Abstract
Changing weather patterns, droughts and competing water demands are dramatically altering the landscape and creating conditions conducive to the production of wind-blown dust and dust storms. In California, such factors are leading to the rapid shrinking of the Salton Sea, a 345 square mile land-locked “sea” situated near the southeastern rural border region known as the Imperial Valley. The region is anticipated to experience a dramatic increase in wind-blown dust and existing studies suggest a significant impact on the health and quality of life for nearby residents of this predominantly low-income, Mexican-American community. The discussion calls attention to the public health dimensions of the Salton Sea crisis. We know little about the possible long-term health effects of exposure to mobilized lakebed sediments or the numerous toxic contaminants that may become respirable on entrained particles. We draw on existing epidemiological literature of other known sources of wind-blown dust, such as desert dust storms, and related health effects to begin to understand the potential public health impact of wind-blown dust exposure. The increased production of wind-blown dust and environmental exposures to such non-combustion related sources of particulate matter are a growing health threat, due in part to drought coupled with increasing pressures on limited water resources. Recent population-based studies have linked dust storms with cardiovascular mortality, asthma hospitalization and decrease in pulmonary function in both adults and children. A growing number of studies provide evidence of the acute health effects of wind-blown dust exposures among children, which with repeated insults have the potential to influence respiratory health over time. The shrinking of the Salton Sea illustrates a public health and environmental justice crisis that requires action and attention to protect the health and well-being of local communities.
Keywords: Salton Sea, children’s health, respiratory, Imperial County, particulate matter
Graphical Abstract
Introduction
A brief history of Salton Sea
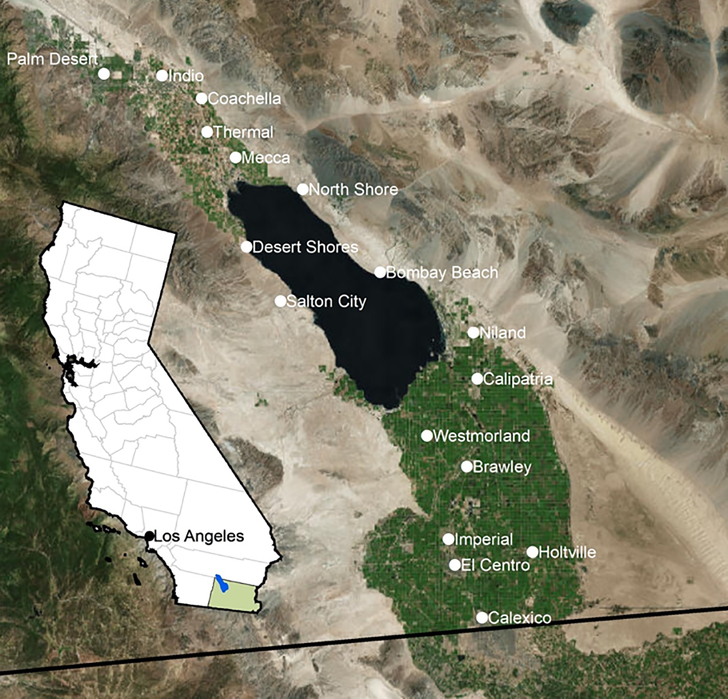
Climate change is predicted to bring increasingly hotter and drier conditions to much of the Southwestern United States (US), creating conditions conducive to the increased production of wind-blown dust (Pu and Ginoux 2017). These factors, coupled with drought and competing water demands, have laid the ground for a human health and ecological disaster-in-the-making in the southeastern border region of California (CA), known as the Imperial Valley. The Salton Sea, a 345-square-mile shallow land-locked “sea” situated in the northern part of Imperial Valley, was formed inadvertently during diversion of the Colorado River in the early 1900s filling a dry salt bed (Figure 1). This shallow terminal lake, which is 35 miles long, 15 miles wide and only an average of 20 feet deep, has been sustained in this arid desert climate largely by irrigation runoff from adjacent agricultural lands. The Sea essentially serves as a repository for irrigation wastewaters, which historically have comprised over 95% of the annual water inflows (Hart et al. 1998; Tompson 2016).
Figure 1.
Map of Imperial Valley and location within state of California.
