.62 (0.86) | 9 | −0.32 (0.98) |
BL baseline assessment, 48-h 24–48 h follow-up assessment, BESS Balance Error Scoring System, BL preseason baseline assessment, CC contact control group, ImPACT Immediate Post-Concussion Assessment and Cognitive Test, SAC Standardized Assessment of Concussion, SCAT3 Sport Concussion Assessment Tool–3, SRC sport-related concussion group,
p < 0.05;
p < 0.01;
p < 0.001
Table 3.
ANCOVA analyses comparing SRC and CC groups on 24–48-h clinical assessment variables (controlling for baseline performance)
| F | df | p | ηp 2 | |
|---|---|---|---|---|
| SCAT3 symptom severity | 19.88 | (1,42) | < 0.001 | 0.321 |
| SAC total | 1.11 | (1,42) | 0.299 | 0.026 |
| BESS total | 5.35 | (1,41) | 0.026 | 0.115 |
| ImPACT memory composite | 10.15 | (1,16) | 0.006 | 0.388 |
| ImPACT speed composite | 0.46 | (1,16) | 0.506 | 0.028 |
ANCOVA analysis of covariance, SRC sport-related concussion group, CC contact control group, η2 P partial eta squared effect size estimates, SCAT3 Sport Concussion Assessment Tool–3, SAC Standardized Assessment of Concussion, BESS Balance Error Scoring System, ImPACT Immediate Post-Concussion Assessment and Cognitive Test
CBF differences
Within 24–48 h post-injury, voxelwise analysis showed significantly lower CBF in the concussion group relative to the control group (p < 0.05 corrected), predominantly in regions including the left inferior parietal lobule (IPL), right supramarginal syrus (SMG), right middle frontal gyrus (MFG), posterior cingulate cortex, left occipital gyrus, and thalamus (Fig. 1a and Table 4). We also evaluated the results by applying a more conservative threshold of corrected p < 0.01. Although the spatial size of significant clusters was attenuated, the less CBF of the SRC group compared to that the CC group in the original analysis remained significant in regions including the right IPL, right MFG, and thalamus (Fig. 1b).
Fig. 1.

Regions (in blue) show significantly less CBF in the SRC group at 24–48 h after injury, relative to the CC group. No region shows significantly more CBF in the SRC group compared to the CC group. a Images reflect familywise error correction at p < 0.05. The color bar indicates the t score; b Images reflect familywise error correction at p < 0.01. The color bar indicates the t score
Table 4.
Clusters showing decreased CBF in concussed subjects at 24–48 h post-injury compared to control subjects (p < 0.05 at cluster size >137 voxels)
| Location | Size | Center | Peak | ||||
|---|---|---|---|---|---|---|---|
| (Voxels) | X | Y | Z | X | Y | Z | |
| R. IPL | 1495 | 32.8 | −75.6 | 38.2 | 46 | −76 | 30 |
| R. SMG | 691 | 52.9 | −44.7 | 39 | 50 | −38 | 46 |
| R. MFG | 506 | 42.3 | 49.2 | −0.5 | 40 | 52 | 12 |
| PCC | 175 | −1.5 | −51.1 | 37.8 | −2 | −48 | 38 |
| L. Occipital Gyrus | 161 | −34.1 | −87.5 | 17.8 | −38 | −86 | 22 |
| Thalamus | 138 | 1 | −12.5 | 5.7 | 4 | −12 | 6 |
CBF cerebral blood flow, R right, L left, IPL inferior parietal lobule, SMG supramarginal gyrus, MFG middle frontal gyrus, PCC posterior cingulate cortex
An additional region-of-interest (ROI) analysis was performed using significant clusters as ROIs, where a voxel-base analysis showed group difference. The ROI-averaged rCBF was compared between groups for the two sites combined and for each site separately (Fig. 2). An ROI analysis from the two sites’ combined data (using the site variable as a covariate) suggested a significant CBF decreased in acute SRC relative to CC subjects (p < 10−6, effect size: 0.461). An ROI analysis of each site separately demonstrated similar group differences, while the UNC site data showed a larger effect size (0.680, p < 10−7) and the UW site showed a relatively smaller effect size (0.262, p = 0.018) (Fig. 2). Moreover, negative correlations were found between the BESS total score at 24–48 h after injury and decreased rCBF (SRC group) in the left occipital gyrus (r = −0.433, p = 0.039), and between symptom duration (in days) of SRC participants and rCBF on two ROIs: right IPL (r = −0.470, p = 0.024) and right SMG (r = −0.444, p = 0.034). At the one site that performed ImPACT (n = 10 with SRC), a positive correlation was found between the ImPACT memory composite score and the rCBF of the right MFG (r = 0.671, p = 0.034), and negative correlations were found between the ImPACT impulse control composite score and the rCBF of the right SMG (r = −0.709, p = 0.022) and right MFG (r = −0.757, p = 0.011) in SRC at 24–48 h post-injury (Fig. 3).
