Abstract
After more than two decades since clinical trials tested the first use of recombinant adeno-associated virus (rAAV) to treat cystic fibrosis (CF) lung disease, gene therapy for this disorder has undergone a tremendous resurgence. Fueling this enthusiasm has been an enhanced understanding of rAAV transduction biology and cellular processes that limit transduction of airway epithelia, the development of new rAAV serotypes and other vector systems with high-level tropism for airway epithelial cells, an improved understanding of CF lung pathogenesis and the cellular targets for gene therapy, and the development of new animal models that reproduce the human CF disease phenotype. These advances have created a preclinical path for both assessing the efficacy of gene therapies in the CF lung and interrogating the target cell types in the lung required for complementation of the CF disease state. Lessons learned from early gene therapy attempts with rAAV in the CF lung have guided thinking for the testing of next-generation vector systems. Although unknown questions still remain regarding the cellular targets in the lung that are required or sufficient to complement CF lung disease, the field is now well positioned to tackle these challenges. This review will highlight the role that next-generation CF animal models are playing in the preclinical development of gene therapies for CF lung disease and the knowledge gaps in disease pathophysiology that these models are attempting to fill.
Keywords: cystic fibrosis, animal models, viral vectors, pathophysiology, gene therapy, cellular targets
Introduction
Cystic fibrosis is a multiorgan disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.1 CFTR is an anion channel that conducts Cl− and HCO3− across several types of epithelium in the lung, intestine, pancreas, liver, and gallbladder. Each of these organs shares commonalties in pathology, including impaired hydration of the epithelial surface, excessive mucus production and obstruction, and reduced pH of secretions.2 In the cystic fibrosis (CF) lung, these abnormalities impair clearance and innate immunity, and they lead to chronic bacterial infections.3–7 Since lung disease is the most life-threatening component of CF, it is considered the primary initial target organ for gene therapies.
The first CF clinical trials with recombinant adeno-associated virus (rAAV) utilized a rAAV-2 serotype to express CFTR in the maxillary sinuses.8 Results from this initial dose-escalation toxicity study demonstrated it was safe and that the vector genome accumulated in the sinus epithelium. A subsequent does-escalation study demonstrated in 2 of 10 patients that the CFTR transgene was expressed and the transepithelial potential difference, an index of CFTR-mediated Cl− transport, was partially corrected.9,10 These studies paved the way for testing the rAAV2-CFTR vector through aerosol administration to the lungs of CF patients11–13 and culminated in a repeat-dosing aerosol administration study, which examined forced expiratory volume (FEV1) as the primary endpoint.13 Although the rAAV2-CFTR vector was proven safe in each of these studies, there was no improvement in the primary endpoint of the repeat-dosing study and it was subsequently terminated. Results from this repeat administration study demonstrated persistence of vector genomes, but no expression of CFTR transgene-derived mRNA.
Reasons for the failure of these initial rAAV2-CFTR clinical trials targeting the lung were difficult to predict at the time, but they emerged with more basic research over the subsequent decade. First, the original rAAV2-CFTR vector system (called tgAAVCF) utilized the inverted terminal repeat (ITR) within the vector genome as the promoter. Although this promoter was weak, it was necessary to fit the full-length CFTR cDNA into the vector without exceeding the packaging capacity.14 Later improvements in vector design utilized short synthetic promoters with the full-length CFTR cDNA15 or smaller mini-gene versions of CFTR with a deleted region that did not alter channel function.16–18 Second, despite efficient entry of rAAV2 from the apical membrane of airway epithelia, this serotype—and many others for that matter—encounters a proteasome-dependent block in nuclear uptake.19–24 This barrier was later circumvented when it was discovered that proteasome inhibitors can significantly enhance apical transduction of rAAV in airway epithelia and thus also enhance complementation of Cl− transport in CF epithelia by using rAAV-CFTR vectors.15,21–24 Lastly, the preclinical rhesus monkey studies that supported the initial rAAV2-CFTR clinical trial demonstrated efficient transduction of the airway,25 but it was later found that the tropism of rAAV2 for the apical surface of polarized human primary airway epithelia was 100-fold lower than that observed in polarized rhesus monkey primary airway epithelia.26 These studies emphasized the need for a concrete understanding of vector/target cell biology and the importance of choosing a preclinical animal species that has a similar vector tropism to humans.
It is clear that the lack of CFTR in the airways leads to impaired mucociliary clearance caused by poor hydration of airway surface liquid (ASL) and that the resultant viscous mucus secretions provide the environment for bacterial colonization. However, a significant knowledge gap that remains for CF lung gene therapy is a clearer understanding of the target cell types required for complementation of disease. CFTR expression in the lung is highly regulated at the cellular level.27–30 For example, submucosal glands (SMGs) embedded beneath the airways express CFTR27 and CFTR-dependent secretions from these structures are important in innate immunity in the airway.31,32 If these structures play a role in CF pathogenesis, as research in CF animal models suggests,33 strategies for CFTR delivery to these structures, which are relatively inaccessible from the airway lumen, may require unique vector systems.
An additional potential unique cellular target includes the newly characterized pulmonary ionocyte,29,30 which expresses the majority of CFTR in the conducting proximal airways and SMG ducts. Ionocytes compose only ∼1–2% of cells on the surface of the large airways and these cells have a very unique cellular anatomy and composite of channels that suggests they are involved in pH regulation and ion transport. Whether ionocytes are a required cell type for complementation of CF lung disease remains unknown, but based on the composition of other channels that are co-expressed with CFTR in these cells, their discovery raises the question as to whether certain functions of CFTR are cell-intrinsic.34 CFTR also physically interacts with other ion channels, such as the epithelial sodium channel (ENaC),35 to regulate ion and fluid movement across airway epithelia.36,37 These studies emphasize that certain functions of CFTR may be dependent on the cellular composite of other channels that control ion movement.
Our current understanding of how CFTR maintains airway clearance and innate immunity in a multicellular airway epithelium is based predominantly on the notion that CFTR functions similarly in any columnar cell type (i.e., cells with an apical and basolateral membrane) to move anions across the epithelium. This notion is supported by the finding that CFTR gene replacement using many types of viral vectors, including rAAV,15,16,38 human bocavirus type 1 (HBoV1),39 lentivirus,40,41 helper-dependent adenovirus (HDAd),42 and human parainfluenza virus,43 can complement CFTR-mediated Cl− currents after transduction of bronchial airway cultures derived from humans with CF that are grown at an air-liquid interface (ALI). If one accepts that this wide array of viral vectors likely has differing cellular specificities for transduction of airway cell types, then the cellular partitioning of CFTR expression in vivo is likely not an obstacle for CF lung gene therapy if Cl− transport is the primary driver of disease.
Puzzling paradoxes, however, exist in translating in vitro findings to the in vivo setting in terms of the cellular specificity of CFTR function. For example, forced expression of CFTR in polarized human CF ALI cultures using human parainfluenza virus, which is believed to primarily target ciliated cells, can correct defects in Cl− transport and mucociliary clearance.43 However, studies in transgenic mice overexpressing CFTR under the control of the Foxj1 promoter (which drives expression specifically in ciliated cells) on a CFTR-knockout (KO) background failed to rescue CFTR-dependent nasal potential difference despite high levels of CFTR mRNA and protein expression.44 These contradictory findings raise two important questions: Is the ciliated cell an effective target for CF gene therapy? Has the heavy reliance on Cl− transport as an endpoint for efficacy in the development of CF gene therapies led to oversimplified thinking about disease complementation in vivo, ignoring the fact that airway fluid dynamics and pH, mucus secretion and clearance, and innate immunity are regulated by specific cell populations, including those in the surface airway epithelium (SAE) and SMGs?6,31–33,45–48 Addressing this in vivo biology has historically been difficult, because mice do not develop lung disease in the absence of CFTR49–52 and this species also lacks SMGs in the cartilaginous airways.53–55
Armed with next-generation viral vector systems and an improved understanding of viral transduction biology in the airway epithelia, the field is now well positioned with effective tools to deliver CFTR to the lung. However, remaining unknowns about CF lung pathogenesis, and how various CFTR-expressing cell types coordinate lung clearance and innate immunity, present potential hurdles for gene therapy. New CF animal models have begun to pave the way to address these challenges and will continue to play an important role in the preclinical development of CF lung gene therapies. This article will review these knowledge gaps, discuss opportunities to address them, and describe their potential impact for future CF lung gene therapy approaches.