The Imperial Valley, which surrounds the Salton Sea’s southern shores, is a highly productive agricultural region that is dependent on water imported from the Colorado River. The Imperial Irrigation District (IID), which provides water to the agricultural industry in Imperial Valley, has historically held the single largest entitlement to freshwater from the Colorado River of any Colorado River use.. Water for the Imperial Valley is diverted from the Colorado River into the All-American Canal, a human-made irrigation channel along the Mexico/California border. Through an extensive network of canals and ditches, approximately 3.2 billion cubic meters of Colorado River water is delivered to over 2,000 square kilometers of Imperial Valley agricultural land per year, although this number is expected to decline in coming years (http://www.iid.com). In 2003, a federally ordered Quantification Settlement Agreement (QSA) stipulated a reduction of Colorado River water imports to the Imperial Valley, to increase water resources for growing urban regions. As part of the negotiations, 15 years of “mitigation water” was allocated to the Sea to provide time for the state to address the impacts of a shrinking Sea, due to the anticipated reduction in agricultural irrigation runoff. The role of water in maintaining the ecological and economic vitality of the Salton Sea was not seen as a direct “beneficial use” within the water reapportionment (Cantor 2016). Thus, as of December 2017, nearly half of all freshwater flowing into the land-locked Salton Sea has been diverted for predominantly urban uses as part of this settlement, precipitating the rapid shrinking of the largest inland water body in CA (King et al. 2011; Tompson 2016).
A crisis in slow motion: Disappearing seas and air pollution
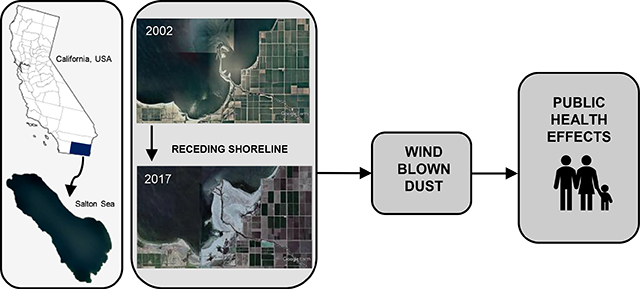
The Salton Sea has been shrinking slowly for years (Barnum et al. 2017). Given the surrounding desert climate and shallowness of the Sea, large swaths of the seabed have and will continue to become exposed as the water levels recede. The retreating shoreline leaves behind exposed playa which has the potential to generate dust that is easily mobilized by strong winds in the area from the vast salt flats (King et al. 2011) (Figure 2). A model from the US Geological Survey estimated that the decline of 3 feet in elevation will expose over 11,000 acres of saline lakebed sediment (Case et al. 2013). A separate study predicts that fugitive wind-blown dust could increase by up to 40 to 80 tons per day after water inflows are reduced in 2018 and the lake will shrink by about 100 square miles by 2030 (Cohen and Hyun 2006). Previous research suggests that these salt-based crusts are already a significant source of dust emissions and predicts that the playa is likely to become an increasingly important source of respirable particulate matter <10 μm in diameter (PM10) in the region (King et al. 2011). Prior to the water transfer, approximately 10% of PM10 in the region was attributable to playa-like soils, a contribution that is estimated to increase during high wind events (Frie et al. 2017).
Figure 2.
Images of three sites located on southern shore of Salton Sea, in 2002 (left panels, a, c, and e) and in 2017 (right panels b, d, and f). Aerial images were obtained using Google Earth and show differences in location of shoreline in relation to farm fields and exposure of lakebed playa over time.
Large scale desertification of previously submerged areas greatly increases the amount of mobilized dust, and coupled with wind events, amplifies the potential for humans to be exposed to such dust (Crooks, 2016). The consequences of rapid desiccation of a lakebed have been previously observed, such as in the case of the Aral Sea (Uzbekistan and Kazakhstan) and Owens Lake (CA). Dust from saline lakebeds is associated with a high proportion of particles <10μm (PM10) containing adsorbed sulfate, chloride, pesticides and heavy metals (Abuduwaili et al. 2010). The drying of Owens Lake, which once occupied an area less than a third of the size of the Salton Sea, led to severe dust emissions and became the largest source of PM10 in the United States prior to mitigation (Gill 1996). The PM emissions from Owens Lake reached concentrations in the thousands of μg/m3 and could be transported hundreds of miles (Gill et al. 2002). The particulates entrained from the dried lakebed were smaller in size (mode of 3.5 μm) than agricultural dust and therefore more easily respirable by humans (Reid et al. 1994). Toxic metals, including lead, arsenic, nickel and chromium as well as high concentrations of salts have been identified in sediments (Gill et al. 2002) and in dust sampled during wind events (Reid et al. 1994). Similar impacts have been observed near the Aral Sea (Wiggs et al. 2003). These cases illustrate that the exposure of the sediments leads to substantial increases in respirable PM and therefore suggest the potential for significant impacts on the health and quality of life of nearby residents (Abuduwaili et al. 2010).