Fig. 2.
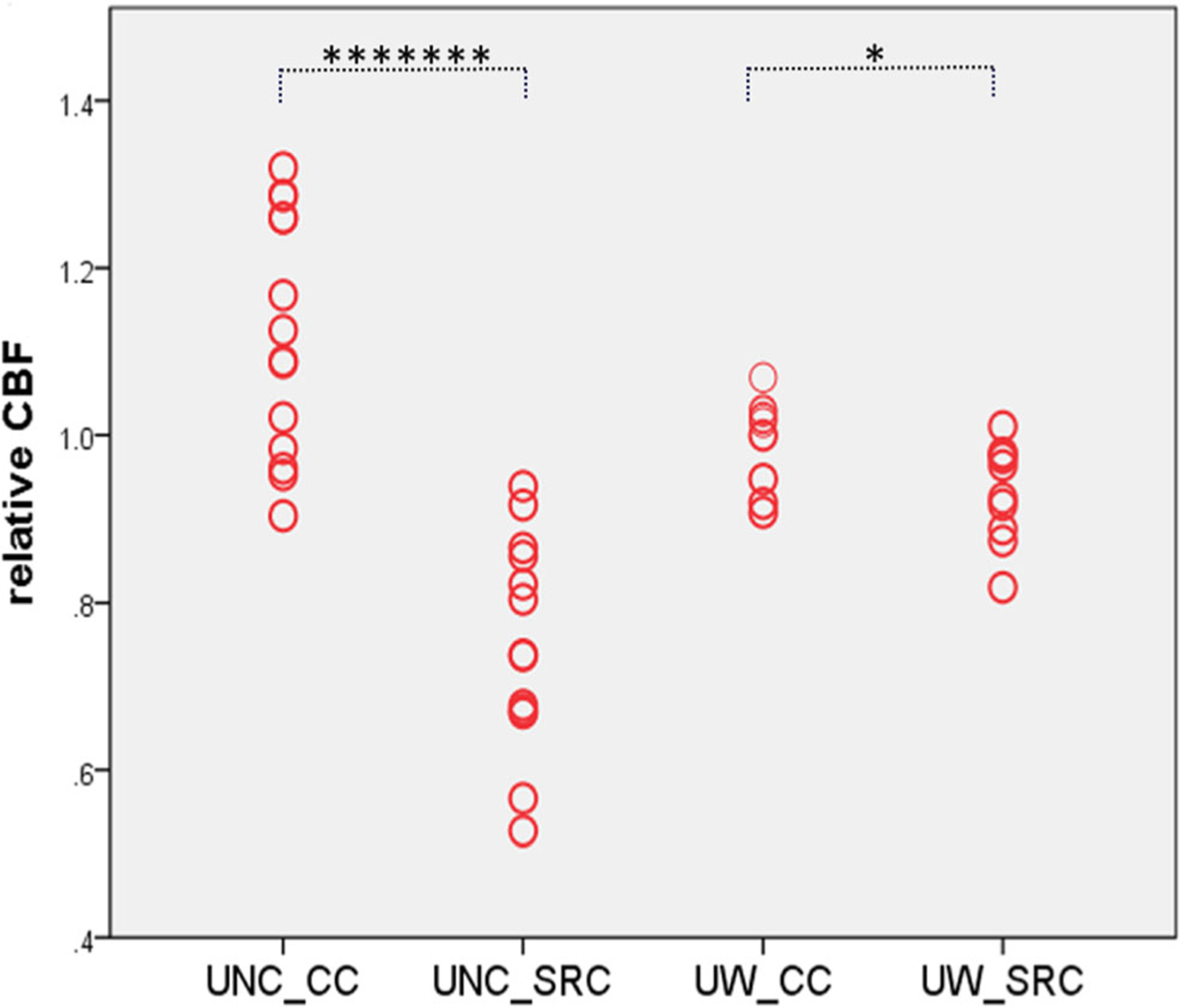
Individual relative CBF value of each group at each site. Note: UNC-CC = Controls from the UNC site; UNC_SRC = SRC sample from the UNC site; UW_CC = Controls from the UW site; UW_SRC = SRC sample from the UW site (******* p < 10−7; * p < 0.01, corrected)
Fig. 3.

