Abstract
Cerebral autoregulation, as measured using the pressure reactivity index (PRx), has been related to global patient outcome in adult patients with traumatic brain injury (TBI). To date, this has been documented without accounting for standard baseline admission characteristics and intracranial pressure (ICP). We evaluated this association, adjusting for baseline admission characteristics and ICP, in a multi-center, prospective cohort. We derived PRx as the correlation between ICP and mean arterial pressure in prospectively collected multi-center data from the High-Resolution Intensive Care Unit (ICU) cohort of the Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI) study. Multi-variable logistic regression models were analyzed to assess the association between global outcome (measured as either mortality or dichotomized Glasgow Outcome Score–Extended [GOSE]) and a range of covariates (IMPACT [International Mission for Prognosis and Analysis of Clinical Trials] Core and computed tomography [CT] variables, ICP, and PRx). Performance of these models in outcome association was compared using area under the receiver operating curve (AUC) and Nagelkerke's pseudo-R2. One hundred ninety-three patients had a complete data set for analysis. The addition of percent time above threshold for PRx improved AUC and displayed statistically significant increases in Nagelkerke's pseudo-R2 over the IMPACT Core and IMPACT Core + CT models for mortality. The addition of PRx monitoring to IMPACT Core ± CT + ICP models accounted for additional variance in mortality, when compared to models with IMPACT Core ± CT + ICP alone. The addition of cerebrovascular reactivity monitoring, through PRx, provides a statistically significant increase in association with mortality at 6 months. Our data suggest that cerebrovascular reactivity monitoring may provide complementary information regarding outcomes in TBI.
Keywords: autoregulation, cerebrovascular reactivity, IMPACT, outcome analysis
Introduction
The continuous monitoring of cerebrovascular reactivity in critically ill adult patients with moderate and severe traumatic brain injury (TBI) has received support from international multi-modal monitoring consensus statements.1–3 Given the common use of intracranial pressure (ICP) monitoring, the use of ICP-derived indices provides the most convenient means of assessing cerebrovascular reactivity in this population and have been cited as an option by the Brain Trauma Foundation (BTF) guidelines.4 Of several indices,1,5,6 The pressure reactivity index (PRx) has received the most attention and is the most widely described cerebrovascular reactivity index in the literature,7,8 with higher values denoting increasing autoregulatory dysfunction.
The biological relevance of PRx and its incorporation into clinical management guidelines are underpinned by its association with outcome in retrospective analysis. These reports demonstrate that during the acute phase, both the magnitude of mean PRx or the duration spent with PRx above a pre-specified threshold are associated with mortality and functional outcome.7,9–11 However, this basis for attributing biological relevance and management utility suffers from some shortcomings.
First, it is unclear whether these PRx-related metrics maintain their strong association with outcome when adjusting for admission characteristics incorporated in recognized clinical outcome prediction tools in TBI, the best known of which are the International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) models.12 The performance of the IMPACT models in predicting outcome, measured using area under the receiver operating curve (AUC), ranges from 0.60 to 0.80 (depending on cohort and the time of the study), and the proportion of outcome association variance they explain, as quantified by Nagelkerke's pseudo-R2, ranges up to 0.35.12,13 A rigorous assessment of PRx in association with outcome would depend on being able to add to any variance explained by the IMPACT models.
Second, we need to understand whether the association between PRx with outcome is not simply because this metric is a non-specific index of post-admission disease course, rather than a specific effect attributable to deterioration in cerebrovascular reactivity. One rational way to address this issue is to examine whether PRx-derived metrics maintain their strong association with outcome when adjusting for a canonical physiological measure of TBI disease course such as ICP.
Finally, the most substantial publications that relate PRx to outcome come from a retrospective analysis of single-center data, without formal blinding of outcome assessment. A rigorous assessment of the outcome association of PRx would require testing on a prospective multi-center patient cohort, with independent and blinded assessment of outcome
The goal of this study was to explore PRx cerebrovascular reactivity monitoring and its association with outcome, adjusting for existing admission IMPACT Core and Core + computed tomography (CT) model variables. In addition, the association between PRx and outcome was assessed while adjusting for canonical metrics of TBI disease course (mean ICP and duration of ICP above classical thresholds). This was accomplished through analysis of a prospectively acquired multi-center data set from critically ill adult patients with moderate/severe TBI (the CENTER-TBI [Collaborative European NeuroTrauma Effectiveness Research in TBI] study).14
Methods
Patient population
All patients from the multi-center CENTER-TBI high-resolution intensive care unit (ICU) monitoring cohort with parenchymal ICP monitoring were included in this analysis. Patients with external ventricular drain–based ICP data were excluded given the interrupted nature of their recordings (i.e., reliable ICP can be recorded only when the drainage is closed). These patients were prospectively recruited between January 2015 and December 2017 from 21 centers in the European Union (EU). All patients were admitted to the ICU for their TBI during the course of the study, with high-frequency digital signals recorded from their ICU monitors during the course of their ICU stay. All patients suffered predominantly from moderate-to-severe TBI (moderate = Glasgow Coma Score [GCS] 9–12; severe = GCS of ≤8). A minority of patients (n = 31) were categorized at the time of admission as suffering from less-severe TBI, but experienced subsequent early deterioration leading to ICU admission for care and monitoring. All patients in this cohort had invasive ICP monitoring conducted in accord with the BTF guidelines.4
Ethics
Data used in these analyses were collected as part of the CENTER-TBI study, which had individual national or local regulatory approval; the UK Ethics approval is provided as an exemplar: (IRAS No: 150943; REC 14/SC/1370). The CENTER-TBI study (EC grant 602150) has been conducted in accord with all relevant laws of the EU, if directly applicable or of direct effect, and all relevant laws of the country where the Recruiting sites were located, including, but not limited to, the relevant privacy and data protection laws and regulations (the “Privacy Law”), the relevant laws and regulations on the use of human materials, and all relevant guidance relating to clinical studies from time to time in force, including, but not limited to, the ICH Harmonized Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95; “ICH GCP”) and the World Medical Association Declaration of Helsinki entitled “Ethical Principles for Medical Research Involving Human Subjects.” Informed consent by the patients and/or the legal representative/next of kin was obtained, accordingly to the local legislations, for all patients recruited in the Core Dataset of CENTER-TBI and documented in the e-CRF.