Objectives
The shrinking of the Salton Sea has both known and likely unforeseen public health implications, including the growing risk of exposure to potentially hazardous wind-blown dust and dust storm events. However, historical efforts and management of the Salton Sea has focused primarily on habitat, ecology and restoration (Barnum et al. 2017). The consequences on the health and well-being of the local communities, who are staged to bear the disproportionate burden of the rural to urban water transfer, have largely been on the periphery of regulatory and legal discussions regarding water use and the future of the Salton Sea. There are nearly 130,000 people living within 15 miles (24 km) of the Salton Sea, of whom one-third are children based on 2010 US Census estimates.
The primary objective of this commentary is to call attention to the public health dimensions of the Salton Sea crisis. We know little about the possible long-term health effects of exposure to mobilized lakebed sediments or the numerous toxic contaminants that may become respirable on entrained particles. However, we can draw on existing epidemiological literature of other known sources of wind-blown dust, such as desert dust storms, and related health effects to begin to understand the potential public health impact of emerging exposure sources. Here, we briefly review what is known about the adverse health effects related to wind-blown dust exposure, in both adults and in children, and we highlight the case of the Salton Sea—a looming environmental crisis that has the potential for broad impacts on the future health of nearby communities, and implications for other communities facing the impacts of drying lakes.
Discussion
A Sea sustained by industrial agriculture leaves a toxic legacy
Agricultural irrigation runoff water flows into the Alamo and New Rivers or through discharge canals, which in turn, flow north and discharge into the Salton Sea (De Vlaming et al. 2004). This irrigation runoff brings agricultural pesticides, such as organophosphorus insecticides, chlorpyrifos, as well as industrial contaminants, into the Sea (De Vlaming et al. 2004). As the Sea’s water quality and impacts on fish and bird life have been a long-standing issue in the region, researchers have measured organochlorines, pesticides and toxic metals in the water, sediments and marine life, over the past four decades (Eccles 1979; Setmire et al. 1990; Bruehler and de Peyster 1999; Sapozhnikova et al. 2004; LeBlanc and Kuivila 2008; Xu et al. 2016). Some compounds partition extensively to sediments and one study of lakebed sediments frequently detected chlorpyrifos, trifluralin and DDE in concentrations that were concluded to be “not insignificant in terms of potential exposure and human health” (LeBlanc and Kuivila 2008). Measured concentrations of lindane, dieldrin, DDE and total PCBs in shoreline sediments of the Salton Sea exceeded PELs (probable effect levels) for sediment quality in freshwater, with the highest concentrations measured in the Southern part of the Sea which receives inflows from 2 rivers and agricultural runoff (Sapozhnikova et al. 2004). Levels of organochlorine pesticides on the southern edge of the Sea were higher in air-exposed sediments compared to submerged sediments (Wang et al. 2012). In addition to pesticides, toxic metals, such as arsenic, cadmium, copper, molybdenum, nickel, zinc and selenium, have been measured in playa sediments at levels of ecological concern (Vogl and Henry 2002; Xu et al. 2016).
As the Sea dries, such toxicants that have been deposited in the playa sediments can become entrained in the air on dust particles, creating the potential for inhalation exposures. As observed at Owens Lake and during large-scale dust events, dust particles can carry a complex heterogeneous mixture of organic and inorganic species that can change across space and time (Kelly and Fussell 2012). According to the Imperial Irrigation District (IID), between 2003 and 2016 the acreage of exposed playa around the Salton Sea increased from 862 to 16,452 (Formation Environmental LLC 2018). It is anticipated that this rate will accelerate in 2018, increasing not only acreage, but also the playa width and therefore the emissions potential. Models of dust potential suggest that southern portion of the shoreline, where the receding is progressing the fastest, has the highest dust emission potential (Breck et al. 2018). At the Salton Sea, soft crusts were found to be significant producers of dust during winter and early spring, as were dry wash areas containing loose particles on the surface year-round (King et al. 2011).