Correlation maps showing (a) negative correlation between BESS total score at 24–48 h after injury and decreased rCBF in left occipital gyrus; b negative correlation between “days from injury to asymptomatic” of SRC and rCBF on right IPL; c negative correlation between “days from injury to asymptomatic” of SRC and rCBF on right SMG; d positive correlation between ImPACT memory composite score and rCBF of right MFG; e negative correlations between ImPACT impulse control composite score and rCBF of right SMG; f negative correlations between ImPACT impulse control composite score and rCBF of right MFG in SRC at 24–48 h post-injury. (Note: IPL = inferior parietal lobule; SMG = supramarginal gyrus; MFG = middle frontal gyrus)
Furthermore, neither voxelwise nor ROI analysis found any significant difference in CBF between UNC CC and UW CC groups. Since our sample is relatively small per group at each site, we have conducted further analysis using SPSS for outlier detection, in order to rule out the possibility that group difference shown above was unduly influenced by individual subject with extreme CBF value. Both Stem-and-Leaf Plot and Q-Q Plots didn’t find any subject with extreme value.
In an additional analysis, we repeated the above voxelwise CBF group comparisons with and without the number of prior concussions as the covariate but found that the number of prior concussions had no significant effect on the final CBF results in this sample.
Discussion
This study included two sites using different ASL sequences on different MRI platforms. As recently reported by our MRI core team (Nencka et al. 2017), two “human phantom” subjects were imaged multiple times at each of the four CARE ARC imaging sites, which utilize equipment from two imaging vendors. Additionally, a control cohort of healthy athletes participating in non-contact sports were enrolled in the study at each CARE ARC site and imaged at four time points. Multiple morphological image contrasts were acquired in each MRI exam; along with quantitative diffusion, functional, perfusion, and relaxometry imaging metrics. Notably, between-subject variance was generally found to be greater than within-subject and between-site variance. These results lend support to the expectation that cross-site and cross-vendor advanced quantitative MRI metrics can be utilized to improve analytic power in assessing sensitive neurological variations; such as those effects hypothesized to occur in sports-related-concussion (Nencka et al. 2017). Importantly, we didn’t find significant difference in CBF on control subjects between two sites of this study.
In this study, concussed contact sport players demonstrated abnormally reduced CBF within 24–48 h after sustaining SRC in comparison with controls. Findings were evident in both voxelwise and ROI analyses. Although the pathogenesis of concussion has yet to be fully elucidated, it is becoming increasingly clear that cerebrovascular alterations play a significant role in the evolution of injury sequelae as well as in the process of post-traumatic brain repair (Dijkhuizen 2011; Len and Neary 2011; Pop and Badaut 2011; Tan et al. 2014; Gardner et al. 2015; Giza and Hovda 2014). Reduced CBF signifies one of the most lasting markers of concussion in animal models of TBI (Pasco et al. 2007; McGoron et al. 2008; Giza and Hovda 2014), while it was shown that CBF decreased immediately after injury in an animal model (Ginsberg et al. 1997; Muir et al. 1992) and that decreased regional CBF persisted after injury (Yamakami and McIntosh 1989).
