Abstract
HIV disclosure is an important behavior with implications for HIV treatment and prevention but understudied among new to HIV care patients who face unique challenges adjusting to a new diagnosis. This study evaluated the factors associated with HIV disclosure status and patterns of HIV disclosure among new to HIV care patients. A cross-sectional study was conducted evaluating the iENGAGE (integrating ENGagement and Adherence Goals upon Entry) cohort. Participants were enrolled in this randomized behavioral trial between December 2013 and June 2016. The primary and secondary outcomes included HIV disclosure status (Yes/No) and patterns of disclosure (Broad, Selective and Nondisclosure), respectively. Logistic and Multinomial Logistic Regression were used to evaluate the association of participant factors with HIV disclosure and patterns of HIV disclosure, respectively. Of 371 participants, the average age was 37 ± 12 years, 79.3% were males, and 62.3% were African Americans. A majority of participants (78.4%) disclosed their HIV status at baseline, 63.1% were broad disclosers and 15.2% were selective disclosers. In multivariable regression, black race, emotional support, and unmet needs predicted any HIV and broad disclosure, whereas males, emotional support, active coping, and acceptance were associated with selective disclosure. Interventions to promote early disclosure should focus on coping strategies and unmet needs, particularly among black and male people living with HIV initiating care.
Keywords: HIV disclosure, new to HIV care, coping, unmet needs, iENGAGE
Introduction
Informing other individual(s) or organization(s) about one's HIV infection status is defined as the process of HIV disclosure.1–4 HIV disclosure is an important behavior with implications for HIV prevention strategies and health outcomes in the lives of people living with HIV (PLWH)5 in resource-rich and poor countries.6 The benefits of HIV disclosure7 include increased opportunities for social support,6,8,9 improved engagement10 and retention in HIV care,11 earlier antiretroviral therapy (ART) initiation,12–14 and better ART adherence.10,15 HIV disclosure is also associated with decreased mental illnesses.15
However, there are chances of undesirable outcomes like discrimination, rejection, and stigma.16–18 Fear of disclosure and anticipated social rejection has also been linked to decline in HIV testing, particularly among young African American women.19 The rates of disclosure vary considerably across resource settings.3,20–22 The average rate of disclosure in developed countries varies from 42% to 100%11,23 and is about 72% in the United States.24
HIV disclosure is identified as a complex selective process25 with social implications.20 Prior studies on HIV disclosure vary considerably in addressing and categorizing HIV disclosure. For instance, disclosure is evaluated as disclosure to specific categories like family members, parents, friends, or sexual partners or to specific individuals like mother, father, partner or female relative.26–29 Disclosure was reported utmost to mothers30 and nonfamily members31,32 and lowest among past or casual sex partners.23 A study conducted among newly diagnosed HIV individuals in the United States showed the choice of disclosure include nondisclosure (disclosed to no one), selective/partial disclosure (disclosed to one person or group), and broad disclosure (disclosed to more than one person or group).17 This study evaluated sociodemographic factors, church attendance, and living arrangement and reported that about 13% of participants chose nondisclosure linked to black race, lower CD4 counts (<200 cells/μL), and living alone.17
Several factors have been associated with HIV disclosure and patterns of disclosure. Age, gender, race, ethnicity, education, marital status, education, time since HIV diagnosis, number of sexual partners, and sexual orientation have been found to correlate with HIV disclosure.8,28,33,34 However, results vary across studies contingent on study design, population, and outcome ascertainment.8,25,35–41
The majority of prior studies on HIV disclosure focus on patients established in HIV care,40,42 men who have sex with men (MSM),43–47 injection drug users (IDU), alcohol users,48 or women;13,30,49,50 few studies have focused on HIV patients who were not established in care.17,39,43,44,51–54 These studies were specific to populations such as ART-naive patients,39 patients initiating ART,55 those diagnosed who had less than 1 year of HIV infection,51,52 which were either focused on specific populations like MSM43,44 or new mothers.53 Also, the focus was on only a few specific factors and their relationship with HIV disclosure like condom use,44 depression,43 stress and coping during disclosure process,51 and CD4 response.55
A study among new to HIV care patients have so far evaluated sociodemographic factors, church attendance, and living arrangement.17 The association of specific coping behaviors, supportive services needed, and HIV treatment self-efficacy with HIV disclosure remains unmapped among patients new to HIV care. Additional studies are required to identify factors associated with disclosure23 especially among new to HIV care patients to achieve better HIV-related outcomes.17 To address these gaps, we evaluated the factors associated with HIV disclosure status and patterns of disclosure among patients new to outpatient HIV care enrolled in the iENGAGE (integrating ENGagement and Adherence Goals upon Entry) study (clinical trials.gov NCT01900236).
Methods
Study design and setting
We conducted a cross-sectional study evaluating the iENGAGE cohort. Participants were enrolled in this randomized behavioral trial between December 2013 and June 2016. The iENGAGE is an NIAID funded randomized controlled behavioral intervention trial evaluating the impact of a four-session counselor-delivered semitailored intervention implemented in a clinic setting (R01 AI 103661 and clinical trials.gov NCT01900236).
Patients new to outpatient HIV care were enrolled within 14 days of their initial primary HIV care provider appointment at four US HIV clinics: the University of Alabama at Birmingham (UAB), the University of North Carolina at Chapel Hill (UNC), the Johns Hopkins University (JHU), and the University of Washington at Seattle (UW). Clinic patients were eligible for study inclusion if they were adults 18 years and older, with documented HIV infection, who were initiating care at one of the four participating sites. Only English speaking, those not planning to move in the next 12 months and able/willing to provide informed consent patients were enrolled. Patients who received prior outpatient HIV care at any other facility or site were excluded. Institutional Review Board (IRB) approvals were obtained at each site for this study. Details of the iENGAGE study can be found elsewhere.56
As a part of the iENGAGE study, participants completed a study assessment at baseline (questionnaires) and at 48 weeks (questionnaire plus blood draw). Questionnaires were completed using CASI (computer-administered self-interview) that asked questions about mental health, alcohol use, substance use, sexual risk assessment, disclosure, social support, unmet needs, coping, and stigma using standardized, validated instruments.
All data were extracted from the iENGAGE database56a and Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) through electronic data queries. CNICS is a clinic-based cohort of HIV-infected patients across eight US HIV clinics and collects comprehensive clinical data using electronic medical records and other established sources at each clinic.57
Participant cohort
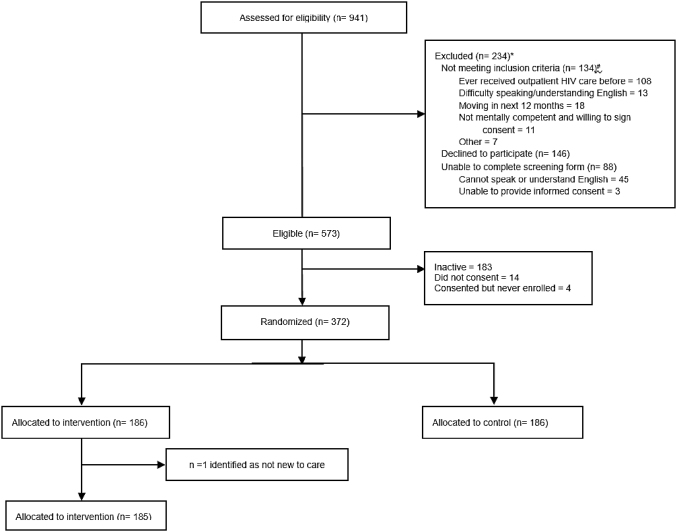
Of the 941 patients screened, 372 new to HIV care participants were enrolled in the iENGAGE study across sites (Fig. 1). One participant was found to be not new to care after being randomized to the intervention arm and was withdrawn from the study due to protocol violation, resulting in a sample size of 371.
FIG. 1.
Participant flow diagram for all new to care patients across four US HIV clinics enrolled in the iENGAGE study during 2013–2016. *93 of the 234 were not new to care participants. #There were 5 questions for participants to meet the inclusion criteria and participants can choose multiple reasons. iENGAGE, integrating ENGagement and Adherence Goals upon Entry.
Outcomes
For the current study, the primary outcome of interest was HIV disclosure status (Yes/No) and the secondary outcome was patterns of HIV disclosure (nondisclosure, selective disclosure, broad disclosure). Our classification of HIV disclosure draws reference from prior study conducted by Elopre et al.,17 which used the same disclosure questionnaire as implemented in the iENGAGE behavioral intervention trial.
Participants completed a three-item HIV disclosure questionnaire as part of the baseline CASI assessment. The following questions were asked to assess if participants disclosed HIV status: Q1—“Have you told anyone about your HIV status, not including your health provider?” (Responses: Yes/No/No response). If the participants responded “Yes” to Q1 they were asked two follow-up questions: Q2—“Have you told more than 1 person about your HIV status?” (Responses: Yes/No) and Q3—“Who have you told about your HIV status?” [Responses: Spouse/significant other, current sexual partner(s), past sexual partner(s), family member(s), friend(s), religious leader(s) (e.g., priest, rabbi, pastor/No response/NA—skip question)].
HIV disclosure status
HIV disclosure was defined as disclosure of HIV status to someone other than health care provider, that is, if participants responded “Yes” to Q1. For data analysis, HIV disclosure status was dichotomized as Yes/No variable.
Patterns of disclosure
Patterns of disclosure were categorized as nondisclosure, selective disclosure, and broad disclosure. Nondisclosure was defined as participants who did not disclose their HIV status to anyone other than the health care provider. Participants who responded “No” to Q1 were categorized as nondisclosers. Selective disclosure was defined as disclosed to only one group from the categorical response items. Participants who responded “No” to Q2, and did not disclose to more than one person and disclosed HIV status to only one group on Q3 [Spouse/significant other only, current sexual partner(s) only, past sexual partner(s) only, family member(s) only, friend(s) only, or religious leader(s) only] were categorized as selective disclosers. Broad disclosure was defined as disclosed to more than one group. Participants who responded “Yes” to Q2 and selected more than one group on Q3 were categorized as broad disclosers.
