Abstract
An estimation method for the Freundlich organic carbon normalized partitioning coefficient (Kfoc) is presented. It is based on regressions developed from batch equilibrium experiments submitted to U.S. EPA for pesticide registration. The regressions represent 18 specific agricultural soils from the U.S. and Europe, and are based on 41 pesticide active ingredients or metabolites. The predictive variable used is the subcooled liquid solubility (Sscl), whose calculation requires the compound’s solubility, and if a solid (mp > 25 °C), its melting point. Sscl represents the compound’s solubility as if it were a liquid at room temperature and does not assume that the compound is neutral. The estimation method was tested on 333 pesticides, and estimated means matched published Kfoc values to within 10× for 83%, 3× for 65%, and 2× for 43%. The estimation method (Kfoc Estimator v0.1) was further tested against data for 94 herbicides, 55 Superfund Priority Pollutants and 22 polychlorinated biphenyls. The estimated mean and median Kfoc values were highly correlated (R2 > 0.63 to 0.87) with the measured values. Matching of reported to estimated values was generally good. The herbicides were 51%–54% within 2×, 74%–77% within 3×, and 93%–94% within 10×. The Priority Pollutants were 22%–29% within 2×, 38%–55% within 3×, and 73%–75% within 10×. The estimates for the PCBs were low, but the maximum estimated Kfoc was consistently within 10 × of the reported Koc. This may be due to the PCB data representing a single soil, rather than the central-tendency estimates produced by Kfoc Estimator v0.1.
Keywords: Freundlich partition coefficient, Pesticides, Estimation, Agriculture, Soil, Kf, Kfoc, Subcooled liquid solubility, Exponent
1. Introduction
A coefficient to describe soil-water partitioning is essential to computer modeling of environmental pollutants, including pesticides and industrial chemicals. The organic carbon normalized partitioning coefficient (Koc) and its Freundlich equivalent (Kfoc) are in common usage for this purpose. In the U.S., these parameters are measured in batch equilibrium studies according to harmonized guideline 835.1230 (U.S. EPA, 2008).
The U.S. EPA Office of Pesticides Programs (OPP) receives dozens of batch equilibrium studies each year, as routine FIFRA (Federal Insecticide, Fungicide and Rodenticide Act) data requirements, for both new pesticides and those under periodic review. Because the soils used in these tests are required to be representative of the area of intended use, particular agricultural soils are used repeatedly in studies submitted to OPP. The pesticide active ingredients that are tested range from neutral organics, to compounds that are ionic, either positively or negatively charged at pH7.
A comprehensive review of the literature for Kfoc and Koc prediction methods is beyond the scope of this article. Estimation methods for Koc or Kfoc have been reviewed by Brusseau (1993), Sabljić et al. (1995), Doucette (2003), Shao et al. (2014), and most recently by Vitale and DiGuardo (2019). Many QSARs (Quantitative Structure-Activity Relationships) for Koc or Kfoc use the octanolwater partitioning coefficient (Kow), Molecular Connectivity Index (MCI) or solubility as the independent variable. In practice, OPP uses the KOCWIN v2.00 program in EPISuite (US EPA, 2012), which gives two estimates, one based on Kow and the other on MCI. The estimates provided do not quantitatively account for ionization of the target compound and are not explicitly related to agricultural soils.
In this paper, Kfoc is regressed against the Sscl, which is essentially an estimate of the solubility of a compound as if it were a liquid at room temperature. The use of Sscl (Liu et al., 2013) as the independent variable is intended to make the QSAR equally applicable to neutral and ionic compounds, and to compounds that are solids or liquids at 25 °C.
This paper is an attempt to use measurements of Kfoc on eighteen agricultural soils to develop a general-purpose QSAR for prediction of both Kfoc and the associated exponent, 1/n. The significance of this work is in the use of Kfoc measurements from commonly-tested agricultural soils, based on a common EPA testing guideline, to give central-tendency estimates of Kfoc for an unmeasured compound from a greater number of soils than otherwise would be submitted. The estimation method is intended to be robust and to work equally well for solids and liquids, as well as neutral and anionic compounds.
2. Materials and methods
Batch equilibrium studies in OPP’s electronic data holdings were searched to find studies that had used the same soils, for at least three different compounds (active ingredients or metabolites thereof). A total of 18 soils with studies for at least three, and as many as ten compounds, were identified (Table 1). Eleven were U.S. soils, and seven were from Europe. Soils were identified by name, for example, the Don Uglem clay loam from North Dakota. The soil identity in different batch equilibrium studies was confirmed by comparing percent sand, silt, and clay content, and well as fraction organic carbon.
Table 1.
