Abstract
Infection with human immunodeficiency virus (HIV) is associated with substantially increased incidence of non-Hodgkin lymphoma (NHL). This risk may be driven, in part, by reduced immune control over viral infections in the setting of acquired immunodeficiency syndrome (AIDS), although the lymphomagenic mechanisms are not yet established. We used bead-based multiplex assays to measure antibody seroreactivity to 32 viral antigens representing 22 different viral infections (human herpesviruses 1–8, hepatitis B and C virus, human T-lymphotropic virus type-1, and human polyomaviruses) in two prospective HIV cohorts. Incident (n = 28) and prevalent (n = 38) AIDS-related NHL cases were matched by age, sex, race, and CD4 count to 67 HIV-positive control individuals without AIDS-NHL. Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations of AIDS-NHL with the number of different viruses to which an individual was seropositive and seroreactivity to individual antigens. Seropositivity to an increasing number of viruses was inversely associated with AIDS-NHL (OR per virus = 0.84, 95% CI = 0.72–0.98). Seroreactivity to herpes simplex virus 2 2mgG unique antigen (OR = 0.47; 95% CI = 0.23–0.97) and to WU polyomavirus viral capsid protein (OR = 0.26, 95% CI = 0.10–0.65) was significantly lower in AIDS-NHL cases compared to controls. In this evaluation of antibodies to multiple viruses, we observed an inverse association between seropositivity to a larger number of viruses and AIDS-NHL. While in need of further evaluation, our data raise the novel hypothesis that insufficient exposures or impaired humoral immune responses to viral infections may be associated with AIDS-related lymphomagenesis.
Keywords: AIDS-related non-Hodgkin lymphoma, antiviral antibodies, multiplex serology
Introduction
Infection with the human immunodeficiency virus (HIV) is a strong risk factor for non-Hodgkin lymphoma (NHL) and development of this malignancy is considered an AIDS-defining condition.1 In the era of modern antiretroviral therapy (ART), HIV-infected individuals have an ∼12-fold increased risk of developing NHL overall compared to the general population.2 This risk varies by NHL histology and is highest for subtypes that are considered AIDS-defining [i.e., diffuse large B cell lymphoma (DLBCL), Burkitt lymphoma (BL), and primary central nervous system (CNS) lymphoma], but is also apparent for several subtypes that occur in immunocompetent individuals and that are not specifically AIDS-defining.3 NHL is currently the most common malignancy in the HIV-infected population, although the burden and incidence rates are likely to decrease with continued improvement in efficacy and uptake of ART.4
AIDS-related NHL is thought to arise from contributions of several processes, including systemic immunosuppression and chronic immune dysregulation, that increase levels of proinflammatory cytokines and B cell activation.5 Reduced immune control over some viral infections due to HIV-associated immunodeficiency is also considered a factor, although precise mechanisms are not fully elucidated. Infection with the Epstein-Barr virus (EBV) and diminished control of EBV-positive B cells has been implicated in the pathogenesis of several AIDS-NHL subtypes, particularly CNS lymphomas for which nearly all tumors are EBV positive in the setting of HIV infection.5 Around 30%–50% of AIDS-related BL and DLBCL also show EBV positivity, although the association of immunosuppression with these histologies is less marked.5,6 In addition to its role in the AIDS-defining condition Kaposi's sarcoma, infection with human herpesvirus 8 [HHV8, Kaposi sarcoma-associated herpesvirus (KSHV)] is also found in primary effusion B cell lymphomas, which predominantly occur in HIV-infected populations.7
Aside from EBV and HHV8, the pathogenic role of other viral infections in AIDS-related lymphomas is not established. Theoretically, HIV-infected individuals may be more susceptible to lymphomagenic effects of common viral infections because of impaired immunity. For example, HIV has been demonstrated to increase hepatitis C virus (HCV) replication in tissue cultures, and coinfection with HCV and/or hepatitis B virus (HBV) has been observed to increase the risk of developing AIDS-NHL in some,8–10 but not in all cohorts.11–14 A recent study that used multiplex technology to measure antibodies to 18 viral, bacterial, and parasitic infections in 199 AIDS-NHL cases and matched HIV-infected controls found seropositivity for the trichodysplasia spinulosa-associated polyomavirus (TSV) to be associated with increased risk of AIDS-NHL, whereas higher antibody levels to some EBV antigens (anti-EBNA1 and anti-ZEBRA) were associated with a reduced risk of AIDS-NHL.15 Notably, the total number of pathogens to which an individual had antibodies was not associated with AIDS-NHL risk [odds ratio (OR) per pathogen = 1.01; 95% confidence interval (CI) 0.91–1.12], but associations for the number of viruses were not reported separately.