Animal Models of CF
After being solely dependent on CF mouse models for 20 years, the past decade has positioned the field with a Noah's Ark of CF animal models (Table 1). Each of these models was initially characterized in the CFTR-KO state. The most well-studied larger animal CF models include the ferret and pig, and each gives rise to the spectrum of multiorgan disease seen in humans with CF. Although CF mice and rats do not develop spontaneous lung infections, the CF rat does develop defects in tracheal mucociliary clearance with age that are associated with SMG hypertrophy.56 Relatively new CF rabbit and sheep models have yet to be fully characterized for their lung phenotype in the adult state. At birth, all CF models lack gross pathology in the lung, and in the case of CF ferrets and pigs, disease in the lung develops with age. Given the focus of this review on the lung, we limit discussion to the CF ferret and pig models for which the lung phenotype has been studied in more depth.
Table 1.
CF animal models
| Species | Year Generated | Spontaneous CF Phenotype by Organ | References |
|---|---|---|---|
| Mouse | 1992 | Intestine (liver mild phenotype) | 49–52 |
| Ferret | 2008 | Lung, pancreas (diabetes), intestine, liver, gallbladder, vas deferens, epididymis. | 57–68 |
| Pig | 2008 | Lung, pancreas (diabetes), intestine, liver, gallbladder, vas deferens | 69–78 |
| Zebrafish | 2013 | Pancreas | 79,80 |
| Rat | 2014 | Intestine, bone (tracheal airway surface fluid defects, but no spontaneous bacterial infection in lung) | 56,81,82 |
| Rabbit | 2016 | Intestine (lung unknown) | Unpublished |
| Sheep | 2018 | Pancreas, intestine, liver, gallbladder, vas deferens, (lung unknown) | 83 |
CF, cystic fibrosis.
Despite the lack of gross pathology in the lungs of newborn CF ferrets and pigs, both species demonstrate dysregulated inflammatory responses after the first exposure to bacteria84,85 and an impaired ability of the lung to eradicate bacteria.74,85 Within weeks to months after birth, both CFTR-KO ferrets and pigs spontaneously acquire bacterial infections in the lung.63,74 Lung disease in both CF ferret and pig models is characterized by excessive mucus that obstructs the airways,60,61,74 impaired mucociliary clearance,33,85 defective SMG secretions,63,86 and polymicrobial bacterial infections.61,74 CF ferrets are extremely sensitive to lung infections early in life before full development of airway SMGs at about 1 month of age63 and thus require aggressive antibiotic treatment to survive.61 When symptomatically treated with antibiotics, CFTR-KO ferrets live longer (average of 105 ± 27 days), but they still succumb to bacterial colonization of the lung.61 When aggressively treated with three antibiotics from birth, CFTR-KO ferrets lived an average of 1,143 ± 77 days (N = 4 females and N = 3 males) and although they do not acquire bacterial lung infection, they still develop structural and mucoinflammatory lung disease.60 The average lifespan was also not significantly different between females (1,144 ± 136 days) and males (1,174 ± 54 days). These findings have led to the concept that there are two separate components of CF lung pathogenesis involving defects in innate immunity and inflammation caused by abnormal mucus and obstruction.87
Both pig and ferret CFTR-KO models have severe gastrointestinal defects before and after birth that has made them particularly difficult to rear; this has limited their utility as preclinical models for gene therapy. For example, all CFTR-KO pigs are born with meconium ileus (in utero obstruction of the intestine)71 and significant exocrine pancreatic disease that is initiated in utero.77 Similarly, a large fraction (80%) of CFTR-KO ferrets have meconium ileus63 and their exocrine pancreatic disease progresses rapidly after birth.59,62 Although meconium ileus in the CFTR-KO pig can be treated surgically,74 this is not possible in CFTR-KO ferrets due to size.
For these reasons, second-generation gut-corrected CFTR-KO ferrets63 and pigs88 have been generated, which express CFTR under the direction of the intestinal fatty acid-binding protein (FABPI) promoter. Although the gut-corrected CF pig model has shown some protection from meconium ileus,88,89 the similar model in ferret did not increase the ease of rearing despite protection from meconium ileus (unpublished). For these reasons, a third-generation CF ferret model was engineered to harbor a CFTR-G551D mutation (CFTRG551D) that is responsive to the CFTR modulator drug VX-770.66
When pregnant female ferrets (jills) harboring CFTRG551D/G551D kits are given VX-770 at embryonic day 28, the CF kits born are protected from pancreatitis and meconium ileus, and when the drug is continued postnatally, the CF kits have normal growth and survival rates. CFTRG551D/KO kits are only partially protected by in utero VX-770 therapy due to reduced (∼50%) expression from the CFTRG551D allele caused by a neomycin expression cassette in the intron that was used to generate the model. Termination of VX-770 at any age reactivates disease in the pancreas, intestine, and lung66; however, when this is done before 2 weeks of age, there is high mortality due to both lung and intestinal pathology. When VX-770 is terminated at 2–3 weeks of age, terminal lung disease with bacterial colonization typically occurs in the majority of animals within 1–3 months. This VX-770-responsive model will greatly expand the utility of the CF ferret for studying disease pathophysiology and the preclinical testing of gene therapies that can either prevent or reverse CF lung disease.90
With the advent of CRISPR/Cas9 gene editing in zygotes, several new ferret models are emerging. For example, a ROSA-26 knock-in has been generated that expresses a CreERT2-responsive LoxP-Tomato-stop-LoxP-EGFP (ROSA-TG) reporter that will enable lineage tracing of stem cells in ferrets.91 Such a ROSA-TG model will also be useful for evaluating gene transfer from Cre-expressing vector systems. Several Cre-driver ferrets have also been generated to enable lineage tracing of stem cells and the conditional deletion of CFTR in a cell-type specific fashion (unpublished). These models will assist in dissecting CF pathogenesis and the cellular targets for gene therapies.
Cellular Targets in the Lung for CF Gene Therapy
The conducting airways are believed to be the predominant target for CF gene therapy and are composed of several unique domains that house a variety of CFTR-expressing cell types important for coordinating innate immunity and clearance in the lung. Recent single-cell RNA sequencing (scRNAseq) data have significantly enhanced the ability to classify various cell types in the human conducting airways.29,30,92,93 In humans, the proximal cartilaginous airways (trachea and bronchi) are composed of the SAE and SMGs. The SAE comprises primarily basal cells, intermediate basal cells (transitional states of basal cell differentiation to secretory and ciliated cells), secretory (goblet) cells, and ciliated cells, but they also contain less abundant neuroendocrine cells, brush cells, and ionocytes (Fig. 1A).30,94–96 SMGs are found in the submucosa of the cartilaginous airways and secrete mucus and fluid that are rich in antibacterial factors.31,32 These structures contain a network of interconnected serous acini and mucous tubules that are surrounded by myoepithelial cells and are secreted into collecting ducts composed of simple columnar cells and ionocytes.27,95–97 Secretions exit SMGs through ciliated ducts that have a similar cellular composition to the SAE but are enriched with ionocytes. Terminal bronchioles contain basal cells, secretory club cells, ciliated cells, neuroendocrine cells, and fewer ionocytes, whereas respiratory bronchioles contain primarily club cells.94–97
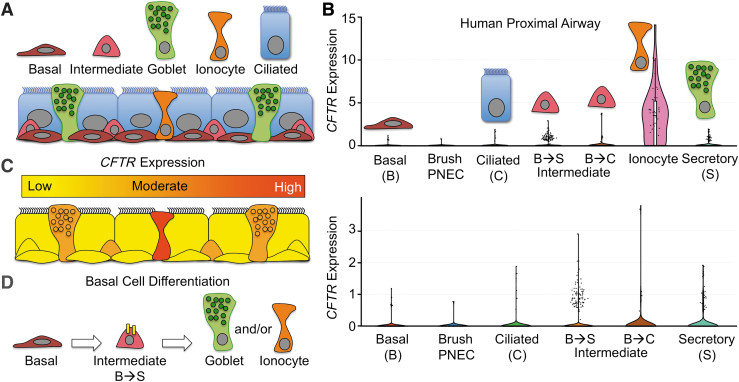
Figure 1.
Cellular expression patterns of CFTR in the proximal human airway. (A) Major cell types in the proximal human cartilaginous airways. Brush cells and PNEC are not shown. Goblet cells are classified at the proximal airway secretory cell. (B) CFTR mRNA expression levels in various proximal human airway cell types as determined by scRNAseq.30 Data were extracted and visualized from the Broad Institute Single Cell Portal. A subset of cells with lower levels of CFTR mRNA expression is visualized with an enlarged y-axis in the lower panel. (C) Schematic summary of CFTR mRNA expression levels for the various cell types marked in (A). (D) CFTR expression (yellow) within a subpopulation of basal intermediate cells, fated to become goblet secretory cells, suggests potential roles for CFTR in differentiation toward other CFTR-expressing cell types. B→S: Basal→Secretory; B→C: Basal→Ciliated. CFTR, cystic fibrosis transmembrane conductance regulator; PNEC, pulmonary neuroendocrine cells; scRNAseq, single-cell RNA sequencing.