Patient-level factors
Sociodemographic variables
Sociodemographic variables included age (years), gender (male, female/transgender), race [white, black, other (Native American, Asian)], and ethnicity (Hispanic, non-Hispanic) were collected at the time of screening.
ART use
ART use at enrollment (Yes/No) was obtained from the CNICS data repository for participants across sites. Participants who started ART before or on the date of enrollment were grouped as “Yes.”
Baseline viral load value
Baseline laboratory value for plasma viral load (VL) was obtained from the CNICS data repository for participants across sites. The closest value to the enrollment date was recorded (preferably −90 days, +14 days). In instances where more than 2 values were available, the highest value was selected with a conservative assumption of higher value representing worse.
Baseline CD4 count
Baseline CD4 count at the time of entering HIV care was obtained using CNICS data repository for participants across sites. The closest value to study enrollment date was recorded (−90 days and +14 days). For data analysis CD4 count was categorized as <200 and ≥200 cells/μL of blood.
Psychosocial factors
At enrollment visit, participants completed questionnaires on psychosocial factors using CASI.56
Depression
The eight-item Patient Health Questionnaire (PHQ-8) was used to assess how often the depressive symptoms bothered participants over the past 2 weeks.58,59 A 4-point Likert-like scale (“not at all” = 0 to “nearly every day” = 3) was used to rate each question and scores ranged from 0 to 24. A score of <10 was considered no depressive disorder, ≥10 was considered major depression, and ≥20 was considered severe major depression. For analysis purposes, we dichotomized depression as yes/no variable.
Anxiety
The five-item PHQ-5 questionnaire was used to assess if participants experienced anxiety (sudden fear or panic) in the past 4 weeks.60 The response options were yes (score of 1)/no (score of 0). The composite score ranged from 0 to 5. Anxiety scores were categorized as no anxiety (score = 0), panic symptoms (score ≤4), and panic syndrome (score = 5). For analysis purposes, we dichotomized anxiety as yes/no variable.
Social support
The four-item abbreviated Medical Outcomes Study Social Support Survey (MOS-4) was used to measure perceived social support.61,62 Each question measured a different type of perceived support (informational, tangible, positive social interaction, affectionate). Items were rated on a 5-point scale ranging from “none of the time” (1) to “all of the time” (5). For data analysis, we used a composite score which ranges from 0 to 100.63 The higher the composite score, the greater the support received.
HIV stigma
HIV stigma was measured using Bunn and Earnshaw instruments.64,65 The domains assessed were enacted stigma, disclosure concerns, negative self-image or internalized stigma, and concerns with public attitudes about PLWH or public stigma.64 A 4-point Likert-like scale ranging from “strongly agree” (1) to “strongly disagree” (4) was used for rating. A composite score was calculated summing responses to all questions.64 Anticipated stigma to family, friends, and health care providers was measured. The responses ranged from “very unlikely” (1) to “very likely” (5).65 The higher the composite scores, the higher the stigma.64,65
Coping
Participant's coping skills were measured using an adapted brief cope questionnaire to assess 9 of the 14 domains: active coping, positive reframing, acceptance, religion, using emotional support, denial, substance use, behavioral disengagement, and self-blame. Each domain was measured using two items.66,67 Using emotional support, positive reframing, acceptance, and religion were perceived as adaptive coping strategies and denial, substance use, behavioral disengagement, and self-blame were perceived as maladaptive coping strategies.68 Items were rated on a 4-point Likert scale ranging from “not doing this at all” (1), to “doing this all the time” (4) and an average score was used for each domain.
Supportive services
Supportive services needed in the last 6 months were assessed using an instrument previously used in the CDC Retention in Care trial.69 Supportive services included counseling, substance use treatment, housing, emergency financial assistance, employment assistance, transportation, food, groceries or meals, benefits assistance, and childcare. For analysis purposes, services were classified into three categories: counseling/substance abuse treatment; housing expenditure (housing, transportation, food, groceries, meals, and childcare); and financial assistance (financial, employment, and benefits assistance).
Quality of life
EuroQOL-5D was used to measure the five health-related quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each quality was measured using a single question. Response for each question ranged from “no problems” (1) to “severe problems” (3).70
Self-efficacy
The 12-item HIV Adherence Self-Efficacy Scale (HIV-ASES) was used to measure self-efficacy in HIV treatment adherence. This questionnaire assessed patient's confidence to carry out important treatment-related behaviors.71 Answer choices ranged from “cannot do it at all” (0) to “certain can do it” (10). In addition, participants had option to select “refuse to answer” or “don't know.” Composite scores were calculated and the higher the score, greater is the adherence self-efficacy.71
Sexual risk factors
Participants completed questionnaires on alcohol use, substance use, and sexual behavior at the enrollment visit and HIV transmission risk factor was obtained using the CNICS data repository.
HIV transmission risk factor
HIV transmission risk factor was recorded as either MSM, IDU, or heterosexuals. For participants who reported multiple risk factors, IDU was given the priority followed by MSM and then heterosexual in the CNICS database.
Alcohol use
The three-item Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire was used to measure alcohol consumption during the past year among participants. AUDIT-C scores were categorized as no risk [score of 0–2 for men (M), 0–1 for women (W)], low risk (score of 3 for M, 2 for W), and high risk (score of 4 for M, 3 for W).72 The transgender patients were treated as females for AUDIT-C scores as males transitioned to females in our participant cohort.
Substance use
The Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) was used to measure substance use (cocaine/crack, amphetamines, opiates, injection drug use).73 For data analysis, substance use was categorized as Never (responded “no” to drug use), Prior (responded “never” used drugs in the past 3 months), and Current (responded used drugs once or twice, weekly, monthly, or daily in the past 3 months).74
Sexual behavior
HIV Risk Assessment for Positives was used to assess the number of sexual partners in the past 6 months. For data analysis, the number of sexual partners was categorized as 0, 1, 2, 3, 4–5, and ≥6 partners based upon the distribution of participant responses. As a smaller number of respondents reported ≥6 partners to be included as an individual count, these participants were collapsed into a single group.
Statistical analyses
Descriptive statistics were calculated as mean and standard deviation for continuous variables, and counts and percentages for categorical variables. Q-Q plots were used to determine normality of continuous variables.
Logistic regression was used to estimate odds ratios (ORs) and their respective 95% confidence intervals (CIs) to evaluate the association of risk factors with HIV disclosure status (Yes/No). To assess the association of risk factors with patterns of disclosure (nondisclosure, selective disclosure, broad disclosure) multinomial logistic regression was used to calculate ORs and their respective CIs.
The variables included in the adjusted models were based on the evidence from the literature, recommendations from expert clinicians, and statistical significance (<0.10) in unadjusted models. We further employed a stepwise method to generate the parsimonious models. Variables were added in the model based on their clinical significance and p-values from the unadjusted model. If the variable was not significant, it was deleted to build a parsimonious model that best explained the relationship of factors with disclosure. A two-sided p-value of <0.05 was considered significant for this analysis. All analyses were done using SAS 9.4 version.
Results
The average age of participants was 37 ± 12 years, 79.3% were males, 62.3% were African Americans, and 94.6% non-Hispanics (Table 1). Overall, 78.4% of the participants disclosed their HIV status at baseline, 63.1% were broad disclosers, and 15.2% were selective disclosers. Among participants who disclosed, 30.8% reported depression and the percentage was similar for broad disclosers (28.7%) compared with selective disclosers (34%) or nondisclosers (34.6%). However, a higher percentage of participants who disclosed their HIV status reported anxiety symptoms (33.2%) compared with participants who did not disclose (25%). Current substance use was reported by a lower percentage of participants who disclosed (16.9%) compared with participants who did not disclose (21.1%). The higher average score indicative of better active coping was reported by broad disclosers (score = 6.8) compared with selective disclosers (score = 7) and nondisclosers (score = 6). Detectable baseline VL value (≥200) was reported by 93.8% and 69.3% reported no baseline ART use (Table 1). Table 2 presents the unadjusted association of factors with HIV disclosure status and patterns of disclosure.
Table 1.
