Abstract
Objective
This study is aimed at evaluating the effects of platelet-rich plasma (PRP) on proliferation, viability, and odontogenic differentiation of neural crest stem-like cells (NCSCs) derived from human dental apical papilla.
Materials and Methods
Cells from apical papillae were obtained and then induced to form neural spheres. The expression of NCSC markers p75NTR and HNK-1 in neural sphere cells was detected by immunofluorescence staining. Human PRP was prepared by a 2-step centrifugation method and activated by CaCl2 and thrombin. The concentrations of PDGF-BB and TGF-β1 in whole blood and PRP were measured by an ELISA kit. PRP in five different concentrations (0%, 2.5%, 5%, 10%, and 25%) was applied to culture NCSCs. On the 1st, 3rd, 5th, and 7th days, cell proliferation was evaluated by CCK8. Cell viability was tested by a live/dead staining kit. mRNA and protein expression of DSPP and BMP4 were analyzed by RT-qPCR and western blot, respectively. Statistical analysis was performed by a one-way analysis of variance (ANOVA) test or t-test.
Results
Dental apical papilla cells formed neural spheres, from which cells displayed positive expression of p75NTR and HNK-1. The concentrations of PDGF-BB and TGF-β1 in PRP were about 3.5-fold higher than those in whole blood. 5% and 10% PRP significantly promoted proliferation of NCSCs, while 25% and 50% PRP inhibited cell proliferation from Day 3 to Day 7. Low-concentration (2.5%, 5%, and 10%) PRP slightly improved viability of NCSCs on Day 7. On the other hand, high-concentration (25% and 50%) PRP significantly inhibited viability of NCSCs from Day 3 to Day 7. RT-qPCR and western blot results indicated that 10% PRP could promote odontogenic differentiation of NCSCs on Day 7. mRNA and protein expression of DSPP and BMP4 were significantly upregulated in the 10% PRP group compared to those in the control group (P < 0.05).
Conclusions
PRP is a simply acquirable blood derivative which contains high concentration of growth factors like PDGF-BB and TGF-β1. PRP in a proper concentration could promote proliferation, viability, and odontogenic differentiation of NCSCs derived from human dental apical papilla.
1. Introduction
The American Association of Endodontics recommends using regenerative endodontic therapy (RET) in immature teeth with necrotic pulp [1, 2]. For dental pulp treatment, “RET,” “revascularization,” and “revitalization” are three synonyms, which are interchangeable [1]. The critical procedure of RET is to induce bleeding in periapical tissue. The blood clots can serve as scaffolds and release growth factors to guide stem cells from apical papilla (SCAP) into root canals for pulp regeneration [2–4]. It was reported that platelet-rich plasma (PRP) could be applied in RET to promote apical closure and root growth in immature permanent teeth [5–8].
PRP contains numerous growth factors, including epidermal growth factor (EGF), transforming growth factor beta 1 (TGF-β1), vascular endothelial growth factor (VEGF), platelet-derived growth factor-BB (PDGF-BB), and insulin-like growth factor (IGF) [9]. PRP is a favorable autologous scaffold that can facilitate cell proliferation and differentiation [10]. Dental pulp stem cells (DPSCs) cultured with a certain concentration of PRP exhibited preferable proliferation, migration, and mineralization [11, 12]. SCAP cultured with PRP expressed a high level of odontogenic markers such as dentin matrix protein 1 (DMP-1) and dentin sialophosphoprotein (DSPP) [13].
Both dental pulp and apical papilla develop from a cranial neural crest [14]. Some studies reported that NCSCs existed in dental pulp and apical papilla [15, 16]. When cultured under neural sphere-forming condition, dental pulp cells formed spheres in which cells expressed NCSC markers (p75NTR, HNK-1, Slug, Snail, Nestin, and Musashi1) [15]. These NCSCs in spheres had multiple differentiation capabilities, as they could differentiate into adipocytes, chondrocytes, osteoblasts, neurons, and smooth muscle cells. NCSCs obtained from apical papilla of immature teeth could differentiate into odontoblasts [16]. However, the influence of PRP on NCSCs is still unclear. Here, we studied effects of PRP on proliferation, viability, and odontogenic differentiation of NCSCs induced from human dental apical papilla.