Multiplex technologies for measuring antiviral antibodies have also been applied in studies within general population-based cohorts of immunocompetent individuals to provide new hypotheses regarding the viral etiology of NHL. Several of these studies have observed that higher prediagnostic antibody levels to certain EBV antigens (e.g., EA-D, ZEBRA, VCA p18) are associated with increased NHL risk,16,17 and novel but inconsistent associations with NHL for serological markers of some polyomavirus infections have also been reported [e.g., John Cunningham virus (JCV), Merkel cell polyomavirus (MCV), and TSV].17–21 A study that pooled three East Asian cohorts also reported an elevated risk of NHL in those with higher prediagnostic serum/plasma antibody levels to human herpesvirus-6 (HHV6) IE1A antigen, although this association was slightly attenuated after further adjusting for EBV serologic markers.17
Multiplex serological assays provide an opportunity to evaluate multiple individual viral agents that may contribute to lymphomagenesis in the HIV/AIDS population. Furthermore, the number of viruses for which an individual is seropositive, which may reflect humoral immune status as well as differences in cumulative exposure to viral infections in individuals infected with HIV, may conceivably be associated with AIDS-NHL, given the effects of HIV on humoral immunity.22 To evaluate these hypotheses, we conducted a nested case–control study utilizing stored plasma/serum samples from two prospective HIV-infected cohorts in the United States, and measured seroreactivity to an extensive set of viral antigens in AIDS-NHL cases and HIV-positive controls.
Materials and Methods
Study population
This case–control study was nested within two prospective cohort studies of HIV-positive men that have been previously described, the District of Columbia/New York Gay Men's Cohort (DCG)23 and the AIDS Cancer Cohort Study (ACC).24 The DCG includes homosexual men who were enrolled in 1982 as patients of three primary care physicians located in Washington, DC and Manhattan, NY. The ACC includes 2,803 persons with an AIDS diagnosis that were enrolled from 24 HIV/AIDS treatment centers throughout the United States. Data on demographics and other lifestyle factors were collected by trained interviewers, and plasma samples were collected from all subjects.
Participants with either incident or prevalent AIDS-NHL were matched to HIV-positive controls who did not have AIDS-NHL by age, sex, race, study cohort, and CD4 count as previously described.25 Two cases and one control did not have sufficient volume of serum/plasma available, leaving a total of 28 incident and 38 prevalent AIDS-NHL cases and 67 HIV-positive controls for the current nested case–control analysis. The 28 prediagnostic blood samples were collected a median of 1.2 (interquartile range, 0.6–1.6) years before NHL diagnosis and from equivalent time points among controls.
Laboratory analyses
Stored plasma or serum samples were tested for antibodies against 32 antigens, representing 22 different viruses, by fluorescent bead-based multiplex assays at the German Cancer Research Center in Heidelberg, Germany (Deutsches Krebsforschungszentrum, DKFZ). This assay platform has been applied to samples from multiple epidemiologic studies, as previously described.16,17,26 In brief, antibodies to multiple viral antigens were simultaneously measured using multiplexed sets of glutathione displaying polystyrene beads (SeroMAP; Luminex) internally labeled with different combinations of fluorescent dyes. Distinct sets of beads carrying affinity-purified glutathione S-transferase fusion protein antigens were mixed with the plasma or serum samples (sample dilution 1:1,000). Antibodies bound to these beads were detected with biotinylated goat–anti human IgG (H+L) secondary antibody and streptavidin-R-phycoerythrin, and the beads were examined in a Luminex 200 analyzer (xMAP; Luminex Corp). The bead sets were identified by their internal fluorescence and the amounts of antibodies that were bound to each viral antigen were quantified based on the median R-phycoerythrin fluorescence intensity (MFI) of at least 100 beads per set.
The antigens on the multiplex panel in this study included the following: herpes simplex virus-1 (HSV1 1gG), HSV2 (2mgG unique), varicella-zoster virus [VZV gE (ORF68) and gI (ORF67)], EBV (EBNA1 peptide, VCA p18, ZEBRA, and EA-D), human cytomegalovirus (CMV pp150 Nter, pp 28, and pp 52), HHV6 (IE1A truncated and IE1B truncated), HHV7 (U14), HHV8/KSHV (LANA 3 long and K8.1), HBV subtype A (HBc and HBe), HCV (1aCore and 1aNS3), human T cell leukemia-lymphoma virus (HTLV1 gag and env), and the viral capsid proteins (VP1) from 10 human polyomaviruses (HPyV; BKV, JCV, KIV, WUV, MCV, HPyV6, HPyV7, TSV, HPyV9, and HPyV10), and lymphotropic polyoma virus (LPV). Seropositivity to specific viruses was defined a priori based on combinations of seropositivity to virus-specific antigens as detailed in Supplementary Table S1.
In addition to the primary study samples, 15 masked duplicate samples were interspersed in the assay batches to evaluate quality control. The coefficients of variation and intraclass correlation coefficients (ICCs) of the quantitative antibody measurements are presented in Supplementary Table S2. Agreement of qualitative seroreactivity was greater than 90% for all 32 antigens.