Baseline Patient Characteristics by HIV Disclosure Status and Patterns of HIV Disclosure at the Four US HIV Clinics Enrolled in the iENGAGE Study During 2013–2016 (n = 371)
| Variables | Overall, n = 371 | HIV disclosure, n = 370 |
Disclosure patterns, n = 369 |
|||
|---|---|---|---|---|---|---|
| Yes, n = 290 | No, n = 80 | Nondisclosure, n = 80 | Selective disclosure, n = 56 | Broad disclosure, n = 233 | ||
| Sociodemographic factors | ||||||
| Age, years | 37.1 ± 12 | 36.8 ± 12 | 38.4 ± 12.1 | 38.4 ± 12.1 | 37.2 ± 13 | 36.6 ± 11.7 |
| Sex | ||||||
| Male | 294 (79.3) | 227 (78.3) | 66 (82.5) | 66 (82.5) | 39 (69.6) | 188 (80.7) |
| Female | 71 (19.1) | 57 (19.7) | 14 (17.5) | 14 (17.5) | 15 (26.8) | 41 (17.6) |
| Transgender | 6 (1.6) | 6 (2.1) | 0 | 0 | 2 (3.6) | 4 (1.7) |
| Race | ||||||
| Black | 231 (62.3) | 163 (56.2) | 67 (83.8) | 67 (83.8) | 41 (73.2) | 121 (52.9) |
| White | 109 (29.4) | 98 (33.8) | 11 (13.8) | 11 (13.8) | 10 (17.9) | 88 (37.8) |
| Other | 31 (8.4) | 29 (10.0) | 2 (2.5) | 2 (2.5) | 5 (8.9) | 24 (10.3) |
| Ethnicity | ||||||
| Hispanic | 20 (5.4) | 19 (6.6) | 1 (1.3) | 1 (1.3) | 3 (5.4) | 16 (6.9) |
| Non-Hispanic | 351 (94.6) | 271 (93.5) | 79 (98.8) | 79 (98.8) | 53 (94.6) | 217 (92.1) |
| Insurance | ||||||
| None | 87 (23.6) | 71 (24.7) | 15 (19) | 15 (19) | 13 (23.2) | 58 (25.1) |
| Private | 107 (29.1) | 87 (30.2) | 20 (25.3) | 20 (25.3) | 19 (33.9) | 68 (29.4) |
| Public | 174 (47.3) | 130 (45.1) | 44 (55.7) | 44 (55.7) | 24 (42.9) | 105 (45.5) |
| ART | ||||||
| Yes | 114 (30.7) | 88 (30.3) | 25 (31.3) | 25 (31.3) | 18 (32.1) | 70 (30) |
| No | 257 (69.3) | 202 (69.7) | 55 (68.8) | 55 (68.8) | 38 (67.9) | 163 (70) |
| Baseline CD4 count, cells/mL of blood | ||||||
| <200 | 85 (24.3) | 65 (24.4) | 20 (26.7) | 20 (26.7) | 10 (18.9) | 55 (25.9) |
| 200–300 | 83 (24.9) | 60 (22.6) | 23 (30.7) | 23 (30.7) | 11 (20.8) | 49 (23.1) |
| ≥350 | 174 (50.9) | 141 (53.0) | 32 (42.7) | 32 (42.7) | 32 (60.4) | 108 (50.9) |
| Baseline VL value, copies/mL of blood | ||||||
| <200 | 16 (4.3) | 15 (5.2) | 1 (1.3) | 1 (1.3) | 2 (3.6) | 13 (5.6) |
| ≥200 | 348 (93.8) | 269 (92.8) | 78 (97.5) | 78 (97.5) | 53 (94.6) | 215 (92.3) |
| Missing | 7 (1.9) | 6 (2.1) | 1 (1.3) | 1 (1.3) | 1 (1.8) | 5 (2.2) |
| Psychosocial factors | ||||||
| Depression | ||||||
| No | 241 (69.3) | 189 (70.3) | 51 (65.4) | 51 (65.4) | 35 (66) | 154 (71.3) |
| Yes | 107 (30.8) | 80 (29.7) | 27 (34.6) | 27 (34.6) | 18 (34) | 62 (28.7) |
| Anxiety | ||||||
| Yes | 113 (31.4) | 94 (33.2) | 19 (25) | 19 (25) | 15 (27.3) | 78 (34.4) |
| No | 247 (68.6) | 189 (66.8) | 57 (75) | 57 (75) | 40 (72.7) | 149 (65.6) |
| Social support score | 57 ± 29.1 | 59.4 ± 29.3 | 48.5 ± 26.9 | 48.5 ± 26.9 | 54.8 ± 27.6 | 60.5 ± 29.6 |
| Quality of life | ||||||
| No mobility | 317 (85.9) | 250 (86.5) | 66 (83.5) | 66 (83.5) | 45 (80.4) | 205 (88.4) |
| No self-care | 358 (97.3) | 280 (97.2) | 77 (97.5) | 77 (97.5) | 54 (98.2) | 225 (97) |
| No usual activities | 300 (81.1) | 236 (81.7) | 63 (78.8) | 63 (78.8) | 47 (83.9) | 188 (81) |
| No pain | 209 (57) | 165 (57.7) | 43 (53.8) | 43 (53.8) | 30 (54.6) | 135 (58.7) |
| No depression/anxiety | 163 (44.7) | 129 (44.8) | 35 (43.8) | 35 (43.8) | 27 (49.1) | 101 (43.5) |
| Stigma | ||||||
| Enacted stigma | 2.2 ± 0.7 | 2.2 ± 0.7 | 2.3 ± 0.7 | 2.3 ± 0.7 | 2.4 ± 0.8 | 2.1 ± 0.7 |
| Disclosure concerns | 3.1 ± 0.6 | 3.0 ± 0.6 | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.3 ± 0.5 | 3.0 ± 0.6 |
| Internalized stigma | 2.3 ± 0.7 | 2.3 ± 0.7 | 2.4 ± 0.8 | 2.4 ± 0.8 | 2.5 ± 0.7 | 2.2 ± 0.7 |
| Public stigma | 2.7 ± 0.7 | 2.7 ± 0.7 | 2.8 ± 0.7 | 2.8 ± 0.7 | 2.9 ± 0.7 | 2.6 ± 0.7 |
| Anticipated stigma | ||||||
| Family | 2.7 ± 1.4 | 2.7 ± 1.4 | 2.8 ± 1.3 | 2.8 ± 1.3 | 2.9 ± 1.4 | 2.6 ± 1.4 |
| Friends | 2.8 ± 1.3 | 2.7 ± 1.2 | 3.1 ± 1.3 | 3.1 ± 1.3 | 3.0 ± 1.3 | 2.6 ± 1.2 |
| Health care provider | 1.8 ± 0.9 | 1.8 ± 0.9 | 1.7 ± 0.9 | 1.7 ± 0.9 | 2.0 ± 1.1 | 1.8 ± 0.9 |
| Coping behavior | ||||||
| Active coping | 6.7 ± 1.7 | 6.9 ± 1.6 | 6 ± 2.2 | 6 ± 2.2 | 7 ± 1.3 | 6.8 ± 1.6 |
| Denial | 3.6 ± 1.9 | 3.5 ± 1.9 | 3.7 ± 1.9 | 3.7 ± 1.9 | 4 ± 1.9 | 3.4 ± 1.9 |
| Substance use | 3.1 ± 1.8 | 3.2 ± 19 | 2.9 ± 1.5 | 2.9 ± 1.5 | 3.0 ± 2.0 | 3.2 ± 1.8 |
| Emotional support | 5.2 ± 2.2 | 5.6 ± 2.0 | 3.6 ± 1.9 | 3.6 ± 1.9 | 4.7 ± 2.1 | 5.8 ± 2 |
| Behavioral disengagement | 2.7 ± 1.2 | 2.7 ± 1.3 | 2.6 ± 1.2 | 2.6 ± 1.2 | 2.5 ± 1.2 | 2.7 ± 1.3 |
| Positive reframing | 5.7 ± 2 | 5.8 ± 1.9 | 5.3 ± 2.0 | 5.3 ± 2.0 | 5.5 ± 2.0 | 5.9 ± 1.9 |
| Acceptance | 6.8 ± 1.5 | 6.9 ± 1.4 | 6.5 ± 1.8 | 6.5 ± 1.8 | 6.5 ± 1.7 | 7 ± 1.4 |
| Religion | 5.5 ± 2.2 | 5.5 ± 2.2 | 5.3 ± 2.3 | 5.3 ± 2.3 | 6.1 ± 2.1 | 5.3 ± 2.2 |
| Self-blame | 4.7 ± 2.1 | 4.7 ± 2.1 | 4.7 ± 2.2 | 4.7 ± 2.2 | 4.6 ± 2.2 | 4.7 ± 2.1 |
| HIV treatment self-efficacy | 9 ± 1.5 | 9 ± 1.5 | 9 ± 1.3 | 9 ± 1.3 | 8.9 ± 1.8 | 9 ± 1.4 |
| Supportive services needed in last 6 months | ||||||
| Financial assistancea | 179 (49.2) | 137 (48.2) | 42 (52.5) | 42 (52.5) | 22 (40) | 114 (50) |
| Household expenditureb | 194 (52.9) | 145 (50.5) | 49 (61.3) | 49 (61.3) | 22 (40.7) | 122 (52.6) |
| Substance use treatment or counseling | 125 (34) | 109 (37.9) | 16 (20) | 16 (20) | 11 (19.6) | 98 (42.3) |
| Sexual risk factors | ||||||
| Transmission risk | ||||||
| MSM | 219 (60) | 175 (61.4) | 43 (54.4) | 43 (54.4) | 35 (62.5) | 218 (60.1) |
| Heterosexual | 117 (32.1) | 87 (30.5) | 30 (38) | 30 (38) | 20 (35.7) | 66 (29) |
| IDU | 29 (8) | 23 (8.1) | 6 (7.6) | 6 (7.6) | 1 (1.8) | 22 (9.7) |
| Sex partners | ||||||
| 0 | 76 (20.5) | 59 (20.3) | 17 (21.25) | 17 (21.3) | 11 (19.6) | 48 (20.6) |
| 1 | 100 (27) | 74 (25.5) | 26 (32.50) | 26 (32.5) | 19 (33.9) | 55 (23.6) |
| 2 | 55 (14.8) | 38 (13.1) | 17 (21.25) | 17 (21.3) | 5 (8.9) | 32 (13.7) |
| 3 | 38 (10.2) | 31 (10.7) | 7 (8.75) | 7 (8.8) | 9 (16.1) | 22 (9.4) |
| 4–5 | 40 (10.8) | 33 (11.4) | 6 (7.50) | 6 (7.5) | 8 (14.3) | 25 (10.7) |
| ≥6 | 62 (16.8) | 55 (19) | 7 (8.75) | 7 (8.8) | 4 (7.1) | 51 (21.9) |
| Alcohol use | ||||||
| No risk | 191 (52.3) | 143 (50.2) | 48 (61.5) | 48 (61.5) | 33 (60) | 110 (48) |
| Low risk | 46 (12.6) | 35 (12.3) | 11 (14.10) | 11 (14.1) | 7 (12.7) | 28 (12.2) |
| High risk | 127 (34.9) | 107 (37.5) | 19 (24.4) | 19 (24.4) | 15 (27.3) | 91 (39.7) |
| Substance use | ||||||
| Never | 198 (55.8) | 148 (53.2) | 50 (65.8) | 50 (65.8) | 38 (69.1) | 110 (49.3) |
| Prior | 93 (26.2) | 83 (29.9) | 10 (13.2) | 10 (13.2) | 10 (18.2) | 73 (32.7) |
| Current | 64 (18) | 47 (16.9) | 16 (21.1) | 16 (21.1) | 7 (12.7) | 40 (17.9) |
| Other factors | ||||||
| Site | ||||||
| UAB | 153 (41.2) | 111 (38.3) | 42 (52.5) | 42 (52.5) | 21 (37.5) | 90 (38.6) |
| UNC | 76 (20.5) | 62 (21.4) | 13 (16.3) | 13 (16.3) | 13 (23.2) | 49 (21) |
| JHU | 78 (21) | 63 (21.7) | 15 (18.8) | 15 (18.8) | 17 (30.4) | 45 (19.3) |
| UW | 64 (17.3) | 54 (18.6) | 10 (12.5) | 10 (12.5) | 5 (8.9) | 49 (21) |
| Study arm | ||||||
| Control | 186 (50.1) | 146 (50.3) | 40 (50) | 40 (50) | 28 (50) | 118 (50.6) |
| Intervention | 185 (49.9) | 144 (49.7) | 40 (50) | 40 (50) | 28 (50) | 115 (49.4) |
Numbers in the table represent n (%) for categorical variables and mean ± standard deviation for continuous variables.