2. Materials and Methods
2.1. Obtain NCSCs from Dental Apical Papilla
The experiment protocol of the current research was approved by the Ethical Review Committee of Sun Yat-sen University. Immature teeth were extracted from patients (aged 16-18 years) and then washed three times with saline to remove residual blood [17–19]. Dental papillae were separated from roots by a dental probe and placed in α-MEM (Gibco, Thermo Fisher Scientific, USA) with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Subsequently, dental apical papillae were cut into small tissue pieces and then treated with collagenase type I and dispase type II for 30 minutes at 37°C. The tissue blocks were filtrated. After that, remaining liquid was centrifuged at 1000 rpm for 5 minutes. The supernatant was removed, and remaining cells were resuspended. The cells isolated from papillae were seeded on cell culture dishes and cultured in a condition of 5% CO2 at 37°C for two weeks. Next, these cells were cultured with neural sphere medium, i.e., DMEM/F12 was supplemented with N-2 (100x), B-27 (50x), 100 U/ml penicillin, 100 μg/ml streptomycin, 20 ng/ml recombinant human EGF, and 20 ng/ml recombinant human FGF2 (Gibco, Thermo Fisher Scientific, USA). One week later, neural spheres were collected and detached into single cells by a StemPro Accutase Cell Dissociation Reagent (Life Technologies, Thermo Fisher Scientific) [20, 21]. These cells were seeded onto 1% Matrigel (Corning, Bedford, MA, USA) precoated dishes for subculture.
2.2. Immunofluorescence Assay
NCSCs at the 2nd passage were digested, centrifuged, and placed on a 6-well culture plate at a density of 2 × 104 per well. After 24 hours, 4% paraformaldehyde was used to fix the cells for 30 minutes, and 0.5% Triton X-100 was used to permeabilize the cells for 20 minutes. Later, the cells were blocked in 5% bovine serum albumin (BSA) for one hour and incubated with primary antibodies against p75NTR (8238S, Cell Signaling) and HNK-1 (ab187274, Abcam) overnight at 4°C. After 12 hours, the cells were probed with Goat Anti-Rabbit Alexa Fluor 488 (ab150077, Abcam) and Goat Anti-Mouse Alexa Fluor 647 (ab150115, Abcam) antibodies for one hour and then washed with phosphate buffer solution (PBS) for three times. Additionally, cell nuclei were counterstained with DAPI. Images were captured with an inverted fluorescence microscope.
2.3. Preparation of Human PRP
A total of four students from Sun Yat-sen University, China, donated their blood. The preparation of human PRP followed a standard protocol [22]. Briefly, whole blood was collected by a 100 ml syringe and centrifuged for 10 minutes at 180 g to separate plasma and blood cells. Next, plasma and blood cells were centrifuged again to three layers: the lower layer was blood cells. The upper layer was poor platelet plasma, and between the two layers was PRP. PRP was activated by adding CaCl2 (22 mM) and thrombin at 37°C for 40 minutes, and then, blood clots were removed. The exudate was centrifuged at 890 g for 10 minutes, and then, supernatant which contained growth factors was cryopreserved at −80°C for further application.
2.4. Growth Factor Concentration Measurement
The concentration of two growth factors (PDGF-BB and TGF-β1) in PRP and whole blood was tested by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, USA). Three repeats were carried out for each test; after that, the absorbance at 450 nm was detected and analyzed.
2.5. Cell Proliferation Assay
The 3rd passage NCSCs were placed on 96-well cell culture plates with initial density of 2 × 103 cells per well. Cell Counting Kit 8 (CCK8) (Dojindo, Tokyo, Japan) was applied to evaluate proliferation of those cells on the 1st, 3rd, 5th, and 7th days after culture [17]. To evaluate the impacts of PRP on NCSC proliferation, five PRP concentrations (0%, 2.5%, 5%, 10%, and 25% PRP) were applied. The optical density (OD) was measured at 450 nm by a microplate reader (Thermo Scientific, UK).
2.6. Live/Dead Staining
The 3rd passage NCSCs were laid upon 6-well plates precoated with 1% Matrigel at density of 5 × 104 cells/well and cultured with neural sphere medium. 0%, 2.5%, 5%, 10%, 25%, and 50% PRP were added into the wells, respectively. On the 1st, 3rd, 5th, and 7th days, a LIVE/DEAD Cell Imaging Kit (R37601, Invitrogen, Thermo Scientific, USA) was used to detect cell viability of NCSCs.