Statistical analysis
Reactivity to each antigen was categorized as seropositive or seronegative based on established MFI cutpoints for each antigen that were defined a priori for this assay (Supplementary Table S1). Correlations among antiviral antibodies in controls were calculated by Spearman statistics (Supplementary Fig. S1). Unconditional logistic regression models were used to estimate the ORs and 95% CIs associating AIDS-NHL with the total number of viruses (up to 22) to which an individual was seropositive and by viral categories of herpesviruses (up to 8), hepatitis viruses (up to 2), human T lymphotropic virus 1 (up to 1), and polyomaviruses (up to 11). These “viral antibody index” measures were analyzed as ordinal variables and using successive dichotomous cutpoints. ORs and 95% CIs were also estimated for AIDS-NHL in relationship to antibody positivity for each individual antigen. Models were adjusted for age (continuous), sex, race/ethnicity (Caucasian, black, and Hispanic), study cohort (DCG, ACC), and CD4 count (<200, ≥200 cells/μL). As a sensitivity analysis, we considered associations with incident and prevalent AIDS-NHL separately. Two-sided p-values <.05 were considered statistically significant. Given the exploratory nature of this study, significance values were not adjusted for multiple comparisons. All analyses were conducted using SAS version 9.4 (SAS, Inc., Cary, NC).
Results
Baseline characteristics of the AIDS-NHL cases and HIV-infected controls without AIDS-NHL are shown in Table 1. The mean [±standard deviation (SD)] age of both the AIDS-NHL cases and the control patients was 41 (±7) years old. Most of the cases and controls were male (89%), Caucasian (67% in cases and 64% in controls), and had CD4 counts below 200 cells/μL (65% in cases and 67% in controls). The distributions of these characteristics did not notably differ between the incident and prevalent AIDS-NHL cases.
Table 1.
Baseline Characteristics of AIDS-Non-Hodgkin Lymphoma Cases and Controls in Two HIV Cohorts
| Controls (n = 67) | AIDS-NHL cases |
|||
|---|---|---|---|---|
| All cases (n = 66) | Incident (n = 28) | Prevalent (n = 38) | ||
| Age in years, mean (SD) | 40.9 (7.4) | 40.5 (7.2) | 41.8 (7.8) | 39.6 (6.7) |
| CD4 cells/μL, mean (SD) | 155 (132.3) | 164.3 (146.1) | 148.3 (162) | 176 (134.2) |
| Study, n (%) | ||||
| AIDS cancer cohort | 59 (88.1) | 57 (86.4) | 19 (67.9) | 38 (100) |
| DC gay male cohort | 8 (11.9) | 9 (13.6) | 9 (32.1) | 0 (0) |
| Sex, n (%) | ||||
| Male | 60 (89.6) | 59 (89.4) | 25 (89.3) | 34 (89.5) |
| Female | 7 (10.4) | 7 (10.6) | 3 (10.7) | 4 (10.5) |
| Ethnicity, n (%) | ||||
| Caucasian | 43 (64.2) | 44 (66.7) | 19 (67.9) | 25 (65.8) |
| Black | 22 (32.8) | 20 (30.3) | 8 (28.6) | 12 (31.6) |
| Hispanic | 2 (3) | 2 (3) | 1 (3.6) | 1 (2.6) |
| CD4 count, n (%) | ||||
| <200 cells/μL | 45 (67.2) | 43 (65.2) | 18 (64.3) | 25 (65.8) |
| ≥200 cells/μL | 22 (32.8) | 23 (34.9) | 10 (35.7) | 13 (34.2) |
NHL, non-Hodgkin lymphoma; SD, standard deviation.
Associations of viral antibody indices and AIDS-NHL
Considering all 22 viruses combined, AIDS-NHL cases were seropositive to fewer viruses (mean ± SD: 9.9 ± 2.5) compared with controls (mean ± SD: 10.9 ± 2.4; Table 2). Overall seropositivity to an increasing number of viral infections was inversely associated with AIDS-NHL (adjusted OR per virus = 0.84, 95% CI = 0.72–0.98). Seropositivity to an increasing number of the 8 herpesviruses (OR = 0.81, 95% CI = 0.61–1.08), the 2 hepatitis viruses (OR = 0.73, 95% CI = 0.40–1.35), and the 11 polyomaviruses (OR = 0.81, 95% CI = 0.65–1.01) were each inversely associated with AIDS-NHL. In analyses that categorized viral antibody index using successive dichotomous cutpoints, a consistent inverse association with AIDS-NHL was observed for overall seropositivity to a larger number of viral infections, regardless of the cutpoint used (Table 3). These inverse associations were comparable for incident and prevalent AIDS-NHL cases, with ORs for overall number of viral infections 0.82 (95% CI = 0.66–1.03) and 0.85 (95% CI = 0.71–1.01), respectively.
Table 2.