Data collection
As part of recruitment to the multi-center high-resolution ICU cohort of CENTER-TBI, all patients had demographics, injury, and imaging data prospectively recorded. Similarly, all patients had high-frequency digital signals from ICU monitoring recorded throughout their ICU stay, with the goal of initiating recording within 24 h of ICU admission. All digital ICU signals were further processed (see Signal Acquisition/Signal Processing). For the purpose of this study, the IMPACT Core and CT variables were extracted from the central study database. They included: age, admission best GCS motor score, and pupillary reactivity (bilaterally reactive, unilateral reactive, bilateral unreactive), Marshall CT classification,15 presence of traumatic subarachnoid hemorrhage (yes, no), presence of an extradural hematoma (yes, no), presence of pre-hospital hypotension (yes, no), and the presence of pre-hospital hypoxia (yes, no). Patient outcomes were assessed at 6 months using the extended Glasgow Outcome Scale–Extended (GOSE). Outcome assessors in TBI and the researchers involved in analysis of high-resolution data were blinded to each other's work. Finally, we used version 2.0 of the CENTER-TBI data set, where missing GOSE measures at 6 months were imputed. As such, CENTER-TBI data version 2.0 was accessed for the purpose of this study, by Opal database software.16
Signal acquisition
Arterial blood pressure (ABP) was obtained through either radial or femoral arterial lines connected to pressure transducers. ICP was acquired from an intraparenchymal strain gauge probe (Codman ICP MicroSensor; Codman & Shurtleff Inc., Raynham, MA), parenchymal fibre optic pressure sensor (Camino ICP Monitor; Integra Life Sciences, Plainsboro, NJ; https://www.integralife.com/). All signals were recorded using digital data transfer or digitized by an analog-to-digital converter (DT9803; Data Translation, Marlboro, MA), where appropriate; sampled at frequency of 100 Hz or higher, using the ICM+ software (Cambridge Enterprise Ltd, Cambridge, UK; http://icmplus.neurosurg.cam.ac.uk) or Moberg CNS Monitor (Moberg Research Inc, Ambler, PA; https://www.moberg.com) or a combination of both. Signal artefacts were removed using both manual and automated methods before further processing or analysis.
Signal processing
Post-acquisition processing of the above signals was conducted using ICM+ (Cambridge Enterprise Ltd; http://icmplus.neurosurg.cam.ac.uk). Cerebral perfusion pressure (CPP) was determined as mean arterial pressure (MAP) – ICP. Ten-second moving averages (updated every 10 sec to avoid data overlap) were calculated for all recorded signals: ICP, ABP (which produced MAP), pulse amplitude of ICP, and CPP. PRx was calculated as the moving correlation coefficient between 30 consecutive 10-sec mean windows of ICP and MAP, updated every minute.
Data were downsampled to minute-by-minute resolution for the entire duration of recording for each patient. Grand mean values of all physiological variables were calculated per patient. In addition, the following post-processing of this physiological data occurred in R (R Core Team [2018]; R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/):
-
1.
ICP: For each patient, the % of time spent above ICP of 20 mm Hg and 22 mm Hg were calculated for the entire recording period.4,11
-
2.
PRx: For each patient, the % of time spent above the following clinically defined thresholds were calculated across the entire recording period: 0, +0.25, and +0.35.11,17 All of these thresholds for PRx have been defined in previous published literature as statistically significant for association with 6-month global outcome in adult TBI patients.
Data were provided in summary sheets for the patient cohort using data from: 1) entire recording and 2) the first 72 h of recording. These two sheets were produced to assess whether there was any difference in outcome association when focusing on more acute physiology, such as that observed during the first 72 h post-injury.
Statistical analysis
All statistical analysis was conducted using R and XLSTAT (Addinsoft, New York, NY; https://www.xlstat.com/en/) add-on package to Microsoft Excel (Microsoft Office 15, Version 16.0.7369.1323; Microsoft Corporation, Redmond, WA). The following analysis was conducted for both the entire recording period and the first 72 h of recording, with similar results. As such, only the entire recording period will be reported in detail, with intermittent reference made to the results from the first 72 h of recording.
Normality of continuous variables was assessed by the Shapiro-Wilks test, where all variables displayed non-parametric characteristics and are hence displayed as median (range) or median (interquartile range; IQR). For all testing described, the alpha was set at 0.05 for significance. GOSE was then dichotomized into the following categories: 1) alive (GOSE 2–8) versus dead (GOSE 1) and 2) favorable (GOSE 5–8) versus unfavorable (GOSE ≤4). IMPACT Core and CT variables, along with physiological variables, were compared between each dichotomized group, using the Mann-Whitney U test or chi-square testing, where appropriate.
Univariate logistic regression (ULR) was first conducted, comparing each IMPACT model variable, and the continuous physiologic variables to the dichotomized outcomes. AUC, 95% confidence intervals (CIs), and p values for the univariate models are reported.
Next, IMPACT Core/Core + CT multi-variable models were created. Using multi-variable logistic regression (MLR) analysis, these models and their association with dichotomized GOSE were assessed. AUC, 95% confidence intervals (CIs), and p values were reported for each model. Finally, the % time spent above threshold for ICP and PRx were sequentially added to the IMPACT Core/Core + CT models. Similar to the IMPACT Core/Core + CT baseline models, AUC, p values, and model Akaike information criteria (AIC) were reported for each dichotomized outcome, with highest AUC, lowest AIC indicating model superiority. Additional accounted variance in association with outcome over the IMPACT Core/Core + CT models was assessed using the relative difference in Nagelkerke's pseudo-R2 (termed delta pseudo-R2). All AUCs and 95% CIs for both ULR and MLR were determined using bootstrapping techniques with 2000 iterations.
