Abstract
Introduction: The optimal management for patients with small, low-risk thyroid cancer is often debated. We aimed to characterize the attitudes and beliefs of providers and patients about management of small, low-risk thyroid cancer and how they relate to overtreatment.
Methods: We conducted 34 semi-structured interviews with surgeons (n = 12), endocrinologists (n = 12), and patients with <1.5 cm papillary thyroid cancer (n = 10). Interviews probed about diagnosis and treatment decision-making, including nonoperative options. We used thematic analysis to identify themes related to overtreatment and created concept diagrams to map observed relationships between themes.
Results: When providers discussed management of small, low-risk thyroid cancer, most felt that overtreatment was a problem, and some brought it up without prompting. Providers often believed that overtreatment results from overdiagnosis and relayed how the process commonly starts with incidental discovery of a thyroid nodule on imaging. Providers viewed biopsy of the nodule as a reflexive or habitual action. They ascribed inappropriate biopsy to lack of adherence to or knowledge of guidelines, radiologist recommendations, and the desire of patients and physicians to minimize diagnostic uncertainty. Providers described subsequent cancer diagnosis as an event that “opens Pandora's box” and often provokes a strong instinctive, culturally rooted need to proceed with surgery—specifically total thyroidectomy. Consequently, most providers felt that it is easier to prevent overdiagnosis than overtreatment and recommended strategies such as improving guideline adherence, resetting patients' expectations, and engaging the media. In contrast, patients did not bring up or openly discuss overtreatment or overdiagnosis. Some patients described the seemingly automatic process from an incidental finding to surgery. Their statements confirmed that the “need to know” was a major motivation for biopsying their nodule. Patients felt that once they had a cancer diagnosis, surgery was a foregone conclusion. Patients admitted their knowledge about thyroid nodules and cancer was low, leaving room for education about the need for biopsy and less extensive treatment options.
Conclusions: Surgeons' and endocrinologists' attitudes and beliefs about overtreatment focus on the automaticity of overdiagnosis. Both patients and providers are cognizant of the cascade of clinical events that propel patients from incidental discovery of a thyroid nodule to surgery.
Keywords: thyroid cancer, overdiagnosis, overtreatment, low-risk, qualitative
Introduction
Thyroid cancer is one of the fastest growing cancer diagnoses in the United States (1–3). Over the past 40 years, the incidence has increased more than threefold (4). A large proportion of this increase is thought to be due to overdiagnosis of small indolent cancers (5). Overdiagnosis is the detection of disease that would never become clinically significant or result in death (5). If undiscovered, many small, ≤1 cm differentiated thyroid cancers will remain asymptomatic and have little to no impact on a person's health, quality of life, or life expectancy (6–8). Moreover, even with the rapidly increasing incidence, the mortality rate for these small thyroid cancers has remained relatively unchanged (4).
When diagnosed, treatment of these small cancers can result in unnecessary surgery and medical care often termed overtreatment. Ultimately, overdiagnosis and overtreatment can have significant adverse effects on patients' quality life and put an undue economic burden on patients and the health care system (9). Overdiagnosis of small differentiated thyroid cancers can also put surgeons and endocrinologists in a difficult position. The patient carries a “cancer” diagnosis, although a variant that may never cause issues during their lifetime. Counseling such patients not to treat a diagnosed cancer can be difficult. Nonetheless, for some very low-risk cancers, surgery, in particular total thyroidectomy, may represent overtreatment. Total thyroidectomy exposes patients to the need for lifelong thyroid hormone replacement and risks of vocal fold paralysis, hypoparathyroidism, and decreased quality of life (10). Recognizing the avoidable risks of overtreatment, the most recent American Thyroid Association (ATA) guidelines endorsed active surveillance as a management option for these patients and recommended restricting surgical treatment to lobectomy (11).
To develop interventions that reduce the overdiagnosis, overtreatment, and the consequences thereof, it is necessary to understand the thought processes and attitudes of providers and patients about detection and treatment of these small thyroid cancers. This window into the overdiagnosis–overtreatment phenomenon provides critically important data to understand what drives overtreatment. The present qualitative study was designed to fill this gap by identifying patients' and physicians' attitudes and beliefs surrounding the diagnosis and treatment of small, low-risk thyroid cancers. We will use themes extracted from interviews to develop a conceptual framework of overtreatment that derives directly from the patient and treatment physician experience.
Materials and Methods
Study population
To capture a broad view of attitudes and beliefs, this qualitative study included participants from three populations: endocrinologists, surgeons, and patients with small, low-risk papillary thyroid cancers. To be eligible, endocrinologists and surgeons had to care for at least five patients with low-risk papillary thyroid cancer per year. This criterion was imposed to ensure some familiarity with diagnostic and treatment decisions. Patients were eligible if they had a preoperative diagnosis of a <1.5 cm papillary thyroid cancer, were node negative, and underwent surgery at the University of Wisconsin (UW) within the previous 5 years. We included patients with tumors up to 1.5 cm in size to capture the views of those who would be potentially eligible for nonsurgical management. All participants had to speak English. The UW Institutional Review Board approved this qualitative study (No. 16079), and participants provided written consent before being interviewed.