Viral Antibody Index in AIDS-Non-Hodgkin Lymphoma Cases and Controls
| Controls (n = 67) |
AIDS-NHL cases (n = 66) |
|||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | OR (95% CI) | p-Value | |
| All viruses | 10.9 (2.4) | 9.9 (2.5) | 0.84 (0.72–0.98) | .02 |
| By virus category | ||||
| Human herpesviruses | 4.3 (1.2) | 4.0 (1.3) | 0.81 (0.61–1.08) | .14 |
| Hepatitis viruses | 0.7 (0.6) | 0.6 (0.6) | 0.73 (0.40–1.35) | .30 |
| Human T Lymphotropic virus | 0 (0.0) | 0 (0.0) | ||
| Human polyomaviruses | 5.9 (1.7) | 5.4 (1.6) | 0.81 (0.65–1.01) | .06 |
Viral antibody index = number of viruses for which an individual is seropositive.
OR and 95% CI adjusted for age, sex, race/ethnicity, cohort, and CD4 count.
CI, confidence interval; OR, odds ratio.
Table 3.
Odds Ratios and 95% Confidence Intervals for Associations Between AIDS-Non-Hodgkin Lymphoma and Viral Antibody Index at Various Cutpoints, Adjusted for Age, Sex, Race/Ethnicity, Cohort, and CD4 Count
| Viral antibody index | Controls (n = 67) |
AIDS-NHL cases (n = 66) |
|
|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | |
| ≥4 vs. <4 | 67 (100.0) | 66 (100.0) | — |
| ≥5 vs. <5 | 67 (100.0) | 64 (97.0) | — |
| ≥6 vs. <6 | 66 (98.5) | 63 (95.5) | 0.31 (0.03–3.20) |
| ≥7 vs. <7 | 64 (95.5) | 58 (87.9) | 0.33 (0.08–1.35) |
| ≥8 vs. <8 | 62 (92.5) | 54 (81.8) | 0.33 (0.10–1.07) |
| ≥9 vs. <9 | 61 (91.0) | 48 (72.7) | 0.24 (0.08–0.69) |
| ≥10 vs. <10 | 48 (71.6) | 44 (66.7) | 0.80 (0.37–1.72) |
| ≥11 vs. <11 | 39 (58.2) | 33 (50.0) | 0.73 (0.36–1.47) |
| ≥12 vs. <12 | 28 (41.8) | 24 (36.4) | 0.74 (0.36–1.54) |
| ≥13 vs. <13 | 14 (20.9) | 6 (9.1) | 0.38 (0.14–1.07) |
| ≥14 vs. <14 | 7 (10.5) | 3 (4.6) | 0.41 (0.10–1.71) |
| ≥15 vs. <15 | 4 (6.0) | 1 (1.5) | 0.23 (0.02–2.33) |
| ≥16 vs. <16 | 3 (4.5) | 0 (0.0) | — |
| ≥17 vs. <17 | 2 (3.0) | 0 (0.0) | — |
| ≥18 vs. <18 | 1 (1.5) | 0 (0.0) | — |
Viral antibody index = number of viruses for which an individual is seropositive.
Seroreactivity to individual viral antigens and AIDS-NHL
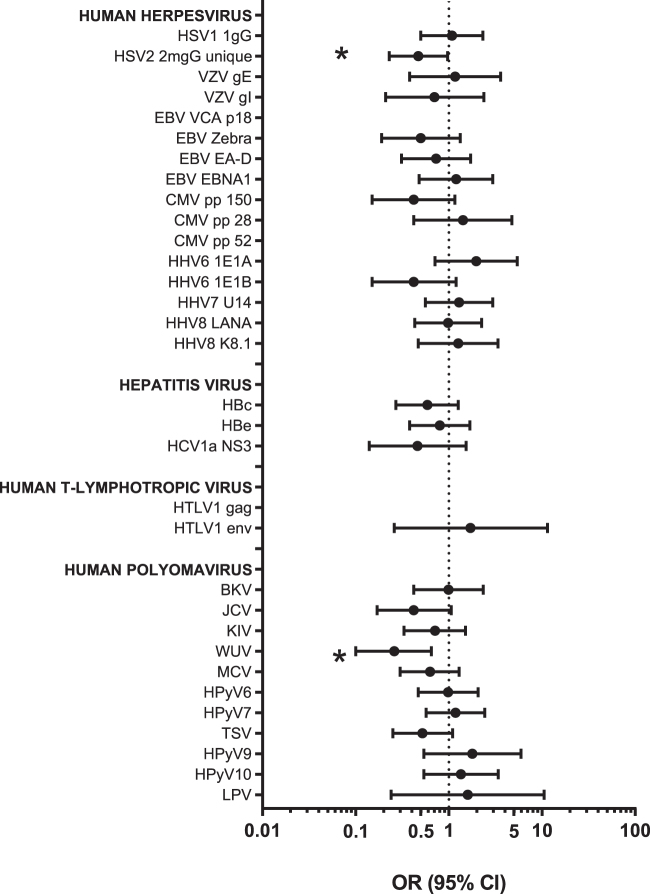
The seroprevalence of individual viral antibodies in cases and controls combined ranged from 2% (HTLV1 gag) to 100% (VCA p18; Supplementary Table S3). Pairwise correlations (rho) among the antibodies in controls ranged from −0.42 to 0.40, with the exception of the two antibodies to HBV antigens (rho = 0.76; Supplementary Fig. S1). The individual associations with AIDS-NHL are shown in Figure 1.