Results
Patient population
At the time of this analysis, a total of 193 patients from the CENTER-TBI high-resolution ICU cohort had complete data sets, including: 6-month GOSE, high-frequency physiological signals containing at least ICP (from parenchymal monitors) and ABP for PRx derivation, and a complete set of IMPACT Core variables. Looking at those with all of the above, plus IMPACT CT variables, our number of patients with complete data at the time of this extraction was 166. Patient demographics for the entire cohort (n = 193) can be found summarized in Table 1. Patient demographics for both the alive/dead and favorable/unfavorable outcome groups can be found in Supplementary Appendix A.
Table 1.
Patient Demographics: Median and IQR
| Demographics | Median (IQR) | |
|---|---|---|
| No. of patients | 193 | |
| Age (years) | 51.0 (30.0–64.0) | |
| Sex | ||
| Male | 149 | |
| Female | 44 | |
| Admission GCS (total) | 6 (3–10) | |
| Admission GCS motor | 4 (1–5) | |
| Admission pupil response | ||
| Bilaterally reactive | 142 | |
| Unilateral unreactive | 15 | |
| Bilaterally unreactive | 36 | |
| Marshall CT grade | 3 (2–6) | |
| No. with traumatic SAH | 145 | |
| No. with extra-axial hematoma | 37 | |
| No. with hypoxia episode | 32 | |
| No. with hypotension episode | 24 | |
| Duration of high-frequency physiological recording (h) | 119.9 (78.3–157.6) | |
| ICP (mm Hg) | 12.6 (9.6–16.6) | |
| CPP (mm Hg) | 70.2 (64.3–76.4) | |
| % time with ICP >20 mm Hg | 5.3 (1.1–19.9) | |
| % time with ICP >22 mm Hg | 2.8 (0.5–14.8) | |
| % time with PRx >0 | 51.7 (38.9–66.4) | |
| % time with PRx >+0.25 | 26.9 (18.5–41.7) | |
| % time with PRx >+0.35 | 19.4 (13.3–31.8) | |
| 6-month GOSE | 4 (3–5) | |
| No. alive: 6 months | 153 | |
| No. dead: 6 months | 40 | |
| No. favorable outcome: 6 months (GOSE 5–8) | 83 | |
| No. unfavorable outcome: 6 months (GOSE 1–4) | 110 | |
CPP, cerebral perfusion pressure; CT, computed tomography; GCS, Glasgow Coma Score; GOSE, Glasgow Outcome Scale-Extended; ICP, intracranial pressure; IQR, interquartile range; MAP, mean arterial pressure; mm Hg, millimeters of mercury; PRx, pressure reactivity index (correlation between ICP and MAP); SAH, subarachnoid hemorrhage.
Logistic regression analysis
ULR results, including AUC and p values, for each of the IMPACT Core, IMPACT CT, and PRx variables can be found in Supplementary Appendix B, with results for both survival and dichotomized 6-month outcomes. Only the results for the entire recording period are reported here, but similar results were found when limiting analysis to the first 72 h of physiological data.
MLR analysis of the IMPACT Core models for alive/dead and favorable/unfavorable outcomes yielded an AUC of 0.707 (95% CI, 0.11–0.798; p < 0.0001) and 0.638 (95% CI, 0.561–0.713; p < 0.0001), respectively. The IMPACT Core + CT models for alive/dead and favorable/unfavorable outcomes yielded an AUC of 0.673 (95% CI, 0.567–0.773; p = 0.015) and 0.652 (95% CI, 0.570–0.732; p = 0.001), respectively.
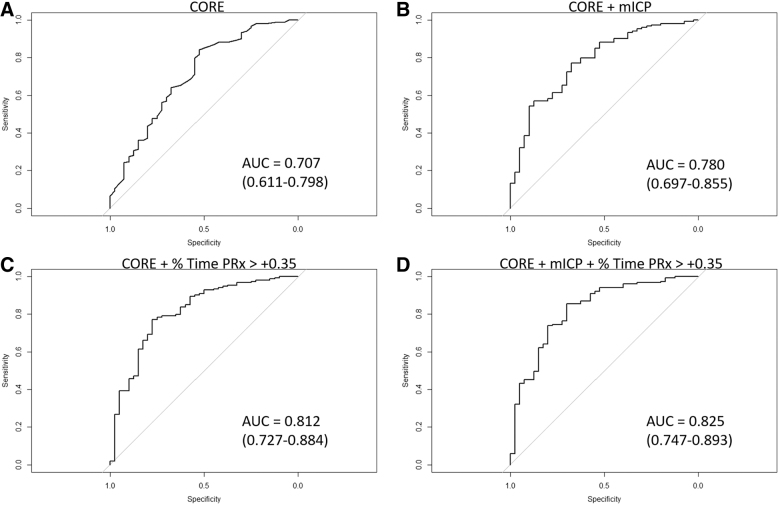
All the IMPACT Core “+” only model AUCs can be found in Table 2, whereas IMPACT Core + CT “+” model AUCs and p values can be seen in Supplementary Appendix C. The results demonstrate that the addition of % time spent with PRx over +0.25 or +0.35, in particular, to both the IMPACT Core and Core + CT, models led to superior AUC values, with lower AIC, for the alive/dead dichotomized outcome over baseline models. This is further exemplified when comparing IMPACT Core + ICP models to IMPACT Core + ICP + PRx variable models (Table 2), where the addition of % time above PRx thresholds of +0.25 (e.g., AUC 0.819, 95% CI 0.735–0.888, AIC 155.6 vs. AUC 0.780, 95% CI 0.697–0.855, AIC 164.9; in mean ICP models) and +0.35 (e.g., AUC 0.825, 95% CI 0.747–0.893, AIC 154.3 vs. AUC 0.780, 95% CI 0.697–0.855, AIC 164.9; in mean ICP models) led to improved AUCs and smaller AIC values. Figure 1 displays the receiver operating curves for the alive/dead dichotomized outcome for IMPACT Core and additional representative models.
Table 2.