CFTR is expressed in several of these cell types at widely divergent levels and this has created controversy over what cell types contribute to CF lung pathogenesis and have the ability to restore lung function after gene therapy.34 For example, ciliated cells have long been considered to be a predominant CFTR-expressing cell type involved in CF pathogenesis and a target for CF gene therapy.43,98 However, some studies have failed to detect both CFTR protein and mRNA in this cell type.27,30 Recently, scRNAseq on human and mouse proximal airway cell types29,30 has demonstrated that the majority of CFTR mRNA in the proximal airway is present within ionocytes, and that ciliated cells express very little in only a small fraction of cells (Fig. 1B, C). scRNAseq has also demonstrated that a subset of intermediate basal cell precursors of secretory cells and differentiated secretory cells express low levels of CFTR in greater numbers than ciliated cells (Fig. 1B, C).29,30
CFTR mRNA and protein expression in intermediate basal cells has been previously shown in human bronchus,27 but the functional significance remains unknown. Notably, after tracheal injury in mice, cycling basal cells increase transcription of CFTR,30 suggesting that CFTR may play a role in basal cell differentiation to secretory cells and/or ionocytes (Fig. 1D). Ionocytes represent only ∼1–2% of proximal conducting airway epithelial cells and were first identified as “jackpot” CFTR-expressing cells enriched in gland ducts and the SAE surround these gland ducts.27 Other cell types known to express CFTR include serous cells of SMGs27,99 and club secretory cells, which demonstrate high levels of expression in the respiratory bronchioles.28,99
The Pulmonary Ionocyte
scRNAseq of human proximal airway epithelial cells demonstrates that 65% of ionocytes express CFTR, whereas only 1.4% of ciliated cells have detectable CFTR mRNA at levels that are 100-fold lower than ionocytes.100 Further, 67% of all CFTR mRNA in the large airways comes from ionocytes.100 These findings are similar to mouse tracheal epithelia cells, where ionocytes express 54% of all CFTR transcripts, whereas ciliated cells express only 1.5%.29 Ciliated cells constitute ∼60–70% of all columnar cell types in the normal human proximal conducting airways101 and only polarized columnar cell types with an apical membrane can contribute to ion movement across an epithelium.
These findings raise an important question about how various cell types in the airway coordinate CFTR-dependent anion movement to control ASL volume, airway pH, innate immunity, and clearance. Do cell-autonomous functions of CFTR exist that are coordinated by other cell-specific apical and basolateral channels that collectively control ion gradients and membrane potential required for the movement of ions and fluid across the epithelium? The answer to this question has important implications for gene therapy. In this regard, pulmonary ionocytes are enriched for a number of specialized ion channels and V-ATPases29,30 that could impart cell-specific CFTR functions as demonstrated in other systems where ionocytes participate in acid-base regulation and salt/water balance.102–106
Consistent with this notion, the abundance of ionocytes in polarized cultures of human bronchial epithelium grown at an ALI correlates with cAMP-inducible Cl− current,30 and the elimination of ionocytes from mouse tracheal epithelial ALI cultures increases the depth of the ASL, the viscosity of the ASL mucus, and also alters the ciliary beat frequency.29 The expression of V-ATPases, which transport H+ within ionocytes of the inner ear, kidney, and epididymis, is driven by Foxi1,107 a transcription factor required for ionocyte specification in mouse, human, and zebrafish.29,30,106 Studies of ionocyte function in the epithelia of amphibians and fish102–106 have implicated three classes that are involved in acid-base regulation and osmoregulation, and one of these is enriched for CFTR.34,105 Inhibiting Foxi1 in the mucociliary skin epithelium of frogs, which ablates ionocytes, leads to altered ciliary beat frequency106—a finding similar to that of Foxi1-KO mouse tracheal cultures.29 Given the importance of fluid and anion transport, as well as pH, in maintaining mucociliary transport (MCT) and innate immunity in the airway,2,69,72,75 the known functions of ionocytes in other systems appear to be well aligned with CF phenotypes. Thus, determining whether ionocytes play a role in CF lung pathogenesis is needed to understand whether this cell type will be a required target for gene therapy.
Potential Mechanisms for Defected pH and Hydration in the CF Airway
The maintenance of an ASL layer with well-hydrated mucin is critical for effective MCT. In the absence of CFTR, ion and fluid transport across the airway epithelium is significantly impaired and this leads to dehydration of the ASL and impaired MCT.6,36 Dehydration of ASL in the CF airway is driven by ENaC, a serine protease-activated and pH-sensitive ENaC that absorbs Na+ from the ASL and thus also drives fluid absorption.108–111 CFTR conducts both Cl− and HCO3−, and defective HCO3− secretion in CF airway epithelia reduces the pH of the ASL.69,72,112 Given that serine proteases in the ASL are more active at a lower pH, the loss of CFTR-dependent HCO3− secretion has been proposed to be responsible for hyperactivity of ENaC in CF and dehydration of the ASL.111 CFTR has also been proposed to inhibit ENaC activity through direct physical interactions.113
Distinguishing these two mechanisms of CFTR-dependent ENaC regulation are noncell autonomous and cell-autonomous modes of action (i.e., whether or not CFTR has to be expressed in the same cell as ENaC). Determining these modes of actions has a direct implication for CF gene therapy approaches and the level of cellular specificity required for CFTR expression to properly regulate ENaC. Defective CFTR-dependent ion transport has also been shown to affect fluid absorption, with CF airway epithelia absorbing fluid more slowly than non-CF epithelia after a small volume challenge.114 The mechanism responsible for this defect includes reduced protease-dependent activation of ENaC in the absence of CFTR.
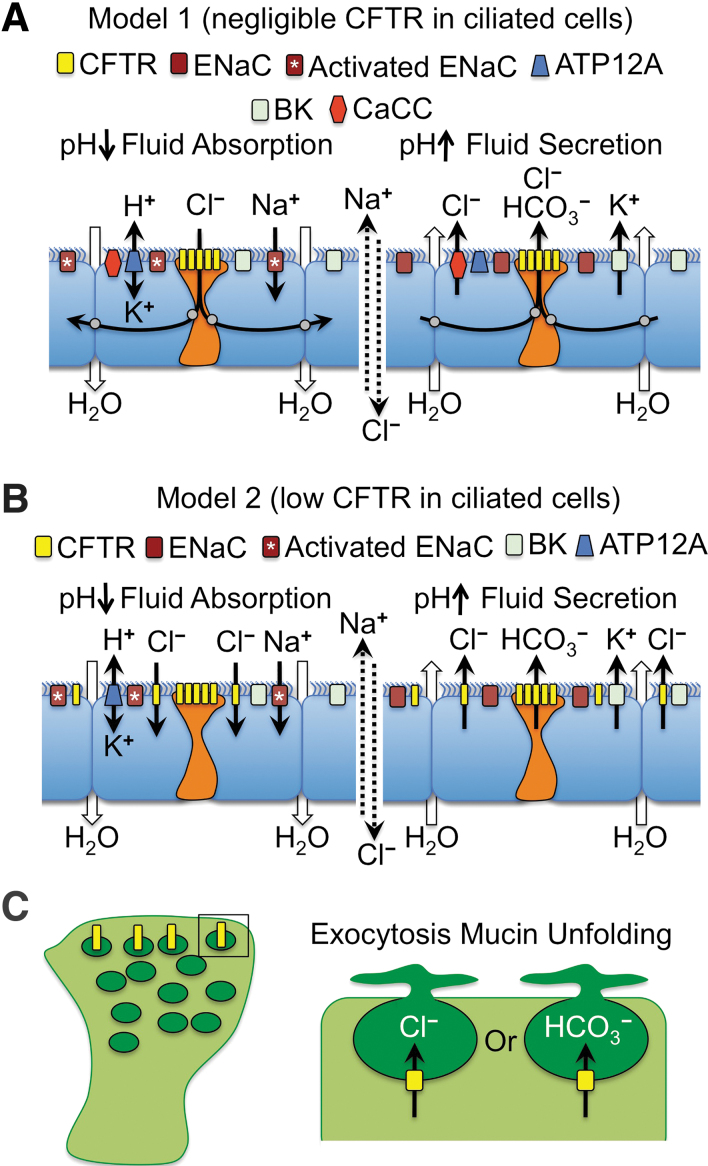
There are many potential mechanisms that may explain why CFTR expression is highly regulated at the cellular level in the airway. We will focus our discussion on two potential models that may control pH-dependent fluid movement in the airway and how they could be particularly important for designing gene therapy approaches (Fig. 2). These proposed mechanisms are based on known channels in the airway epithelium that participate in transepithelial ion and fluid transport together with CFTR36,115–117 and on scRNAseq data on the cell types that express CFTR.29,30 For simplicity, we have focused on the apical channels that may participate in ion transport and fluid movement at the airway surface, but we recognize that the activity of basolateral channels and the partitioning of their expression in various cell types of the airway is equally important in establishing electrical and chemical driving forces for channel activation.36,115,116 Both models propose that ionocytes participate in regulating ASL pH through CFTR-dependent HCO3− secretion to control ENaC activity. These two models also assume that fluid absorption and secretion are opposing forces that maintain ASL height at steady state in a normal airway epithelium.118
Figure 2.