FIG. 1.
Associations between antibody seroreactivity and risk of AIDS-related non-Hodgkin lymphoma. OR and 95% CI adjusted for age, sex, ethnicity, study, and CD4 count. *p-value <.05. CI, confidence interval; OR, odds ratios.
Among the herpesviruses, the seroprevalence of antibodies against the HSV2 2mgG unique antigen was significantly lower in the AIDS-NHL cases (44%) compared to the controls (61%) (OR = 0.47; 95% CI = 0.23–0.97; p = .04; Fig. 1). Antibody positivity to EBV VCA p18 was 100% in both cases and controls. AIDS-NHL cases had lower seropositivity against EBV ZEBRA (79%) and EA-D (76%) relative to controls (88% and 81%, respectively) and higher seroreactivity to EBNA1 (83% vs. 81%), but none of these differences was statistically significant (Fig. 1). Although not statistically significant, noteworthy associations with AIDS-NHL were also observed for seropositivity to the HHV6 antibodies. A positive association with AIDS-NHL was observed for seropositivity to HHV6 IE1A (OR = 1.97; 95% CI = 0.71–5.42), whereas an inverse association was observed for seropositivity to HHV6 1E1B (OR = 0.42; 95% CI = 0.15–1.20) as well as to CMV pp 150 (OR = 0.42, 95% CI = 0.15–1.16).
AIDS-NHL cases had a lower seroprevalence of all measured anti-HBV antibodies (HBc: 58% vs. 69%; HBe: 55% vs. 60%) and anti-HCV antibodies (HCV-1a NS3: 8% vs. 15%). The adjusted ORs for these associations were not statistically significant, but ranged from 0.46 to 0.80 (Fig. 1).
Among the 11 evaluated antibodies to polyomaviruses, the seroprevalence of anti-WUV VP1 was significantly lower in AIDS-NHL cases overall (64%) compared to controls (85%) (OR = 0.26, 95% CI = 0.10–0.65; p = .004). Furthermore, the seroprevalence of antibodies against BKV, JCV, KIV, MCV, HPyV6, and TSV antigens were all nonsignificantly lower in overall AIDS-NHL cases compared to controls, whereas the seroprevalence of HPyV7, HPyV9, HPyV10, and LPV was nonsignificantly higher in the AIDS-NHL cases (Supplementary Table S3 and Fig. 1). In the adjusted models, a suggestive inverse association with AIDS-NHL was observed for JCV (OR = 0.42; 95% CI = 0.17–1.06) and TSV (OR = 0.52; 95% CI = 0.25–1.10) antibodies (Fig. 1).
There were no individuals classified as seropositive for HTLV1 infection based on our a priori definition of seroreactivity to both antigens, although two controls and no cases had antibodies to gag, while two controls and three cases had antibodies to env.
Incident and prevalent AIDS-NHL cases had generally similar antibody prevalence, with significant differences for only 3 of the 32 antibodies: EBV EBNA1, BKV, and WUV (Supplementary Table S3).
Discussion
To provide additional insight into the pathogenesis of AIDS-related NHL, we conducted a nested case–control study utilizing stored blood samples from two prospective HIV cohorts. Antibodies to 32 viral antigens reflecting 22 distinct viral infections were measured using a multiplex serological assay in cases of AIDS-NHL and HIV-infected control individuals without AIDS-NHL. We observed that overall seropositivity to a larger number of viral infections was inversely associated with AIDS-NHL. For individual antigens, we observed significant inverse associations of AIDS-NHL with seropositivity against HSV2 2mgG unique and WUV VP1 antigens. While our findings need to be replicated in other cohorts, our data raise the novel hypothesis that insufficient exposure or impaired humoral responses to viral infections may be associated with AIDS-related NHL.
Typically, antibody seropositivity is considered to reflect exposure to a given virus and, thus, the viral antibody index would be a measure of cumulative viral exposure. Our data could be interpreted as indicating that insufficient exposure to other viruses increases risk of HIV-related NHL. Alternatively, among individuals with HIV infection, the viral antibody index may reflect the general integrity of humoral immune responses to these other viral infections. Experimental evidence has suggested that antibody production and intact humoral responses contribute to long-term control of some persistent viral infections.27 Importantly, HIV infection has been shown to reduce humoral immune responses to HCV, potentially contributing to accelerated liver disease and risk of hepatocellular carcinoma in coinfected patients.28 By extension, the association of reduced viral antibody index with risk of AIDS-NHL may reflect an impaired immune response that contributes to lymphomagenesis, perhaps through loss of viral control.