Multi-Variable Logistic Regression Analysis: IMPACT Core Model Plus Cerebrovascular Reactivity
| Model | AUC A/D (95% CI) | AIC | p value | AUC F/U (95% CI) | AIC | p value |
|---|---|---|---|---|---|---|
| CORE | 0.707 (0.611–0.798) | 176.3 | <0.0001 | 0.638 (0.561–0.713) | 236.5 | <0.0001 |
| CORE + mean ICP | 0.780 (0.697–0.855) | 164.9 | <0.0001 | 0.651 (0.574–0.729) | 237.2 | 0.0001 |
| CORE + % time ICP >20 mm Hg | 0.811 (0.734–0.881) | 158.7 | <0.0001 | 0.647 (0.571–0.724) | 235.7 | 0.0001 |
| CORE + % time ICP >22 mm Hg | 0.811 (0.727–0.884) | 160.5 | <0.0001 | 0.648 (0.570–0.725) | 236.3 | 0.0001 |
| % time above PRx thresholds | ||||||
| CORE + % time PRx >0 | 0.781 (0.694–0.865) | 163.3 | <0.0001 | 0.654 (0.575–0.728) | 236.3 | 0.0002 |
| CORE + % time PRx >+0.25 | 0.803 (0.721–0.877) | 157.4 | <0.0001 | 0.661 (0.584–0.758) | 235.5 | 0.0001 |
| CORE + % time PRx >+0.35 | 0.812 (0.727–0.884) | 155.2 | <0.0001 | 0.661 (0.584–0.737) | 235.3 | 0.0001 |
| Mean ICP + % time above PRx thresholds | ||||||
| CORE + mean ICP + % time PRx >0 | 0.807 (0.724–0.883) | 158.7 | <0.0001 | 0.653 (0.575–0.729) | 237.6 | 0.0001 |
| CORE + mean ICP + % time PRx >+0.25 | 0.819 (0.735–0.888) | 155.6 | <0.0001 | 0.659 (0.582–0.731) | 237.1 | 0.0001 |
| CORE + mean ICP + % time PRx >+0.35 | 0.825 (0.747–0.893) | 154.3 | <0.0001 | 0.661 (0.583–0.733) | 237.0 | 0.0001 |
| % time ICP >20 mm Hg + % time above PRx thresholds | ||||||
| CORE + % time ICP >20 mm Hg + % time PRx >0 | 0.812 (0.729–0.883) | 154.9 | <0.0001 | 0.647 (0.570–0.730) | 236.4 | 0.0001 |
| CORE + % time ICP >20 mm Hg + % time PRx >+0.25 | 0.818 (0.744–0.893) | 152.6 | <0.0001 | 0.651 (0.566–0.730) | 236.1 | 0.0001 |
| CORE + % time ICP >20 mm Hg + % time PRx >+0.35 | 0.822 (0.742–0.890) | 151.6 | <0.0001 | 0.649 (0.571–0.721) | 236.1 | 0.0001 |
| % time ICP >22 mm Hg + % time above PRx thresholds | ||||||
| CORE + % time ICP >22 mm Hg + % time PRx >0 | 0.810 (0.726–0.885) | 156.8 | <0.0001 | 0.648 (0.570–0.723) | 237.1 | 0.0001 |
| CORE + % time ICP >22 mm Hg + % time PRx >+0.25 | 0.814 (0.735–0.886) | 154.4 | <0.0001 | 0.647 (0.570–0.720) | 236.7 | 0.0001 |
| CORE + % time ICP >22 mm Hg + % time PRx >+0.35 | 0.817 (0.736–0.889) | 153.3 | <0.0001 | 0.647 (0.570–0.720) | 236.6 | 0.0001 |
CORE model consisted of age, admission Glasgow Coma Scale motor score, and pupil response (normal bilaterally, unilateral unreactive, or bilaterally unreactive).
A/D, alive/dead; AIC, Akaike information criteria; AUC, area under the receiver operating curve; CPP, cerebral perfusion pressure; CI, confidence interval; F/U, favorable/unfavorable outcome (i.e., favorable = Glasgow Outcome Scale of 5–8; unfavorable = Glasgow Outcome Scale of 1–4); ICP, intracranial pressure; IMPACT, International Mission for Prognosis and Analysis of Clinical Trials; MAP, mean arterial pressure; PRx, pressure reactivity index (correlation between ICP and MAP).
FIG. 1.
ROC for alive/dead dichotomized outcome: IMPACT Core, Core + mean ICP, Core + % time PRx >+0.35, and Core + mean ICP + % time PRx >0.35. (A) IMPACT Core variables alone. (B) IMPACT Core + mean ICP. (C) IMPACT Core + % time with PRx >+0.35. (D) IMPACT Core + mean ICP + % time with PRx >+0.35. ICP, intracranial pressure; MAP, mean arterial pressure; mICP, mean ICP; PRx, pressure reactivity index (correlation between ICP and MAP); AUC, area under the curve; ROC, receiver operating curve.
However, for favorable/unfavorable dichotomized 6-month outcome, there was little to no change in the AUC and AIC for models containing PRx or ICP, above and beyond the IMPACT Core and Core + CT base models. This is in keeping with previous literature suggesting that ICP is a stronger predictor of mortality, compared to functional outcome.2,4,18,19
Additional explanation of outcome variance
All MLR models were then compared using Nagelkerke's pseudo-R2, assessing for additional account of variance in outcome association for both dichotomized outcomes. The results were similar for both the entire recording period and first 72 h of recording. The IMPACT Core + PRx models were initially compared to the IMPACT Core baseline model, demonstrating that the addition of % time above PRx of +0.25 and +0.35 provided statistically significant increases in accounted variance in outcome association over the IMPACT Core model alone (up to 19.3% for alive/dead outcome; p < 0.0001). This held true only for alive/dead dichotomized outcome. Similar results occurred for the IMPACT Core + CT models, where the addition of % time with PRx above +0.25 and +0.35 produced statistically significant increases in pseudo-R2 (up to 19.2% for alive/dead outcome; p < 0.0001).
Similarly, IMPACT Core + ICP + PRx models were compared to IMPACT Core + ICP models (i.e., IMPACT Core + mean ICP; IMPACT Core + % time with ICP >20 mm Hg; and % time with ICP >22 mm Hg), demonstrating statistically significant additional accounted variance in outcome association for the models with % time with PRx >+0.25 and >+0.35 (for alive/dead). Table 3 outlines the pseudo-R2 values in comparing various models for the alive/dead dichotomized outcome for the IMPACT Core only models. Supplementary Appendix D outlines the pseudo-R2 values comparing various IMPACT Core + CT models.