Models for the multicellular integration of CFTR function in ion and fluid movement across the proximal airway epithelium. (A) Model-1 in which CFTR-expressing ionocytes perform the majority of Cl– and HCO3– transport required for regulating fluid movement. As proposed, ionocytes are electrically coupled with ciliated cells through gap junctions (gray circles). (B) Model-2 in which ciliated cells (or alternatively goblet secretory cells, not shown) participate in CFTR-dependent Cl– movement across the epithelium, whereas CFTR in ionocytes functions to transport HCO3–. In both models, pH regulation of the ASL controls ENaC activation, through either ionocyte CFTR-dependent HCO3– secretion to raise the ASL pH or ATP12A-dependent H+ secretion to lower the ASL pH. Lower ASL pH leads to ENaC activation and fluid absorption (left panels of A, B) and higher ASL pH leads to ENaC inactivation and fluid secretion (right panels of A, B). (C) Potential model for CFTR involvement in mucin exocytosis and unfolding in goblet secretory cells. The model proposes that CFTR (yellow) expression in mucin secretory vesicles may control Cl– and/or HCO3– movement required for exocytosis and/or mucin unfolding. ASL, airway surface liquid; ATP12A, nongastric H+/K+ ATPase; BK, voltage-dependent K+ channel; CaCC, calcium-activated chloride channel; ENaC, epithelial sodium channel.
The first model proposes that fluid absorption and secretion defects in CF are associated with CFTR defects in ionocytes (Fig. 2A). Under conditions of fluid absorption (Fig. 2A, left panel), this mechanism proposes that the nongastric H+/K+ ATPase (ATP12A) drives acidification of the ASL73 and the pH-dependent proteolytic activation of ENaC. Active Na+ absorption by ENaC creates an electrical driving force for Cl− absorption through ionocyte CFTR. We propose that CFTR mediates Cl− absorption since fluid absorption after a small volume challenge is impaired in CF ALI cultures.114 Since ionocytes are at such low abundance in the airway, we propose that they may be electrically coupled with ciliated cells through gap junctions that are capable of passing Cl− between cells, as previously suggested.119 Under conditions of fluid secretion (Fig. 2A, right panel), CFTR-dependent HCO3− secretion by ionocytes drives alkalinization of the ASL and inactivation of ENaC, as previously suggested.111 Cl− secretion could occur through ionocyte CFTR and/or the calcium-activated chloride channel (CaCC),36,115,116 whereas K+ secretion by the voltage-dependent K+ channels (BK) provides the counter ion for the movement of salt and fluid into the airway lumen.117 Importantly, when BK channels are inhibited in polarized airway epithelium, through either channel blockers or knockdown of the BK alpha subunit (KCNMA1), ASL dehydrates similarly to CF cultures.117
An alternative second model proposes that low-level CFTR expression in ciliated cells is functionally relevant to fluid homeostasis at the airway surface (Fig. 2B). Similar to Model 1, this model proposes that fluid absorption is driven by ATP12A-dependent acidification of the ASL and activation of ENaC to absorb Na+ (Fig. 2B, left panel). However, this model proposes that the overall low level of CFTR in ciliated cells collectively absorbs the predominance of Cl− and that ionocytes primarily function to secrete HCO3−, which is inactive during fluid absorption conditions. When fluid absorption passes the optimal ASL height and becomes slightly dehydrated, Model 2 proposes that ionocytes are activated to secrete HCO3−, potentially though mechanical forces,36 and the ASL pH rises leading to inactivation of ENaC (Fig. 2B, right panel). Cl− and K+ secretion through ciliated cell CFTR and BK channels leads to salt and fluid movement into the airways, raising ASL volume.
Although the two models just mentioned were chosen as provocative examples representing two cell types with extremely different levels of CFTR expression (ionocytes>>ciliated cells), secretory cells may also participate in transepithelial Cl− movement as they express CFTR at higher levels than ciliated cells based on scRNAseq. Secretory cells in the cartilaginous airways (i.e., goblet cells) also differ in their biology to those in the bronchioles (i.e., club cells) and thus CFTR may play different roles in these two types of secretory cells. For example, vesicular CFTR expression in goblet cells may play a role in exocytosis of mucin granules and the proper hydration and unfolding of mucin (Fig. 2C). By contrast, given that ionocyte abundance appears to decrease in the distal airways, CFTR expression at the apical membrane in club cells may play an important role in pH regulation and hydration of the ASL. Further, scRNAseq120 and in situ hybridization28 demonstrated that alveolar Type II (AT2) cells also express CFTR. Given their large numbers, AT2 cells collectively express the greatest total amount of CFTR in the lung. Although CFTR within AT2 cells is known to control fluid absorption in the newborn lung and in pulmonary edema,121–123 whether AT2 cells contribute to CF lung pathogenesis remains largely unknown.
Although these are only two potential models and many other possibilities exist that can explain how cell type-specific CFTR expression could impact innate immunity and clearance in the airway, they emphasize several key points regarding complementation of CF airway defects by gene therapy. For example, will it be necessary to express or correct CFTR within ionocytes to obtain proper airway pH and thus also regulation of ENaC? Can ciliated cells adopt ionocyte-like functions if CFTR is overexpressed using gene therapy? Or do ciliated cells not have the proper channel composition to properly regulate HCO3− secretion even if high levels of CFTR are expressed. Further, if CFTR plays a role in mucin secretion as a Cl− and/or HCO3− channel within mucin granules, complementation of this cell autonomous function would likely require CFTR expression within goblet and/or club cells.
CF Gene Therapy in Ferret and Pig Models of CF
Given the robust lung phenotype in CF ferrets and pigs, these models provide opportunities for the preclinical testing of gene therapy vector systems. Several recombinant vector systems have been evaluated in both species, including lentivirus, HDAd, bocavirus (BoV), and AAV. Each vector system has advantages and disadvantages. For example, lentiviruses are integrating viruses and thus provide longer-term persistence, whereas HDAd, BoV, and AAV have episomal genomes that dilute with cell division. AAV vectors suffer from packaging limitations, which are significant when delivering the large CFTR cDNA, whereas a related parvovirus vector using HBoV1 capsids has increased packaging capacity to enable the production of virus with the intact CFTR cDNA and a strong promoter. HDAd vectors have a very large packaging capacity, but carry significantly more foreign protein into cells, which may enhance immunogenicity. Recently, HDAd and rAAV vectors have been adapted to allow for integration of their genomes by using the PiggyBac transposase. Each of these vector systems and their application in ferret and pig models is discussed in more detail later.
rAAV vectors
The only viral requirements for rAAV vectors are two 145-nucleotide ITRs. However, the coding sequence of full-length CFTR is 4430 nucleotides. With the addition of the ITRs, the recombinant AAV genome exceeds its native size of 4,680 nucleotides, leaving little room for a promoter and polyadenylation sequence. Although rAAV vectors can effectively package up to 4,900 nucleotides, vector genomes containing CFTR greater than this size incur small deletions within the ends of the genome.16 Two approaches have been used to enhance the capacity of rAAV vectors to deliver CFTR. The first approach created CFTR minigenes that produced channels with similar functional characteristics to full-length CFTR.17,124 One of the better-characterized CFTR minigenes included a 156-nucleotide deletion in the CFTR regulatory (R) domain and was capable of rescuing intestinal obstruction in CF mice.125
The second approach to circumvent packaging limitations has been to reduce the size of promoters that drive CFTR expression and the use of small synthetic poly-A sequences.15,16,18,126 Promoters that can be packaged with a shortened CFTR minigene have included a minimal human cytomegalovirus (CMV) promoter containing core elements from the CMV immediate early promotor18 and thymidine kinase126 promoters/enhancers as well as synthetic promoter/enhancer elements.15,16 Using the latter approach, a synthetic enhancer-element screen identified a 100-nucleotide sequence (F5) that gave expression from an 83-nucleotide synthetic promoter (tg83) at levels equivalent to the CMV/beta-actin promoter/enhancer.16 When the F5tg83 promoter was used in conjunction with the CFTR-ΔR cDNA, the rAAV-CFTR-ΔR vector gave near normal levels of CFTR-mediated Cl− current in human CF airway epithelia.