Recruitment
We recruited providers at a national conference (the 86th Annual ATA Meeting in Denver, CO) to increase the demographic, geographic, practice, and training diversity of participants. Providers on the conference program were recruited via e-mail before the meeting. Further recruitment was performed at the meeting using flyers placed in open conference areas and snowball sampling (i.e., interviewees were asked to give half-page flyers to other providers interested in participating). To identify eligible patients, we used the UW endocrine surgery database. Eligible patients received a mailed invitation to participate from their surgeon and a follow-up phone call from a study team member. All participants received a cash incentive for participating in the study.
Interview and data collection
Two members of the research team with advanced training in qualitative interviewing (MCS and EMW) performed the semi-structured interviews. All interviews were conducted in a private location or over video conferencing if the patient (n = 4) no longer lived in the area. For providers, all interviews were conducted at the national meeting and analyzed later. We planned follow-up interviews if necessary, but additional data collection was not needed. For patients, interviews were concluded when we achieved thematic saturation (12).
Interview guides included open-ended questions and one case-based clinical vignette. Development was iterative and included input from stakeholders consisting of survivors of thyroid cancer, their families, endocrinologists, surgeons, a radiologist, and social scientists with qualitative research expertise. A separate, but complementary guide was developed for each group (endocrinologists, surgeons, and patients). We revised guides as needed to maximize subsequent interviews. Example questions included:
Providers (for both endocrinologists and surgeons)
What management options would you discuss with a 50-year-old female referred to you with a 0.8 cm nodule in the right lobe that is positive for papillary thyroid cancer on fine needle aspiration (FNA)?
In your opinion, what does overtreatment look like in [patients with small, low-risk thyroid cancers?]
Patients
What was your reaction when you first heard your diagnosis?
What treatment options did you consider based on what you learned before you saw your surgeon?
We did not bring up the terms overdiagnosis and overtreatment with patients to avoid psychological harm. Rather, we described a real story from the news about a woman who chose active surveillance for her small, low-risk thyroid cancer. We then elicited patients' thoughts about this nonoperative option and whether they would have considered choosing it for themselves.
Analysis
Interviews were transcribed verbatim, with personal identifiers removed from transcripts before analysis. We used thematic analysis to evaluate interview transcripts and NVivo 11 (QSR International, Melbourne, Australia) to catalog the coding scheme (13). Team members with backgrounds in sociology, thyroid surgery, and population health analyzed a balanced subset of interview transcripts (providers = 3; patients = 3) using an inductive strategy and open coding to identify emergent themes. We used constant comparison to refine the coding taxonomy and resolved disagreements between coders by group consensus. We analyzed all provider interviews, although thematic saturation was reached after coding ∼18 interviews.
We used memo writing to focus and develop emerging themes (14). For higher level analysis, we used concept diagrams to map proposed relationships between overdiagnosis and overtreatment. Discussion of the codes and data often inspired ideas about overarching themes and broader processes. Continual refinement of concept diagrams and integration of memos progressed until the data were adequately described.
Results
We conducted 34 semi-structured interviews (median length 60 minutes, range 30–120 minutes) between September 2016 and April 2017 to identify providers' and patients' attitudes and beliefs about diagnosis and treatment of small, low-risk thyroid cancers. Participants included 12 endocrinologists and 12 surgeons, as well as 10 patients with small, low-risk papillary thyroid cancer. The providers were from all regions of the United States and were 72% male, 88% white, 88% in academic practice, and had a median age of 43 years (range 34–68 years). Most (68%) saw at least 10 patients annually with thyroid cancer measuring ≤1 cm. The patients were 80% female, 90% white, 90% underwent total thyroidectomy, and their median age was 48 years (interquartile range 21–77 years). The demographics of all participants are shown in Table 1.
Table 1.
Demographic Data
| n (%) | |
|---|---|
| Providers | |
| Age, median (range) | 43 (34–68) |
| Female | 7 (29) |
| Caucasian | 21 (88) |
| Academic practice | 21 (88) |
| Location | |
| East/Northeast | 10 (42) |
| South | 4 (17) |
| Midwest | 8 (33) |
| West | 2 (8) |
| Treat >10 PTMC/year | 16 (68) |
| >50% of endocrinology practice is thyroid cancer | 6 (50) |
| >20 Thyroid surgeries/year | 12 (100) |
| Member of the ATA | 24 (100) |
| Read all of 2015 ATA guidelines | 16 (68) |
| Read at least part of 2015 ATA guidelines | 33 (92) |
| Patients | |
| Age, median (range) | 48 (21–77) |
| Female | 8 (80) |
| Caucasian | 9 (90) |
| Currently employed | 8 (80) |
| Education | |
| High school | 1 (10) |
| Some college/associates degree | 4 (40) |
| College degree | 3 (30) |
| Postgraduate degree | 2 (20) |
| Underwent total thyroidectomy | 9 (90) |
ATA, American Thyroid Association; PTMC, papillary microcarcinoma.