Financial assistance category includes financial, employment, benefits assistance.
Household expenditure category includes housing, transportation, food, groceries, meals, and childcare.
ART, antiretroviral therapy; IDU, injection drug users; iENGAGE, integrating ENGagement and Adherence Goals upon Entry; JHU, Johns Hopkins University at Baltimore; MSM, men who have sex with men; UAB, University of Alabama at Birmingham; UNC, University of North Carolina at Chapel Hill; UW, University of Washington at Seattle; VL value, viral load value.
Table 2.
Unadjusted Logistic and Multinomial Logistic Regression Models for HIV Disclosure Status and Patterns of HIV Disclosure at the Four US HIV Clinics Enrolled in the iENGAGE Study During 2013–2016
| Variables | HIV disclosure (n = 370) |
HIV disclosure patterns (n = 369) |
|
|---|---|---|---|
| Yes, OR (95% CI) | Selective disclosure, OR (95% CI) | Broad disclosure, OR (95% CI) | |
| Sociodemographic factors | |||
| Age, years, 10 unit change | 0.90 (0.73–1.10) | 0.99 (0.97–1.02) | 0.99 (0.97–1.01) |
| Gender | |||
| Male | 0.76 (0.40–1.45) | 0.49 (0.22–1.10) | 0.89 (0.46–1.72) |
| Female/transgender | Ref. | Ref. | Ref. |
| Race | |||
| Black | 0.28 (0.14–0.54) | 0.67 (0.26–1.72) | 0.23 (0.11–0.45) |
| Other | 1.63 (0.34–7.77) | 2.75 (0.43–17.49) | 1.5 (0.31–7.23) |
| White | Ref. | Ref. | Ref. |
| Ethnicity | |||
| Hispanic | 5.54 (0.73–42.02) | 4.47 (0.45–44.14) | 5.83 (0.76–44.64) |
| Non-Hispanic | Ref. | Ref. | Ref. |
| Insurance | |||
| None | 1.09 (0.52–2.28) | 0.91 (0.35–2.41) | 1.14 (0.53–2.42) |
| Public | 0.68 (0.38–1.23) | 0.57 (0.26–1.28) | 0.70 (0.38–1.29) |
| Private | Ref. | Ref. | Ref. |
| ART | |||
| Yes | 0.96 (0.56–1.64) | 1.04 (0.50–2.17) | 0.95 (0.55–1.64) |
| No | Ref. | Ref. | Ref. |
| Baseline CD4 count, cells/mL of blood | |||
| 200–350 | 0.80 (0.40–1.60) | 0.96 (0.34–2.72) | 0.78 (0.38–1.58) |
| ≥350 | 1.36 (0.72–2.55) | 2.00 (0.81–4.93) | 1.23 (0.64–2.34) |
| <200 | Ref. | Ref. | Ref. |
| Baseline VL value, copies/mL of blood | |||
| ≥200 | 0.23 (0.03–1.77) | 0.34 (0.03–3.84) | 0.21 (0.03–1.65) |
| Missing | 0.40 (0.02–7.48) | 0.50 (0.01–19.56) | 0.39 (0.02–7.40) |
| <200 | Ref. | Ref. | Ref. |
| Psychosocial factors | |||
| Depression | |||
| Yes | 0.80 (0.47–1.37) | 0.97 (0.47–2.03) | 0.76 (0.44–1.32) |
| No | Ref. | Ref. | Ref. |
| Anxiety | |||
| Yes | 1.49 (0.84–2.65) | 1.13 (0.51–2.48) | 1.57 (0.87–2.8) |
| No | Ref. | Ref. | Ref. |
| Social support score | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | 1.02 (1.01–1.02) |
| Quality of life | |||
| No mobility | 1.26 (0.64–2.51) | 0.81 (0.33–1.96) | 1.50 (0.73–3.07) |
| No self-care | 0.91 (0.19–4.37) | 1.40 (0.12–15.81) | 0.84 (0.17–4.11) |
| No usual activities | 1.20 (0.65–2.22) | 1.41 (0.58–3.44) | 1.15 (0.62–2.16) |
| No pain | 1.17 (0.71–1.93) | 1.03 (0.52–2.06) | 1.22 (0.73–2.04) |
| No depression/anxiety | 1.04 (0.63–1.72) | 1.24 (0.62–2.47) | 0.99 (0.59–1.66) |
| Stigma | |||
| Enacted stigma | 0.81 (0.56–1.17) | 1.23 (0.74–2.05) | 0.74 (0.50–1.08) |
| Disclosure concerns | 0.68 (0.44–1.07) | 1.52 (0.79–2.94) | 0.57 (0.36–0.92) |
| Internalized stigma | 0.82 (0.58–1.16) | 1.33 (0.82–2.16) | 0.73 (0.51–1.05) |
| Public stigma | 0.85 (0.58–1.23) | 1.36 (0.80–2.33) | 0.75 (0.51–1.11) |
| Anticipated stigma | |||
| Family | 0.92 (0.76–1.10) | 1.01 (0.79–1.31) | 0.89 (0.74–1.08) |
| Friends | 0.79 (0.65–0.97) | 0.98 (0.74–1.30) | 0.76 (0.62–0.93) |
| Health care provider | 1.10 (0.83–1.45) | 1.29 (0.90–1.84) | 1.05 (0.79–1.39) |
| Coping | |||
| Active coping | 1.29 (1.11–1.49) | 1.35 (1.07–1.70) | 1.27 (1.10–1.48) |
| Denial | 0.97 (0.84–1.11) | 1.09 (0.90–1.31) | 0.94 (0.81–1.08) |
| Substance use | 1.10 (0.95–1.29) | 1.06 (0.86–1.30) | 1.12 (0.95–1.32) |
| Emotional support | 1.62 (1.40–1.88) | 1.33 (1.10–1.61) | 1.71 (1.47–2.00) |
| Behavioral disengagement | 1.09 (0.87–1.36) | 0.96 (0.69–1.34) | 1.12 (0.89–1.40) |
| Positive reframing | 1.17 (1.02–1.33) | 1.06 (0.88–1.28) | 1.19 (1.04–1.36) |
| Acceptance | 1.19 (1.02–1.39) | 1.01 (0.82–1.26) | 1.25 (1.06–1.47) |
| Religion | 1.03 (0.92–1.16) | 1.18 (1.00–1.39) | 1.00 (0.89–1.12) |
| Self-blame | 1.00 (0.89–1.13) | 0.98 (0.83–1.15) | 1.00 (0.89–1.13) |
| HIV treatment self-efficacy | 0.99 (0.84–1.18) | 0.95 (0.76–1.19) | 1.01 (0.85–1.21) |
| Supportive service needs in last 6 months | |||
| Financial assistancea | 1.03 (0.68–1.56) | 0.60 (0.30–1.21) | 0.91 (0.54–1.51) |
| Household expenditureb | 0.65 (0.39–1.07) | 0.44 (0.22–0.88) | 0.70 (0.42–1.18) |
| Substance use treatment or counseling | 2.44 (1.34–4.43) | 0.98 (0.42–2.30) | 2.93 (1.60–5.37) |
| Sexual risk factors | |||
| Transmission risk | |||
| MSM | 1.40 (0.82–2.39) | 1.22 (0.59–2.51) | 1.48 (0.85–2.57) |
| IDU | 1.32 (0.49–3.56) | 0.25 (0.03–2.24) | 1.67 (0.61–4.53) |
| Heterosexual | Ref. | Ref. | Ref. |
| Sex partners | |||
| 1 | 0.82 (0.41–1.65) | 1.13 (0.43–2.96) | `0.75 (0.36–1.55) |
| 2 | 0.64 (0.29–1.41) | 0.46 (0.13–1.59) | 0.67 (0.30–1.50) |
| 3 | 1.28 (0.48–3.41) | 1.99 (0.57–6.90) | 1.11 (0.40–3.07) |
| 4–5 | 1.59 (0.57–4.41) | 2.06 (0.56–7.58) | 1.48 (0.52–4.21) |
| ≥6 | 2.26 (0.87–5.88) | 0.88 (0.21–3.74) | 2.58 (0.98–6.77) |
| 0 | Ref. | Ref. | Ref. |
| Alcohol use | |||
| Low risk | 1.07 (0.50–2.27) | 0.93 (0.33–2.64) | 1.11 (0.51–2.41) |
| High risk | 1.89 (1.05–3.40) | 1.15 (0.51–2.58) | 2.09 (1.15–3.81) |
| No risk | Ref. | Ref. | Ref. |
| Substance use | |||
| Prior | 2.80 (1.35–5.82) | 1.32 (0.50–3.48) | 3.31 (1.58–6.96) |
| Current | 0.99 (0.52–1.90) | 0.58 (0.22–1.54) | 1.14 (0.58–2.22) |
| Never | Ref. | Ref. | Ref. |
| Other factors | |||
| Site | |||
| UAB | 0.49 (0.23–1.05) | 1.00 (0.30–3.30) | 0.44 (0.20–0.95) |
| UNC | 0.88 (0.36–2.18) | 2.00 (0.53–7.49) | 0.77 (0.31–1.92) |
| JHU | 0.78 (0.32–1.87) | 2.27 (0.63–8.14) | 0.61 (0.25–1.50) |
| UW | Ref. | Ref. | Ref. |
| Study arm | |||
| Intervention | 0.99 (0.60–1.62) | 1.0 (0.51–1.98) | 0.98 (0.59–1.62) |
| Control | Ref. | Ref. | Ref. |
Financial assistance category includes financial, employment, benefits assistance.