A previous study utilizing bead-based multiplex technology did not find an association between AIDS-NHL and the number of pathogens to which an individual was reactive, but differences in the antigen panel used in that study and in our study may partially explain the conflicting findings.15 Notably, while our panel of antigens focused exclusively on viruses, the previous study included antibodies associated with bacterial (Helicobacter pylori, Chlamydia trachomatis, and Mycoplasma genitalium) and parasitic infections (Toxoplasma gondii) as well. The viruses considered in the two studies also differed, as we evaluated antibodies associated with nine additional viruses that were not included in the previous study.
In analyses of individual antigens, we found significantly lower antibody prevalence to the polyomavirus WUV VP1 antigen in the AIDS-NHL cases compared to controls. Polyomaviruses are a family of nonenveloped DNA viruses with prevalence that ranges from 35% to 90% in the general population.29 WUV was first identified in 2007 from respiratory tract specimens using shotgun sequencing.30 WUV antibodies have been detected at a higher frequency in HIV-positive individuals and the frequency of WUV reactivation may be similar to that of other polyomaviruses in immunodeficient states.31–33
While the differences were borderline statistically significant, AIDS-NHL cases had lower prevalence of antibodies to the polyomaviruses JCV and TSV. JCV has previously been detected at a significantly higher frequency in DLBCL tumor samples compared to control lymph tissues.34 In studies of NHL in the general population, antibodies to JCV were inversely associated with the disease in a case–control study,18 and nonsignificantly lower in cases nested within a prospective cohort.19 On the contrary, antibodies to TSV were nonsignificantly higher in nested NHL cases in a U.S. prospective cohort20 and in a pooled analysis of three Asian cohorts.17 In a previous study of AIDS-NHL, seropositivity for TSV was significantly associated with increased AIDS-NHL risk whereas seropositivity to JCV was nonsignificantly lower in AIDS-NHL cases relative to controls.15 The correlations between the polyomaviruses in our study were low, suggesting that our observed associations in the context of AIDS-NHL are independent.
The strongest finding in our study was that AIDS-NHL cases had significantly lower prevalence of antibodies to HSV2 2mgG unique antigen, compared to control individuals. HSV2 is a sexually transmitted herpesvirus that infects the genital mucosa and persists in the body for life following primary infection. Compared to the 16% seroprevalence in the general U.S. population,35 our data are consistent with other reports showing that HSV2 is a relatively common coinfection with HIV.36 A higher prevalence of anti-HSV2 antibodies has been reported in pediatric patients with acute lymphoblastic leukemia,37 but higher HSV2 antibody prevalence was not observed among NHL patients in a case–control study conducted in South Africa.38
The 2mgG antigen is 1 of 11 glycoproteins expressed by HSV2 and is the only known protein that provides a high degree of HSV2 specificity.39 HSV2 2mgG has been shown to elicit a type-specific CD4+ T cell response, and in vitro studies have implicated 2mgG involvement in the extracellular release of infectious particles.40 Mice immunized with 2mgG and CpG have been demonstrated to have reduced infectious HSV2 particles and 2mgG has been proposed as a promising HSV2 vaccine antigen.41 Our findings showing lower prevalence of antibodies against this antigen in AIDS-NHL cases may reflect a reduced capacity for a T cell-mediated immune response against HSV2 in these patients. Although we cannot rule out the possibility that the HSV2 association may be confounded by another coinfection, the correlations between antibodies against the 2mgG antigen and other herpesviruses antibodies were not high in our study.
We did not observe significant differences between AIDS-NHL cases and control individuals in our study for antibodies to other herpesviruses. In a previous study, EBV ZEBRA antibodies were inversely associated with risk of AIDS-NHL diagnosed ≥4 years after blood collection.15 Two general population-based cohort studies found that EBV seropositivity16 or prediagnostic serum levels of anti-EBV ZEBRA and EA-D antibodies17 were inversely associated with NHL in the initial period following blood collection, but positively associated at longer intervals. However, these associations in both studies were based on small number of seronegative cases and were not statistically significant. Whether or not these EBV antibody patterns are distinctly different in AIDS-NHL compared to the pathogenesis of NHL in the general population, or if the associations reflect study-specific differences in time between blood collection and diagnosis, will need further evaluation in other prospective studies.
Our study had several strengths and limitations. A notable strength of this study was the use of a multiplex panel that enabled the measurement of a relatively large number of antibodies associated with 22 different viral infections. All samples were measured concurrently in the same laboratory, in which personnel were blinded to the case–control status of the samples. The antibody measurements were further observed to be highly reproducible based on the calculated assay CVs and ICCs of the masked quality control replicates. The primary limitation of our study was the small sample size and, consequently, the possibility of missing a true association. Some of our samples were from prevalent cases with possible reverse causality, but we note that the associations of the antiviral antibody index with incident and prevalent AIDS-NHL were similar in magnitude. Also, the samples from incident cases were collected relatively close to NHL diagnosis, which may limit interpretations as it relates to deciphering etiologic versus disease-induced patterns. Finally, we were unable to evaluate associations with specific AIDS-NHL subtypes given the size of our study. Risk factors may differ by NHL subtype, and differences in subtype distributions between studies could underlie observed differences in antibody associations. Larger studies would be needed to evaluate potential subtype-specific associations.