When discussing diagnosis and treatment for a small, low-risk thyroid cancer, themes emerged among providers and patients in four key areas: overdiagnosis, overtreatment, treatment decision-making, and strategies to reduce overdiagnosis and overtreatment. The results are organized according to these overarching themes and integrate provider and patient data for each. Analysis revealed that both provider specialties had significant variability with respect to their attitudes and beliefs. Therefore, we present the results of “providers” as a group and identify the specialty of providers for quotes and any areas where discrepancies emerged.
Overdiagnosis
While providers were not prompted to discuss overdiagnosis, many believed that overdiagnosis fuels overtreatment and discussed it without prompt. Providers made statements such as “overdiagnosis is to blame more than overtreatment” (Surgeon 9). Both groups of providers emphasized biopsy as a critical point for intervention. In contrast, patients did not use the terms “overdiagnosis” but described a seemingly automatic process that led them from discovery of the nodule to biopsy to diagnosis and then surgery.
Incidental discovery of thyroid nodules
Many providers believed that overdiagnosis starts with the incidental discovery of a thyroid nodule on cross-sectional imaging (e.g., computed tomography [CT], magnetic resonance imaging) performed for an unrelated reason. Discovery of the incidental nodule resulted in a predictable cascade of events ultimately leading to surgery and overtreatment (Fig. 1). Endocrinologist 1 described overdiagnosis as “biopsying a sub-centimeter nodule on a routine basis” and followed that “we are inviting overtreatment because it's opening that whole door.” Endocrinologists were more likely than surgeons (6:1) to state that they would not have biopsied a subcentimeter nodule in the first place. Providers described feeling trapped or “left in a situation” by incidental findings that forced them to proceed with further diagnostic testing. For example, Endocrinologist 11 acknowledged that “If a patient has a car accident, they go for a CT scan and you see small tiny nodules [in the thyroid], you're stuck. Now you have to do an ultrasound, and then you would have to decide to do an FNA or not to do an FNA.”
FIG. 1.
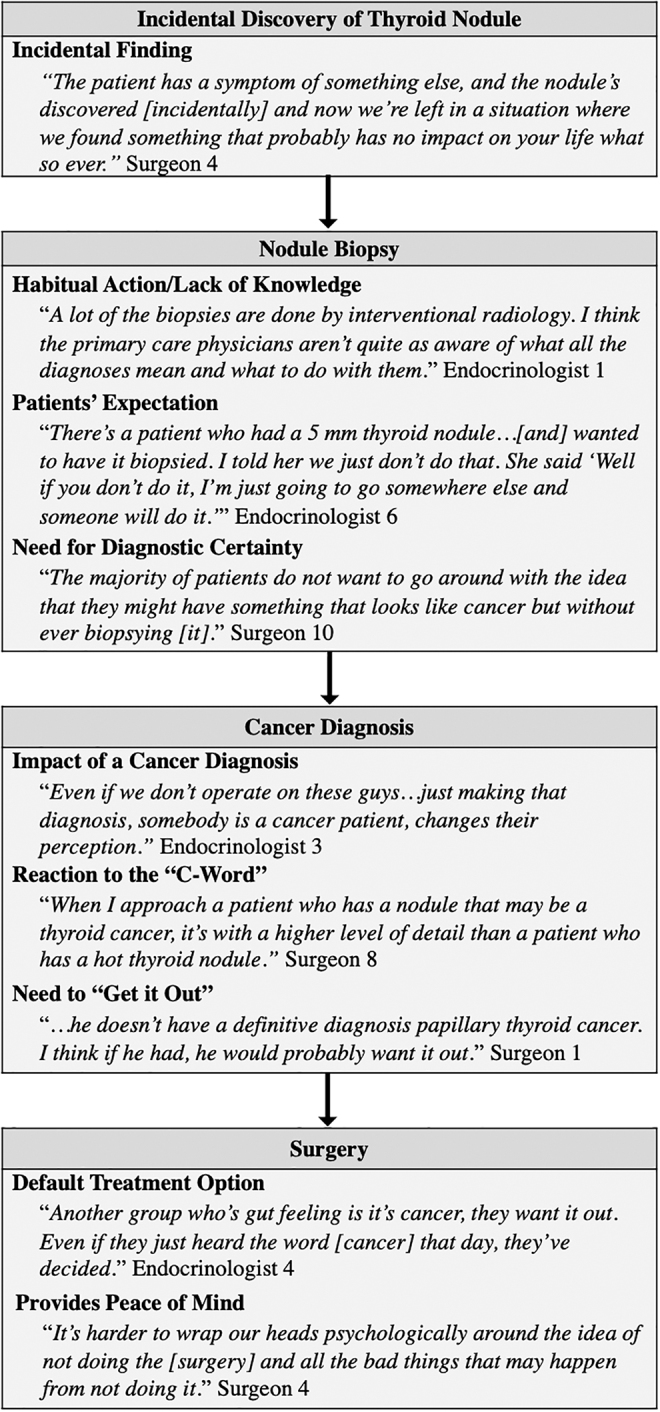
Provider exemplary quotes describing the clinical pathway leading to overdiagnosis and overtreatment.