2.7. Quantitative Real-Time PCR (RT-qPCR)
Seven days after PRP treatment, total RNA was extracted by using a TRIzol reagent (Invitrogen, USA). The cDNA was synthesized by a RevertAid First Strand cDNA Synthesis Kit (Invitrogen, USA). RT-qPCR was completed by iQ SYBR Green Supermix (BioRad Laboratories, USA) with iQ5 thermal iCycler (BioRad Laboratories, USA). GAPDH was the control. The sequences of the target primers were the following: DSPP, forward 5′-TTTGGGCAGTAGCATGGGC-3′ and reverse 5′-CCATCTTGGGTATTCTCTTGCCT-3′; BMP4, forward 5′-ATGATTCCTGGTAACCGAATGC-3′ and reverse 5′-CCCCGTCTCAGGTATCAAACT-3′; and GAPDH, forward 5′-AAGGTGAAGGTCGGAGTCAA-3′ and reverse 5′-AATGAAGGGGTCATTGATGG-3′.
2.8. Western Blot
After culturing for seven days, cells in two groups were lysed in 0.1 ml RIPA buffer with PMSF (Invitrogen, Rockford, IL, USA) for 30 minutes and then centrifuged to collect supernatant. Target proteins were separated on 10% SDS-polyacrylamide gels (Beyotime Institute of Biotechnology, China) and transferred to PVDF membranes (Thermo Fisher Scientific, Carlsbad, CA, USA). PVDF membranes were incubated with primary antibodies at 4°C overnight. The primary antibodies were BMP4 (sc-12721, Santa Cruz Biotechnology Inc., Dallas, TX, USA) and DSPP (sc-73632). Later, PVDF membranes were incubated with secondary antibody (sc-516102) at 37°C for 2 hours. GAPDH (sc-47724) was set as the control.
2.9. Statistical Analysis
All data were presented as the mean ± standard deviation (SD). The one-way analysis of variance (ANOVA) test or t-test was performed. Statistical significance was defined as P < 0.05.
3. Results
3.1. Dental Apical Papilla Cells Formed Neural Spheres
The pink apical papillae were gently separated from teeth (Figure 1(a)). The dental apical papilla cells formed colonies one week after culture (Figure 1(b)). In sphere-forming medium containing FGF2, EGF, B-27, and N-2 supplements, the 3rd passage dental papilla cells formed spheres within one week (Figure 1(c)).
Figure 1.
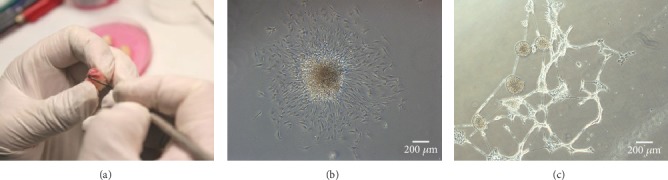
(a) Pink dental apical papillae were obtained. (b) Dental apical papilla cells formed colonies in one week. (c) Neural spheres were observed one week after induction.
3.2. Expression of NCSC Markers in Sphere-Forming Cells
Sphere-forming cells were visualized by immunofluorescence and verified positive expression of NCSC surface markers, including p75NTR and HNK-1 (Figure 2). These results indicated that the sphere-forming cells were NCSCs.
Figure 2.
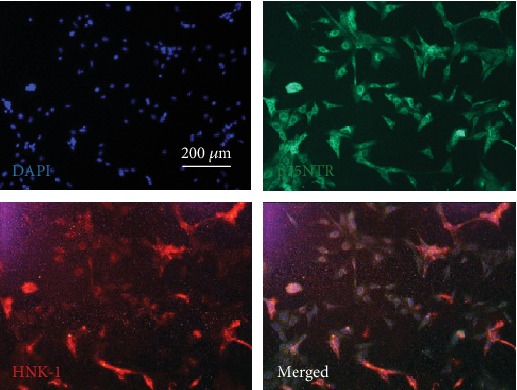
Neural sphere cells expressed p75NTR and HNK-1 in immunofluorescence assay.
3.3. Character of Human PRP
Platelet counting in whole blood was 1.72 ± 0.13 × 108/ml, while that in PRP was 7.02 ± 0.68 × 108/ml. The number of platelets in PRP was almost 4.1-fold compared to that in whole blood. Mean red blood cell (RBC) counting reduced from 4.29 ± 0.85 × 106/ml in whole blood to 0.13 ± 0.03 × 106/ml in PRP (P < 0.01).