Household expenditure category includes housing, transportation, food, groceries, meals, and childcare.
ART, antiretroviral therapy; CI, confidence interval; IDU, injection drug users; iENGAGE, integrating ENGagement and Adherence Goals upon Entry; JHU, Johns Hopkins University at Baltimore; MSM, men who have sex with men; OR, odds ratio; UAB, University of Alabama at Birmingham; UNC, University of North Carolina at Chapel Hill; UW, University of Washington at Seattle; VL value, viral load value.
HIV disclosure status
In the final parsimonious logistic regression model, blacks had significantly lower odds of disclosure compared with whites (OR = 0.28; 95% CI = 0.13–0.58). Greater use of emotional support as coping behavior was associated with significantly higher disclosure (OR = 1.62; 95% CI = 1.39–1.89). The odds of disclosure was double among participants receiving substance use or counseling services in the last 6 months (OR = 2.07; 95% CI = 1.05–4.07) compared with those who did not (Table 3). MSM individuals were more likely to disclose compared with heterosexuals as shown in the unadjusted analysis but the results were not statistically significant (Table 2) and transmission risk factor variable was not eventually added to the parsimonious model.
Table 3.
Adjusted Logistic Regression Models for HIV Disclosure Status at the Four US HIV Clinics Enrolled in the iENGAGE Study During 2013–2016
| Variables | HIV disclosure (Yes/No) Adjusted model, n = 223 |
HIV disclosure (Yes/No) Parsimonious model, n = 348 |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Age | 0.98 (0.94–1.02) | |
| Gender | ||
| Male | 0.14 (0.03–0.75) | |
| Female | Ref. | |
| Race | ||
| Black | 0.46 (0.15–1.43) | 0.28 (0.13–0.58) |
| Othera | 1.24 (0.15–9.96) | 1.77 (0.35–9.01) |
| White | Ref. | Ref. |
| Ethnicity | ||
| Hispanic | 3.43 (0.24–49.16) | |
| Non-Hispanic | Ref. | |
| Substance use | ||
| Prior | 4.07 (1.02–16.14) | |
| Current | 0.30 (0.07–1.33) | |
| Never | Ref. | |
| Alcohol use | ||
| Low risk | 1.18 (0.28–4.98) | |
| High risk | 2.31 (0.79–6.72) | |
| No risk | Ref. | |
| Depression | ||
| Yes | 0.83 (0.26–2.65) | |
| No | Ref. | |
| Anxiety | ||
| Yes | 1.21 (0.41–3.53) | |
| No | Ref. | |
| Supportive services in last 6 months | ||
| Substance use treatment or counseling | 2.59 (0.91–7.40) | 2.07 (1.05–4.07) |
| Housing expenditureb | 0.71 (0.27–1.82) | |
| Baseline CD4 count, cells/mL of blood | ||
| 200–350 | 0.69 (0.19–2.59) | |
| >350 | 0.79 (0.24–2.66) | |
| <200 | Ref. | |
| Coping behavior | ||
| Active coping | 1.37 (1.04–1.82) | |
| Use of emotional support | 1.61 (1.22–2.11) | 1.62 (1.39–1.89) |
| Acceptance | 0.89 (0.64–1.23) | |
| Positive reframing | 0.78 (0.57–1.07) | |
| Anticipated Stigma from friends | 1.01 (0.67–1.50) | |
| Social support score | 1.01 (0.99–1.02) | |
| Transmission risk | ||
| MSM | 1.80 (0.50–6.44) | |
| IDU | 3.45 (0.44–27.08) | |
| Heterosexual | Ref. | |
| Sex partners | ||
| 1 | 0.30 (0.06–1.40) | |
| 2 | 0.38 (0.08–1.89) | |
| 3 | 0.40 (0.06–2.62) | |
| 4–5 | 0.68 (0.12–3.93) | |
| ≥6 | 0.39 (0.07–2.27) | |
| 0 | Ref. | |
| Site | ||
| UAB | 0.77 (0.20–3.03) | |
| UNC | 3.27 (0.64–16.55) | |
| JHU | 2.07 (0.42–10.24) | |
| UW | Ref | |
Other race category includes Native American, Asian, or other.
Household expenditure category includes housing, transportation, food, groceries, meals, and childcare.
CI, confidence interval; IDU, injection drug users; iENGAGE, integrating ENGagement and Adherence Goals upon Entry; JHU, Johns Hopkins University at Baltimore; MSM, men who have sex with men; OR, odds ratio; UAB, University of Alabama at Birmingham; UNC, University of North Carolina at Chapel Hill; UW, University of Washington at Seattle.
Bold values indicate statistical significance where a two-sided p-value was <0.05.
Patterns of disclosure
In the final parsimonious multinomial logistic regression model, blacks had significantly lower odds of broad disclosure (OR = 0.23; 95% CI = 0.10–0.53) compared with whites. Participants who reported use of emotional support as coping behavior (OR = 1.75; 95% CI = 1.45–2.12) and need for substance use treatment or counseling in the past 6 months (OR = 2.47; 95% CI = 1.12–5.51) had significantly higher odds of broad disclosure. Greater use of emotional support (OR = 1.42; 95% CI = 1.13–1.79) and active coping (OR = 1.43; 95% CI = 1.07–1.90) were associated with higher selective disclosure, whereas greater use of acceptance (OR = 0.73; 95% CI = 0.55–0.96) was associated with lower selective disclosure. Males (OR = 0.28; 95% CI = 0.09–0.85) were associated with lower odds of selective disclosure (Table 4).
Table 4.
Adjusted Multinomial Logistic Regression Models for Patterns of HIV Disclosure at the Four US HIV Clinics Enrolled in the IENGAGE Study During 2013–2016
| Variables | Patterns of HIV disclosure Adjusted model, n = 234 |
Patterns of HIV disclosure Parsimonious model, n = 300 |
||
|---|---|---|---|---|
| Selective disclosure, OR (95% CI) | Broad disclosure, OR (95% CI) | Selective disclosure, OR (95% CI) | Broad disclosure, OR (95% CI) | |
| Age | 0.98 (0.93–1.03) | 0.98 (0.95–1.02) | ||
| Gender | ||||
| Male | 0.27 (0.05–1.43) | 0.39 (0.12–1.60) | 0.28 (0.09–0.85) | 0.54 (0.21–1.42) |
| Female | Ref. | Ref. | Ref. | Ref. |
| Race | ||||
| Black | 0.62 (0.13–3.05) | 0.30 (0.10–0.94) | 0.66 (0.22–2.03) | 0.23 (0.10–0.53) |
| Othera | 3.21 (0.26–40.19) | 1.21 (0.15–10.15) | 4.75 (0.67–33.61) | 1.74 (0.32–9.30) |
| White | Ref. | Ref. | Ref. | Ref. |
| Ethnicity | ||||
| Hispanic | 0.70 (0.02–23.16) | 3.40 (0.25–46.75) | ||
| Non-Hispanic | Ref. | Ref. | ||
| Social support score | 1.01 (0.99–1.04) | 1.00 (0.98–1.02) | ||
| Alcohol use | ||||
| Low risk | 0.78 (0.13–4.72) | 0.79 (0.20–3.18) | ||
| High risk | 1.16 (0.34–3.95) | 1.74 (0.68–4.43) | ||
| Supportive services needed in last 6 months | ||||
| Substance use treatment or counseling | 0.48 (0.11–2.08) | 2.49 (0.95–6.53) | 0.58 (0.19–1.84) | 2.47 (1.12–5.51) |
| Household expenditureb | 0.69 (0.21–2.24) | 0.92 (0.37–2.30) | ||
| Baseline CD4 count (cells/mL of blood) | ||||
| 200–350 | 0.64 (0.10–4.05) | 0.75 (0.22–2.49) | ||
| >350 | 2.92 (0.65–13.14) | 0.73 (0.24–2.22) | ||
| <200 | Ref. | Ref. | ||
| Coping | ||||
| Active coping | 1.34 (0.90–1.99) | 1.19 (0.91–1.55) | 1.43 (1.07–1.90) | 1.07 (0.88–1.32) |
| Use of emotional support | 1.36 (0.97–1.89) | 1.73 (1.34–2.23) | 1.42 (1.13–1.79) | 1.75 (1.45–2.12) |
| Behavioral disengagement | 0.94 (0.32–2.76) | 1.62 (0.79–3.31) | ||
| Acceptance | 0.59 (0.37–0.92) | 0.80 (0.56–1.13) | 0.73 (0.55–0.96) | 0.95 (0.75–1.19) |
| Positive reframing | 0.82 (0.55–1.21) | 0.88 (0.66–1.18) | ||
| Religion | 1.88 (0.9–3.64) | 1.01 (0.64–1.60) | ||
| Anticipated stigma from friends | 1.10 (0.57–2.12) | 0.92 (0.58–1.46) | ||
| Site | ||||
| UAB | 0.41 (0.05–3.18) | 0.83 (0.22–3.15) | ||
| UNC | 1.32 (0.16–11.32) | 2.99 (0.63–14.15) | ||
| JHU | 0.78 (0.10–6.10) | 0.90 (0.21–3.87) | ||
| UW | Ref. | Ref. | ||
| Stigma | ||||
| Disclosure concerns | 2.10 (0.57–7.69) | 0.89 (0.35–2.26) | ||
| Negative self-image | 0.97 (0.38–2.48) | 0.58 (0.27–1.24) | ||
Other includes Native American, Asian, and other race.