There are several possible pathophysiologic mechanisms of decreased CBF after mTBI (Len and Neary 2011; Pop and Badaut 2011; Giza and Hovda 2014). Following TBI, a primary injury at the moment of impact damages brain tissue by disrupting blood vessels; this event facilitates secondary injury cascades affecting the neurovascular unit (NVU) physiology (Pop and Badaut 2011). The NVU is a physiological entity that is structurally defined by interactions occurring between endothelial cells, pericytes, smooth muscle cells, astrocytes, and neurons (Iadecola and Nedergaard 2007; Pop and Badaut 2011). The post-traumatic changes in the NVU are mostly observed during the first week after injury, although the evolution of these changes in the NVU over a long period of time is still unknown (Pop and Badaut 2011). Moreover, cerebral autoregulation, the intrinsic ability of the brain to maintain a constant CBF in response to variations in systemic blood pressure, has been found to be lost or impaired following mTBI for up to 14 days (Junger et al. 1997; Strebel et al. 1997; Rangel-Castilla et al. 2008; Len and Neary 2011). In addition, research has illustrated that the autonomic and cardiovascular systems become uncoupled following acute brain injury (Goldstein et al. 1998), and it has been suggested that deficits in neuroautonomic control after brain injury are correlated with abnormal cerebrovascular responses (Zhang et al. 2002). CBF changes after TBI may also be related to changes in the basic properties of the cerebral vasculature, and decreased CBF soon after injury is a common signature of TBI (Pop and Badaut 2011).
Our findings of decreased CBF in acute SRC are in line with our most recent report from another study showing significantly lower CBF in acute and subacute SRC in a sample of high school and Division III college football players (Wang et al. 2016). Our results are also consistent with other MRI perfusion studies of mTBI (Wang et al. 2015; Liu et al. 2013; Meier et al. 2015), although the exact regions showing decreased CBF in mTBI varied across reports, which might be due to different sample characteristics and/or the use of different methodology in assessments. The most reliable patterns of parenchymal changes after TBI have been observed in the frontal, temporal, and cingulate regions, whereas effects were observed to varying degrees in nearly every brain region (Levine et al. 2008). More importantly, our results showed a significant association between decreased CBF in acute SRC and clinical assessments, including the BESS total score, ImPACT memory composite and impulse control composite scores, and symptom duration. It should be noted that SRC represents the mildest end of the continuum of TBI. Our finding of a CBF deficit in the context of mild concussion might be a call for an additional study of more severe mTBI to look for a CBF dose response based on injury severity (Wang et al. 2016).
Due to the availability of product ASL sequences on different MR scanner platforms, the two study sites applied different ASL protocols. While the 3D pCASL technique with a higher signal-to-noise ratio and efficiency has been recommended (Chen et al. 2011; Vidorreta et al. 2013; Wu et al. 2007; Alsop et al. 2015), only very limited research to date has applied this advanced MRI technique in concussion research (Meier et al. 2015; Barlow et al. 2017; Wang et al. 2016). In contrast, the 2D PASL is still widely available and has demonstrated good test-retest reliability (Wang et al. 2011). One advantage of 2D PASL is that the single-shot acquisition is immune to inconsistency between excitations due to motion that can affect multishot methods such as 3D pCASL (Alsop et al. 2015). To control for the disparity of ASL imaging protocols between sites, we have applied several approaches including estimation of rCBF and partial volume correction. Notably, both study sites have shown similar patterns despite using different ASL sequences. Even though the UM site sample showed smaller SRC versus CC differences, robust correlations were observed between reduced rCBF and ImPACT memory composite and impulse control composite scores, supporting the validity of this rCBF measure.