The activated PRP contained more PDGF-BB and TGF-β1 than whole blood (P < 0.01). The concentration of PDGF-BB in activated PRP (7.42 ± 0.44 ng/ml) was 3.4-fold higher than that in whole blood (2.20 ± 0.11 ng/ml). The concentration of TGF-β1 in PRP (41.38 ± 4.77 ng/ml) was 3.5-fold higher than that in whole blood (11.81 ± 1.70 ng/ml).
3.4. Influence of PRP on Proliferation of NCSCs
As shown in Figure 3, average optical density (OD) indicated that the cell number was similar among five groups on Day 1. From Day 3 to Day 7, 5% and 10% PRP significantly promoted proliferation of NCSCs, while 25% and 50% PRP significantly inhibited cell proliferation. On Days 5 and 7, the OD value from high to low was 10%, 5%, 2.5%, 0%, 25%, and 50% PRP group, respectively.
Figure 3.
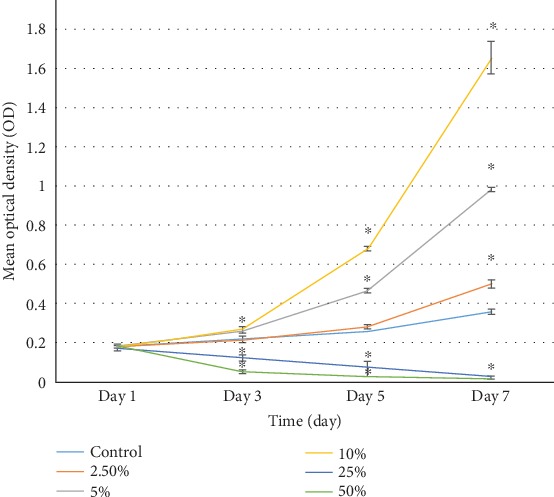
Influence of PRP concentration on NCSC proliferation.
3.5. Influence of PRP on Viability of NCSCs
The fluorescent images of live/dead cells are shown in Figure 4(a), in which live cells were presented as green and dead cells as red. In Figure 4(b), 0%, 2.5%, 5%, and 10% PRP groups maintained significantly better cell viability than 25% and 50% PRP groups (P < 0.05) from Day 3 to Day 7. Low-concentration (2.5%-10%) PRP slightly improved viability of NCSCs compared with the control group on Day 7. Less than 10% NCSCs survived in the 50% PRP group from Day 3 to Day 7. Similarly, the viability decreased from 90% to 30% in the 25% PRP group over time. This result suggested that high-concentration (>25%) PRP decreased cell viability and was not recommended for NCSC culture.
Figure 4.
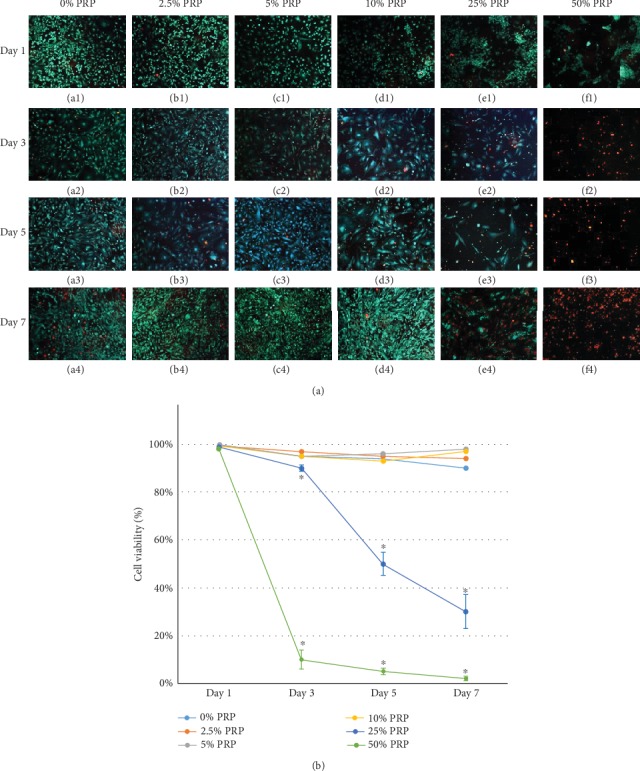
Influence of PRP on cell viability of NCSCs.