Properties of soils and chemicals used in regressions.
| Soil | Soil properties | Chemical Properties | |||||
|---|---|---|---|---|---|---|---|
| Avg f-oc | %sand | %silt | %clay | No. Of chemicals | solid sol. (mg/L) | mp range (K) | |
| CA Fresno Atwater loamy sand | 0.0036 | 82 | 10 | 8 | 3 | 0.492 to 429 | 373 to 456 |
| FRG Laacher Hof Axxa sandy loam | 0.0173 | 72.4 | 22.6 | 5 | 5 | 0.029 to 436 | 378 to 490 |
| FRG Hoefchen am Hohenseh silt | 0.021 | 8.5 | 81.3 | 10.2 | 5 | 0.029 to 42,000 | 378 to 503 |
| NC Pikeville loamy sand | 0.014 | 77 | 20 | 3 | 4 | 10.9 to 71,000 | 384 to 474 |
| ND Ostlie East sandy clay loam | 0.032 | 52 | 24 | 24 | 4 | 0.087 to 2100 | 337 to 453 |
| NJ Penn silt loam | 0.014 | 25 | 52 | 23 | 3 | 14 to 2100 | 377 to 463 |
| WA Ephrata loamy sand | 0.0042 | 75.7 | 20.5 | 3.8 | 3 | 0.03 to 42,000 | 490 to 503 |
| SWTZ Gartenacker loam/silt loam | 0.021 | 45 | 43 | 12 | 3 | 0.98 to 119,000 | 337 to 369 |
| ND Roger Myron sandy loam | 0.012 | 77 | 10 | 13 | 5 | 0.087 to 37,100 | 298 to 453 |
| ND Mutchler sandy (clay) loam | 0.018 | 59 | 20 | 21 | 4 | 1 to 37,100 | 359 to 453 |
| ND Horse Camp Bridge silt loam | 0.043 | 21 | 54 | 25 | 5 | 436 to 69,100 | 365 to 489 |
| FRG Speyer/LUFA2.2 loamy sand | 0.022 | 82 | 13.6 | 4.4 | 10 | 0.07 to 8.86E+5 | 369 to 510 |
| FRG LUFA/Speyer2.1 sand | 0.0072 | 89 | 7 | 4 | 6 | 0.08 to 400 | 369 to 510 |
| SWTZ Vetroz silt loam | 0.039 | 18.2 | 58.5 | 23.3 | 4 | 0.02 to 2.5 | 332 to 442 |
| SWTZ Les Evouettes silt loam | 0.014 | 31.9 | 61.1 | 7 | 3 | 0.046 to 2.5 | 332 to 442 |
| MS Bosket sandy loam | 0.0038 | 49 | 44 | 7 | 3 | 0.07 to 25,700 | 375 to 447 |
| SWTZ Illarsaz humicsoil | 0.197 | humic | 3 | 0.02 to 4 | 332 to 442 | ||
| ND Don Uglem clay loam | 0.039 | 39 | 30 | 31 | 5 | 0.087 to 2100 | 298 to 453 |
Table 1 also summarizes the number of compounds tested for each of the soils, along with the range of solubility (of the solid), and the range of melting points (Kelvins) (Lewis et al., 2016). At least 3, and as many as 10 compounds were included in the regressions for each soil. For each regression, estimates of Kfoc were compared to the measured values to ensure that the estimates were reasonable (data not shown). Overall, 41 different active ingredients or degradates were included in the 18 regressions.
In order to calculate the Sscl, only compounds with reported solubility and melting point were retained for the regressions. Sscl was calculated by the method presented in Liu et al. (2013) using the assumption of 56.5 kJ/mol/K for the entropy of fusion. The uncertainty introduced by the use of Sscl is in its estimation for solid compounds. The further a compound’s melting point is from 25 °C, the greater the possible extrapolation in Sscl from the solubility from of the solid-state compound. The assumption for the entropy of fusion may over- or under-estimate the actual value, so the error is believed to largely cancel out.
Reported Kf values, not normalized for the fraction of organic carbon in the soil, were regressed versus calculated Sscl values and plotted on a log-log scale. A power regression was run for each soil, because of the very wide range of Sscl (8 orders of magnitude in Fig. 1) and Kf (over 3 orders of magnitude) values, so that a straight line was obtained on the log-log plot. Power regressions were used for all soils to be consistent. The resulting slopes, exponents and R2 values were recorded for each soil.
Fig. 1.
Kf as a function of subcooled liquid solibility for Speyer 2.2 loamy sand (R2=0.75).
The regression models for the 18 soils, in the form of the Kfoc Estimator v0.1 Excel spreadsheet, were used to estimate Kfoc for 333 pesticides from the Footprint database (Lewis et al., 2016), 94 herbicides (Ahrens, 1994), 55 Superfund Priority Pollutants (US EA, 1996) and 22 Polychlorinated biphenyls (Hansen et al., 1999).
3. Results
Fig. 1 presents the plot for the Speyer 2.2 loamy sand soil from Germany, which had the most data. Note that the relationship between Kf and Sscl spans 8 orders of magnitude of Sscl. Table 2 presents the slope and exponent of the power function describing the log-log plot of Kf versus sub-cooled liquid solubility for each soil. These are the regression parameters used in the Kfoc Estimator v0.1 model to produce the 18 individual Kfoc estimates.
Table 2.