Relevant limitations of this study require acknowledgment. A modest sample size is the primary limitation of this preliminary investigation. Also, due to a limited number of female participants, we were unable to examine gender differences. Although the ARC project is ongoing, it would be very important to follow up with existing subjects to link evolution of ASL metrics to recovery following SRC, in order to detect a complete neuropathophysiological recovery course after SRC. In this study, we recruited athletes from four different contact sports. Because the different types of contact sport, sport-specific characteristics (e.g., helmet use) may have different effects on brain structure and function after injury (Harmon et al. 2013), further research is warranted to evaluate such effects on SRC. A multimodal approach to combine ASL with other imaging methods such as functional and diffusion MRI would be very useful to characterize the physiological effects of concussion. Future studies may demonstrate that such advanced imaging modalities could potentially direct rehabilitation after concussion and possibly aid in determining when it is safe for an athlete to return to play, although there is no evidence at the present time to support such conclusions (Dashnaw et al. 2012).
Acknowledgements
This publication was made possible, in part, with support from the Grand Alliance Concussion Assessment, Research, and Education (CARE) Consortium, funded, in part by the National Collegiate Athletic Association (NCAA) and the Department of Defense (DOD). The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Psychological Health and Traumatic Brain Injury Program under Award NO W81XWH-14-2-0151. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense (DHP funds). The authors would also like to thank Jody Harland, Janetta Matesan, Larry Riggen (Indiana University); Ashley Rettmann (University of Michigan); Melissa Koschnitzke (Medical College of Wisconsin); Michael Jarrett, Vibeke Brinck and Bianca Byrne (Quesgen); Thomas Dompier, Melissa Niceley Baker, and Sara Dalton (Datalys Center for Sports Injury Research and Prevention); and the research and medical staff at each of the participating sites.
Funding This study was funded as part of the National Collegiate Athletic Association–Department of Defense Grand Alliance.
Footnotes
Conflicts of interest The authors declare that they have no conflicts of interest.
Research involving human participants This study was approved by MCW IRB.
Informed consent All subjects provided written informed consent before participating in the study.
References
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, et al. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine, 73(1), 102–116. 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan S, & Lu H (2010). On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magnetic Resonance Imaging, 28(7), 928–935. 10.1016/j.mri.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Borogovac A, & Brown TR (2008). Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magnetic Resonance in Medicine, 60(6), 1362–1371. 10.1002/mrm.21670. [DOI] [PubMed] [Google Scholar]
- Asllani I, Habeck C, Borogovac A, Brown TR, Brickman AM, & Stern Y (2009). Separating function from structure in perfusion imaging of the aging brain. Human Brain Mapping, 30(9), 2927–2935. 10.1002/hbm.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, Jansen HM, Otte A, Peremans K, Vervaet M, Crombez R, de Ridder L, van Heeringen C, Thirot J, Dierckx R, & Korf J (2003). Imaging of mild traumatic brain injury using 57Co and 99mTc HMPAO SPECT as compared to other diagnostic procedures. Medical Science Monitor, 9(10), MT112–MT117. [PubMed] [Google Scholar]
- Barlow KM, Marcil LD, Dewey D, Carlson HL, MacMaster FP, Brooks BL, & Lebel RM (2017). Cerebral perfusion changes in post-concussion syndrome: A prospective controlled cohort study. Journal of Neurotrauma, 34(5), 996–1004. 10.1089/neu.2016.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C., & Prevention. (2007). Nonfatal traumatic brain injuries from sports and recreation activities–United States, 2001–2005. MMWR. Morbidity and Mortality Weekly Report, 56(29), 733–737. [PubMed] [Google Scholar]