In conclusion, this study represents an evaluation of the most viral antibody associations with AIDS-NHL reported to date. We found an inverse association with the number of viral infections to which an individual was seropositive, potentially implicating insufficient exposure or impaired humoral response to viral infections in AIDS lymphomagenesis. While our findings need to be replicated and extended in other studies, these data provide new hypotheses regarding the pathogenesis of NHL in the setting of HIV infection.
Supplementary Material
Author Disclosure Statement
No potential conflicts of interest were disclosed.
Funding Information
This study was supported, in part, by the Intramural Research Program of the National Cancer Institute (1ZIACP010212-09).
Supplementary Material
References
- 1. Cesarman E: Pathology of lymphoma in HIV. Curr Opin Oncol 2013;25:487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA: Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. Lancet HIV 2017;4:e495–e504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA: Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: A population-based study. AIDS 2014;28:2313–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA: Projected cancer incidence rates and burden of incident cancer cases in HIV-infected adults in the United States through 2030. Ann Intern Med 2018;168:866–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Epeldegui M, Vendrame E, Martinez-Maza O: HIV-associated immune dysfunction and viral infection: Role in the pathogenesis of AIDS-related lymphoma. Immunol Res 2010;48:72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaidano G, Capello D, Carbone A: The molecular basis of acquired immunodeficiency syndrome-related lymphomagenesis. Semin Oncol 2000;27:431–441 [PubMed] [Google Scholar]
- 7. Jenner RG, Maillard K, Cattini N, et al. : Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphoma has a plasma cell gene expression profile. Proc Natl Acad Sci U S A 2003;100:10399–10404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rotman Y, Liang TJ: Coinfection with hepatitis C virus and human immunodeficiency virus: Virological, immunological, and clinical outcomes. J Virol 2009;83:7366–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Besson C, Lancar R, Prevot S, et al. : Outcomes for HIV-associated diffuse large B-cell lymphoma in the modern combined antiretroviral therapy era. AIDS 2017;31:2493–2501 [DOI] [PubMed] [Google Scholar]
- 10. Aromaa A, Kosunen TU, Knekt P, et al. : Circulating anti-Helicobacter pylori immunoglobulin A antibodies and low serum pepsinogen I level are associated with increased risk of gastric cancer. Am J Epidemiol 1996;144:142–149 [DOI] [PubMed] [Google Scholar]
- 11. Bonnet F, Balestre E, Thiebaut R, et al. : Factors associated with the occurrence of AIDS-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: Aquitaine Cohort, France. Clin Infect Dis 2006;42:411–417 [DOI] [PubMed] [Google Scholar]
- 12. Franceschi S, Polesel J, Rickenbach M, et al. : Hepatitis C virus and non-Hodgkin's lymphoma: Findings from the Swiss HIV Cohort Study. Br J Cancer 2006;95:1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waters L, Stebbing J, Mandalia S, et al. : Hepatitis C infection is not associated with systemic HIV-associated non-Hodgkin's lymphoma: A cohort study. Int J Cancer 2005;116:161–163 [DOI] [PubMed] [Google Scholar]
- 14. Jou E, Gligich O, Chan AC, et al. : Viral co-infections and paraproteins in HIV: Effect on development of hematological malignancies. Ann Hematol 2016;95:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halec G, Waterboer T, Brenner N, et al. : Serological assessment of 18 pathogens and risk for AIDS-associated Non-Hodgkin lymphoma. J Acquir Immune Defic Syndr 2019;80:e53–e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teras LR, Rollison DE, Pawlita M, et al. : Epstein-Barr virus and risk of non-Hodgkin lymphoma in the cancer prevention study-II and a meta-analysis of serologic studies. Int J Cancer 2015;136:108–116 [DOI] [PubMed] [Google Scholar]
- 17. Bassig BA, Willhauck-Fleckenstein M, Shu XO, et al. : Serologic markers of viral infection and risk of non-Hodgkin lymphoma: A pooled study of three prospective cohorts in China and Singapore. Int J Cancer 2018;143:570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engels EA, Rollison DE, Hartge P, et al. : Antibodies to JC and BK viruses among persons with non-Hodgkin lymphoma. Int J Cancer 2005;117:1013–1019 [DOI] [PubMed] [Google Scholar]
- 19. Rollison DE, Engels EA, Halsey NA, Shah KV, Viscidi RP, Helzlsouer KJ: Prediagnostic circulating antibodies to JC and BK human polyomaviruses and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2006;15:543–550 [DOI] [PubMed] [Google Scholar]