Regression Parameters for Kf vs. Sscl and 1/n vs. Melting Point.
| Soil | Kf vs. Sscl (power regression) | 1/n vs. Melting Point (linear regression) | |||
|---|---|---|---|---|---|
| intercept | exponent | R2 | slope | intercept | |
| CA Fresno Atwater loamy sand | 2.3734 | −0.421 | 0.87 | 0.0007 | 0.5481 |
| FRG Laacher Hof Axxa sandy loam | 32.53 | −0.336 | 0.81 | 0.0009 | 0.5041 |
| FRG Hoefchen am Hohenseh silt | 21.079 | −0.333 | 0.79 | 0.0005 | 0.6845 |
| NC Pikeville loamy sand | 18.507 | −0.167 | 0.75 | −0.0005 | 1.1234 |
| ND Ostlie East sandy clay loam | 111.43 | −0.379 | 0.79 | −0.0005 | 1.0405 |
| NJ Penn silt loam | 89.923 | −0.502 | 0.97 | 0.0007 | 0.5885 |
| WA Ephrata loamy sand | 18.318 | −0.381 | 0.78 | −0.0081 | 5.0037 |
| SWTZ Gartenacker loam/silt loam | 80.412 | −0.477 | 0.99 | −0.0015 | 1.4041 |
| ND Roger Myron sandy loam | 51.163 | −0.312 | 0.70 | −0.0007 | 1.1279 |
| ND Mutchler sandy (clay) loam | 91.721 | −0.367 | 0.92 | 0.0005 | 0.6751 |
| ND Horse Camp Bridge silt loam | 4.9906 | −0.124 | 0.03 | −0.0002 | 0.9694 |
| FRG Speyer/LUFA2.2 loamy sand | 78.719 | −0.356 | 0.75 | −0.0027 | 2.0066 |
| FRG LUFA/Speyer2.1 sand | 26.7 | −0.287 | 0.72 | −0.0018 | 1.6124 |
| SWTZ Vetroz silt loam | 502.43 | −0.877 | 0.98 | 0.0006 | 0.719 |
| SWTZ Les Evouettes silt loam Switz | 128.02 | −1.092 | 0.98 | 0.0012 | 0.4282 |
| MS Bosket sandy loam | 80.248 | −0.378 | 0.93 | −0.0001 | 0.9898 |
| SWTZ lllarsaz humic soil | 19471947.8 | −0.757 | 0.97 | 0.0004 | 0.82 |
| ND clay loam Don Uglem | 250.43 | −0.372 | 0.71 | −0.0006 | 1.0761 |
Equations for the Freundlich exponent 1/n were estimated by regressing, within the data set for each of the 18 soils, the reported 1/n value versus the melting point of each compound. These results are also reported in Table 2.
In general, the regression of 1/n versus melting point gave an intercept less than one with a shallow positive slope, or an intercept greater than one with a shallow negative slope, so that a value of 1/n = 1 was reached at some melting point. With a few exceptions, the intercepts were close to one (0.5–2). Larger intercepts (Ephrata loamy sand) had correspondingly steeper slopes. Only two soils (Horse Camp Bridge and Bosket sandy loam) had intercepts less than one and a negative slope, but in both cases the slope was very close to one and the R2 value was low (0.002–0.0561), indicating that 1/n was close to one in all cases. Overall, these results indicate that 1/n is not a strong function of melting point, and that use of a value of 1/n = 1 would not be unreasonable.
A calculator (Kfoc Estimator v. 0.1) was constructed from the results in Tables 1 and 2 to estimate Kfoc and 1/n from the melting point and solubility of 333 pesticide active ingredients found in the Footprint database (Lewis et al., 2016). Each of the 333 pesticides had reported Kfoc, melting point, and solubility data, and were generally organic chemicals. No attempt was made to segregate the 333 pesticides by structural class. Values of pKa were recorded to test whether Kfoc predictions were affected by chemicals being ionized rather than neutral. The calculator estimates Kfoc for each of the 18 soils, then reports the median, average, standard deviation, minimum and maximum values of Kfoc, and calculates 1/n for each soil based on the melting point regression.
Fig. 2 shows a plot of the predicted versus reported average Kfoc values for the 333 pesticides. The 1:1 line is indicated, showing general good agreement, with some scatter as expected since many different structural classes, not all expected to partition to organic carbon, are included. Predictions were within a factor of 10 for 83%, within a factor of 3 for 65%, and within a factor of 2 for 43% of the 333 pesticides. The worst predictions were obtained for quaternary ammonium compounds (e.g., paraquat) since these cationic compounds are not expected to partition to organic carbon.
Fig. 2.
Footprint Avg kfoc vs predict Avg kfoc N=333.
To test whether the Kfoc of ionic compounds was predicted well, the ratio of the predicted and reported Kfoc values was plotted versus the reported pKa for each compound (data not shown). The ratio of predicted Kfoc to reported Kfoc was 1 or less for 99 compounds, and >1 for 74 compounds; 65 were within 2×. The lack of an apparent trend in the data suggests that pKa had little influence on the quality of the prediction.