3.6. 10% PRP Promoted Odontogenic Differentiation of NCSCs
10% PRP was selected to assess NCSC differentiation because it achieved optimal cell proliferation and viability as mentioned above. RT-qPCR was conducted to analyze key gene expression (DSPP and BMP4) involved in odontogenic differentiation of NCSCs (Figure 5(a)). Both DSPP and BMP4 mRNA expressions were significantly upregulated in the 10% PRP group compared with those in the control group (P < 0.05). Similarly, western blot results displayed that 10% PRP significantly promoted DSPP and BMP4 expression in the protein level (P < 0.05) (Figure 5(b)). Both RT-qPCR and western blot results suggested that 10% PRP promoted odontogenic differentiation of NCSCs in vitro.
Figure 5.
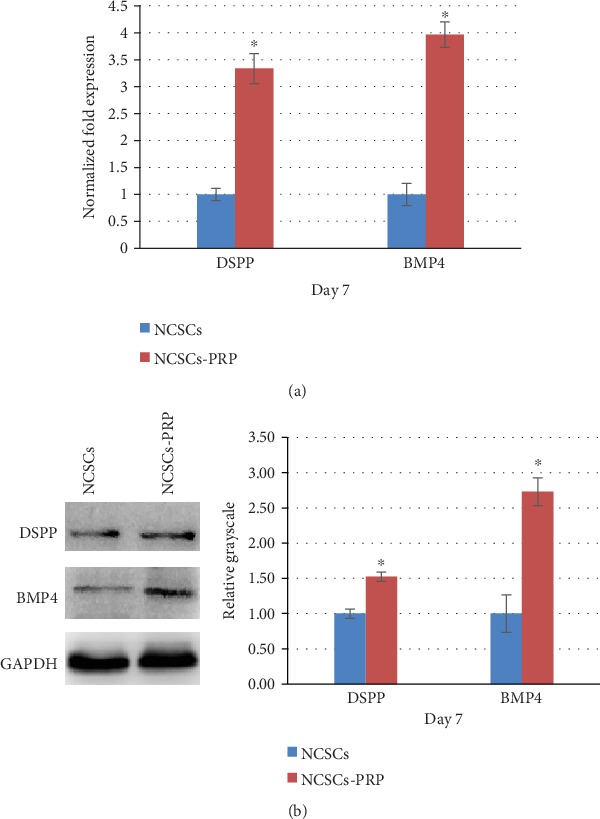
10% PRP promoted odontogenic differentiation of NCSCs. (a) In the NCSC-PRP group, the mRNA expression of DSPP and BMP4 increased 3.3-fold and 3.9-fold, respectively (P < 0.05). (b) In the NCSC-PRP group, the protein expression of DSPP and BMP4 increased 1.5-fold and 2.7-fold, respectively (P < 0.05).
4. Discussion
SCAP are progenitor cells of odontoblasts, which are crucial for dentin formation and root completion [23]. SCAP obtained from human third molars exhibited characteristics of neural crest-derived progenitor cells [16]. In the current study, the results displayed that SCAP formed neural spheres after directional induction using neural sphere-forming medium for one week. The similar phenomenon was observed in dental pulp cells using neural sphere-forming medium [15]. In our study, the neural sphere cells derived from apical papillae expressed p75NTR and HNK-1 which were classic markers of NCSCs [24, 25]. These results indicated that neural sphere cells derived from apical papillae were NCSCs.
Various studies reported that proliferation of dental cells could be influenced by different concentrations of PRP. A researcher pointed out that 1%-20% PRP significantly enhanced human DPSC proliferation in six days [26]. 2% to 10% PRP derived from human umbilical cord blood significantly stimulated proliferation of human DPSCs, while 2% PRP led to the highest proliferation in three days [27]. In another study, 0.5 and 1% PRP promoted proliferation of human DPSCs, but 5% PRP inhibited cell proliferation in five days [28]. Similar results were also observed in rat DPSCs; 1% and 10% PRP enhanced proliferation of DPSCs in ten days, but 50% PRP inhibited cell proliferation [12]. In the current experiment, 2.5%-10% PRP increased proliferation of NCSCs derived from the dental apical papilla, while 25% and 50% PRP inhibited cell proliferation in seven days. This result explained that low-concentration (2.5%-10%) PRP could promote NCSC proliferation, while high-concentration (25% and 50%) PRP gave an opposite result. The effects of PRP concentration on NCSCs are similar to DPSCs as mentioned above, but variance of PRP concentration might be attributed to the source of PRP and cell types.