Patients and providers similarly expressed that the process from diagnosis to treatment seemed automatic and inevitable once the incidental nodule was identified. Many patients confirmed that their cancers were found when doctors were evaluating something else (Fig. 2). Patient 2 (24 year-old female) recalled, “It was actually an incidental finding. I was having these really bad, debilitating headaches … so they wanted me to get a CT. The CT turned out fine, but they saw nodules on my thyroid.”
FIG. 2.
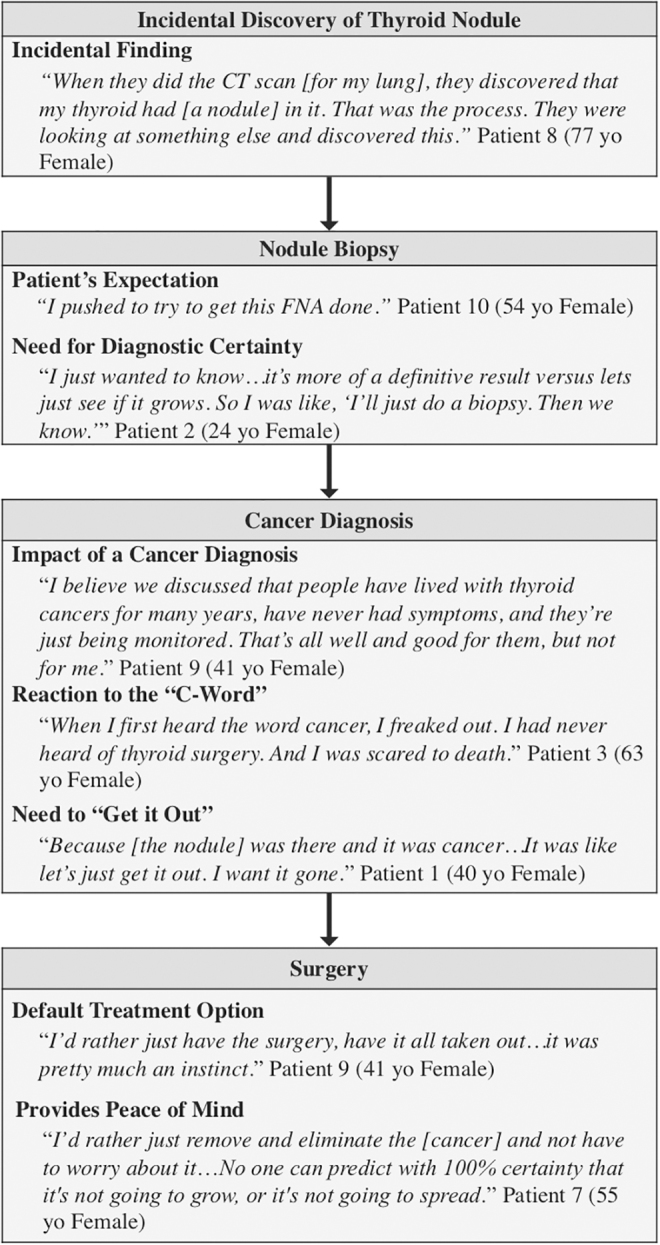
Patient exemplary quotes describing the clinical process from incidental discovery of a thyroid nodule to surgery.
Biopsy as a habit and expectation
While providers identified incidental discovery of a nodule as the catalyst for overdiagnosis, they described the subsequent biopsy as a habitual action (Fig. 1). Surgeon 10 explained, “If I see a small nodule on an ultrasound, that looks suspicious, what am I going to do? Not biopsy it? The patient wants to know, and they trust me. I can biopsy it.” Providers ascribed the reflex to biopsy to lack of adherence to or knowledge of guidelines, recommendations by radiology, and the desire of patients and physicians to minimize diagnostic uncertainty. They also acknowledged that patients expect a biopsy regardless of how the nodule was discovered. Some providers felt that radiology reports create “patient hysteria” because patients worry the nodule is cancer. Endocrinologist 1 said, “… the ultrasound [report] says things like, ‘Recommend FNA biopsy.’ Then patients come in expecting that they're going to have a biopsy … [and they're] super anxious about this nodule until they get a biopsy.”