- Chen Y, Wang DJ, & Detre JA (2011). Test-retest reliability of arterial spin labeling with common labeling strategies. Journal of Magnetic Resonance Imaging, 33(4), 940–949. 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Nath AR, Beauchamp MS, & Cox RW (2012). FMRI group analysis combining effect estimates and their variances. Neuroimage, 60(1), 747–765. 10.1016/j.neuroimage.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Horovitz SG, Carr WS, Picchioni D, Coddington N, Fukunaga M, Xu Y, Balkin TJ, Duyn JH, & Braun AR (2013). Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proceedings of the National Academy of Sciences of the United States of America, 110(25), 10300–10305. 10.1073/pnas.1217691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connectivity, 7(3), 152–171. 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashnaw ML, Petraglia AL, & Bailes JE (2012). An overview of the basic science of concussion and subconcussion: Where we are and where we are going. Neurosurgical Focus, 33(6), E5: 1–9. 10.3171/2012.10.FOCUS12284. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, & Rao H (2009). Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Current Opinion in Neurology, 22(4), 348–355. 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM (2011). Advances in MRI-based detection of cerebrovascular changes after experimental traumatic brain injury. Translational Stroke Research, 2(4), 524–532. 10.1007/s12975-011-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Lu H, Jones CK, Pekar JJ, & van Zijl PC (2006). An account of the discrepancy between MRI and PETcerebral blood flow measures. A high-field MRI investigation. NMR in Biomedicine, 19(8), 1043–1054. 10.1002/nbm.1075. [DOI] [PubMed] [Google Scholar]
- Gardner AJ, Tan CO, Ainslie PN, van Donkelaar P, Stanwell P, Levi CR, & Iverson GL (2015). Cerebrovascular reactivity assessed by transcranial Doppler ultrasound in sport-related concussion: A systematic review. British Journal of Sports Medicine, 49(16), 1050–1055. 10.1136/bjsports-2014-093901. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Zhao W, Alonso OF, Loor-Estades JY, Dietrich WD, & Busto R (1997). Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol, 272(6 Pt 2), H2859–2868. 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- Giza CC, & Hovda DA (2014). The new neurometabolic cascade of concussion. Neurosurgery, 75(Suppl 4), S24–S33. 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Toweill D, Lai S, Sonnenthal K, & Kimberly B (1998). Uncoupling of the autonomic and cardiovascular systems in acute brain injury. The American Journal of Physiology, 275(4 Pt 2), R1287–R1292. [DOI] [PubMed] [Google Scholar]
- Gowda NK, Agrawal D, Bal C, Chandrashekar N, Tripati M, Bandopadhyaya GP, Malhotra A, & Mahapatra AK (2006). Technetium Tc-99m ethyl cysteinate dimer brain single-photon emission CT in mild traumatic brain injury: A prospective study. AJNR. American Journal of Neuroradiology, 27(2), 447–451. [PMC free article] [PubMed] [Google Scholar]
- Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Halstead M, Herring SA, Kutcher JS, Pana A, Putukian M, & Roberts WO (2013). American medical Society for Sports Medicine position statement: Concussion in sport. British Journal of Sports Medicine, 47(1), 15–26. 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- Iadecola C, & Nedergaard M (2007). Glial regulation of the cerebral microvasculature. Nature Neuroscience, 10(11), 1369–1376. 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Brooks BL, Collins MW, & Lovell MR (2006). Tracking neuropsychological recovery following concussion in sport. Brain Injury, 20(3), 245–252. 10.1080/02699050500487910. [DOI] [PubMed] [Google Scholar]
- Jordan BD (2013). The clinical spectrum of sport-related traumatic brain injury. Nature Reviews. Neurology, 9(4), 222–230. 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Junger EC, Newell DW, Grant GA, Avellino AM, Ghatan S, Douville CM, et al. (1997). Cerebral autoregulation following minor head injury. Journal of Neurosurgery, 86(3), 425–432. 10.3171/jns.1997.86.3.0425. [DOI] [PubMed] [Google Scholar]