There is only one direct evidence about the role of PRP on cell viability in the dental field. DPSCs exposed to 10% and 20% PRP acquired significantly (P < 0.05) higher viability than the cells cultured in 10% FBS on Day 4 [29]. Similar results were also observed in adipose tissue-derived mesenchymal stem cells, of which viability significantly increased when cultured with 5% and 20% PRP for 16 hours [30]. For Schwann cells, 2.5-10% PRP significantly promoted cell viability on Day 3 and Day 7 [31]. Besides, 5% PRP significantly increased viability of periodontal cells, while 50% PRP inhibited viability on Day 2 and Day 3 [32]. Creeper et al. pointed out that the high concentration of PRP could cause cytotoxic effect on periodontal ligament cells [33]. In our study, high-concentration (25% and 50%) PRP resulted in obvious low-level cell viability, while low-concentration (2.5%-10%) PRP slightly improved viability of NCSCs.
A few studies pointed out that PRP could improve odontogenic/osteogenic differentiation [13, 34, 35] and mineralization [11, 12] of dental stem cells. PRP promoted mRNA and protein expression of DSPP and DMP-1 in SCAP [13]. Because DSPP and DMP-1 are markers of odontoblasts, this result indicated that PRP could be a candidate for inducing SCAP into odontoblasts. Previous studies also reported that BMP4 acted as a crucial mesenchymal odontogenic signal during early tooth development [36]. Our results demonstrated that 10% PRP-treated NCSCs obtained higher expression of odontogenic differentiation markers, such as DSPP and BMP4. This result was consistent with the previous studies, which means NCSCs treated by certain concentration of PRP could obtain better odontogenic differentiation potential.
5. Conclusions
In this study, we have elucidated that PRP isolated from humans is a simply acquirable blood derivative which contains high concentration of growth factors like PDGF-BB and TGF-β1. Moreover, we found that PRP in proper concentration (2.5%-10%) could promote cell proliferation, viability, and odontogenic differentiation of NCSCs derived from human dental apical papilla. Therefore, these findings provide considerable evidence and valuable information to apply PRP in regenerative endodontic therapy.
Acknowledgments
This study was supported by the Guangdong Basic and Applied Basic Research Foundation (2016A030313275 and 2020A1515010239), National Natural Science Foundation of China (81500825), Medical Scientific Research Foundation of Guangdong Province (A2015423), Special Fund for Public Welfare Research and Capacity Building of Guangdong Province (2014A020212211), and Science and Technology Program of Guangzhou (201607010205).
Contributor Information
Xiaolei Zhang, Email: zhangxl35@mail.sysu.edu.cn.
Wen Zhang, Email: zhangwn8@mail.sysu.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There are no conflicts of interest from any author.