The patients also discussed how they expected a biopsy of their nodule and acknowledged that, in some cases, their physician ordered the biopsy without their input. Patient 10 (54 years old female) stated, “I pushed to try to get this FNA done … because just the way they said, ‘It probably isn't cancer’ … that weighs on your mind.” The “need to know” and discomfort with the potential for the nodule to be cancerous also drove patients to want a biopsy. Patient 2 (24 years old female) explained that s/he was “wanting a more definitive result versus waiting to see if it grows … If it's growing, it could be spreading.”
Overtreatment
When discussing diagnosis and treatment of small, low-risk thyroid cancer, some providers brought up overtreatment unprompted. However, the significant majority of providers expressed concern about overtreatment. They also relayed the difficulties in not treating a diagnosed cancer. When asked to describe overtreatment, most providers equated overtreatment to “over-operating,” while others felt that overtreatment consisted of any intervention that did not provide some benefit to the patient. Surgeon 9 stated, “we're not really improving people's life expectancies by operating on these little things” and further elaborated that for some patients “It's really hard on them … We have changed their life.”
Although most providers were concerned about overtreatment, a subset of providers did not view surgery as overtreatment. These providers discussed overdiagnosis, but felt that once cancer is confirmed, total thyroidectomy is a reasonable treatment option. Endocrinologist 5 stated, “Once you've made the diagnosis, I don't think you can say that treatment, the surgical treatment, is overtreatment.” Overall, we did not observe systematic differences between endocrinologists and surgeons with regard to their views on management of these patients or overtreatment.
Treatment decision-making
Impact of cancer diagnosis
While providers discussed the incidental discovery and seemingly inevitable biopsy of a thyroid nodule as initiating events leading to overtreatment, they also discussed several factors that affect the decision to proceed with surgery (Fig. 1). Providers described the cancer diagnosis as “letting the cat out of the bag” and “opening Pandora's box.” Endocrinologist 3 said, “It's insane the way that [the diagnosis] changes behavior. The same 5 mm nodule that you would yell at the primary care doctor for ordering the ultrasound and tell the patient not to even biopsy … is now cancer, and the patient has no choice but to go to surgery. That's intellectual craziness. And we're being driven by the biopsy.”
Reaction to the “C word”—“Get it out”
Providers acknowledged that a cancer diagnosis provokes a strong instinctive and culturally rooted need to proceed with active treatment, specifically surgery. The majority of providers discussed the strongly held notion that you cannot “treat cancer by watching.” Surgeon 1 explained, “The biggest barrier is my own anxiety. I think that anxiety is matched by my patients … It comes from being in a culture where cancer is bad and needs to get out.” Overall, providers felt that, for most patients, the concept of cancer is “very scary” and “they just want it out.” Some providers recognized that not all patients experience fear and anxiety after their diagnosis. “Patients are very different in how they react to it. Some have already done a lot of reading and know that it's no big deal. Others are devastated by the word cancer” (Endocrinologist 4).
Patients also discussed a strong negative reaction to the “C word” (Fig. 2). For most patients, “just the word cancer” activated a gut reaction to “get it out.” Patient 7 (55 years old female) felt that “No one can predict with 100% certainty that it's not going to grow or spread. That's why I lean more towards, let's just get it out.” However, some patients recognized that their cancer was a “very slow growing cancer.” Patients who understood the relatively benign biology of thyroid cancer recognized that “if [the cancer] wasn't found for another year, it probably wouldn't have made a difference” (Patient 10, 54 years old female).
Surgery provides peace of mind
Providers explained how surgery provides an emotional benefit by removing the cancer (Fig. 1). Surgeon 5 stated, “The primary driving factor for all of these patients, anybody who has cancer … or an indeterminate biopsy … they feel relieved to have it over with. Their primary concern is to have the … potential danger removed.” However, Endocrinologist 3 discussed that not all patients receive the anticipated emotional benefit. “I used to think if I took all of those [patients] to surgery I would help them. Nah, I take them to surgery, and they are still worried.”
Patients echoed the sentiment that surgery puts their “mind at ease” (Fig. 2). In general, patients felt that “there was some comfort in getting [surgery] done so that the thyroid is out, and you don't have to worry about it” (Patient 8, 77 years old female).
Surgery as an automatic next step for cancer treatment
While fear and anxiety evoked by the “C word” clearly played a role in actively treating small thyroid cancers with surgery, so did the assumption that surgery is the default treatment (Fig. 1). Surgeon 6 described, “It's definitely difficult not to do something. I think that's in our culture. We want to fix things.”
Patients also held the belief that surgery was the default (Fig. 2). Some patients discussed how the referral process reinforced the idea that surgery was the only option. Patient 1 (40 years old female) explained, “I had the biopsy … it [showed the nodule] was cancer. They set up an appointment with the surgeon … I just assumed surgery was the option.” Patient 4 (25 years old female) bluntly described the process as, “biopsy, you have thyroid cancer, get your thyroid out.”