The composition of dust may strongly influence toxicity, which is important from a biological, public health and regulatory standpoint. Research is only beginning to examine the components and sources of dust in Imperial Valley, but the presence of multiple contaminants in Salton Sea sediments indicates that pesticides and metals could be carried on particles and inhaled by nearby residents. Prior work has suggested additional risks to respiratory health associated with exposure to toxicants carried by dust particles, including some evidence that metals carried in fine PM may contribute to respiratory hospital admissions among children (Ostro et al. 2009) and to increased blood pressure and decreased lung function in young adults (Cakmak et al. 2014). However, to date researchers have not evaluated the health risks associated with the inhalation of dust originated from these potentially toxic Salton Sea sediment mixtures among residents of nearby communities.
A drying Sea and the next generation: Effects of wind-blown dust on public health
Epidemiological studies have begun to explore the health effects of non-combustion related sources of PM exposure (reviewed in Tables 1 and 2). Among adults, a number of studies have observed that desert dust transported to Europe from Saharan dust storms was associated with increased mortality ( Tobias et al. 2011; Perez et al. 2012; Neophytou, 2013), with the strongest effects associated with exposure to coarse PM10 desert dust particles (Mallone et al. 2011; Stafoggia et al. 2015). While US-based studies are few, a recent analysis found a significant association between dust storms and increases in lagged non-accidental and cardiovascular mortality (Crooks et al. 2016). Others have reported associations between dust storms and increased emergency room visits and hospitalizations due to asthma or chronic obstructive pulmonary disease, COPD (Kanatani, 2010; Tam et al. 2012; Thalib and Al-Taiar, 2012; Ma et al 2016). Studies of exposure to Asian dust storm particles reported associations with decreased pulmonary function in adult asthmatics and increased reporting of lower respiratory tract symptoms (Watanabe et al. 2015). One study of respiratory symptoms in adults living near a desiccated lake in Canada compared to those living farther away measured an increased prevalence of cough and wheeze at the time of assessment, as well as chronic symptoms of cough, wheeze, eye irritation, and nasal irritation in the exposed population (Gomez et al. 1992). Chamber experiments with asthmatic adults found that exposure to PM10 at levels equivalent to dust storms reduced FEV1 (forced expiratory volume in one second), up to an hour post-exposure (Gupta et al.2012). Evidence from an experimental study in animals suggests that exposure to desert dust may provoke inflammatory injury in the lower respiratory tract by inducing oxidative stress and the release of pro-inflammatory mediators in the respiratory epithelium (Ghio et al. 2014).
Table 1.
Wind-blown dust and health in adult populations.
| Author | Study Location | Study Group | Study Design | Sample Size | Exposure Assessment | Outcomes | Significant Findings |
|---|---|---|---|---|---|---|---|
| Gomez et al., 1992 | Southern Saskatchewan (Canada) | Residents of all ages | Cross-sectional | N = 300 | Airborne dust concentrations were determined by drawing ambient air through open-face filter cassettes, containing pre-weighed glass fiber filters. PM was collected at the (1) lake bed surface 1.6km from the shore, (2) wind-blown surface deposits at a shore edge, and (3) wind-blown surface deposits from a pasture nearby were analyzed for physical characteristics. | Adverse respiratory health effects measured using a self-administered respiratory health questionnaire. Height, weight, blood pressure, and pulmonary function were recorded. | The individuals at greatest risk for adverse health effects were farmers and outdoor blue-collar workers. The exposed population experienced an increased prevalence of current cough and wheeze, as well as chronic symptoms of cough, wheeze, eye irritation, and nasal irritation. Smoking adjusted odds ratios were consistent with the prevalence ratios. |