References
- 1.Kim S. G., Malek M., Sigurdsson A., Lin L. M., Kahler B. Regenerative endodontics: a comprehensive review. International Endodontic Journal. 2018;51(12):1367–1388. doi: 10.1111/iej.12954. [DOI] [PubMed] [Google Scholar]
- 2.Iwaya S. I., Ikawa M., Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dental Traumatology. 2001;17(4):185–187. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 3.Lovelace T. W., Henry M. A., Hargreaves K. M., Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. Journal of Endodontics. 2011;37(2):133–138. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Jadhav G., Shah N., Logani A. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: a pilot clinical study. Journal of Endodontics. 2012;38(12):1581–1587. doi: 10.1016/j.joen.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Murray P. E. Platelet-rich plasma and platelet-rich fibrin can induce apical closure more frequently than blood-clot revascularization for the regeneration of immature permanent teeth: a meta-analysis of clinical efficacy. Frontiers in Bioengineering and Biotechnology. 2018;6(1):p. 139. doi: 10.3389/fbioe.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torabinejad M., Turman M. Revitalization of Tooth with Necrotic Pulp and Open Apex by Using Platelet- rich Plasma: A Case Report. Journal of Endodontics. 2011;37(2):265–268. doi: 10.1016/j.joen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Bezgin T., Yilmaz A. D., Celik B. N., Kolsuz M. E., Sonmez H. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. Journal of Endodontics. 2015;41(1):36–44. doi: 10.1016/j.joen.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Narang I., Mittal N., Mishra N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: a clinical study. Contemporary Clinical Dentistry. 2015;6(1):63–68. doi: 10.4103/0976-237X.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng C., Zhu Q., Liu X., et al. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. Journal of Tissue Engineering and Regenerative Medicine. 2016;10(5):428–436. doi: 10.1002/term.1756. [DOI] [PubMed] [Google Scholar]
- 10.Gaviño Orduña J. F., Caviedes-Bucheli J., Manzanares Céspedes M. C., et al. Use of platelet-rich plasma in endodontic procedures in adults: regeneration or repair? A report of 3 cases with 5 years of follow-up. Journal of Endodontics. 2017;43(8):1294–1301. doi: 10.1016/j.joen.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Altaii M., Kaidonis X., Koblar S., Cathro P., Richards L. Platelet rich plasma and dentine effect on sheep dental pulp cells regeneration/revitalization ability (in vitro) Australian Dental Journal. 2017;62(1):39–46. doi: 10.1111/adj.12426. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L., Xie Y. H., Lin B. R. Effects of washed platelets vs platelet-rich plasma on the proliferation and mineralization of rat dental pulp cells. Genetics and Molecular Research. 2015;14(3):9486–9496. doi: 10.4238/2015.August.14.12. [DOI] [PubMed] [Google Scholar]
- 13.Yeom K. H., Ariyoshi W., Okinaga T., et al. Platelet-rich plasma enhances the differentiation of dental pulp progenitor cells into odontoblasts. International Endodontic Journal. 2016;49(3):271–278. doi: 10.1111/iej.12443. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y., Zhang P., Gu R. L., Liu Y. S., Zhou Y. S. Origin and clinical applications of neural crest-derived dental stem cells. The Chinese Journal of Dental Research. 2018;21(2):89–100. doi: 10.3290/j.cjdr.a40435. [DOI] [PubMed] [Google Scholar]
- 15.Abe S., Hamada K., Miura M., Yamaguchi S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biology International. 2012;36(10):927–936. doi: 10.1042/CBI20110506. [DOI] [PubMed] [Google Scholar]
- 16.Gosau M., Götz W., Felthaus O., Ettl T., Jäger A., Morsczeck C. Comparison of the differentiation potential of neural crest derived progenitor cells from apical papilla (dNC-PCs) and stem cells from exfoliated deciduous teeth (SHED) into mineralising cells. Archives of Oral Biology. 2013;58(6):699–706. doi: 10.1016/j.archoralbio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W., Zhang X., Li J., et al. Foxc2 and BMP2 induce osteogenic/odontogenic differentiation and mineralization of human stem cells from apical papilla. Stem Cells International. 2018;2018:10. doi: 10.1155/2018/2363917.2363917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Zhang X., Ling J., Wei X., Jian Y. Osteo-/odontogenic differentiation of BMP2 and VEGF gene-co-transfected human stem cells from apical papilla. Molecular Medicine Reports. 2016;13(5):3747–3754. doi: 10.3892/mmr.2016.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W., Zhang X., Ling J., et al. Proliferation and odontogenic differentiation of BMP2 gene‑transfected stem cells from human tooth apical papilla: an in vitro study. International Journal of Molecular Medicine. 2014;34(4):1004–1012. doi: 10.3892/ijmm.2014.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q., Nguyen P. D., Shi S., et al. Neural crest stem-like cells non-genetically induced from human gingiva-derived mesenchymal stem cells promote facial nerve regeneration in rats. Molecular Neurobiology. 2018;55(8):6965–6983. doi: 10.1007/s12035-018-0913-3. [DOI] [PubMed] [Google Scholar]