Strategies to reduce overdiagnosis and overtreatment
Providers suggested several strategies to prevent overdiagnosis and overtreatment including education of patients and physicians to promote adherence to guidelines, working in multidisciplinary teams, re-naming of low-risk malignancies, changing reimbursement models, and resetting patients' expectations by reframing the diagnostic treatment paradigm and engaging mass media (Table 2). Surgeon 6 also noted that “There needs to be a cultural change to thinking that we could be harming [patients] more by doing the surgery.”
Table 2.
Strategies Suggested by Providers to Prevent Overdiagnosis and Overtreatment
| Strategy | Exemplary quote(s) |
|---|---|
| Provider-Focused and Patient-Focused Education | “… Education, both at the patient level and the provider level. With the surgeons and the endocrinologists through continuing education.” Surgeon 3 |
| Adherence to Guidelines | “First of all, we need people to follow the guidelines … don't biopsy things smaller than a centimeter. I think that will prevent a lot of the overdiagnosis.” Endocrinologist 9 |
| Multidisciplinary Approach to Diagnosis and Treatment | “There are radiology guidelines, there are ATA guidelines, there will be AAES guidelines … There are lots of different people having opinions, so having a multidisciplinary approach … is important.” Surgeon 6 |
| Re-classification as Low-Risk Malignancy | “Number one you gotta stop making a diagnosis. Number two is finding a better name for this. I understand it's a cancer from the biology standpoint. But it is not a cancer the way the U.S. uses this word.” Endocrinologist 3 |
| Changing Reimbursement Models | “Eventually what has to happen … is that insurance carriers, Medicare, etc. need to stop reimbursing for biopsies done on asymptomatic patients with nodules less than 1 cm.” Surgeon 4 |
| Resetting Patient Expectations | “I think if I can offer [patients] a really clear recommendation … this [nodule] should not be biopsied … biopsying it is not going to improve your quality of life.” Surgeon 9 |
| Engaging the Mass Media | “I mean people pay attention to social media. They pay attention to the news. We can get the message out there—if we carefully word it.” Endocrinologist 8 |
AAES, American Association of Endocrine Surgeons.
Some providers felt that engaging the media could play a role in patient education and changing peoples' beliefs about cancer. Endocrinologist 1 acknowledged, “People read about cancers in general and that colors their decision-making … but not all cancers are the same, and many patients don't understand the differences.” Providers also recommended resetting patients' expectations by putting patient-oriented education on the ATA, ThyCa, Inc., and American Association of Endocrine Surgeons (AAES) websites, such as “what [they] should expect, the questions to ask, and the things your doctor should be talking to you about” (Surgeon 3).
Patients indicated openness to receiving such education and information about their disease and management options. They expressed significant trust in their providers and willingness to consider nonsurgical management options if recommended. Patient 6 (65 years old male) said, “I guess in a lot of ways, I would rely on the particular doctor that I'd feel comfortable with, would help me make that [treatment] decision.”
Discussion
This study explored patients' and providers' attitudes and beliefs about diagnosis and treatment of small, low-risk thyroid cancer. Endocrinologists and surgeons believed that overtreatment results directly from overdiagnosis. Patients and providers described the cascade from incidental diagnosis of a thyroid nodule to surgical intervention as automatic. Participants felt that once a thyroid nodule is discovered, biopsy is habitual and expected. They acknowledged how diagnosis of the “C word” elicits fear and anxiety that in turn provokes a gut instinct to “get it out.” Patients and providers also believed that surgery is the default treatment and provides peace of mind by removing the cancer. Together, these findings reveal that habits, beliefs (about cancer), and default thinking all play a role in overdiagnosis and overtreatment of low-risk thyroid cancer. These areas provide targets for educational and behavioral interventions at the system, provider, and patient levels to reduce overdiagnosis and overtreatment.
An unexpected finding of this study was the role of habit as a major driver of the overdiagnosis–overtreatment paradigm. In the cascade from incidental finding of a thyroid nodule to surgery, habit describes the process by which a stimulus—identification of a thyroid nodule—automatically generates an impulse toward action—biopsy (15). The “nodule–biopsy” relationship is likely a learned stimulus–response association that is cultivated over time as providers go through training and on to practice. A study from Australia by Nickel et al. also described biopsy as an “almost automatic recommendation” from clinicians after identification of a thyroid nodule (16). Thus, as study participants noted, intervening by increasing the threshold for biopsy outlined in the 2015 ATA guidelines and the American College of Radiology Thyroid Imaging Reporting and Data System (TI-RADS™) is a critical first step in addressing the “nodule–biopsy” habit (10,17). However, habits can be hard to change and are a well-described barrier to guideline adoption (18–21). Therefore, further educational and behavioral interventions will likely be necessary to changing providers' habits. These interventions could include changes in reimbursement, “hard stops” in electronic ordering, quick and free access to guidelines (through pocket cards or apps), and physician feedback with score cards. Engaging other national organizations of primary care providers and/or radiologists to further disseminate guidelines may also be helpful.