Table 3.
Added Variance in A/D Outcome Association at 6 Months with Cerebrovascular Reactivity Monitoring Over IMPACT Core Models
| CORE (n = 193) | Δ Nagelkerke's pseudo-R2 |
|
|---|---|---|
| CORE | ||
| + % time PRx >0 | 0.128 | |
| + % time PRx >+0.25 | 0.176 | |
| + % time PRx >+0.35 | 0.193 | |
| CORE (n = 193) | Δ Nagelkerke's pseudo-R2 |
|
|---|---|---|
| CORE + mean ICP | ||
| + mean ICP |
+ % time PRx >0 |
0.075 |
| + % time PRx >+0.25 |
0.104 |
|
| + % time PRx >+0.35 |
0.115 |
|
| CORE + % time ICP >20 mm Hg | ||
| + % time ICP >20 mm Hg |
+ % time PRx >0 |
NS |
| + % time PRx >+0.25 |
0.077 |
|
| + % time PRx >+0.35 |
0.086 |
|
| CORE + % time ICP >22 mm Hg | ||
| + % time ICP >22 mm Hg | + % time PRx >0 |
NS |
| + % time PRx >+0.25 |
0.076 |
|
| + % time PRx >+0.35 | 0.086 | |
CORE model consisted of age, admission Glasgow Coma Scale motor score, and pupil response (normal bilaterally, unilateral unreactive, or bilaterally unreactive). CT variables consisted of admission Marshall CT grade, presence of traumatic subarachnoid hemorrhage, and presence of extradural hematoma. All numbers reported for Nagelkerke's pseudo-R2 are statistically significant (i.e., p < 0.05) increases in accounted variance in outcome association over the CORE or CORE + ICP models.
A/D, alive/dead dichotomized outcome; CPP, cerebral perfusion pressure; CT, computed tomography; ICP, intracranial pressure; IMPACT, International Mission for Prognosis and Analysis of Clinical Trials; MAP, mean arterial pressure; NS, non-significant; PRx, pressure reactivity index (correlation between ICP and MAP).
However, despite the association with mortality, evaluating favorable/unfavorable dichotomized outcome displayed no significant differences between models when assessed using Nagelkerke's pseudo-R2, in keeping with the similar AUC and AIC values identified during MLR analysis, for both the Core and Core + CT models.
Discussion
Using the multi-center prospectively collected CENTER-TBI high-resolution ICU cohort, we have been able to demonstrate that metrics derived from cerebrovascular reactivity monitoring (PRx) maintain a strong association with mortality at 6 months, when adjusting for baseline admission characteristics (IMPACT Core and Core + CT variables) and ICP monitoring. These results were replicated using both the entire recording period and first 72 h of recording.
In particular, this is the first study to demonstrate the potential additional benefit to outcome association of cerebrovascular reactivity monitoring in TBI. The percentage of time spent over threshold for PRx of +0.25 and +0.35, when added to the IMPACT Core and Core + CT models, provided improved AUCs, lower AIC values, and statistically significant increases in accounted variance in outcome association for alive/dead dichotomized outcome, with up to 19.3% additional accounted variance in some cases. This provides strong evidence to support this type of monitoring in TBI patients, validating the strong associations with mortality that have been observed in previous large retrospective studies, where adjusting for baseline admission characteristics was not possible.1,11,17
We recognized that the improved mortality association achieved by incorporating PRx metrics may have simply represented the availability of data beyond initial presentation, thus providing information regarding disease evolution, rather than implying a specific biological impact of abnormal cerebrovascular reactivity. In order to test this question, first we asked whether PRx-derived data provided incremental improvements in explaining mortality outcome variance beyond that provided by a more conventional marker of abnormal physiology in this population—ICP. We found that the addition of PRx-derived data provided improved mortality association beyond that provided by incorporating ICP data alone. The AUCs observed for those models with % time PRx over +0.25 or +0.35 trended higher than those models that included both IMPACT Core variables and ICP, with lower AIC values. Second, we evaluated the first 72 h of monitoring, with results from this analysis confirming those from the entire monitoring period. These results confirm that PRx monitoring maintains its association with mortality when adjusting for baseline characteristics and ICP, potentially providing added value. However, given only 193 patients for the Core models and 166 patients for the Core + CT models in this study, these results do require further validation.
To confirm the added benefit of PRx monitoring above and beyond ICP monitoring in this cohort, we produced full models containing IMPACT Core/Core + CT and added ICP variables. We then tested the additional benefit of % time above PRx threshold to these models. The addition of PRx monitoring, through the % time above +0.25 and +0.35, produced improved AUCs and statistically significant relative increases in pseudo-R2 (up to 11.5% in some cases), indicating that the addition of PRx to ICP monitoring provides statistically significant added mortality association. These results are the first of their kind, highlighting the added benefit of PRx monitoring in moderate and severe TBI patients.
Finally, in keeping with the literature supporting that ICP is a stronger predictor of mortality (over functional outcome) in TBI,2,4,18,19 IMPACT Core and Core + CT models performed similar to those with ICP and PRx variables included, when evaluating the association with favorable/unfavorable dichotomized outcome. This was highlighted by the similar AUC and AIC values, with no significant difference on Nagelkerke's pseudo-R2 testing. This emphasizes the role of dysautoregulation (and the use of PRx) in associations with mortality. It is unknown whether other cerebrovascular reactivity indices would perform better for favorable/unfavorable outcome association when adjusting for IMPACT and ICP variables. Given the lack of strong association between impaired cerebrovascular reactivity and functional outcome identified in this study, we must acknowledge the results are disappointing. This carries potential implications for ongoing works in PRx/optimal cerebral perfusion pressure (CPPopt)–directed physiological targets, though the link between CPPopt and functional outcome has yet to be clearly identified, and is the focus of ongoing phase II studies.20 There is the possibility that the findings here may translate to a lack of association between CPPopt parameters and functional outcome, which one might argue may be more important than mortality as an outcome metric.