- Len TK, & Neary JP (2011). Cerebrovascular pathophysiology following mild traumatic brain injury. Clinical Physiology and Functional Imaging, 31(2), 85–93. 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- Levine B, Kovacevic N, Nica EI, Cheung G, Gao F, Schwartz ML, & Black SE (2008). The Toronto traumatic brain injury study: Injury severity and quantified MRI. Neurology, 70(10), 771–778. 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang B, Wolfowitz R, Yeh PH, Nathan DE, Graner J, Tang H, Pan H, Harper J, Pham D, Oakes TR, French LM, & Riedy G (2013). Perfusion deficits in patients with mild traumatic brain injury characterized by dynamic susceptibility contrast MRI. NMR in Biomedicine, 26(6), 651–663. 10.1002/nbm.2910. [DOI] [PubMed] [Google Scholar]
- Manley GT, & Maas AI (2013). Traumatic brain injury: An international knowledge-based approach. JAMA, 310(5), 473–474. 10.1001/jama.2013.169158. [DOI] [PubMed] [Google Scholar]
- McCrea M, & Guskiewicz K (2014). Evidence-based management of sport-related concussion. Progress in Neurological Surgery, 28, 112–127. 10.1159/000358769. [DOI] [PubMed] [Google Scholar]
- McCrea M, Kelly JP, Kluge J, Ackley B, & Randolph C (1997). Standardized assessment of concussion in football players. Neurology, 48(3), 586–588. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, & Kelly JP (2003). Acute effects and recovery time following concussion in collegiate football players: The NCAA concussion study. JAMA, 290(19), 2556–2563. 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- McCrea M, Prichep L, Powell MR, Chabot R, & Barr WB (2010). Acute effects and recovery after sport-related concussion: A neurocognitive and quantitative brain electrical activity study. The Journal of Head Trauma Rehabilitation, 25(4), 283–292. 10.1097/HTR.0b013e3181e67923. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz K, Randolph C, Barr WB, Hammeke TA, Marshall SW, Powell MR, Woo Ahn K, Wang Y, & Kelly JP (2013). Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. Journal of the International Neuropsychological Society, 19(1), 22–33. 10.1017/S1355617712000872. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. (2013). Consensus statement on concussion in sport: The 4th international conference on concussion in sport held in Zurich, November 2012. British Journal of Sports Medicine, 47(5), 250–258. 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- McGoron AJ, Capille M, Georgiou MF, Sanchez P, Solano J, Gonzalez-Brito M, & Kuluz JW (2008). Post traumatic brain perfusion SPECT analysis using reconstructed ROI maps of radioactive microsphere derived cerebral blood flow and statistical parametric mapping. BMC Medical Imaging, 8, 4 10.1186/1471-2342-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polanski DW, & Mayer AR (2015). Recovery of cerebral blood flow following sports-related concussion. JAMA Neurology, 72(5), 530–538. 10.1001/jamaneurol.2014.4778. [DOI] [PubMed] [Google Scholar]
- Metting Z, Spikman JM, Rodiger LA, & van der Naalt J (2014). Cerebral perfusion and neuropsychological follow up in mild traumatic brain injury: Acute versus chronic disturbances? Brain and Cognition, 86(0), 24–31, 10.1016/j.bandc.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Muir JK, Boerschel M, & Ellis EF (1992). Continuous monitoring of posttraumatic cerebral blood flow using laser-Doppler flowmetry. Journal of Neurotrauma, 9(4), 355–362. 10.1089/neu.1992.9.355. [DOI] [PubMed] [Google Scholar]
- Nencka AS, Meier TB, Wang Y, Muftuler LT, Wu YC, Saykin AJ, Harezlak J, Brooks MA, Giza CC, Difiori J, Guskiewicz KM, Mihalik JP, LaConte SM, Duma SM, Broglio S, McAllister T, McCrea MA, & Koch KM (2017). Stability of MRI metrics in the advanced research core of the NCAA-DoD concussion assessment, research and education (CARE) consortium. Brain Imaging and Behavior, 12, 1121–1140. 10.1007/s11682-017-9775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, Birdsill AC, Palotti M, Wharton W, Hermann BP, LaRue A, Bendlin BB, Rowley HA, Asthana S, Sager MA, & Johnson SC (2014). Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cerebral Cortex, 24(4), 978–988. 10.1093/cercor/bhs381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco A, Lemaire L, Franconi F, Lefur Y, Noury F, Saint-Andre JP, et al. (2007). Perfusional deficit and the dynamics of cerebral edemas in experimental traumatic brain injury using perfusion and diffusion-weighted magnetic resonance imaging. Journal of Neurotrauma, 24(8), 1321–1330. 10.1089/neu.2006.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V, & Badaut J (2011). A neurovascular perspective for long-term changes after brain trauma. Translational Stroke Research, 2(4), 533–545. 10.1007/s12975-011-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, & Robertson CS (2008). Cerebral pressure autoregulation in traumatic brain injury. Neurosurgical Focus, 25(4), E7 10.3171/FOC.2008.25.10.E7. [DOI] [PubMed] [Google Scholar]