| Mallone et al., 2011 | Rome (Italy) | Daily counts of mortality for residents from 2001 to 2004 | Time-stratified case-crossover | N = 80,423 | PM10 measured by the Lazio Environmental Agency. PM2.5 and PM2.5–10 data collected from a monitoring station 2km east of the city center. | Daily counts of mortality (natural, cardiac, cerebrovascular, and respiratory) provided by the Regional Register of Causes of Deaths. | Interquartile range increases in PM2.5–10 (10.8 μg/m3) and PM10 (19.8 μg/m3) were associated with increased mortality due to natural, cardiac, cerebrovascular, and respiratory causes, with estimated effects ranging from 2.64% to 12.65% (95% CI: 1.18–25.42%) for the association between PM2.5–10 and respiratory mortality (0- to 5-day lag). |
| Tobias et al., 2011 | Madrid (Spain) | Daily counts of mortality for residents from 2003 to 2005 | Case-crossover | N = 1096 | Saharan dust days, as defined by levels of PM10 and PM2.5 collected from the automated network of the Madrid’s City Comprehensive Air-Pollution Monitoring, Forecasting and Information System | Daily counts of total mortality provided by Madrid’s mortality registry | During Saharan dust days, an increase of 10 μg/m3 of PM10 was associated with a 2.8% increase in total mortality compared with 0.6% during non-dust days (P-value for interaction = 0.0165). |
| Samoli et al., 2011 | Athens (Greece) | Daily counts of mortality for residents from 2001 to 2006 | Case-crossover | N = 11,249 | PM10, PM2.5, NO2, SO2, and O3, measured by the monitoring network operated by the Ministry of Environment, Energy and Climate Change of Greece | Cardiovascular and respiratory mortality collected from the Greek National Statistical Service | A 10 μg/m3 increase in PM10 was associated with a 0.71% (95% CI: 0.42– 0.99%) increase in all deaths. The effects for total and cause specific mortality was greater for those ≥75 years of age, while for total mortality higher effects were observed among females. The main effect of desert dust days and its interaction with PM10 concentrations were significant in all cases except for respiratory mortality and cardiovascular mortality among those <75 years. |
| Gupta et al., 2012 | Jaipur (India), using dust samples from 4 regions of Rajasthan | Adults with stable, mild asthma | Randomized single-blind placebo-conrolled crossover study | N = 20 | Four samples of dust from sandstorm-prone areas of Rajasthan, or placebo were administered randomly over 5 study days at levels equivalent to PM10 of 1500–2000 μg/m3 for 5 sec per 2 min. Particle size and adhesion were measured by laser diffraction and unconfined compressive strength tests. | Serial FEV1 measures at 5, 15, 30 and 60 minute intervals post-exposure | The maximal decline in FEV1 was observed 15 minutes post-exposure with all dust samples. Mean ΔFEV1 ranged from 0.69 ± 0.08 to 0.32 ± 0.04 liters for dust aerosol samples. Decline in FEV1 correlated with volume of dust particles with size <10 μm (PM10) and adhesiveness of the dust particles from the four different collection locations. |
| Perez et al., 2012 | Barcelona (Spain) | Daily counts of mortality for residents from 2003 to 2007 | Case-crossover | N = 1524 | Saharan dust days defined by a back-trajectory analysis using information obtained from satellite images and by days when levels of PM10 measured at a remote rural monitoring site reached at least 50% of the PM10 levels measured at the urban sampling site in Barcelona. PM1, PM2.5, | Daily counts of cardiovascular, respiratory, and cerebrovascular mortality obtained from the Catalan mortality registry | During non-Saharan dust days, an IQR increase in PM10 was associated with statistically significantly increases in cardiovascular (OR: 1.033, 95% CI: 1.006–1.060) and respiratory mortality (OR: 1.044, 95% CI: 1.001–1.089). During Saharan dust days, strongest cardiovascular effects were observed with PM10 (OR: 1.085, 95% CI: 1.017–1.158). |