Past studies have demonstrated the outcome relevance of abnormal physiology as recorded by multi-modal monitoring, but their explanatory power has not been shown to be additional to that of well-established covariates, such as those included in the IMPACT models. The additional explanation of mortality variance that we demonstrate with PRx has implications for refined prognostication. However, the real aim of continuous monitoring of brain signals is not an outcome prediction, but timely and wisely reactions to a temporary crisis.
Limitations
Despite the interesting and reassuring results of the above analysis, some important limitations deserve attention.
First, despite this being a multi-center prospective data set, the overall patient numbers are relatively low at 193, with only 166 having full Core and CT IMPACT variables. The specific requirements for available data (i.e., presence of complete IMPACT Core/Core + CT variables, high-frequency digital physiologic signals from parenchymal ICP monitoring, and a recorded outcome at 6 months) limited our patient numbers to 193 and 166 for the Core and Core + CT cohorts, respectively. This was secondary to missing data for the admission CT characteristics. However, it must be acknowledged, despite the limited numbers, our results were statistically significant. As such, the ability to extrapolate the results of this study to other larger TBI populations may be limited; thus, future dedicated studies with this type of high-resolution data sets are needed to provide validation of these results.
Second, given that these data were collected as a multi-center, prospective, observational study, there exists the potential impact of patients, injury, and treatment heterogeneity on both the recorded physiological signals and patient outcomes. However, if anything, we would rather expect this additional heterogeneity to dilute the studied effects.
Third, the association with outcome was much stronger for alive/dead dichotomization, as opposed to favorable/unfavorable. This may be a function of the small patient numbers, or the fact that PRx reflects pressure reactivity more accurately in conditions of low cerebral compliance, associated with high ICP, which in turn is well known for its stronger association with mortality over morbidity.2,4,18,19 In further work, we will investigate whether other ICP-derived cerebrovascular reactivity indices, such as pulse amplitude index5 or RAC,6 which evaluate other facets of autoregulation and/or intracranial compliance, can provide explanation of variation in favorable/unfavorable outcome, when added to existing IMPACT variables. The main limitation of these indices is the need for high-frequency digital physiological waveforms for analysis of ICP pulse. This will be explored in future studies using the CENTER-TBI high-resolution ICU cohort.14
Fourth, given that this was a preliminary multi-center analysis of the association between cerebrovascular reactivity and outcome, adjusting for baseline characteristics, we are limited in our ability to comment on what exact period of monitoring after TBI displays the strongest association with mortality. Given that the goal was not to build prognostic models for use, but merely to explore whether the association between PRx and outcome was preserved when accounting for baseline admission characteristics, this was beyond the scope of this project. It is possible that specific periods of monitoring post-injury are stronger predictors of outcome. Such analysis, while controlling for baseline characteristics, would require extensive daily, or even high-resolution, analysis of outcome association. Given the current limitations with complete data sets, this is something we plan on exploring in the future using amalgamated data from CENTER-TBI and ongoing high-frequency physiology data collection schemes from partner institutions.
Fifth, evaluating PRx over the entire recording period and first 72 h may suggest that PRx is relatively stable over time. This is far from the case, given that we know that PRx fluctuates widely in the setting of moderate/severe TBI. Similarly evaluating PRx over such periods, one could argue that the impaired PRx values observed simply reflect the severity of primary injury and not a fluctuating, targetable parameter. We know that PRx varies over the course of ICU stay and between patients. Instead of using grand average data in the analysis, we utilized % time above thresholds in attempt to capture some of this variability over time. However, we must acknowledge that future work is required evaluating temporal response patterns of PRx over time. It remains unknown whether impaired cerebrovascular reactivity is a “targetable” physiological entity. To date, studies evaluating treatment impact on PRx have demonstrated little-to-no impact of current TBI therapies on cerebrovascular reactivity.21,22 With that said, one cannot completely rule out the potential for other, more novel therapeutic strategies for impaired cerebrovascular reactivity in TBI. This aspect is the ongoing work of various TBI research programs globally, integrating proteomic and genomics with high-resolution physiological data, with the goal of uncovering therapeutic targets for prevention and treatment of impaired reactivity.
Finally, on multi-variable analysis, the Core + CT models performed worse than the Core models, with lower absolute AUCs for the Core + CT models. The trend for Core + CT models performing slightly worse may be reflected in the smaller patient cohort (n = 166 vs. n = 193) for those with complete non-imputed Core + CT data. Future larger multi-center data sets with high-frequency physiological data will be required to definitively answer questions surrounding cerebrovascular reactivity monitoring and its role in TBI care.
Conclusion
PRx maintains its strong association with mortality in adult TBI when adjusting for baseline admission characteristics (IMPACT Core and CT variables) and ICP. The addition of cerebrovascular reactivity monitoring, through PRx, provides a statistically significant increase in mortality association at 6 months, when added to the IMPACT Core + ICP and Core + CT + ICP models. Our data suggest that cerebrovascular reactivity monitoring may provide complementary information regarding mortality association in TBI.
Supplementary Material
Acknowledgments
Data used in preparation of this article were obtained in the context of CENTER-TBI, a large collaborative project with the support of the European Union 7th Framework program (EC grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA), and from Integra LifeSciences Corporation (USA).