Additional advances in rAAV transduction of airway epithelia have stemmed from the identification of alternative capsids that have high tropism for the apical surface of human airway epithelia38,127,128 and an improved understanding that AAV encounters an intracellular proteasome-dependent block during movement to the nucleus21,22,129 that can be overcome by co-administration of proteasome inhibitors.23,130 Directed evolution with an AAV2/AAV5 capsid library after multiple rounds of apical infection of ALI cultures has generated a novel AAV capsid variant (AAV2.5T) that is highly tropic for the apical surface of well-differentiated human airway epithelia in vitro.38
Collectively, these advances in rAAV vector systems have solved major obstacles encountered in the first rAAV2 CF clinical trials, including: (1) expression using small highly active promoters, (2) packaging capacity using a CFTR minigene, and (3) tropism to the airway surface using evolved capsids. In addition, the apical postentry barriers limiting productive rAAV transduction of polarized airway epithelium can be largely overcome by transient inhibition of the proteasome during or after the infection period.
Several of these rAAV vector systems have been tested in ferret and pig models and have demonstrated additional barriers to translating studies from human airway epithelium in vitro to animal models in vivo. For example, rAAV1 is highly effective at transducing well-differentiated human and ferret airway epithelia in the presence of proteasome inhibitors.127,131 rAAV1 can also transduce the neonatal ferret lung.24 However, when ferrets reach 1 month of age, rAAV1 transduction of the lung declines significantly due to airway secretions that inhibit postentry processing of the virus, which is refractory to proteasome inhibition.24 Similarly, AAV2.5T, which is highly effective at transducing well-differentiated human airway epithelium in vitro,38 does not transduce well-differentiated pig airway epithelium in vitro or in vivo. For these reasons, the group evolved an rAAV vector in pig airway in vivo,132 using a shuffled capsid library generated from AAV-1, -2, -4, -5, -6, -8, and -9 cap genes,133 an error-prone AAV2 library,134 and a diversified AAV2 capsid library generated by peptide replacement at four capsid surface loops.135 The isolated virus, called AAVH22, was highly tropic for pig airways.132 Further, when AAVH22 was used to package the F5tg83-pCFTR-ΔR expression cassette, it was effective at correcting Cl− currents, ASL pH, and bacterial killing in CF pigs.132 More recently, a system was developed to enhance the persistence of rAAV-CFTR genomes by incorporating PiggyBac transposase binding sites into the AAV vector and delivering a second AAV vector carrying the PiggyBac transposase.136 Although the complete system has yet to be tested in vivo, the CFTR-containing vector complemented disease endpoints when aerosolized in the lungs of CF pigs.
HBoV1 vectors
HBoV1 is an autonomously replicating human parvovirus commonly associated with acute respiratory tract infections in infants and young children.137,138 Unique to HBoV1 is its ability to reinfect children after seroconversion from a primary infection.139 HBoV1 has a genome size of 5,543 nucleotides that is considerably larger than the 4,700-nucleotide AAV genome. This provided opportunities to engineer a recombinant HBoV1 vector that could carry the full-length CFTR cDNA driven by a strong promoter. To this end, methods were developed to package the rAAV2 genome into the HBoV1 capsid. The resultant rAAV2/HBoV1 hybrid vector was highly tropic for well-differentiated human airway epithelia after apical infection; however, proteasome inhibitors were still required for maximal transduction.39 This vector system has the capacity to package viral genomes up to 5,800 nucleotides and thus may also be useful for gene editing approaches that require a larger payload and RNA-guided nuclease. Further, improved packaging systems are now enabling production of rAAV2/HBoV1 on par with that of rAAV2.140
Humans are the only known host for HBoV1 respiratory tract infections and this presents challenges for preclinical animal studies using the rAAV2/HBoV1-based vector systems. However, studies evaluating rAAV2/HBoV1 transduction in the ferret lung have proved promising and suggest that the CF ferret model could potentially be used to develop rAAV2/HBoV1 as a gene therapy vehicle.141 This study in wild-type ferrets demonstrated that repeat rAAV2/HBoV1 dosing to the lungs of 7-day- and 29-day-old animals was effective. Further, ferrets exposed to the virus at 7 days of age gave rise to fivefold higher levels of transduction after reinfection at 29 days of age, as compared with that observed in naive infected 29-day-old animals. The ability of HBoV1 capsids to reinfect ferrets is reminiscent of findings in young children.139
Lentiviral vectors
Unlike rAAV and rAAV/HBoV1 vectors, lentiviral vectors have the advantage of integrating into the target cell genome and thus may persist longer if stem or progenitor cells are transduced. A wide array of lentiviruses have been proposed for gene therapy of CF lung disease, including: human immunodeficiency virus,142–144 simian immunodeficiency virus,41,145 feline immunodeficiency virus (FIV),40,146,147 and equine infectious anemia virus (EIAV).148,149 Although these lentiviruses are not typically tropic for the airway epithelium, advances in pseudotyping the lentiviral envelope proteins have allowed for the development of efficient vector systems. For example, pseudotyping EIAV with influenza virus hemagglutinin148,149 and FIV with baculovirus GP64146 produces recombinant lentiviruses with high-level tropism for the apical surface of the airway. Improvements to the airway tropism of lentiviruses have also been made by using libraries of GP64 envelope mutants.150
Preclinical testing of lentiviral vectors has also been performed in pigs and ferrets. For example, recombinant EIAV pseudotyped with influenza A virus subtype H7 hemagglutinin highly transduces ferret bronchial and bronchiolar airway epithelium.151 FIV pseudotyped with GP64 also efficiently transduces ferret bronchiolar epithelium.151 Although these studies in ferrets have thus far only evaluated lentiviral transduction using reporter genes, in vivo studies in CF pigs have evaluated lentiviral-mediated CFTR delivery and the complementation of CFTR defects in excised tissues.152 These studies have demonstrated complementation of transepithelial cAMP-stimulated current, ASL pH, and bacterial killing defects observed in CF pig airways.
HDAd and Piggybac/adenovirus vectors
The first CF gene therapy trials targeting the nasal and lung epithelium were performed by using E1-deleted recombinant adenoviral vectors.153–160 CFTR gene transfer in these studies was low and the approach using first-generation E1-deleted adenovirus was subsequently abandoned due to concerns about safety caused by innate and acquired immunity against viral genes that remained within the vector. More recently, HDAd has been developed for CF lung gene therapy. HD-Ad has the advantage that all virally encoded genes are deleted from the vector. Although deletion of viral genes reduces adaptive T cell responses against virally infected cells, incoming viral capsid proteins can still mount a CD8+ T cell response through the presentation of viral capsid epitopes by infected dendritic cells.161 This limitation is universal to all protein capsid viruses (including rAAV), but since the adenovirus capsid is larger than AAV, the acquired immune responses to HD-Ad will likely be more severe. HD-Ad vectors have been tested in both pigs and ferrets and demonstrated to be highly efficient in gene transfer to the airways when tight junctions are disrupted with lysophosphatidylcholine to expose the basolateral coxsackievirus and adenovirus receptor for infection.151,162
Additional modifications to adenoviral vector systems have included the use of transposase-mediated integration of a transgene cassette. Using the DNA transposon piggyBac, a hybrid piggyBac/adenovirus vector can highly transduce the large and small conducting airways of pigs with a GFP reporter cassette when lysophosphatidylcholine is co-administered.163 Basal cells, a known stem/progenitor cell in the conducting airways, were also highly transduced, suggesting that this vector system could potentially provide long-term persistence when a transposase is co-delivered. Further, the delivery of an adenoviral vector expressing CFTR to CF pig airways corrected anion channel activity, ASL pH, and bacterial killing defects observed in the CF pig airways.163 Future work is needed to demonstrate the utility of this vector system to integrate CFTR.
Conclusions and Future Prospects
Thirty-six CF gene therapy clinical studies or trials have occurred worldwide in the 30 years since the CFTR gene was identified.164,165 Of these, only two clinical trials were designed and powered with a sufficient number of patients to assess efficacy by using pulmonary function tests, including rAAV213 and nonviral/plasmid.166 This review has focused on viral vectors for CF lung gene therapy and the role that larger CF animal models have in the development of these approaches.