Household expenditure category includes housing, transportation, food, groceries, meals, and childcare.
CI, confidence interval; iENGAGE, integrating ENGagement and Adherence Goals upon Entry; JHU, Johns Hopkins University at Baltimore; OR, odds ratio; UAB, University of Alabama at Birmingham; UNC, University of North Carolina at Chapel Hill; UW, University of Washington at Seattle.
Bold values indicate statistical significance where a two-sided p-value was <0.05.
Discussion
Early disclosure is an important HIV prevention and treatment strategy.75,76 Prior studies have demonstrated that in developed countries with rich resource settings, PLWH choose to disclose their HIV status to obtain psychosocial support and eliminate the stress of keeping HIV diagnosis private,77–79 whereas in developing countries with poor resource settings, HIV disclosure allows patients to have financial, material, and emotional support, including help with access to care and counseling.6,33,80–83 Yet little is known about factors associated with early HIV disclosure among new to care patients.17,23 In this cross-sectional study among new to HIV care patients enrolled within 14 days of their HIV primary care appointment across four US HIV clinics, we found that the odds of any HIV and broad disclosure were significantly lower in blacks (p = 0.001) and higher among patients who reported unmet needs of substance use treatment or counseling (p = <0.004). Greater use of emotional support was significantly associated with higher HIV and broad disclosure (p = <0.001). The odds of selective disclosure was significantly lower among men compared with women (p = 0.02). Greater use of emotional support (p = 0.003) and active coping (p = 0.01) were associated with higher selective disclosure, whereas greater use of acceptance (p = 0.02) was associated with lower use of selective disclosure. Coping behaviors were found to be associated with all types of disclosure, and may represent a modifiable factor for behavioral interventions to enhance disclosure among new to care PLWH.
We observed that men were 46% less likely to broadly disclose and 72% less likely to selectively disclose their HIV status compared with females after adjusting for other variables in the analysis. Our results are consistent with prior studies among new patients seeking HIV care.17,52 Furthermore, the fear of being perceived as a homosexual, which may not be accepted culturally may result in nondisclosure.8 A study conducted among patients within 6 months of HIV diagnosis showed that about 55.6% of males did not disclose their HIV status.84 Buma (2015) showed that only 15% of males disclosed their HIV status before starting ART.39 Our results contradict the findings of another cross-sectional study conducted among HIV patients enrolled within a year of diagnosis, where there was no difference in the odds of disclosure among males and females.85 The difference in results is likely due to differences in populations engaged in the study. Additionally, in the unadjusted analysis, we examined transmission risk factors and found that MSM were more likely to disclose their HIV status compared with the heterosexual group, although the results were not significant. These results may suggest that males who are MSM were more likely to disclose as suggested by another prior study.17
Black race was associated with lower odds of HIV disclosure to anyone (72%), broad (77%), and selective disclosure (34%). Results from a prior study showed that blacks were four times more likely to nondisclosure and about two times more likely to selective disclosure compared with broad disclosure.17 Blacks are more susceptible to stigma from cultural context,51 especially anticipated social rejection being a key reason among black women19 resulting in nondisclosure. Greater racial diversity in the neighborhood was shown to be associated with lesser internalized stigma and perceived health care discrimination among women (cohort with 59% black women) with HIV infection or at risk for HIV infection in the United States.86,86a,87 Brief disclosure intervention (BDI) by Greene and colleagues, which includes disclosure advice and disclosure strategies, increasing patient awareness and empowerment to navigate disclosure decisions and interventions tailored to reduce stigma, including component of peer group support86a,88,89 may be beneficial in reducing the disparity. In addition, blacks have increased depressive symptoms from the stress of the new diagnosis and adjustment disorder resulting in nondisclosure and social isolation.43 Our results were in a similar direction with other studies, except for some differences in the magnitude of results, which could be attributed to diverse geographic HIV clinic data used in our study, which was the single site for the prior study.
To our knowledge, this is the first study to assess the association of need for supportive services and HIV disclosure among new to HIV care patients. The initial year of HIV care is challenging and it is important to address unmet needs in this cohort. In this study, the need for substance use treatment or counseling services was significantly associated with almost two times the odds of disclosure to anyone and broad disclosure. PLWH face significant challenges related to substance use90 and mental health issues.91–93 Furthermore, among new to care patients, these challenges are exaggerated with the added stress of coming to frequent medical appointments, taking regular medications and learning the skills to navigate through the diagnosis during this initial year. Hence, they may choose to disclose to more people to gain social support and help with other necessities. Our results suggest that addressing unmet needs during initial HIV primary care appointment is important. It is possible that HIV-infected individuals in need of substance use or counseling services may be dealing with multiple health-related issues and disclosed to the social network broadly rather being selective to be able to get all the help required to address different issues. Future studies among the larger cohort of new to HIV care patients may provide more insight on the role of unmet needs and its association with the early disclosure; addressing these needs during the initial visit would be beneficial and unmet needs be a focus for intervention targeted for new to HIV care patients.
Interestingly, the trends for higher use of different adaptive coping strategies were toward better disclosure. There was significant increase in disclosure to anyone, broad and selective disclosure compared with nondisclosure for increased use of active coping and emotional support in unadjusted analysis. In adjusted analysis, the results remained statistically significant for use of emotional support for all disclosures and active coping for selective disclosure. It is probably because a patient actively trying to cope with the diagnosis seeks support by disclosing. We found that the higher the acceptance, the lower the disclosure. One possible explanation is participants who may have accepted their HIV diagnosis may not have felt the need to disclose their HIV status to gain support. Based on the unadjusted and adjusted results of this study, focusing on adaptive and maladaptive coping strategies may motivate newly diagnosed patients to disclose their serostatus early and achieve better HIV-related outcomes. Use of different coping strategies may depend on the outcomes of disclosure. One prior study indicated that nondisclosure actually became a coping strategy after experiencing negative outcomes from initial disclosure.51 Nevertheless, our results are supportive of using coping strategies as a part of the intervention for early HIV disclosure for patients new to HIV to aid disclosure. Individual- and community-level interventions, including health care agencies, community-based organizations etc. to promote culturally competent practices to care and support for PLWH,94 or adopting prior coping interventions with components of HIV informational support, seeking social support (family, friends), networking, and maintaining positive attitude after HIV diagnosis, have been reported to be effective.95 We recommend future studies to explore the relationship of type and magnitude of each coping strategy and early HIV disclosure among new to HIV care patients. Studies evaluating patient/partner intervention models96 with focus on adaptive coping strategies would be an interesting step forward.
Our study provides an understanding of the association of several factors with HIV disclosure status and patterns of disclosure and adds to the existing literature among new to HIV care patients. The results of our study add to the future efforts to build HIV disclosure-specific interventions for new to HIV care patients. We have a geographically diverse sample population and a geographically diverse cohort of individuals who have never received outpatient HIV care before.
We recognize limitations of this study. The cross-sectional design of this study did not allow for assessment of temporal relationship and no inferences on causality can be made. However, our associations can gauge the strength of effect and possibility of potential factors to consider. Patterns of HIV disclosure results, particularly broad disclosure, should be interpreted with context, as the disclosure questionnaire used for the iENGAGE study did not capture information on the number of individual participants disclosed to in each category which is valuable information and needs to be captured in future studies for in-depth insights. Results of the study may not be generalized beyond the geographic areas covered by the iENGAGE study, but the sites used for study implementation are representative of national estimates. Association of HIV disclosure to retention in care and VL suppression was not assessed but is an important next step, which our group is working on. Data collected during the iENGAGE study is self-reported and there is a possibility of recall bias or information bias. However, prior studies have shown that self-reported data are acceptable for capturing HIV behaviors.97
Our study found that black race, emotional support, and unmet needs were associated with any HIV and broad disclosure, and males, emotional support, active coping, and acceptance were associated with selective disclosure. Interventions of early HIV disclosure targeted for new to HIV care patients may require a multifaceted approach and focus on coping strategies and unmet needs as intervention components. Future studies on early HIV disclosure in larger cohorts of PLWH may provide insight on evidence-based intervention recommendations for new to HIV care patients.
Compliance with Ethical Standards
Ethics approval
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study, as approved by the local Institutional Review Boards at each study site.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The iENGAGE study was supported by the National Institutes of Allergy and Infectious Diseases (NIAID) grant (5R01AI103661-05). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. Michael J. Mugavero has received grant support (to the University of Alabama at Birmingham) from Bristol-Myers Squibb and consulting fees from Gilead Sciences.