Another key finding of this study is the role of patients' and providers' beliefs about cancer in driving the processes from overdiagnosis to overtreatment. While providers commonly acknowledged the role of cancer fear and anxiety in decision-making by patients, they also discussed their own fears and biases. Meanwhile, patients described their seemingly automatic, emotional reactions in response to finding a thyroid nodule or being diagnosed with the “C word.” This reaction evoked a need to proceed with biopsy or surgery and “get it out.” Nobel Laureate and decision psychologist Daniel Kahneman describes these automatic thoughts as fast and intuitive but warns that impulses, judgements, and decisions that result from these reactions are prone to bias, errors, or other cognitive illusions (22). Studies have shown that patients who report higher levels of anxiety following a diagnosis of cancer are more likely to choose a surgical treatment option (23,24). Intervening by changing the name and removing the term “cancer” encourages both patients and providers to pursue less invasive treatment options (25). While the thyroid cancer community has already intervened by introducing NIFTP (noninvasive follicular thyroid neoplasm with papillary-like nuclear features), additional interventions will likely be needed to address cancer fear and associated beliefs. Such interventions may include slowing down the process of treatment decision-making or using decision aids that improve knowledge. A widespread educational media campaign about the indolent nature of low-risk thyroid cancer may also be an avenue to reduce overdiagnosis and overtreatment and was suggested by the providers interviewed.
This qualitative analysis also reveals that default thinking likely contributes to overtreatment. While similar to habit and so-called “fast thinking,” default thoughts represent simplified streamlined mental process whereby a person automatically selects the “default” option (i.e., biopsy or total thyroidectomy), unless a viable alternative is specified (22). The power of default thinking, particularly with respect to changing clinical practice and guideline adoption, comes from the tendency for people to leverage solutions from the past when they are faced with problems or challenges (26). Thus, as we observed, patients' and providers' defaults with respect to cancer diagnosis is active treatment with surgery, more specifically total thyroidectomy. Cultural norms certainly perpetuate the assumption that all cancer is bad and needs to be treated with surgery (27). Future educational efforts like a large media campaign aimed at the general public need to promote a cultural shift toward recognizing the indolent nature of low-risk thyroid cancer and the negative impact of overdiagnosis on overall quality of life. Other educational efforts may include promoting thyroid cancer awareness through schools and improving online resources about thyroid cancer to provide accurate patient-centered information.
To the best of our knowledge, this study is the first to qualitatively synthesize the attitudes and beliefs of patients with very low-risk thyroid cancer together with those of providers when examining treatment decisions and overtreatment. While combining the findings from both patients and providers is a strength, it is also a limitation. There are other limitations, such as selection bias, that should be considered as well. For one, interviews with providers were completed at a national conference within a year of the release of the 2015 ATA and included a pool of thyroidologists and high-volume thyroid surgeons. While this strategy captured geographic and racial/ethnic diversity as well as a broad scope of clinical and surgical practices, providers' attitudes and beliefs may change over time. Providers who do not attend the ATA meetings or have lower volume practices may describe differing experiences. In terms of patients, the sample size was small and participants were recruited from a single academic medical institution with high-volume surgeons, which may affect the experience with regard to the surgery itself. In addition, most patients were Caucasian. It is possible that patients with other backgrounds or in other settings may have different attitudes or beliefs. Our patient participants did report similar experiences to those in other well-developed countries (16). Another limitation is the potential for recall bias. To minimize this limitation, we recruited patients who had surgery in the past five years. Finally, the background and theoretical orientation of the research team may have impacted results if participants shared only experiences and thoughts that they felt researchers wanted to hear.
To conclude, in this study, providers who care for patients with small, low-risk thyroid cancers and patients themselves describe an established clinical pathway that leads from incidental discovery of a thyroid nodule to surgery. Providers suggest that overdiagnosis of thyroid nodules predisposes patients to overtreatment by triggering this cascade of clinical events. The present findings reveal that provider habits, patient reactions to the “C word,” and default thinking all play a role in overdiagnosis and overtreatment of small, low-risk thyroid cancers. These findings can be used to guide development of future educational and behavior interventions designed for clinicians as well as patients with the ultimate goal of mitigating the negative impact of both overdiagnosis and overtreatment. Multiple interventions are possible and will need to be studied.