Regardless of the vector used for CF lung gene therapy, there are shared knowledge gaps. Larger CF animal models that faithfully reproduce the human CF lung phenotype will play an important role addressing these questions. For example, will it be necessary to reconstitute the native cellular CFTR expression patterns in the lung to delay disease progression? What are the pathophysiologically most important cellular targets in the CF lung? Are SMGs required gene therapy targets for the treatment of CF lung disease? Recent advances in the ability to genetically engineer ferrets that are capable of conditional inactivation or reactivation of CFTR in a cell-specific manner will aid in understanding the cellular targets that are most critical for CF lung gene therapy.
Although not discussed in this review, the immune response to viral vector administration to the lung will likely become a major focus of research over the next decade as gene therapy trials for CF commence. For example, lessons from systemic delivery of AAV vectors have demonstrated that both humoral and cellular immunity against viral antigens can limit vector readministration and persistence of transgene expression, respectively. Solutions to these immunologic barriers may include transient immunosuppression and tolerization strategies using engineered host T cells. Larger animal models, which more accurately reflect the immune responses of humans and the CF disease state, will likely also have a significant role in developing these targeted immunosuppression strategies.
Lastly, the development of viral vector systems that perform similarly in humans and the CF animal models used to test efficacy of gene therapies may require alternative approaches, such as interspecies-directed evolution. The landscape of gene therapy is rapidly changing with the development of new gene editing tools and such an approach targeted at airway stem cells would solve potential technical hurdles if CFTR expression needs to be regulated at the cellular level for therapeutic efficacy. Although these newer technologies are not discussed in this review, they also rely heavily on the development of effective gene delivery systems. Thus, successful CFTR gene replacement therapies will likely inform next-generation gene editing approaches. Despite these challenges, there is tremendous excitement for CF lung gene therapy and the prospects of a mutation-agnostic cure for all CF patients.
Acknowledgments
The authors gratefully thank Dr. Jennifer Barr for editorial assistance in the preparation of this review.
Author Disclosure
Z.Y. and J.F.E. receive income for consulting with Spirovant Science. J.F.E. has sponsored research with Vertex Pharmaceuticals and Spirovant Science.
Funding Information
This work was supported by grants from the NIH (HL051670 and DK047967 to J.F.E.), Cystic Fibrosis Foundation (to J.F.E. and Z.Y.), the University of Iowa Center for Gene Therapy (DK054759 to J.F.E.), and the Roy J. Carver Chair in Molecular Medicine (to J.F.E.).
References
- 1. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073 [DOI] [PubMed] [Google Scholar]
- 2. Kunzelmann K, Schreiber R, Hadorn HB. Bicarbonate in cystic fibrosis. J Cyst Fibros 2017;16:653–662 [DOI] [PubMed] [Google Scholar]
- 3. Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 2015;372:351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wine JJ, Hansson GC, Konig P, et al. Progress in understanding mucus abnormalities in cystic fibrosis airways. J Cyst Fibros 2018;17:S35–S39 [DOI] [PubMed] [Google Scholar]
- 5. Zhou-Suckow Z, Duerr J, Hagner M, et al. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res 2017;367:537–550 [DOI] [PubMed] [Google Scholar]
- 6. Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 2007;58:157–170 [DOI] [PubMed] [Google Scholar]
- 7. Ratjen F, Bell SC, Rowe SM, et al. Cystic fibrosis. Nat Rev Dis Primers 2015;1:15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet 1998;351:1702–1703 [DOI] [PubMed] [Google Scholar]
- 9. Wagner JA, Messner AH, Moran ML, et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999;109:266–274 [DOI] [PubMed] [Google Scholar]
- 10. Wagner JA, Nepomuceno IB, Messner AH, et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther 2002;13:1349–1359 [DOI] [PubMed] [Google Scholar]
- 11. Aitken ML, Moss RB, Waltz DA, et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther 2001;12:1907–1916 [DOI] [PubMed] [Google Scholar]
- 12. Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004;125:509–521 [DOI] [PubMed] [Google Scholar]
- 13. Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther 2007;18:726–732 [DOI] [PubMed] [Google Scholar]
- 14. Flotte TR, Afione SA, Solow R, et al. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem 1993;268:3781–3790 [PubMed] [Google Scholar]
- 15. Zhang LN, Karp P, Gerard CJ, et al. Dual therapeutic utility of proteasome modulating agents for pharmaco-gene therapy of the cystic fibrosis airway. Mol Ther 2004;10:990–1002 [DOI] [PubMed] [Google Scholar]
- 16. Yan Z, Sun X, Feng Z, et al. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum Gene Ther 2015;26:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Wang D, Fischer H, et al. Efficient expression of CFTR function with adeno-associated virus vectors that carry shortened CFTR genes. Proc Natl Acad Sci U S A 1998;95:10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostedgaard LS, Rokhlina T, Karp PH, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A 2005;102:2952–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duan D, Yue Y, Yan Z, et al. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther 1998;9:2761–2776 [DOI] [PubMed] [Google Scholar]
- 20. Berry GE, Asokan A. Cellular transduction mechanisms of adeno-associated viral vectors. Curr Opin Virol 2016;21:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding W, Zhang L, Yan Z, et al. Intracellular trafficking of adeno-associated viral vectors. Gene Ther 2005;12:873–880 [DOI] [PubMed] [Google Scholar]
- 22. Duan D, Yue Y, Yan Z, et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000;105:1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan Z, Zak R, Luxton GW, et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol 2002;76:2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan Z, Sun X, Evans IA, et al. Postentry processing of recombinant adeno-associated virus type 1 and transduction of the ferret lung are altered by a factor in airway secretions. Hum Gene Ther 2013;24:786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conrad CK, Allen SS, Afione SA, et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther 1996;3:658–668 [PubMed] [Google Scholar]
- 26. Liu X, Luo M, Trygg C, et al. Biological differences in rAAV transduction of airway epithelia in humans and in old world non-human primates. Mol Ther 2007;15:2114–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engelhardt JF, Yankaskas JR, Ernst SA, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 1992;2:240–248 [DOI] [PubMed] [Google Scholar]
- 28. Engelhardt JF, Zepeda M, Cohn JA, et al. Expression of the cystic fibrosis gene in adult human lung. J Clin Invest 1994;93:737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montoro DT, Haber AL, Biton M, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018;560:319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plasschaert LW, Zilionis R, Choo-Wing R, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018;560:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc 2004;1:47–53 [DOI] [PubMed] [Google Scholar]
- 32. Dajani R, Zhang Y, Taft PJ, et al. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol 2005;32:548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoegger MJ, Fischer AJ, McMenimen JD, et al. Cystic fibrosis. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014;345:818–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang Q, Engelhardt JF. Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur J Hum Genet 1998;6:12–31 [DOI] [PubMed] [Google Scholar]
- 35. Berdiev BK, Cormet-Boyaka E, Tousson A, et al. Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J Biol Chem 2007;282:36481–36488 [DOI] [PubMed] [Google Scholar]
- 36. Wu D, Boucher RC, Button B, et al. An integrated mathematical epithelial cell model for airway surface liquid regulation by mechanical forces. J Theor Biol 2018;438:34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shei RJ, Peabody JE, Kaza N, et al. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis. Curr Opin Pharmacol 2018;43:152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Excoffon KJ, Koerber JT, Dickey DD, et al. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc Natl Acad Sci U S A 2009;106:3865–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan Z, Keiser NW, Song Y, et al. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol Ther 2013;21:2181–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang G, Slepushkin V, Zabner J, et al. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest 1999;104:R55–R62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mitomo K, Griesenbach U, Inoue M, et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol Ther 2010;18:1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cao H, Ouyang H, Ip W, et al. Testing gene therapy vectors in human primary nasal epithelial cultures. Mol Ther Methods Clin Dev 2015;2:15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang L, Button B, Gabriel SE, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol 2009;7:e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ostrowski LE, Yin W, Diggs PS, et al. Expression of CFTR from a ciliated cell-specific promoter is ineffective at correcting nasal potential difference in CF mice. Gene Ther 2007;14:1492–1501 [DOI] [PubMed] [Google Scholar]
- 45. Henderson AG, Ehre C, Button B, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 2014;124:3047–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramsey BW, Banks-Schlegel S, Accurso FJ, et al. Future directions in early cystic fibrosis lung disease research: an NHLBI workshop report. Am J Respir Crit Care Med 2012;185:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ballard ST, Trout L, Bebok Z, et al. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol 1999;277:L694–L699 [DOI] [PubMed] [Google Scholar]