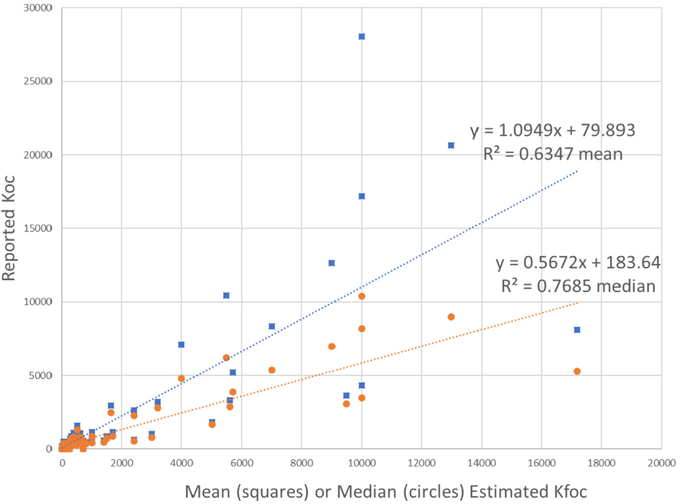
The Kfoc Estimator v. 0.1 program was further tested against published data for Koc for 95 diverse herbicides (WSSA, 1994), 56 Superfund Priority Pollutants (U.S. EPA, 1996), and 22 PCBs (Hansen et al., 1999) measured in one soil. For the herbicides (Fig. 3), the estimated Kfoc values were well-correlated (R2 = 0.7685 vs. median estimates, R2 = 0.6347 vs. mean estimates).
Fig. 3.
kfoc estimated vs WSS Handbook (Ahrens,1994) koc.
Quaternary ammonium compounds and arsenic-containing compounds were not well-predicted, presumably because their sorption behavior is not correlated to organic carbon content. For the Priority Pollutants (Fig. 4), measured values were predicted with R2 values of 0.8737 vs. median predicted and 0.8664 vs. mean predicted. Finally, the Koc values for the PCBs (Fig. 5) were estimated with R2 values of 0.8316 vs. median estimated and 0.8303 vs median estimated.
Fig. 4.
Superfund priority poputant Geometrie mean koc vs Estimate mean vs median koc.
Fig. 5.
PCB congener logkoc (Hansen, et al. 1999) vs Estimate mean and median lofkoc.
As can be seen in Figs. 3 to 5, the accuracy of the mean and median estimates differs for the three data sets. In Fig. 3, the mean estimates have a slope near 1, while the median estimates have a slope near 0.5. In Fig. 4 for Superfund Priority Pollutants, the regression lines are reasonably near the 1:1 values, with estimates underpredicting to some degree as the values approach 100,000. In Fig. 5, for PCBs, while the estimates are highly correlated with the measured values, there is considerable underestimation. This is probably because the measured values are from a single soil, and so do not represent an ‘average’ soil in the way that the Kfoc Estimator v0.1 attempts to do. Literature and estimated values for Figs. 3–5 are in the Appendix.
4. Discussion
The calculated Sscl was well-correlated with the reported Kf values, even over 8 orders of magnitude of Sscl for the Speyer 2.2 soil (see Fig. 1). R2 values for the 18 soils ranged from 0.70 to 0.99, with one exception, indicating that Sscl is a useful independent variable for prediction of Kf.
The correlation between mean and median predicted Kfoc values from the Kfoc Estimator v0.1 is shown in Figs. 2 to 5 for different data sets. The data in Fig. 2 (333 pesticide active ingredients) show a fair correlation between the mean predicted Kfoc and the reported values (R2 = 0.39). This data set includes compounds such as quaternary ammonium cations, whose very strong partitioning behavior does not reflect their very high solubility. These compounds’ Kfoc values are predicted poorly by Kfoc Estimator v0.1, which contributes to the relatively low R2. Even so, predictions were within a factor of 10 for 83%, within a factor of 3 for 65%, and within a factor of 2 for 43% of the 333 pesticides.
Fig. 3 shows the results for a set of 94 herbicides. The correlations between reported Koc estimated mean Kfoc (R2 = 0.63) and estimated median Kfoc (R2 = 0.77) are good. The estimated means are within a factor of 2 for 51% of the 94 herbicides, within a factor of 3 for 77% and within a factor of 10 for 94%. The estimated median Kfoc values are within 2× for 54%, 3× for 74% and 10× for 93% of the 94 herbicides. This indicates generally good agreement between reported and estimated values.
Fig. 4 shows the correlation between reported geometric mean Koc for 55 Superfund Priority Pollutants, and the mean and median estimated Kfoc values from Kfoc Estimator v0.1. Correlations are good (mean, R2 = 0.86; median, R2 = 0.87). The estimated mean Kfoc values are with 2× of reported Koc values for 22%, within 3× for 38% and within 10× for 75% of the 55 compounds. The estimated median Kfocs are within 2× for 29%, within 3× for 55% and within 10× for 73% of the 55 compounds. Agreement is not as good as for the 94 herbicides. This may be due to the structural diversity of the 55 Priority Pollutants, and due to the structural differences from pesticides in general.
Fig. 5 shows the correlation between reported logKoc values for 22 PCB congeners tested on a single soil, and the mean and median estimated Kfoc values. Correlations are good (R2 = 0.83 for both mean and median). However, the values estimated by Kfoc Estimator v0.1 are significantly lower than the reported values. The mean estimates run from 2 to 14% of reported Koc, and the median values run from 0.65 to 10.4% of reported Koc. Seven estimated means and only one estimated median are within 10× of reported Koc. Estimated maximum Kfoc values were within a factor of 10 of the reported Koc. The low estimates for this data set are attributed to the fact that the reported values are for a single soil, while Kfoc Estimator v0.1 gives the central tendency for estimates from 18 soils. These central tendency values are not intended to reflect any one soil.