Acknowledgments
We would like to acknowledge Elizabeth M. Wendt, MPH, for performing interviews and Linn Jennings, MS, Huda J. Khokhar, BS, and Esra Alagoz, PhD, for their assistance coding.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Support for this research included the American Association of Endocrine Surgeons Paul LoGerfo Research Award, the National Cancer Institute of the National Institutes of Health award number K08CA230204, and the National Institutes of Health award number T35DK062709. The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, Ogilvie JB, Terris DJ, Netterville J, Wong RJ, Randolph G; AACE Endocrine Surgery Scientific Committee. 2015. American Association of Clinical Endocrinologists and American College of Endocrinology Disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract 21:686–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies L, Welch HG. 2014. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322 [DOI] [PubMed] [Google Scholar]
- 3. Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2006. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 4. SEER cancer statistics factsheets: thyroid cancer. National Cancer Institute, Bethesda, MD. Available at http://seer.cancer.gov/statfacts/html/thyro.html (accessed June4, 2019)
- 5. Welch GH. 2017. Cancer screening, overdiagnosis, and regulatory capture. JAMA Intern Med 177:915–916 [DOI] [PubMed] [Google Scholar]
- 6. Takano T. 2017. Natural history of thyroid cancer [review]. Endocr J 64:237–244 [DOI] [PubMed] [Google Scholar]
- 7. Welch HG, Doherty GM. 2018. Saving thyroids—overtreatment of small papillary cancers. N Engl J Med 379:310–312 [DOI] [PubMed] [Google Scholar]
- 8. Applewhite MK, James BC, Kaplan SP, Angelos P, Kaplan EL, Grogan RH, Aschebrook-Kilfoy B. 2016. Quality of life in thyroid cancer is similar to that of other cancers with worse survival. World J Surg 40:551–561 [DOI] [PubMed] [Google Scholar]
- 9. Ramsey S, Blough D, Kirchhoff A, Kreizenbeck K, Fedorenko C, Snell K, Newcomb P, Hollingworth W, Overstreet K. 2013. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 32:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang TS, Goffredo P, Sosa JA, Roman SA. 2014. Papillary thyroid microcarcinoma: an over-treated malignancy? World J Surg 38:2297–2303 [DOI] [PubMed] [Google Scholar]
- 11. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guest G, Bunce A, Johnson L. 2006. How many interviews are enough? An experiment with data saturation and variability. Field Method 18:59–82 [Google Scholar]
- 13. Hsieh H, Shannon S. 2005. Three approaches to qualitative content analysis. Qual Health Res 15:1277–1288 [DOI] [PubMed] [Google Scholar]
- 14. Charmaz K 2006 Memo-Writing. Constructing Grounded Theory. Sage Publishing, Thousand Oaks, CA, pp 72–94
- 15. Wood W, Neal DT. 2009. The habitual consumer. J Consum Psychol 19:579–592 [Google Scholar]
- 16. Nickel B, Brito JP, Moynihan R, Barratt A, Jordan S, McCaffery K. 2018. Patients' experiences of diagnosis and management of papillary thyroid microcarcinoma: a qualitative study. BMC Cancer 18:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, Hammers LW, Hamper UM, Langer JE, Reading CC, Scoutt LM, Stavros AT. 2017. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol 14:587–595 [DOI] [PubMed] [Google Scholar]
- 18. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. 1999. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 282:1458–1465 [DOI] [PubMed] [Google Scholar]
- 19. Davis DA, Taylor-Vaisey A. 1997. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ 157:408–416 [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. 2016. Barriers and strategies in guideline implementation—a scoping review. Healthcare (Basel) 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wood W, Quinn JM, Kashy DA. 2002. Habits in everyday life: thought, emotion, and action. J Pers Soc Psychol 83:1281–1297 [PubMed] [Google Scholar]
- 22. Kahneman D. 2011. Thinking, Fast and Slow. Farrar, Straus and Giroux, New York [Google Scholar]
- 23. Heath I. 2014. Role of fear in overdiagnosis and overtreatment—an essay by Iona Heath. BMJ 349:g6123. [DOI] [PubMed] [Google Scholar]
- 24. Volk RJ, McFall SL, Cantor SB, Byrd TL, Le YC, Kuban DA, Mullen PD. 2014. “It's not like you just had a heart attack”: decision-making about active surveillance by men with localized prostate cancer. Psychooncology 23:467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nickel B, Barratt A, McGeechan K, Brito JP, Moynihan R, Howard K, McCaffery K. 2018. Effect of a change in papillary thyroid cancer terminology on anxiety levels and treatment preferences: a randomized crossover trial. JAMA Otolaryngol Head Neck Surg 144:867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elliot S, Young T. 2016. Nature by default in early childhood education for sustainability. Aust J Environ Educ 32:57–64 [Google Scholar]
- 27. Vrinten C, McGregor LM, Heinrich M, von Wagner C, Waller J, Wardle J, Black GB. 2017. What do people fear about cancer? A systematic review and meta-synthesis of cancer fears in the general population. Psychooncology 26:1070–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]