- 48. Wang X, Zhang Y, Amberson A, et al. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol 2001;24:195–202 [DOI] [PubMed] [Google Scholar]
- 49. Semaniakou A, Croll RP, Chappe V. Animal models in the pathophysiology of cystic fibrosis. Front Pharmacol 2018;9:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilke M, Buijs-Offerman RM, Aarbiou J, et al. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros 2011;10 Suppl 2:S152–S171 [DOI] [PubMed] [Google Scholar]
- 51. Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev 1999;79:S193–S214 [DOI] [PubMed] [Google Scholar]
- 52. Guilbault C, Saeed Z, Downey GP, et al. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 2007;36:1–7 [DOI] [PubMed] [Google Scholar]
- 53. Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat 2000;197 Pt 3:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pack RJ, Al-Ugaily LH, Morris G, et al. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res 1980;208:65–84 [DOI] [PubMed] [Google Scholar]
- 55. Widdicombe JH, Chen LL, Sporer H, et al. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat 2001;198:207–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Birket SE, Davis JM, Fernandez CM, et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 2018;3 DOI: 10.1172/jci.insight.97199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fisher JT, Zhang Y, Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol 2011;742:311–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med 2011;17:478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olivier AK, Yi Y, Sun X, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 2012;122:3755–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rosen BH, Evans TIA, Moll SR, et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med 2018;197:1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun X, Olivier AK, Liang B, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol 2014;50:502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun X, Olivier AK, Yi Y, et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am J Pathol 2014;184:1309–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun X, Sui H, Fisher JT, et al. Disease phenotype of a ferret CFTR knockout model of cystic fibrosis. J Clin Invest 2010;120:3149–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun X, Yi Y, Liang B, et al. Incretin dysfunction and hyperglycemia in cystic fibrosis: role of acyl-ghrelin. J Cyst Fibros 2019;18:557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sun X, Yi Y, Xie W, et al. CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Endocrinology 2017;158:3325–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun X, Yi Y, Yan Z, et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci Transl Med 2019;11 DOI: 10.1126/scitranslmed.aau7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yi Y, Sun X, Gibson-Corley K, et al. A transient metabolic recovery from early life glucose intolerance in cystic fibrosis ferrets occurs during pancreatic remodeling. Endocrinology 2016;157:1852–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peng X, Alfoldi J, Gori K, et al. The draft genome sequence of the ferret (Mustela putorius furo) facilitates study of human respiratory disease. Nat Biotechnol 2014;32:1250–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012;487:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rogers CS, Hao Y, Rokhlina T, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest 2008;118:1571–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rogers CS, Stoltz DA, Meyerholz DK, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008;321:1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shah VS, Ernst S, Tang XX, et al. Relationships among CFTR expression, HCO3- secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc Natl Acad Sci U S A 2016;113:5382–5387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shah VS, Meyerholz DK, Tang XX, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 2016;351:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stoltz DA, Meyerholz DK, Pezzulo AA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2010;2:29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang XX, Ostedgaard LS, Hoegger MJ, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest 2016;126:879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xie Y, Ostedgaard L, Abou Alaiwa MH, et al. Mucociliary transport in healthy and cystic fibrosis pig airways. Ann Am Thorac Soc 2018;15:S171–S176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abu-El-Haija M, Ramachandran S, Meyerholz DK, et al. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Am J Pathol 2012;181:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Uc A, Olivier AK, Griffin MA, et al. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Navis A, Bagnat M. Loss of cftr function leads to pancreatic destruction in larval zebrafish. Dev Biol 2015;399:237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Navis A, Marjoram L, Bagnat M. Cftr controls lumen expansion and function of Kupffer's vesicle in zebrafish. Development 2013;140:1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tuggle KL, Birket SE, Cui X, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One 2014;9:e91253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stalvey MS, Havasi V, Tuggle KL, et al. Reduced bone length, growth plate thickness, bone content, and IGF-I as a model for poor growth in the CFTR-deficient rat. PLoS One 2017;12:e0188497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fan Z, Perisse IV, Cotton CU, et al. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight 2018;3 DOI: 10.1172/jci.insight.123529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bartlett JA, Ramachandran S, Wohlford-Lenane CL, et al. Newborn cystic fibrosis pigs have a blunted early response to an inflammatory stimulus. Am J Respir Crit Care Med 2016;194:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Keiser NW, Birket SE, Evans IA, et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol 2015;52:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Joo NS, Cho HJ, Khansaheb M, et al. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest 2010;120:3161–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mall MA, Danahay H, Boucher RC. Emerging concepts and therapies for mucoobstructive lung disease. Ann Am Thorac Soc 2018;15:S216–S226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stoltz DA, Rokhlina T, Ernst SE, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest 2013;123:2685–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ballard ST, Evans JW, Drag HS, et al. Pathophysiologic evaluation of the transgenic Cftr “Gut-Corrected” porcine model of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 2016;311:L779–L787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yan Z, McCray PB Jr., Engelhardt JF. Advances in gene therapy for cystic fibrosis lung disease. Hum Mol Genet 2019;28:R88–R94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yu M, Sun X, Tyler SR, et al. Highly efficient transgenesis in ferrets using CRISPR/Cas9-mediated homology-independent insertion at the ROSA26 locus. Sci Rep 2019;9:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vieira Braga FA, Kar G, Berg M, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med 2019;25:1153–1163 [DOI] [PubMed] [Google Scholar]
- 93. Travaglini KJ, Nabhan AN, Penland L, et al. A molecular cell atlas of the human lung from single cell RNA sequencing. 2019. bioRxiv DOI: 10.1101/742320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 2011;27:493–512 [DOI] [PubMed] [Google Scholar]
- 95. Liu X, Driskell RR, Engelhardt JF. Airway glandular development and stem cells. Curr Top Dev Biol 2004;64:33–56 [DOI] [PubMed] [Google Scholar]
- 96. Liu X, Driskell RR, Engelhardt JF. Stem cells in the lung. Methods Enzymol 2006;419:285–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu X, Engelhardt JF. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc 2008;5:682–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kreda SM, Mall M, Mengos A, et al. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell 2005;16:2154–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tizzano EF, O'Brodovich H, Chitayat D, et al. Regional expression of CFTR in developing human respiratory tissues. Am J Respir Cell Mol Biol 1994;10:355–362 [DOI] [PubMed] [Google Scholar]
- 100. Carraro G, Mulay A, Yao C, et al. Single cell reconstruction of human basal cell diversity in normal and IPF lung. Am J Respir Crit Care Med 2020. (submitted, in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mercer RR, Russell ML, Roggli VL, et al. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol 1994;10:613–624 [DOI] [PubMed] [Google Scholar]
- 102. Quigley IK, Stubbs JL, Kintner C. Specification of ion transport cells in the Xenopus larval skin. Development 2011;138:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yan JJ, Hwang PP. Novel discoveries in acid-base regulation and osmoregulation: a review of selected hormonal actions in zebrafish and medaka. Gen Comp Endocrinol 2019;277:20–29 [DOI] [PubMed] [Google Scholar]
- 104. Hwang PP. Ion uptake and acid secretion in zebrafish (Danio rerio). J Exp Biol 2009;212:1745–1752 [DOI] [PubMed] [Google Scholar]
- 105. Conte FP. Origin and differentiation of ionocytes in gill epithelium of teleost fish. Int Rev Cell Mol Biol 2012;299:1–25 [DOI] [PubMed] [Google Scholar]
- 106. Dubaissi E, Papalopulu N. Embryonic frog epidermis: a model for the study of cell-cell interactions in the development of mucociliary disease. Dis Model Mech 2011;4:179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vidarsson H, Westergren R, Heglind M, et al. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS One 2009;4:e4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Myerburg MM, Butterworth MB, McKenna EE, et al. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem 2006;281:27942–27949 [DOI] [PubMed] [Google Scholar]
- 109. Tan CD, Hobbs C, Sameni M, et al. Cathepsin B contributes to Na+ hyperabsorption in cystic fibrosis airway epithelial cultures. J Physiol 2014;592:5251–5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 2009;284:792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Garland AL, Walton WG, Coakley RD, et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci U S A 2013;110:15973–15978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Coakley RD, Grubb BR, Paradiso AM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A 2003;100:16083–16088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stutts MJ, Canessa CM, Olsen JC, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science 1995;269:847–850 [DOI] [PubMed] [Google Scholar]