5. Conclusions
A calculator (Kfoc Estimator v. 0.1) for the Freundlich Organic Carbon-normalized partition coefficient (Kfoc) and its associated exponent (1/n) has been constructed from studies submitted to the U.S. EPA pesticides program. The calculator is based on regressions for 41 active ingredients and metabolites in 18 specific agricultural soils. It produces 18 separate estimates, so soil-to-soil variability may be assessed; mean and median results represent central tendencies across the 18 soils. The use of the subcooled liquid solubility (Sscl) as the independent variable makes the calculator applicable to both neutral and anionic organic compounds. Predictions of Kfoc for 333 active ingredients were within 10× for 83%, 3× for 65%, and 2× for 43%. Predictions are well-correlated with published values for diverse sets of compounds, including herbicides, Priority Pollutants and polychlorinated biphenyls. Testing shows that the Kfoc Estimator is not applicable to quaternary ammonium or organometallic compounds. A copy of Kfoc Estimator v. 0.1 is available from the author.
HIGHLIGHTS.
Freundlich soil-water partition coefficient (Kfoc) is predicted from subcooled liquid solubility.
Predictions are based on measured values from 41 pesticides in 18 agricultural soils, giving robust central-tendency estimates.
Mean and Median Kfoc estimates give estimates within a factor of 10 of literature values for a majority of compounds from 3 of 4 data sets.
Acknowledgments
Copyright
This is a work of the U.S. Government and is not subject to copyright protection in the U.S. It has been subjected to review by the Office of Pesticide Programs and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsemenst or recommendation for use.
Appendix. Measured and Estimated Koc/Kfoc Values from Hansen (1999), U.S. EPA (1996) and Ahrens (1994). Values estimated by Kfoc Estimator v. 0.1 marked as “this work.”
| CAS No | name | Water solubility mg/L | mp,°C | Geomean Koc | This Work | |
|---|---|---|---|---|---|---|
| Ea Mean Kfoc | Est Median Kfoc | |||||
| 50-32-5 | Benzo(a)pyrene | 162E-03 | 179 | 968,774 | 37,561 | 11,909 |
| 72-43-5 | Methoxychlor | 4,50E-02 | 82.5 | 80,000 | 16,551 | 8039 |
| 50-29-3 | DDT | 2,50E-02 | 108.5 | 677,934 | 16,493 | 8025 |
| 56-55-3 | Benz(a)anthracene | 9.40E-03 | 155 | 357,537 | 15,591 | 7798 |
| 117-81-7 | Bis(2-ethylhexyl)phthalate | 3.40E-01 | −50 | 111,123 | 10,367 | 6188 |
| 57-74-9 | Chlordane | 5.60E-02 | 105 | 51,310 | 10,233 | 6137 |
| 72-54-8 | DDD | 9.00E-02 | 109.5 | 45,800 | 7225 | 4885 |
| 76-44-8 | Heptachlor | 1.80E-01 | 95.5 | 9,528 | 5844 | 4129 |
| 309-00-2 | Aldrin | 1.80E-01 | 104.3 | 48,685 | 5237 | 3902 |
| 206-44-0 | Fluoranthene | 2.06E-01 | 107 | 49,096 | 4716 | 3654 |
| 8001-35-2 | Toxaphene | 7.40E-01 | 65 | 95,816 | 3394 | 3284 |
| 129-00-0 | Pyrene | 1.35E-01 | 156 | 67,992 | 3316 | 2896 |
| 85-68-7 | Butyl benzyl phthalate | 2.69E+00 | −35 | 13,746 | 3308 | 2892 |
| 120-12-7 | Anthracene | 4.34E-02 | 218 | 23,493 | 2900 | 2440 |
| 60-57-1 | Dieldrin | 1.95E-01 | 175.5 | 25,546 | 2267 | 2043 |
| 86-73-7 | Fluorene | 1.98E+00 | 116 | 7,707 | 1494 | 1225 |
| 83-32-9 | Acenaphthene | 4.24E+00 | 95 | 4,898 | 1329 | 1045 |
| 72-20-8 | Endrin | 2.50E-01 | 228 | 10,811 | 1224 | 934 |
| 115-29-7 | Endosulfan | 5.10E-01 | 209 | 2,040 | 1093 | 825 |
| 319-84-6 | a-HCH (a-BHC) | 2.00E+00 | 158 | 1,762 | 1008 | 779 |
| 58-89-9 | g -HCH (Lindane) | 6.80E+00 | 112.5 | 1,352 | 937 | 739 |