| Tam et al., 2012 | Hong Kong (China) | Adult emergency hospital patients from 1998 to 2002 | Case-crossover | N = 462,308 | Hourly concentrations of NO2, SO2, O3, PM10, and PM2.5 collected from 11 air quality monitoring stations | Emergency hospital admissions for respiratory disease collected from the Hospital Authority, a Hong Kong public institution that provides more than 90% of all the hospital beds in the city | Significant increases in emergency hospital admission due to COPD were found 2 days after a dust storm episode. A 10 μg/m3 increase of PM10 (lag 2 days) was associated with an increased risk of COPD admission RR=1.05 (95% CI: 1.01–1.09). |
| Thalib et al., 2012 | Kuwait | Patients of all ages who sought emergency room care for respiratory problems or asthma | Time-series design | N = 88,267 | PM10, temperature, humidity, and dust storm days collected by the Environment Public Authority of Kuwait | Daily emergency asthma admissions and admissions due to respiratory causes from 1996 to 2000 obtained from the Department of Vital Statistics of the Ministry of Health, Kuwait | During the five-year study period, 569 (33.6%) days had dust storm events. These events were associated with an increased risk of same-day asthma and respiratory admission, adjusted RR = 1.07 (95% CI: 1.02–1.12) and 1.06 (95% CI: 1.04–1.08), respectively. |
| Neophytou et al., 2013 | Nicosia (Cyprus) | Daily counts of mortality of residents from 2004 to 2007 | Time-series design | -- | Hourly concentrations of PM10 and O3 were collected from ambient air quality monitoring stations in both urban and rural locations. Hourly measurements of NO2, NOx, SO2 and CO were collected from an urban monitoring station. Daily air temperature and relative humidity measurements were obtained from the meteorological station in closest proximity to the urban quality monitoring station | Non-trauma, cardiovascular, and respiratory daily mortality collected from the Health Monitoring Unit of the Ministry of Health via the Cyprus Statistical Services | There was a 2.43% (95% CI: 0.53, 4.37) increase in daily cardiovascular mortality associated with each 10-μg/m3 increase in PM10 concentrations on dust days. Associations for total (0.13% increase, 95% CI: −1.03, 1.30) and respiratory mortality (0.79% decrease, 95% CI: −4.69, 3.28) on dust days and all PM10 and mortality associations on non-dust days were not significant. |
| Stafoggia et al., 2015 | 13 European cities: Barcelona, Madrid (Spain), Marseille (France), Bologna, Milan, Modena Palermo, Parma, Reggio Emilia, Rome, Turin (Italy), Athens,Thessaloniki (Greece) | City residents from 2001 to 2010 | Time-stratified case-crossover | -- | PM10, PM2.5, PM2.5–10 collected from the regional Environmental Protection Agencies. Desert dust advection days were identified in using a combination of tools including meteorological products, aerosol maps, air masses back-trajectories, and satellite images. | Daily counts of mortality for natural, cardiovascular, and respiratory mortality collected from each city’s mortality registry. Hospital emergency admissions collected from the hospital discharge databases of each country. | Increases of 10 μg/m3 in non-desert and desert PM10 (lag 0–1 days) were associated with increases in natural mortality of 0.55% (95% CI: 0.24, 0.87%) and 0.65% (95% CI: 0.24, 1.06%), respectively. Similar associations were estimated for cardio-respiratory mortality and hospital admissions. |
| Watanabe et al., 2015 | Yonago, Matsue, Sakaiminato, and Yasugi (Japan) | Residents of all ages | Longitudinal panel study design | N = 137 | Daily information on Asian Dust Storm events was obtained from the Japan Meteorological Agency. Concentrations of SMP, SO2, NO2, and O3 from fixed monitors. LIDAR data for sand dust particles and aerosolized air pollutants. | Daily lower respiratory tract symptoms measured using a questionnaire indicating on a scale of 0 to 3 the extent of lower respiratory tract symptoms including cough, sputum, dyspnea, and wheezing. PEF measured daily by each child in the morning | Sand dust particles were significantly associated with worsened lower respiratory tract symptoms in adult patients with asthma, but not with pulmonary function. Elevated sand dust particle levels were significantly associated with the symptom score (0.04; 95% CI: 0.03, 0.05), and increases persisted for 5 days. There was no significant association between PEF and heavy dust exposure (0.01 L/min; 95% CI, −0.62, 0.11). |