- Riemann BL, & Guskiewicz KM (2000). Effects of mild head injury on postural stability as measured through clinical balance testing. Journal of Athletic Training, 35(1), 19–25. [PMC free article] [PubMed] [Google Scholar]
- Schatz P, & Maerlender A (2013). A two-factor theory for concussion assessment using ImPACT: Memory and speed. Archives of Clinical Neuropsychology, 28(8), 791–797. 10.1093/arclin/act077. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Gay M, Johnson B, & Zhang K (2012). Concussion in athletics: Ongoing clinical and brain imaging research controversies. Brain Imaging and Behavior, 6(2), 224–243. 10.1007/s11682-012-9167-2. [DOI] [PubMed] [Google Scholar]
- Strebel S, Lam AM, Matta BF, & Newell DW (1997). Impaired cerebral autoregulation after mild brain injury. Surgical Neurology, 47(2), 128–131. [DOI] [PubMed] [Google Scholar]
- Tan CO, Meehan WP 3rd, Iverson GL, & Taylor JA (2014). Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology, 83(18), 1665–1672. 10.1212/WNL.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidorreta M, Wang Z, Rodriguez I, Pastor MA, Detre JA, & Fernandez-Seara MA (2013). Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage, 66, 662–671. 10.1016/j.neuroimage.2012.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, & Detre JA (2002). Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magnetic Resonance in Medicine, 48(2), 242–254. 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, & Detre JA (2003a). Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine, 49(5), 796–802. 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, Zimmerman RA, & Detre JA (2003b). Pediatric perfusion imaging using pulsed arterial spin labeling. Journal of Magnetic Resonance Imaging, 18(4), 404–413. 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, Kim S, & Hutchins GD (2011). Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage, 54(2), 1188–1195. 10.1016/j.neuroimage.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Alger JR, Qiao JX, Hao Q, Hou S, Fiaz R, et al. (2012). The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: Comparison with dynamic susceptibility contrast-enhanced MRI. Stroke, 43(4), 1018–1024. 10.1161/STROKEAHA.111.631929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, West JD, Bailey JN, Westfall DR, Xiao H, Arnold TW, Kersey PA, Saykin AJ, & McDonald BC (2015). Decreased cerebral blood flow in chronic pediatric mild TBI: An MRI perfusion study. Developmental Neuropsychology, 40(1), 40–44. 10.1080/87565641.2014.979927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nelson LD, LaRoche AA, Pfaller AY, Nencka AS, Koch KM, & McCrea MA (2016). Cerebral blood flow alterations in acute sport-related concussion. Journal of Neurotrauma, 33(13), 1227–1236. 10.1089/neu.2015.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, & Wang J (2007). A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magnetic Resonance in Medicine, 58(5), 1020–1027. 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- Wu WC, Lin SC, Wang DJ, Chen KL, & Li YD (2013). Measurement of cerebral white matter perfusion using pseudocontinuous arterial spin labeling 3T magnetic resonance imaging - an experimental and theoretical investigation of feasibility. PLoS One, 8(12), UNSP e82679. 10.1371/journal.pone.0082679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakami I, & McIntosh TK (1989). Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. Journal of Cerebral Blood Flow and Metabolism, 9(1), 117–124. 10.1038/jcbfm.1989.16. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, & Levine BD (2002). Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation, 106(14), 1814–1820. [DOI] [PubMed] [Google Scholar]