| 91-20-3 | Naphthalene | 3.10E+01 | 80.2 | 1,191 | 693 | 596 |
| 106-46-7 | 1,4-Dichlorobenzene | 7.38E+01 | 53.1 | 616 | 631 | 536 |
| 95-50-1 | 1,2-Dichlorobenzene | 1.56E+02 | −17 | 379 | 606 | 511 |
| 108-38-3 | m-Xylene | 1.61E+02 | −47.4 | 196 | 599 | 504 |
| 319-85-7 | b-HCH (b-BHC) | 2.40E-01 | 312 | 2,139 | 591 | 496 |
| 100-41-4 | Ethylbenzene | 1.69E-02 | −95 | 204 | 588 | 494 |
| 95-47-6 | o-Xylene | 1.78E+02 | −25 | 241 | 577 | 482 |
| 106-42-3 | p-Xylene | 1.85E+02 | 13 | 311 | 569 | 474 |
| 127-18-4 | Tetrachloroethylene | 2.00E+02 | −22 | 265 | 552 | 458 |
| 120-82-1 | 1,2,4-Trichlorobenzene | 3.00E+02 | 17 | 1,659 | 476 | 384 |
| 100-42-5 | Styrene | 3.10E+02 | −30 | 912 | 470 | 378 |
| 108-90-7 | Chlorobenzene | 4.72E+02 | −45 | 224 | 403 | 315 |
| 108-88-3 | Toluene | 5.26E+02 | −94 | 140 | 388 | 300 |
| 118-74-1 | Hexachlorobenzene | 6.20E+00 | 228 | 80,000 | 362 | 277 |
| 56-23-5 | Carbon tetrachloride | 7.93E-r02 | −23 | 152 | 335 | 251 |
| 84-66-2 | Diethylphthalate | 1.08E+03 | −40.5 | 82 | 300 | 220 |
| 79-01-6 | Trichloroethylene | 1.10E+03 | −87 | 94 | 298 | 218 |
| 71-55-6 | 1,1,1-Trichloroethane | 1.33E+03 | −35 | 135 | 278 | 201 |
| 71-43-2 | Benzene | 1.75E+03 | 5 | 62 | 253 | 179 |
| 98-95-3 | Nitrobenzene | 2.09E+03 | 6 | 119 | 238 | 168 |
| 75-35-4 | 1,1 -Dichloroethylene | 2.25E+03 | −122 | 65 | 232 | 164 |
| 78-87-5 | 1,2-Dichloropropane | 2.80E+03 | −100.3 | 47 | 215 | 151 |
| 79-34-5 | 1,1,2,2-Tetrachloroethane | 2.97E+03 | −36 | 79 | 210 | 148 |
| 75-25-2 | Bromoform | 3.10E+03 | 7.5 | 126 | 207 | 146 |
| 79-00-5 | 1,1,2-Trichloroethane | 4.42E-i-03 | −37 | 75 | 183 | 128 |
| 75-34-3 | 1,1-Dichloroethane | 5.06E-f03 | −97 | 53 | 175 | 122 |
| 156-60-5 | trans-1,2-Dichloroethylene | 6.30E+03 | −50 | 38 | 162 | 113 |
| 67-66-3 | Chloroform | 7.92E-f-03 | −63 | 53 | 150 | 104 |
| 107-06-2 | 1,2-Dichloroethane | 8.52E-r03 | −35 | 38 | 147 | 101 |
| 75-09-2 | Methylene chloride | 1.30E+04 | −95.1 | 10 | 127 | 87 |
| 74-83-9 | Methyl bromide | 1.52E+04 | −94 | 9 | 121 | 82 |
| 111-44-4 | Bis(2-chloroethyl)ether | 1.72E+04 | −50 | 76 | 116 | 79 |
| PCB# | Log Koc | This Work | Sol mg/L | mp °C | This Work | ||
|---|---|---|---|---|---|---|---|
| Log est mean | Log est median | Est mean Kfoc | Est median Kfoc | ||||
| 183 | 6.32 | 5.06 | 4.29 | 4.90E-03 | 83 | 114,096 | 19,631 |
| 138 | 6.2 | 4.84 | 4.20 | 7.29E-03 | 80.5 | 68,990 | 15,718 |
| 180 | 6.37 | 4.81 | 4.18 | 3.85E-03 | 112 | 64,527 | 15,248 |
| 153 | 6.19 | 4.58 | 4.08 | 9.14E-03 | 103 | 37,945 | 11,920 |
| 151 | 6.02 | 4.47 | 4.03 | 1.36E-02 | 100 | 29,375 | 10,611 |
| 101 | 5.78 | 4.43 | 4.01 | 2.63E-02 | 77 | 26,612 | 10,133 |
| 118 | 5.99 | 4.41 | 4.00 | 1.34E-02 | 109 | 25,474 | 9927 |
| 87 | 5.82 | 4.37 | 3.98 | 2.84E-02 | 81 | 23,449 | 9543 |
| 44 | 5.1 | 4.22 | 3.91 | 1.00E-01 | 47 | 16,677 | 8070 |
| 49 | 5.09 | 4.16 | 3.87 | 7.84E-02 | 67 | 14,398 | 7484 |
| 70 | 5.26 | 4.13 | 3.86 | 3.62E-02 | 106 | 13,372 | 7200 |
| 95 | 5.62 | 4.09 | 3.84 | 5.41 E-02 | 94 | 12,250 | 6866 |
| 28 | 4.98 | 4.05 | 3.82 | 1.43E-01 | 57 | 11,303 | 6533 |
| 31 | 4.96 | 3.99 | 3.78 | 1.43E-01 | 67 | 9770 | 5959 |
| 66 | 5.38 | 3.98 | 3.77 | 3.68E-02 | 128 | 9568 | 5880 |
| 18 | 4.79 | 3.93 | 3.74 | 2.99E-01 | 44 | 8576 | 5476 |
| 52 | 5.1 | 3.93 | 3.74 | 1.13E-01 | 87 | 8537 | 5460 |
| 60 | 5.38 | 3.88 | 3.71 | 3.89E-02 | 142 | 7634 | 5070 |
| 40 | 5.14 | 3.82 | 3.66 | 8.07E-02 | 121 | 6603 | 4594 |
| 8 | 4.76 | 3.79 | 3.64 | 5.38E-01 | 43 | 6187 | 4392 |
| 15 | 4.79 | 3.74 | 3.60 | 6.00E-02 | 149 | 5467 | 4024 |