| Crooks et al., 2016 | Arizona, California, Nevada, Utah, Texas, Kansas, Oklahoma, Nebraska, Colorado, New Mexico, Idaho, Washington, Oregon, Montana (United States (USA)) | Residents of selected US states from 1993 to 2005 | Time-stratified case-crossover | -- | Dust storms, as reported by the US National Weather Service. Ambient monitor data was used to calculate 24-hr average PM10, PM2.5 and O3 for 1993–2010. | Daily counts of non-accidental mortality collected from the National Center for Health Statistics | Increases in lagged non-accidental mortality was associated with dust storms. Total non-accidental mortality increased by 7.4% (95% CI: 1.6, 13.5) and 6.7% (95% CI: 1.1, 12.6) at 2- and 3-day lags, respectively, and by an average of 2.7% (95% CI: 0.4, 5.1) over lags 0–5, compared with referent days. |
| Kanatani et al. 2016 | Kyoto, Toyama, and Tottori (Japan) | Pregnant women | Prospective cohort study | N =3,327 | Asian dust days defined as days during which the LIDAR detected more than 70μg/cm3 of total suspended matter of desert dust. | Allergy symptoms measured using a mobile self-administered questionnaire evaluating allergy score. | Pregnant women had an increased risk of allergic symptoms on high desert-dust days (adjusted OR: 1.10; 95% CI, 1.04–1.18). The increased OR was mostly driven by those who showed positive IgE to Japanese cedar pollen when pollen simultaneously dispersed (adjusted OR: 1.25; 95% CI, 1.13–1.38), whereas no clear risk increase was observed in the absence of pollen or for participants with negative IgE to Japanese cedar pollen. The risk elevation was observed from low levels of desert dust in a dose-dependent manner even on control days. |
| Watanabe et. Al 2016 | Yonago, Matsue, Sakaiminato, Yasugi, and Saihaku (Japan) | Residents of all ages | Panel study design | N = 231 | Concentrations of SPM, SO2, NO2, and Ox monitored at fixed site locations. Data for PM2.5 and data for non-spherical and spherical particles from LIDAR. Heavy Asian Dust days were defined as having a level of dust particles >0.032 km−1 (i.e. the average plus 1 SD during the study period) | PEF measured daily 3x/ daily using a peak flow meter before the patients inhaled corticosteroids or took oral drugs. Each patient recorded the best value from the three attempts. At the end of each month, the Japanese version of the Asthma Control Test scores were recorded. | Increases in the interquartile range of Asian dust particles (0.018 km−1) led to changes in PEF of −0.42 L/min (95% CI: −0.85 to 0.01). An increase of 11.8 μg/m3 in suspended particulate matter and 6.9 μg/m3 in PM2.5 led to decreases of −0.17 L/min (95% CI: −0.53 to 0.21) and 0.03 L/min (95% CI: −0.35 to 0.42), respectively. Six heavy Asian dust days were identified. Change in PEF after a heavy Asian dust day was −0.97 L/min (95% CI: −1.90 to −0.04). |
| Ma et al. 2016 | Lanzhou (China) | Patients of all ages who went to the emergency room for respiratory problems | Time-series design | N = 30,650 | Daily ambient concentrations of PM10, SO2, and NO2 measured by four fixed monitoring stations located in the urban district. | Emergency room visits for respiratory diseases from 2007 to 2011 collected from three large-scale comprehensive hospitals in Lanzhou | Significant associations were found between outdoor air pollution concentrations and respiratory diseases, as expressed by daily ER visits in the spring dust season. The association between air pollution and ER visits appeared to be more evident on dust days than non-dust days. Relative risks (95% CIs) per 10 μg/m3 increase in 3-day PM10 (L3), 5-day SO2 (L5), and the average of current and previous 2-day NO2 (L1) were RR: 1.140 (1.071–1.214), RR: 1.080 (0.967–1.205), and RR: 1.298 (1.158–1.454), respectively, on dust days. |
Abbreviations used: CO: Carbon monoxide; FENO: Fraction of exhaled nitrogen oxide; ISAAC: International Study of Asthma and Allergies in Childhood; LIDAR: Light Detection and Ranging System; NO2: Nitrogen dioxide; NOx: Nitrogen oxide; O3: Ozone; OR: odds ratio; PM10: any particle measuring less than 10 μm in diameter; PM2.5: any particle measuring less than 2.5μm in diameter; RR: relative risk; SO2: Sulphur dioxide; 95% CI: 95% confidence interval.
Table 2.