References
- 1. Adejumo AO. Perceived HIV stigmatization, HIV/AIDS cognition and personality as correlates of HIV self-disclosure among people living with HIV in Ibadan, Nigeria. Gend Behav 2011;9:3854–3869 [Google Scholar]
- 2. Klopper CE. Factors Influencing HIV Status Disclosure. Stellenbosch: Stellenbosch University, 2011. [Google Scholar]
- 3. Philogene J. Patterns of HIV Serostatus Disclosure Among HIV-Positive Young Adults in Haiti: A Mixed-Methods Investigation. Durham, NC: Duke University, 2014 [Google Scholar]
- 4. The Well Project. Disclosure and HIV. Available at: www.thewellproject.org/hiv-information/disclosure-and-hiv (Last accessed April19, 2018)
- 5. Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: UNAIDS, 2014 [Google Scholar]
- 6. Obermeyer CM, Baijal P, Pegurri E. Facilitating HIV disclosure across diverse settings: A review. Am J Public Health 2011;101:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atuyambe LM, Ssegujja E, Ssali S, et al. HIV/AIDS status disclosure increases support, behavioural change and, HIV prevention in the long term: A case for an Urban Clinic, Kampala, Uganda. BMC Health Serv Res 2014;14:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouillon K, Lert F, Sitta R, Schmaus A, Spire B, Dray-Spira R. Factors correlated with disclosure of HIV infection in the French Antilles and French Guiana: Results from the ANRS-EN13-VESPA-DFA Study. AIDS 2007;21(Suppl 1):S89–S94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogoina D, Ikuabe P, Ebuenyi I, Harry T, Inatimi O, Chukwueke O. Types and predictors of partner reactions to HIV status disclosure among HIV-infected adult Nigerians in a tertiary hospital in the Niger Delta. Afr Health Sci 2015;15:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: A systematic review. AIDS 2012;26:2059–2067 [DOI] [PubMed] [Google Scholar]
- 11. Maman S, Medley A. Gender Dimensions of HIV Status Disclosure to Sexual Partners: Rates, Barriers and Outcomes. Geneva: World Health Organization, 2004. [Google Scholar]
- 12. Ding Y, Li L, Ji G. HIV disclosure in rural China: Predictors and relationship to access to care. AIDS Care 2011;23:1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gari T, Habte D, Markos E. HIV positive status disclosure among women attending art clinic at Hawassa University Referral Hospital, South Ethiopia. East Afr J Public Health 2010;7:87–91 [PubMed] [Google Scholar]
- 14. Go VF, Latkin C, Le Minh N, et al. Variations in the role of social support on disclosure among newly diagnosed HIV-infected people who inject drugs in Vietnam. AIDS Behav 2016;20:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stirratt MJ, Remien RH, Smith A, et al. The role of HIV serostatus disclosure in antiretroviral medication adherence. AIDS Behav 2006;10:483–493 [DOI] [PubMed] [Google Scholar]
- 16. Derlega VJ, Winstead BA, Greene K, Serovich J, Elwood WN. Perceived HIV-related stigma and HIV disclosure to relationship partners after finding out about the seropositive diagnosis. J Health Psychol 2002;7:415–432 [DOI] [PubMed] [Google Scholar]
- 17. Elopre L, Westfall AO, Mugavero MJ, et al. Predictors of HIV disclosure in infected persons presenting to establish care. AIDS Behav 2016;20:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siegel K, Lune H, Meyer IH. Stigma management among gay/bisexual men with HIV/AIDS. Qual Sociol 1998;21:3–24 [Google Scholar]
- 19. Cheong J, Tucker JA, Chandler SD. Reasons for accepting and declining free HIV testing and counseling among young African American women living in disadvantaged southern urban communities. AIDS Patient Care STDS 2019;33:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, de Wit J, Qiao S, Sherr L. HIV disclosure to children in low-and middle-income countries: Towards effective interventions. AIDS 2015;29(Suppl 1):S1–S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramos JV, Mmbaga BT, Turner EL, et al. Modality of primary HIV disclosure and association with mental health, stigma, and antiretroviral therapy adherence in Tanzanian youth living with HIV. AIDS Patient Care STDS 2018;32:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. UNAIDS. The Gap Report. 2014. Available at: https://www.un.org/en/africa/osaa/pdf/unsystemfolder/2014/unaids2014.pdf (Last accessed January27, 2014).
- 23. World Health Organization. HIV Status Disclosure to Sexual Partners: Rates, Barriers and Outcomes for Women. Geneva, Switzerland: World Health Organization, 2004 [Google Scholar]
- 24. Mattson CL, Freedman M, Beer L, Sullivan P, Skarbinski J. Prevalence and Predictors of HIV Disclosure Among Adults Receiving Medical Care in the United States. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2009 [Google Scholar]
- 25. Sullivan KM. Male self-disclosure of HIV-positive serostatus to sex partners: A review of the literature. J Assoc Nurses AIDS Care 2005;16:33–47 [DOI] [PubMed] [Google Scholar]
- 26. Mayfield Arnold E, Rice E, Flannery D, Rotheram-Borus MJ. HIV disclosure among adults living with HIV. AIDS Care 2008;20:80–92 [DOI] [PubMed] [Google Scholar]
- 27. Elopre L, Hook EW, Westfall AO, et al. The role of early HIV status disclosure in retention in HIV care. AIDS Patient Care STDS 2015;29:646–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lan CW, Li L, Lin C, Feng N, Ji G. Community disclosure by people living with HIV in rural China. AIDS Educ Prev 2016;28:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiener L, Mellins CA, Marhefka S, Battles HB. Disclosure of an HIV diagnosis to children: History, current research, and future directions. J Dev Behav Pediatr 2007;28:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armistead L, Morse E, Forehand R, Morse P, Clark L. African-American women and self-disclosure of HIV infection: Rates, predictors, and relationship to depressive symptomatology. AIDS Behav 1999;3:195–204 [Google Scholar]
- 31. Hays RB, McKusick L, Pollack L, Hilliard R, Hoff C, Coates TJ. Disclosing HIV seropositivity to significant others. AIDS 1993;7:425–432 [DOI] [PubMed] [Google Scholar]
- 32. Simoni JM, Mason HR, Marks G, Ruiz MS, Reed D, Richardson JL. Women's self-disclosure of HIV infection: Rates, reasons, and reactions. J Consult Clin Psychol 1995;63:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akani C, Erhabor O. Rate, pattern and barriers of HIV serostatus disclosure in a resource-limited setting in the Niger delta of Nigeria. Trop Doct 2006;36:87–89 [DOI] [PubMed] [Google Scholar]
- 34. Simon Rosser BR, Horvath KJ, Hatfield LA, Peterson JL, Jacoby S, Stately A. Predictors of HIV disclosure to secondary partners and sexual risk behavior among a high-risk sample of HIV-positive MSM: Results from six epicenters in the US. AIDS Care 2008;20:925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalichman SC, Nachimson D. Self-efficacy and disclosure of HIV-positive serostatus to sex partners. Health Psychol 1999;18:281. [DOI] [PubMed] [Google Scholar]
- 36. Bingman CR, Marks G, Crepaz N. Attributions about one's HIV infection and unsafe sex in seropositive men who have sex with men. AIDS Behav 2001;5:283–289 [Google Scholar]
- 37. Crepaz N, Marks G. Serostatus disclosure, sexual communication and safer sex in HIV-positive men. AIDS Care 2003;15:379–387 [DOI] [PubMed] [Google Scholar]
- 38. Mucheto P, Chadambuka A, Shambira G, Tshimanga M, Gombe N, Nyamayaro W. Determinants of nondisclosure of HIV status among women attending the prevention of mother to child transmission programme, Makonde district, Zimbabwe, 2009. Pan Afr Med J 2011;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buma D, Bakari M, Fawzi W, Mugusi F. The influence of HIV-status disclosure on adherence, immunological and virological outcomes among HIV-infected patients started on antiretroviral therapy in Dar-es-Salaam, Tanzania. J HIV AIDS 2015;1:111 [Google Scholar]
- 40. Daskalopoulou M, Lampe FC, Sherr L, et al. Non-disclosure of HIV status and associations with psychological factors, ART non-adherence, and viral load non-suppression among people living with HIV in the UK. AIDS Behav 2017;21:184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marks G, Richardson JL, Maldonado N. Self-disclosure of HIV infection to sexual partners. Am J Public Health 1991;81:1321–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Rosa CJ, Marks G. Preventive counseling of HIV-positive men and self-disclosure of serostatus to sex partners: New opportunities for prevention. Health Psychol 1998;17:224. [DOI] [PubMed] [Google Scholar]
- 43. Abler L, Sikkema KJ, Watt MH, Hansen NB, Wilson PA, Kochman A. Depression and HIV serostatus disclosure to sexual partners among newly HIV-diagnosed men who have sex with men. AIDS Patient Care STDS 2015;29:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crosby RA, Mena L, Arnold T. Disclosure of newly diagnosed HIV infection and condom use at first sex after diagnosis: A study of young Black men who have sex with men. Sex Health 2017;14:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hart TA, Wolitski RJ, Purcell DW, Parsons JT, Gomez CA, Seropositive Urban Men's Study Team. Partner awareness of the serostatus of HIV-seropositive men who have sex with men: Impact on unprotected sexual behavior. AIDS Behav 2005;9:155–166 [DOI] [PubMed] [Google Scholar]
- 46. Hightow-Weidman LB, Jones K, Phillips G, 2nd, Wohl A, Giordano TP, Group YoCSIS. Baseline clinical characteristics, antiretroviral therapy use, and viral load suppression among HIV-positive young men of color who have sex with men. AIDS Patient Care STDS 2011;25(Suppl 1):S9–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hightow-Weidman LB, Phillips G, 2nd, Outlaw AY, et al. Patterns of HIV disclosure and condom use among HIV-infected young racial/ethnic minority men who have sex with men. AIDS Behav 2013;17:360–368 [DOI] [PubMed] [Google Scholar]