Contributor Information
Collaborators: and the CENTER-TBI High Resolution ICU (HR ICU) Sub-Study Participants and Investigators, Audny Anke, Ronny Beer, Raimund Helbok, Bo-Michael Bellander, David Nelson, Andras Buki, Giorgio Chevallard, Giuseppe Citerio, Arturo Chieregato, Endre Czeiter, Bart Depreitere, George Eapen, Shirin Frisvold, Stefan Jankowski, Daniel Kondziella, Lars-Owe Koskinen, Geert Meyfroidt, Kirsten Moeller, Anna Piippo-Karjalainen, Rahul Raj, Andreea Radoi, Juan Sahuquillo, Arminas Ragauskas, Saulius Rocka, Jonathan Rhodes, Rolf Rossaint, Ana Stevanovic, Oliver Sakowitz, Nina Sundström, Riikka Takala, Tomas Tamosuitis, Olli Tenovuo, Peter Vajkoczy, Alessia Vargiolu, Rimantas Vilcinis, Stefan Wolf, and Alexander Younsi
CENTER-TBI High Resolution Sub-Study Participants and Investigators
Audny Anke, Department of Physical Medicine and Rehabilitation, University Hospital Northern Norway; Ronny Beer, Raimund Helbok, Department of Neurology, Neurological Intensive Care Unit, Medical University of Innsbruck, Innsbruck, Austria; Bo-Michael Bellander, David Nelson, Department of Neurosurgery & Anesthesia & Intensive Care Medicine, Karolinska University Hospital, Stockholm, Sweden; Andras Buki, Department of Neurosurgery, University of Pecs and MTA-PTE Clinical Neuroscience MR Research Group and Janos Szentagothai Research Centre, University of Pecs, Hungarian Brain Research Program, Pecs, Hungary; Giorgio Chevallard, Arturo Chieregato, NeuroIntensive Care, Niguarda Hospital, Milan, Italy; Giuseppe Citerio, NeuroIntensive Care Unit, Department of Anesthesia & Intensive Care, ASST di Monza, Monza, Italy and School of Medicine and Surgery, Università Milano Bicocca, Milano, Italy; Endre Czeiter, Department of Neurosurgery, University of Pecs and MTA-PTE Clinical Neuroscience MR Research Group and Janos Szentagothai Research Centre, University of Pecs, Hungarian Brain Research Program (Grant No. KTIA 13 NAP-A-II/8), Pecs, Hungary; Bart Depreitere, Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium; George Eapen†; Shirin Frisvold, Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway; Stefan Jankowski, Neurointensive Care, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom; Daniel Kondziella, Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark; Lars-Owe Koskinen Department of Clinical Neuroscience, Neurosurgery, Umea University, Umea, Sweden; Geert Meyfroidt, Intensive Care Medicine, University Hospitals Leuven, Leuven, Belgium; Kirsten Moeller, Department Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark; Anna Piippo-Karjalainen, Rahul Raj, Helsinki University Central Hospital, Helsinki, Finland; Andreea Radoi, Juan Sahuquillo, Department of Neurosurgery, Vall d'Hebron University Hospital, Barcelona, Spain; Arminas Ragauskas, Saulius Rocka, Department of Neurosurgery, Kaunas University of Technology and Vilnius University, Vilnius, Lithuania; Jonathan Rhodes, Department of Anaesthesia, Critical Care & Pain Medicine NHS Lothian & University of Edinburg, Edinburgh, United Kingdom; Rolf Rossaint, Ana Stevanovic, Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany; Oliver Sakowitz, Klinik für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany and Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany; Nina Sundström, Department of Radiation Sciences, Biomedical Engineering, Umea University, Umea, Sweden; Riikka Takala, Perioperative Services, Intensive Care Medicine, and Pain Management, Turku University Central Hospital and University of Turku, Turku, Finland; Tomas Tamosuitis, Neuro-intensive Care Unit, Kaunas University of Health Sciences, Kaunas, Lithuania; Olli Tenovuo, Rehabilitation and Brain Trauma, Turku University Central Hospital and University of Turku, Turku, Finland; Peter Vajkoczy, Neurologie, Neurochirurgie und Psychiatrie, Charité–Universitätsmedizin Berlin, Berlin, Germany; Alessia Vargiolu, NeuroIntensive Care Unit, Department of Anesthesia & Intensive Care, ASST di Monza, Monza, Italy; Rimantas Vilcinis, Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania; Stefan Wolf, Interdisciplinary Neuro Intensive Care Unit, Charité–Universitätsmedizin Berlin, Berlin, Germany; and Alexander Younsi, Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany.
Funding Information
D.K.M. was also supported by funding from the National Institute for Health Research (NIHR, UK) through a Senior Investigator award and the Cambridge Biomedical Research Centre at the Cambridge University Hospitals NHS Foundation Trust. The study also received additional support from the NIHR Clinical Research network. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care, UK.
F.A.Z. receives research support from the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS), University of Manitoba Thorlakson Chair in Surgical Research Establishment Fund, the University of Manitoba VPRI Research Investment Fund (RIF), the University of Manitoba Rudy Falk Clinician-Scientist Professorship, and the Health Sciences Centre Foundation Winnipeg.
E.P.T. has received salary support from the Swedish Society of Medicine and Swedish Society for Medical Research.
Author Disclosure Statement
Data used in preparation of this article were obtained in the context of CENTER-TBI, a large collaborative project with the support of the European Union 7th Framework program (EC grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA), and from Integra LifeSciences Corporation (USA).
P.S. and M.C. receive part of licensing fees for the software ICM+ (Cambridge Enterprise Ltd, UK) used for data collection and analysis in this study.
D.K.M. has consultancy agreements and/or research collaborations with GlaxoSmithKline Ltd, Ornim Medical, Shire Medical Ltd, Calico Inc., Pfizer Ltd, Pressura Ltd, Glide Pharma Ltd, and NeuroTraumaSciences LLC.
M.C. is supported by the NIHR Cambridge Centre.