- 20. Teras LR, Rollison DE, Pawlita M, et al. : Prediagnostic circulating polyomavirus antibody levels and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2015;24:477–480 [DOI] [PubMed] [Google Scholar]
- 21. Robles C, Poloczek A, Casabonne D, et al. : Antibody response to Merkel cell polyomavirus associated with incident lymphoma in the Epilymph case-control study in Spain. Cancer Epidemiol Biomarkers Prev 2012;21:1592–1598 [DOI] [PubMed] [Google Scholar]
- 22. Shen X, Tomaras GD: Alterations of the B-cell response by HIV-1 replication. Curr HIV/AIDS Rep 2011;8:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goedert JJ, Biggar RJ, Melbye M, et al. : Effect of T4 count and cofactors on the incidence of AIDS in homosexual men infected with human immunodeficiency virus. JAMA 1987;257:331–334 [PubMed] [Google Scholar]
- 24. Nawar E, Mbulaiteye SM, Gallant JE, et al. : Risk factors for Kaposi's sarcoma among HHV-8 seropositive homosexual men with AIDS. Int J Cancer 2005;115:296–300 [DOI] [PubMed] [Google Scholar]
- 25. Landgren O, Goedert JJ, Rabkin CS, et al. : Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol 2010;28:773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waterboer T, Sehr P, Michael KM, et al. : Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem 2005;51:1845–1853 [DOI] [PubMed] [Google Scholar]
- 27. Sanna PP, Burton DR: Role of antibodies in controlling viral disease: Lessons from experiments of nature and gene knockouts. J Virol 2000;74:9813–9817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Netski DM, Mosbruger T, Astemborski J, Mehta SH, Thomas DL, Cox AL: CD4+ T cell-dependent reduction in hepatitis C virus-specific humoral immune responses after HIV infection. J Infect Dis 2007;195:857–863 [DOI] [PubMed] [Google Scholar]
- 29. Dalianis T, Hirsch HH: Human polyomaviruses in disease and cancer. Virology 2013;437:63–72 [DOI] [PubMed] [Google Scholar]
- 30. Kleines M, Hausler M, Kruttgen A, Scheithauer S: WU polyomavirus (WUPyV): A recently detected virus causing respiratory disease? Viruses 2009;1:678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharp CP, Norja P, Anthony I, Bell JE, Simmonds P: Reactivation and mutation of newly discovered WU, KI, and Merkel cell carcinoma polyomaviruses in immunosuppressed individuals. J Infect Dis 2009;199:398–404 [DOI] [PubMed] [Google Scholar]
- 32. Miller MA, Weibel C, Ferguson D, Landry ML, Kahn JS: WU polyomavirus in patients infected with HIV or hepatitis C virus, Connecticut, USA, 2007. Emerg Infect Dis 2009;15:1095–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Babakir-Mina M, Ciccozzi M, Farchi F, et al. : KI and WU polyomaviruses and CD4+ cell counts in HIV-1-infected patients, Italy. Emerg Infect Dis 2010;16:1482–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hogfeldt T, Jaing C, Loughlin KM, et al. : Differential expression of viral agents in lymphoma tissues of patients with ABC diffuse large B-cell lymphoma from high and low endemic infectious disease regions. Oncol Lett 2016;12:2782–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bradley H, Markowitz LE, Gibson T, McQuillan GM: Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis 2014;209:325–333 [DOI] [PubMed] [Google Scholar]
- 36. Kouyoumjian SP, Heijnen M, Chaabna K, et al. : Global population-level association between herpes simplex virus 2 prevalence and HIV prevalence. AIDS 2018;32:1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loutfy SA, Alam El-Din HM, Ibrahim MF, Hafez MM: Seroprevalence of herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus in children with acute lymphoblastic leukemia in Egypt. Saudi Med J 2006;27:1139–1145 [PubMed] [Google Scholar]
- 38. Berrington de Gonzalez A, Urban MI, Sitas F, et al. : Antibodies against six human herpesviruses in relation to seven cancers in black South Africans: A case control study. Infect Agent Cancer 2006;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jun W, Hu R, Hyland L, et al. : Expression and characterization of the soluble form of recombinant mature HSV-2 glycoprotein G for use in anti-HSV-2 IgG serodiagnostic immunoassay. J Virol Methods 2018;252:65–69 [DOI] [PubMed] [Google Scholar]
- 40. Eriksson K, Bellner L, Gorander S, et al. : CD4(+) T-cell responses to herpes simplex virus type 2 (HSV-2) glycoprotein G are type specific and differ in symptomatic and asymptomatic HSV-2-infected individuals. J Gen Virol 2004;85(Pt 8):2139–2147 [DOI] [PubMed] [Google Scholar]
- 41. Gorander S, Harandi AM, Lindqvist M, Bergstrom T, Liljeqvist JA: Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J Virol 2012;86:7544–7553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.