| 2 | 4.58 | 3.73 | 3.60 | 9.00E-01 | 32 | 5340 | 3956 |
| compound | water solubility mg/L | mp, °C | Koc | This Work | |
|---|---|---|---|---|---|
| mean est Kfoc | median est Kfoc | ||||
| fluridone | 12 | 154–155 | 1000 | 526 | 433 |
| oxyfluorfen | 0.1 | 76–80 | 5483 | 10,444 | 6217 |
| lactofcn | 0.1 | 419–455 | 10000 | 17,170 | 8190 |
| fluazifop Butyl Ester | 1.1 | 10 | 5700 | 5222 | 3894 |
| fenoxaprop Ethyl Ester | 0.5 | 89–91 | 9490 | 3646 | 3089 |
| benefin | 0.1 | 65–66.5 | 9000 | 12,631 | 6985 |
| trifluralin | 0.3 | 46–47 | 7000 | 8327 | 5371 |
| ethalfluralin | 0.3 | 57–59 | 4000 | 7088 | 4822 |
| isopropalin | 0.08 | no data | 10,000 | 28,040 | 10,384 |
| prodiamine | 0.013 | 122.5–124 | 13,000 | 20,643 | 8973 |
| cinmethylin | 6.3 | no data | 300 | 855 | 692 |
| pendimethalin | 0.275 | 47–57 | 17,200 | 8083 | 5267 |
| oxadiazon | 0.7 | 87 | 3200 | 3196 | 2804 |
| sethoxydim pH7 | 4390 | no data | 100 | 184 | 129 |
| acifluorfen | 120 | 142–160 | 113 | 253 | 180 |
| bifenox | 0.398 | 84–86 | 10,000 | 4337 | 3466 |
| tridiphane | 1.8 | 428 | 5600 | 3296 | 2884 |
| napropamide | 73 | 748–755 | 700 | 525 | 432 |
| norflurazon | 28 | 177 | 700 | 320 | 238 |
| bensulide | 25 | 344 | 1000 | 1132 | 846 |
| desmedipham | 7 | 120 | 1500 | 865 | 698 |
| phenmedipham | 10 | 143–144 | 2400 | 618 | 523 |
| imazaquin | 60 | 219–224 | 20 | 171 | 120 |
| butylate | 44 | no data | 400 | 984 | 766 |
| clomazone | 1100 | 25 | 300 | 297 | 218 |
| thiobencarb | 30 | 3.3 | 1700 | 1146 | 857 |
| fomesafen Na | 600,000 | 220–221 | 60 | 10 | 5 |
| haloxyfop acid | 433 | 107–108 | 60 | 487 | 394 |
| isoxaben | 1 | 176–179 | 380 | 1117 | 838 |
| dithiopyr | 1.38 | 65 | 1638 | 2936 | 2483 |
| triclopyr BE | 23 | 148–150 | 780 | 432 | 342 |
| triallate | 4 | 29–30 | 2400 | 2619 | 2268 |
| naptalam | 200 | 185 | 20 | 152 | 105 |
| oryzalin | 2.6 | 141–142 | 600 | 1058 | 806 |
| prometryn | 33 | 118–120 | 400 | 486 | 394 |
| diallate | 14 | 25–30 | 500 | 1569 | 1308 |
| DCPA | 0.5 | 156 | 5000 | 1818 | 1648 |
| metolachlor | 488 | −40 | 200 | 398 | 310 |
| ametryn | 200 | 84–85 | 300 | 338 | 254 |
| triasulfuron pH7 | 815 | 186 | 121 | 94 | 63 |
| pronamide | 15 | 155–156 | 800 | 480 | 389 |
| linuron | 75 | 93–94 | 400 | 447 | 357 |
| siduron | 18 | 133–138 | 420 | 527 | 434 |
| chlorsulfuron | 31,800 | 174–178 | 40 | 32 | 20 |
| alachlor | 200 | 39.5–41.5 | 124 | 486 | 394 |
| pebulate | 60 | <25 | 430 | 871 | 702 |
| vernolate | 108 | no data | 260 | 695 | 597 |
| fluometuron | 110 | 163–164.5 | 100 | 219 | 155 |
| flumetsulam acid | 5600 | 253 | 700 | 32 | 20 |
| atrazine | 33 | 175–177 | 100 | 307 | 226 |
| propachlor | 580 | 77 | 112 | 245 | 174 |
| ethofumesate | 110 | 70–72 | 340 | 467 | 376 |
| molinate | 970 | <25 | 190 | 311 | 230 |
| propanil | 500 | 85–89 | 149 | 239 | 169 |
| EPTC | 370 | no data | 200 | 440 | 350 |
| simazine | 6.2 | 225–227 | 130 | 368 | 282 |
| prometon | 720 | 91–92 | 150 | 204 | 143 |
| cyanazine | 160 | 1673–169 | 190 | 187 | 131 |
| hexazinone | 33X100 | 115–117 | 54 | 48 | 30 |
| cycloate | 85 | no data | 650 | 762 | 637 |
| diuron | 42 | 158–159 | 48CJ | 323 | 241 |
| 2,4DB acid | 46 | 120–121 | 440 | 427 | 337 |
| MCPB Na salt | 200,000 | 100–101 | 20 | 31 | 19 |
| triflusulfuron pH7 | 110 | 150–154 | 80 | 241 | 171 |
| dazomet | 2000 | 100–106 | 10 | 131 | 90 |
| diethatyl | 105 | 49–50 | 1400 | 572 | 478 |
| mefluidide | 180 | 183–185 | 200 | 160 | 111 |
| bromacil | 815 | 158–159 | 32 | 116 | 79 |