- 48. Conserve DF, King G, Devieux JG, Jean-Gilles M, Malow R. Determinants of HIV serostatus disclosure to sexual partner among HIV-positive alcohol users in Haiti. AIDS Behav 2014;18:1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clum GA, Czaplicki L, Andrinopoulos K, et al. Strategies and outcomes of HIV status disclosure in HIV-positive young women with abuse histories. AIDS Patient Care STDS 2013;27:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Comer LK, Henker B, Kemeny M, Wyatt G. Illness disclosure and mental health among women with HIV/AIDS. J Community Appl Soc Psychol 2000;10:449–464 [Google Scholar]
- 51. Hult JR, Wrubel J, Branstrom R, Acree M, Moskowitz JT. Disclosure and nondisclosure among people newly diagnosed with HIV: An analysis from a stress and coping perspective. AIDS Patient Care STDS 2012;26:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olley BO, Seedat S, Stein DJ. Self-disclosure of HIV serostatus in recently diagnosed patients with HIV in South Africa. Afr J Reprod Health 2004;8:71–76 [PubMed] [Google Scholar]
- 53. Skunodom N, Linkins RW, Culnane ME, et al. Factors associated with non-disclosure of HIV infection status of new mothers in Bangkok. Southeast Asian J Trop Med Public Health 2006;37:690–703 [PubMed] [Google Scholar]
- 54. Winchester MS, McGrath JW, Kaawa-Mafigiri D, et al. Early HIV disclosure and nondisclosure among men and women on antiretroviral treatment in Uganda. AIDS Care 2013;25:1253–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trinh TT, Yatich N, Ngomoa R, et al. Partner disclosure and early CD4 response among HIV-infected adults initiating antiretroviral treatment in Nairobi Kenya. PLoS One 2016;11:e0163594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Modi R, Amico KR, Knudson A, et al. Assessing effects of behavioral intervention on treatment outcomes among patients initiating HIV care: Rationale and design of iENGAGE intervention trial. Contemp Clin Trials 2018;69:48–54 [DOI] [PubMed] [Google Scholar]
- 56a. Modi RA, Mugavero MJ, Amico KR, et al. A web-based data collection platform for multisite randomized behavioral intervention trials: Development, key software features, and results of a user survey. JMIR Res Protoc 2017;6:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: The Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol 2008;37:948–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173 [DOI] [PubMed] [Google Scholar]
- 59. Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med 2009;24:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA 1999;282:1737–1744 [DOI] [PubMed] [Google Scholar]
- 61. Gjesfjeld CD, Greeno CG, Kim KH. A confirmatory factor analysis of an abbreviated social support instrument: The MOS-SSS. Res Soc Work Pract 2008;18:231–237 [Google Scholar]
- 62. Dour HJ, Wiley JF, Roy-Byrne P, et al. Perceived social support mediates anxiety and depressive symptom changes following primary care intervention. Depress Anxiety 2014;31:436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. RAND Health Care. Social Support Survey Instrument Scoring Instructions. Available at: https://www.rand.org/health/surveys_tools/mos/social-support.html (Last accessed January27, 2020)
- 64. Bunn JY, Solomon SE, Miller C, Forehand R. Measurement of stigma in people with HIV: A reexamination of the HIV Stigma Scale. AIDS Educ Prev 2007;19:198–208 [DOI] [PubMed] [Google Scholar]
- 65. Earnshaw VA, Quinn DM, Kalichman SC, Park CL. Development and psychometric evaluation of the Chronic Illness Anticipated Stigma Scale. J Behav Med 2013;36:270–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carver CS. You want to measure coping but your protocol' too long: Consider the brief cope. Int J Behav Med 1997;4:92. [DOI] [PubMed] [Google Scholar]
- 67. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. J Pers Soc Psychol 1989;56:267–283 [DOI] [PubMed] [Google Scholar]
- 68. Meyer B. Coping with severe mental illness: Relations of the brief COPE with symptoms, functioning, and well-being. J Psychopathol Behav Assess 2001;23:265–277 [Google Scholar]
- 69. Katz MH, Cunningham WE, Mor V, et al. Prevalence and predictors of unmet need for supportive services among HIV-infected persons: Impact of case management. Med Care 2000;38:58–69 [DOI] [PubMed] [Google Scholar]
- 70. Mathews WC, May S. EuroQol (EQ-5D) measure of quality of life predicts mortality, emergency department utilization, and hospital discharge rates in HIV-infected adults under care. Health Qual Life Outcomes 2007;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: Validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES). J Behav Med 2007;30:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): Validation in a female Veterans Affairs patient population. Arch Intern Med 2003;163:821–829 [DOI] [PubMed] [Google Scholar]
- 73. World Health Organization. Management of Substance Abuse: The WHO ASSIST Project. Geneva: World Health Organization, 2006. [Google Scholar]
- 74. World Health Organization. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Manual for Use in Primary Care. Geneva: World Health Organization, 2010. [Google Scholar]
- 75. Chaudoir SR, Fisher JD, Simoni JM. Understanding HIV disclosure: A review and application of the Disclosure Processes Model. Soc Sci Med 2011;72:1618–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pinkerton SD, Galletly CL. Reducing HIV transmission risk by increasing serostatus disclosure: A mathematical modeling analysis. AIDS Behav 2007;11:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kalichman SC, DiMarco M, Austin J, Luke W, DiFonzo K. Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. J Behav Med 2003;26:315–332 [DOI] [PubMed] [Google Scholar]
- 78. Krumbach U, Sarker M, Neuhann F. To disclose or not to disclose: The interaction between disclosure of HIV status, adherence and treatment outcome: A literature review. In: 17th International AIDS Conference. Mexico City, 2008 [Google Scholar]
- 79. Levy A, Laska F, Abelhauser A, et al. Disclosure of HIV seropositivity. J Clin Psychol 1999;55:1041–1049 [DOI] [PubMed] [Google Scholar]
- 80. Chandra PS, Deepthivarma S, Jairam KR, Thomas T. Relationship of psychological morbidity and quality of life to illness-related disclosure among HIV-infected persons. J Psychosom Res 2003;54:199–203 [DOI] [PubMed] [Google Scholar]
- 81. Li L, Sun S, Wu Z, Wu S, Lin C, Yan Z. Disclosure of HIV status is a family matter: Field notes from China. J Fam Psychol 2007;21:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Miller AN, Rubin DL. Motivations and methods for self-disclosure of HIV seropositivity in Nairobi, Kenya. AIDS Behav 2007;11:687–697 [DOI] [PubMed] [Google Scholar]
- 83. Sethosa E, Peltzer K. Evaluation of HIV counselling and testing, self-disclosure, social support and sexual behaviour change among a rural sample of HIV reactive patients in South Africa. Curationis 2005;28:29–41 [DOI] [PubMed] [Google Scholar]
- 84. Halperin J, Pathmanathan I, Richey LE. Disclosure of HIV status to social networks is strongly associated with increased retention among an urban cohort in New Orleans. AIDS Patient Care STDS 2013;27:375–377 [DOI] [PubMed] [Google Scholar]
- 85. Amoran OE. Predictors of disclosure of sero-status to sexual partners among people living with HIV/AIDS in Ogun State, Nigeria. Niger J Clin Pract 2012;15:385–390 [DOI] [PubMed] [Google Scholar]
- 86. Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women's Interagency HIV Study (WIHS). Int J Epidemiol 2018;47:393–394i [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86a. Greene K, Carpenter A, Catona D, Magsamen-Conrad K. The Brief Disclosure Intervention (BDI): Facilitating African Americans' disclosure of HIV. J Commun 2013;63:138–158 [Google Scholar]
- 87. Crockett KB, Edmonds A, Johnson MO, et al. Neighborhood racial diversity, socioeconomic status, and perceptions of HIV-related discrimination and internalized HIV stigma among women living with HIV in the United States. AIDS Patient Care STDS 2019;33:270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sengupta S, Banks B, Jonas D, Miles MS, Smith GC. HIV interventions to reduce HIV/AIDS stigma: A systematic review. AIDS Behav 2011;15:1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rao D, Kemp CG, Huh D, et al. Stigma reduction among African American women with HIV: UNITY Health Study. J Acquir Immune Defic Syndr 2018;78:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Campbell AN, Tross S, Calsyn DA. Substance use disorders and HIV/AIDS prevention and treatment intervention: Research and practice considerations. Soc Work Public Health 2013;28:333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001;58:721–728 [DOI] [PubMed] [Google Scholar]
- 92. Edwards M, Quinlivan EB, Bess K, et al. Implementation of PHQ-9 depression screening for HIV-infected patients in a real-world setting. J Assoc Nurses AIDS Care 2014;25:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pence BW, Gaynes BN, Williams Q, et al. Assessing the effect of Measurement-Based Care depression treatment on HIV medication adherence and health outcomes: Rationale and design of the SLAM DUNC Study. Contemp Clin Trials 2012;33:828–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wu L, Li X. Community-based HIV/AIDS interventions to promote psychosocial well-being among people living with HIV/AIDS: A literature review. Health Psychol Behav Med 2013;1:31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Khakha D, Kapoor B. Effect of coping strategies intervention on the coping styles adopted by People living with HIV/AIDS at a tertiary care hospital in the national capital. Nurs Midwifery Res J 2015;11:45–56 [Google Scholar]
- 96. Fife BL, Scott LL, Fineberg NS, Zwickl BE. Promoting adaptive coping by persons with HIV disease: Evaluation of a patient/partner intervention model. J Assoc Nurses AIDS Care 2008;19:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Napper LE, Fisher DG, Reynolds GL, Johnson ME. HIV risk behavior self-report reliability at different recall periods. AIDS Behav 2010;14:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]