- 114. Evans TI, Joo NS, Keiser NW, et al. Glandular proteome identifies antiprotease cystatin C as a critical modulator of airway hydration and clearance. Am J Respir Cell Mol Biol 2016;54:469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol 2017;313:L859–L872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med 2012;2:a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Manzanares D, Gonzalez C, Ivonnet P, et al. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem 2011;286:19830–19839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shamsuddin AKM, Quinton PM. Concurrent absorption and secretion of airway surface liquids and bicarbonate secretion in human bronchioles. Am J Physiol Lung Cell Mol Physiol 2019;316:L953–L960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Johnson LG, Olsen JC, Sarkadi B, et al. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet 1992;2:21–25 [DOI] [PubMed] [Google Scholar]
- 120. Xu Y, Mizuno T, Sridharan A, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 2016;1:e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2005;2:206–213 [DOI] [PubMed] [Google Scholar]
- 122. Fang X, Fukuda N, Barbry P, et al. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 2002;119:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Laube M, Bossmann M, Thome UH. Glucocorticoids distinctively modulate the CFTR channel with possible implications in lung development and transition into extrauterine life. PLoS One 2015;10:e0124833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ostedgaard LS, Zabner J, Vermeer DW, et al. CFTR with a partially deleted R domain corrects the cystic fibrosis chloride transport defect in human airway epithelia in vitro and in mouse nasal mucosa in vivo. Proc Natl Acad Sci U S A 2002;99:3093–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ostedgaard LS, Meyerholz DK, Vermeer DW, et al. Cystic fibrosis transmembrane conductance regulator with a shortened R domain rescues the intestinal phenotype of CFTR−/− mice. Proc Natl Acad Sci U S A 2011;108:2921–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang D, Fischer H, Zhang L, et al. Efficient CFTR expression from AAV vectors packaged with promoters—the second generation. Gene Ther 1999;6:667–675 [DOI] [PubMed] [Google Scholar]
- 127. Yan Z, Lei-Butters DC, Liu X, et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J Biol Chem 2006;281:29684–29692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yan Z, Lei-Butters DC, Keiser NW, et al. Distinct transduction difference between adeno-associated virus type 1 and type 6 vectors in human polarized airway epithelia. Gene Ther 2013;20:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ding W, Zhang LN, Yeaman C, et al. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther 2006;13:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yan Z, Zak R, Zhang Y, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol 2004;78:2863–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liu X, Luo M, Guo C, et al. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther 2007;14:1543–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Steines B, Dickey DD, Bergen J, et al. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight 2016;1:e88728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jang JH, Koerber JT, Kim JS, et al. An evolved adeno-associated viral variant enhances gene delivery and gene targeting in neural stem cells. Mol Ther 2011;19:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Maheshri N, Koerber JT, Kaspar BK, et al. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol 2006;24:198–204 [DOI] [PubMed] [Google Scholar]
- 135. Koerber JT, Klimczak R, Jang JH, et al. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol Ther 2009;17:2088–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cooney AL, Thornell IM, Singh BK, et al. A Novel AAV-mediated gene delivery system corrects CFTR function in pigs. Am J Respir Cell Mol Biol 2019;61:747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007;44:904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Deng Y, Gu X, Zhao X, et al. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One 2012;7:e34353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Meriluoto M, Hedman L, Tanner L, et al. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg Infect Dis 2012;18:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yan Z, Zou W, Feng Z, et al. Establishment of a high-yield recombinant adeno-associated virus/human bocavirus vector production system independent of bocavirus nonstructural proteins. Hum Gene Ther 2019;30:556–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yan Z, Feng Z, Sun X, et al. Human bocavirus type-1 capsid facilitates the transduction of ferret airways by adeno-associated virus genomes. Hum Gene Ther 2017;28:612–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Farrow N, Donnelley M, Cmielewski P, et al. Role of basal cells in producing persistent lentivirus-mediated airway gene expression. Hum Gene Ther 2018;29:653–662 [DOI] [PubMed] [Google Scholar]
- 143. Limberis M, Anson DS, Fuller M, et al. Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum Gene Ther 2002;13:1961–1970 [DOI] [PubMed] [Google Scholar]
- 144. Kobinger GP, Limberis MP, Somanathan S, et al. Human immunodeficiency viral vector pseudotyped with the spike envelope of severe acute respiratory syndrome coronavirus transduces human airway epithelial cells and dendritic cells. Hum Gene Ther 2007;18:413–422 [DOI] [PubMed] [Google Scholar]
- 145. Griesenbach U, Inoue M, Meng C, et al. Assessment of F/HN-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am J Respir Crit Care Med 2012;186:846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sinn PL, Burnight ER, Hickey MA, et al. Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J Virol 2005;79:12818–12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sinn PL, Hickey MA, Staber PD, et al. Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor alpha. J Virol 2003;77:5902–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Patel M, Giddings AM, Sechelski J, et al. High efficiency gene transfer to airways of mice using influenza hemagglutinin pseudotyped lentiviral vectors. J Gene Med 2013;15:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McKay T, Patel M, Pickles RJ, et al. Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Ther 2006;13:715–724 [DOI] [PubMed] [Google Scholar]
- 150. Sinn PL, Hwang BY, Li N, et al. Novel GP64 envelope variants for improved delivery to human airway epithelial cells. Gene Ther 2017;24:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yan Z, Stewart ZA, Sinn PL, et al. Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Hum Gene Ther Clin Dev 2015;26:38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cooney AL, Abou Alaiwa MH, Shah VS, et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight 2016;1:e88730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Boucher RC, Knowles MR, Johnson LG, et al. Gene therapy for cystic fibrosis using E1-deleted adenovirus: a phase I trial in the nasal cavity. The University of North Carolina at Chapel Hill. Hum Gene Ther 1994;5:615–639 [DOI] [PubMed] [Google Scholar]
- 154. Crystal RG, Jaffe A, Brody S, et al. A phase 1 study, in cystic fibrosis patients, of the safety, toxicity, and biological efficacy of a single administration of a replication deficient, recombinant adenovirus carrying the cDNA of the normal cystic fibrosis transmembrane conductance regulator gene in the lung. Hum Gene Ther 1995;6:643–666 [DOI] [PubMed] [Google Scholar]
- 155. Crystal RG, Mastrangeli A, Sanders A, et al. Evaluation of repeat administration of a replication deficient, recombinant adenovirus containing the normal cystic fibrosis transmembrane conductance regulator cDNA to the airways of individuals with cystic fibrosis. Hum Gene Ther 1995;6:667–703 [DOI] [PubMed] [Google Scholar]
- 156. Knowles MR, Hohneker KW, Zhou Z, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med 1995;333:823–831 [DOI] [PubMed] [Google Scholar]
- 157. Welsh MJ, Zabner J, Graham SM, et al. Adenovirus-mediated gene transfer for cystic fibrosis: part A. Safety of dose and repeat administration in the nasal epithelium. Part B. Clinical efficacy in the maxillary sinus. Hum Gene Ther 1995;6:205–218 [DOI] [PubMed] [Google Scholar]
- 158. Wilson JM, Engelhardt JF, Grossman M, et al. Gene therapy of cystic fibrosis lung disease using E1 deleted adenoviruses: a phase I trial. Hum Gene Ther 1994;5:501–519 [DOI] [PubMed] [Google Scholar]
- 159. Zabner J, Ramsey BW, Meeker DP, et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Invest 1996;97:1504–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zabner J, Couture LA, Gregory RJ, et al. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 1993;75:207–216 [DOI] [PubMed] [Google Scholar]
- 161. Kushwah R, Cao H, Hu J. Characterization of pulmonary T cell response to helper-dependent adenoviral vectors following intranasal delivery. J Immunol 2008;180:4098–4108 [DOI] [PubMed] [Google Scholar]
- 162. Cao H, Ouyang H, Grasemann H, et al. Transducing airway basal cells with a helper-dependent adenoviral vector for lung gene therapy. Hum Gene Ther 2018;29:643–652 [DOI] [PubMed] [Google Scholar]
- 163. Cooney AL, Singh BK, Loza LM, et al. Widespread airway distribution and short-term phenotypic correction of cystic fibrosis pigs following aerosol delivery of piggyBac/adenovirus. Nucleic Acids Res 2018;46:9591–9600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Donnelley M, Parsons DW. Gene therapy for cystic fibrosis lung disease: overcoming the barriers to translation to the clinic. Front Pharmacol 2018;9:1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Griesenbach U, Pytel KM, Alton EW. Cystic fibrosis gene therapy in the UK and elsewhere. Hum Gene Ther 2015;26:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Alton EW, Armstrong DK, Ashby D, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 2015;3:684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]