| methazole | 42 | 123–124 | 3000 | 1029 | 791 |
| metribuzin | 1100 | 1255–1263 | 60 | 134 | 92 |
| terbacil | 710 | 175–177 | 55 | 106 | 72 |
| mecoprop acid | 620 | 94–95 | 20 | 209 | 148 |
| tebuthiuron | 2500 | 161.5–164 | 80 | 77 | 51 |
| picloram | 430 | no data | 90 | 416 | 327 |
| sodium chlorate | 1,000,000 | 248 | 10 | 8 | 3 |
| 2,4-D acid | 900 | 135–138 | 20 | 133 | 91 |
| MCPA acid | 825 | 118–119 | 110 | 157 | 1(19 |
| primisulfuron pH7 | 243 | 203 | 50 | 124 | 85 |
| dicamba acid | 4500 | 114–116 | 2 | 91 | 61 |
| asulam | 534,000 | 135–137 | 40 | 18 | 10 |
| sulfometuron pH7 | 300 | 203–205 | 78 | 114 | 78 |
| metsulfuron | 2790 | 158 | 35 | 78 | 51 |
| tribenuron pH7 | 2040 | 141 | 46 | 98 | 65 |
| clopyralid | 1000 | 151–152 | 33 | 115 | 78 |
| endothall | 100 | 144 | 120 | 266 | 190 |
| thifensulfuron pH7 | 2240 | 186 | 45 | 68 | 44 |
| bentazon | 500 | 137–139 | 34 | 160 | 111 |
| chlorimuron | 1200 | 185–187 | 110 | 83 | 55 |
| glufosinate | 1,370000 | 215 | 100 | 9 | 4 |
| nicosulfuron pH7 | 12,200 | 172–173 | 30 | 44 | 27 |
| maleic hydrazide acid | 4500 | 300 | 250 | 25 | 15 |
| amitrole | 280,000 | 159 | 100 | 19 | 11 |
| acrolein | 237.628 | −87 | 05 | 49 | 31 |
| fosamine | 1,790000 | 175 | 150 | 10 | 5 |
References
- Ahrens WH, 1994, seventh ed.. Herbicide Handbook, Weed Science Society of America; Champaign, IL, 1994. [Google Scholar]
- Brusseau M.l., 1993. Using QSAR to evalsuate phenomenonological models for sorption of organic compounds by soil. Environ. Toxicol. Chem 12, 1835–1846, 1993. [Google Scholar]
- Doucette WJ, 2003. Annual Review. Quantitative structure-activity relationships for predicting soil-sediment sorption coefficients for organic chemicals. Environ. Toxicol. Chem 22,1771–1788, 2003. [DOI] [PubMed] [Google Scholar]
- Hansen BG, et al. , 1999. QSARs for Kow and Koc of PCB congeners: a critical examination of data, assumptions and statistical approaches. Chemosphere 39 (13), 2209–2228, 1999. [Google Scholar]
- Lewis KA, Tzilivakis J, Warner D, Green A, 2016. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J 22 (4), 1050–1064. 10.1080/10807039.2015.1133242. [DOI] [Google Scholar]
- Liu L, et al. , 2013. Determination of the subcooled liquid solubilities of PAHs in partitioning batch experiments. Geosci. Front 4, 123–126, 2013. [Google Scholar]
- Sabljić A, et al. , 1995. QSAR modelling of soil sorption. Improvements and systematics oflog Kocvs. log Kowcorrelations. Chemosphere 3111/12, 4489–4514, 1995. [Google Scholar]
- Shao Y, et al. , 2014. Integrated QSPR models to predict the soil sorption coeficient for a large diverse set of compounds by using different modeling methods. Atmos. Environ 88, 2014 212–218. [Google Scholar]
- U.S. EPA Office of Solid Waste and Emergency Response, 1996. Superfund Soil Screening Guidance. Part 5, Chemical Specific Parameters. https://semspub.epa.gov/work/HQ/175235.pdf.
- U.S, EPA, 2008. Guideline 835.1230, Adsorption/Desorption (Batch Equilibrium). Found at: https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-835-fate-transport-and-transformation-test.
- US EPA, 2012. Estimation Programs Interface Suite™ for Microsoft® Windows, 4.11. United States Environmental Protection Agency, Washington, DC, USA. [Google Scholar]
- Vitale CM, DiGuardo A, 2019. A review of the predictive models estimating association of neutral and ionizable organic chemicals with dissolved organic carbon. Sci. Total Environ 666, 1022–1032 [DOI] [PubMed] [Google Scholar]