- 21.Shi H., Gong Y., Qiang L., et al. Derivation of Schwann cell precursors from neural crest cells resident in bone marrow for cell therapy to improve peripheral nerve regeneration. Biomaterials. 2016;89(1):25–37. doi: 10.1016/j.biomaterials.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Etulain J., Mena H. A., Meiss R. P., et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Scientific Reports. 2018;8(1, article 1513) doi: 10.1038/s41598-018-19419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang G. T., Sonoyama W., Liu Y., Liu H., Wang S., Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. Journal of Endodontics. 2008;34(6):645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schürmann M., Brotzmann V., Bütow M., et al. Identification of a novel high yielding source of multipotent adult human neural crest-derived stem cells. Stem Cell Reviews and Reports. 2018;14(2):277–285. doi: 10.1007/s12015-017-9797-2. [DOI] [PubMed] [Google Scholar]
- 25.Kaku M., Komatsu Y., Mochida Y., Yamauchi M., Mishina Y., Ko C. C. Identification and characterization of neural crest-derived cells in adult periodontal ligament of mice. Archives of Oral Biology. 2012;57(12):1668–1675. doi: 10.1016/j.archoralbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao S. N., Liu W. F., Zhang Z. T. Effect of platelet-rich plasma on cell proliferation and osteogenic activity of pulp stem cells. Zhonghua Kou Qiang Yi Xue Za Zhi. 2013;48(3):177–182. [PubMed] [Google Scholar]
- 27.Lee J. Y., Nam H., Park Y. J., et al. The effects of platelet-rich plasma derived from human umbilical cord blood on the osteogenic differentiation of human dental stem cells. In Vitro Cellular & Developmental Biology - Animal. 2011;47(2):157–164. doi: 10.1007/s11626-010-9364-5. [DOI] [PubMed] [Google Scholar]
- 28.Lee U. L., Jeon S. H., Park J. Y., Choung P. H. Effect of platelet-rich plasma on dental stem cells derived from human impacted third molars. Regenerative Medicine. 2011;6(1):67–79. doi: 10.2217/rme.10.96. [DOI] [PubMed] [Google Scholar]
- 29.Bindal P., Gnanasegaran N., Bindal U., et al. Angiogenic effect of platelet-rich concentrates on dental pulp stem cells in inflamed microenvironment. Clinical Oral Investigations. 2019;23(10):3821–3831. doi: 10.1007/s00784-019-02811-5. [DOI] [PubMed] [Google Scholar]
- 30.D'Esposito V., Passaretti F., Perruolo G., et al. Platelet-rich plasma increases growth and motility of adipose tissue-derived mesenchymal stem cells and controls adipocyte secretory function. Journal of Cellular Biochemistry. 2015;116(10):2408–2418. doi: 10.1002/jcb.25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sowa Y., Kishida T., Tomita K., Adachi T., Numajiri T., Mazda O. Involvement of PDGF-BB and IGF-1 in activation of human Schwann cells by platelet-rich plasma. Plastic and Reconstructive Surgery. 2019;144(6):1025e–1036e. doi: 10.1097/PRS.0000000000006266. [DOI] [PubMed] [Google Scholar]
- 32.Tavassoli-Hojjati S., Sattari M., Ghasemi T., Ahmadi R., Mashayekhi A. Effect of platelet-rich plasma concentrations on the proliferation of periodontal cells: an in vitro study. European Journal of Dentistry. 2016;10(4):469–474. doi: 10.4103/1305-7456.195165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creeper F., Lichanska A. M., Marshall R. I., Seymour G. J., Ivanovski S. The effect of platelet-rich plasma on osteoblast and periodontal ligament cell migration, proliferation and differentiation. Journal of Periodontal Research. 2009;44(2):258–265. doi: 10.1111/j.1600-0765.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 34.Otero L., Carrillo N., Calvo-Guirado J. L., Villamil J., Delgado-Ruíz R. A. Osteogenic potential of platelet-rich plasma in dental stem-cell cultures. The British Journal of Oral & Maxillofacial Surgery. 2017;55(7):697–702. doi: 10.1016/j.bjoms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Wen J., Li H. T., Li S. H., Li X., Duan J. M. Investigation of modified platelet-rich plasma (mPRP) in promoting the proliferation and differentiation of dental pulp stem cells from deciduous teeth. Brazilian Journal of Medical and Biological Research. 2016;49(10, article e5373) doi: 10.1590/1414-431x20165373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon H. J. E., Jia S., Lan Y., Liu H., Jiang R. Activin and Bmp4 signaling converge on Wnt activation during odontogenesis. Journal of Dental Research. 2017;96(10):1145–1152. doi: 10.1177/0022034517713710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