Deceased
References
- 1. Czosnyka M., Smielewski P., Kirkpatrick P., Laing R.J., Menon D., and Pickard J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41, 11–17; discussion, 17-19 [DOI] [PubMed] [Google Scholar]
- 2. Le Roux P., Menon D.K., Citerio G., Vespa P., Bader M.K., Brophy G., Diringer M.N., Stocchetti N., Videtta W., Armonda R., Badjatia N., Bösel J., Chesnut R., Chou S., Claassen J., Czosnyka M., De Georgia M., Figaji A., Fugate J., Helbok R., Horowitz D., Hutchinson P., Kumar M., McNett M., Miller C., Naidech A., Oddo M., Olson D., O'Phelan K., Provencio J.J., Puppo C., Riker R., Roberson C., Schmidt M., and Taccone F. (2014). The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit. Care 21, Suppl. 2, S282–S296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Czosnyka M., Miller C., and Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. (2014). Monitoring of cerebral autoregulation. Neurocrit. Care 21, Suppl. 2, S95–S10225208679 [Google Scholar]
- 4. Carney N., Totten A.M., O'Reilly C., Ullman J.S., Hawryluk G.W.J., Bell M.J., Bratton S.L., Chesnut R., Harris O.A., Kissoon N., Rubiano A.M., Shutter L., Tasker R.C., Vavilala M.S., Wilberger J., Wright D.W., and Ghajar J. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
- 5. Aries M.J.H., Czosnyka M., Budohoski K.P., Kolias A.G., Radolovich D.K., Lavinio A., Pickard J.D., and Smielewski P. (2012). Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit. Care 17, 67–76 [DOI] [PubMed] [Google Scholar]
- 6. Zeiler F.A., Donnelly J., Menon D., Smieleweski P., Hutchinson P.J., and Czosnyka M. (2018). A description of a new continuous physiologic index in TBI using the correlation between pulse amplitude of ICP and cerebral perfusion pressure. J. Neurotrauma. February 9. doi: 10.1089/neu.2017.5241. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Zeiler F.A., Donnelly J., Calviello L., Smielewski P., Menon D.K., and Czosnyka M. (2017). Pressure Autoregulation Measurement Techniques in Adult Traumatic Brain Injury, Part II: A Scoping Review of Continuous Methods. J. Neurotrauma 34, 3224–3237 [DOI] [PubMed] [Google Scholar]
- 8. Needham E., McFadyen C., Newcombe V., Synnot A.J., Czosnyka M., and Menon D. (2017). Cerebral perfusion pressure targets individualized to pressure-reactivity index in moderate to severe traumatic brain injury: a systematic review. J. Neurotrauma 34, 963–970 [DOI] [PubMed] [Google Scholar]
- 9. Zeiler F.A., Donnelly J., Calviello L., Menon D.K., Smielewski P., and Czosnyka M. (2017). Pressure autoregulation measurement techniques in adult traumatic brain injury, part I: a scoping review of intermittent/semi-intermittent methods. J. Neurotrauma 34, 3207–3223 [DOI] [PubMed] [Google Scholar]
- 10. Sorrentino E., Budohoski K.P., Kasprowicz M., Smielewski P., Matta B., Pickard J.D., and Czosnyka M. (2011). Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit. Care 14, 188–193 [DOI] [PubMed] [Google Scholar]
- 11. Sorrentino E., Diedler J., Kasprowicz M., Budohoski K.P., Haubrich C., Smielewski P., Outtrim J.G., Manktelow A., Hutchinson P.J., Pickard J.D., Menon D.K., and Czosnyka M. (2012). Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care 16, 258–266 [DOI] [PubMed] [Google Scholar]
- 12. Steyerberg E.W., Mushkudiani N., Perel P., Butcher I., Lu J., McHugh G.S., Murray G.D., Marmarou A., Roberts I., Habbema J.D.F., and Maas A.I.R. (2008). Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 5, e165; discussion, e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lingsma H., Andriessen T.M.J.C., Haitsema I., Horn J., van der Naalt J., Franschman G., Maas A.I.R., Vos P.E., and Steyerberg E.W. (2013). Prognosis in moderate and severe traumatic brain injury: external validation of the IMPACT models and the role of extracranial injuries. J. Trauma Acute Care Surg. 74, 639–646 [DOI] [PubMed] [Google Scholar]
- 14. Maas A.I.R., Menon D.K., Steyerberg E.W., Citerio G., Lecky F., Manley G.T., Hill S., Legrand V., and Sorgner A.; CENTER-TBI Participants and Investigators. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 [DOI] [PubMed] [Google Scholar]
- 15. Marshall L.F., Marshall S.B., Klauber M.R., Van Berkum Clark M., Eisenberg H., Jane J.A., Luerssen T.G., Marmarou A., and Foulkes M.A. (1992). The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 9, Suppl. 1, S287–S292 [PubMed] [Google Scholar]
- 16. Doiron D., Marcon Y., Fortier I., Burton P., and Ferretti V. (2017). Software Application Profile: Opal and Mica: open-source software solutions for epidemiological data management, harmonization and dissemination. Int. J. Epidemiol. 46, 1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeiler F.A., Donnelly J., Smieleweski P., Menon D., Hutchinson P.J., and Czosnyka M. (2018). Critical Thresholds of ICP Derived Continuous Cerebrovascular Reactivity Indices for outcome prediction in Non-Craniectomized TBI Patients: PRx, PAx and RAC. J. Neurotrauma 35, 1107–1115 [DOI] [PubMed] [Google Scholar]
- 18. Helbok R., Olson D.M., Le Roux P.D., and Vespa P.; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. (2014). Intracranial pressure and cerebral perfusion pressure monitoring in non-TBI patients: special considerations. Neurocrit. Care 21, Suppl. 2, S85–S94 [DOI] [PubMed] [Google Scholar]
- 19. Nourallah B., Zeiler F.A., Calviello L., Smielewski P., Czosnyka M., and Menon D.K. (2018). Critical thresholds for intracranial pressure vary over time in non-craniectomised traumatic brain injury patients. Acta Neurochir. (Wien) 160, 1315–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beqiri E., Smielewski P., Robba C., Czosnyka M., Cabeleira M.T., Tas J., Donnelly J., Outtrim J.G., Hutchinson P., Menon D., Meyfroidt G., Depreitere B., Aries M.J., and Ercole A. (2019). Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open 9, e030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donnelly J., Czosnyka M., Adams H., Cardim D., Kolias A.G., Zeiler F.A., Lavinio A., Aries M., Robba C., Smielewski P., Hutchinson P.J.A., Menon D.K., Pickard J.D., and Budohoski K.P. (2019). Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: a retrospective, single-center analysis. Neurosurgery 85, E75–E82 [DOI] [PubMed] [Google Scholar]
- 22. Zeiler F.A., Ercole A., Beqiri E., Cabeleira M., Aries M., Zoerle T., Carbonara M., Stocchetti N., Smieleweski P., Czosnyka M., and Menon D.K. (2019). Cerebrovascular reactivity is not associated with therapeutic intensity in adult traumatic brain injury: a CENTER-TBI analysis. Acta Neurochir. (Wien) 161, 1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.