Wind-blown dust, drying lakes and health in child populations.
| Author | Study Location | Study Group | Study Design | Sample Size | Exposure Assessment | Outcomes | Significant Findings |
|---|---|---|---|---|---|---|---|
| Kaneko et al. 2003 | Kazalinsk and Zhanakorgan (Kazakhstan) | Children 6 to 15 years old | Cross-sectional | N = 392 | Exposure was defined by proximity to the Aral Sea, such that children living in Kazalinsk (close to the Sea) were compared to children living in Zhanakorgan (far from the Aral Sea) | Prevalence of renal tubulopathy, measured by levels of N-acetyl-b-D-glycosaminidase (NAG) and beta-2 macroglobulin (BMG) in urine samples | Mean urinary NAG and BMG were both significantly higher in Kazalinsk than in Zhanakorgan (p < 0.01). The number of children with abnormal values of NAG (1.5 U/mmol Cr) was significantly higher in Kazalinsk than in Zhanakorgan (7.9% and 2.6%, respectively, p < 0.05). |
| Kunii et al. 2003 | Kazalinsk and Kzyl-Orda (Kazakhstan) | Children 6 to 15 years old | Cross-sectional | N = 815 | Exposure was defined by proximity to the Aral Sea, such that children living within 200km of the Aral Sea were compared to less exposed children 500km from the Aral Sea | Respiratory symptoms were measured using questionnaires. Pulmonary function defined by FVC was measured by spirometer. | Prevalence of current cough and wheezing were higher among the exposed participants, as was restrictive pulmonary dysfunction (10.6%) as compared to the reference group (2.6%). FVC% predicted was lower in the exposed group (median= 96.6%) than reference (median= 100.5%). |
| Wiggs et al. 2003 | Autonomous of Republic Karakalpakstan (Uzbekistan) | Children 7 to 11 years old | Case-crossover | N = 1499 | Dust deposition measured on a monthly basis at 16 sites. PM10 was measured using pump samplers at three sites. | Adverse respiratory health effects assessed by a health survey. The questionnaire covered socio-demography, respiratory symptoms, and living conditions | Children living in the north of the country, where aeolian dust deposition rates are greater, show a lower frequency of respiratory problems. Children located closest to the Aral Sea have fewer respiratory health problems than children living in the main agricultural and urban regions to the south. |
| Bennion et al., 2007 | Autonomous Republic of Karakalpakstan (Uzbekistan) | Children 7 to 11 years old | Cross-sectional | N =100 | Dust deposition, as measured by amounts collected from dust traps. | Respiratory symptoms were collected by questionnaire. Lung function was assessed using a portable spirometer | Overall prevalence of wheeze was low at 4.2%, but varied by region. No association was observed between local annual dust deposition and specific respiratory symptoms. Predicted FEV1 was inversely related to dust exposure during the summer months (−1.465, 95% CI: −2.519 to −0.412) change in predicted FEV1 per 1000kg/ha annual dust deposition. |
| Hong et al. 2010 | Seoul (South Korea) | Elementary school children all 9 years old | Case-crossover | N = 110 | Filter-based gravimetric assessment of PM2.5, PM10, and metal components in PM | Pulmonary function, as measured by the PEF, using a peak flow meter to record measurements 3x/ day at 9:00, 12:00, and 20:00. | Ambient concentrations of PM2.5 and PM10 were not significantly associated with PEF in school children, except asthmatics (p < 0.05). Metal concentrations bound to the particulates were significantly associated with decrease of the children's PEF (p < 0.05). |
| Samoli et al. 2011 | Athens (Greece) | Children 0 to 14 years old | Cross-sectional | N = 3601 | PM10 (daily average), SO2 (daily average), NO2 (1-hour max), and O3 (8 hours) calculated from monitors that provided data for at least 75% of the days in the analyzed period | Pediatric asthma exacerbation measured by daily counts of pediatric asthma emergency admissions. | A 10 mg/m3 increase in PM10 was associated with a 2.54% increase (95% CI: 0.06%, 5.08%) in the number of pediatric asthma hospital admissions. Statistically significant PM10 effects were higher during winter and during desert dust days. |
| Chien et al., 2012 | Taipei (Taiwan) | Preschool (≤ 6 years old) and elementary school children (7 to 14 years old) | Case-crossover | Asian Dust Storm events, as identified by the Department of Atmospheric Science at Chinese Culture University and the Taiwan Environmental Protection Agency | Spatiotemporal distributions of clinic visits for respiratory disease, as measured by daily clinic visits among preschool and elementary school children registered in 12 districts of Taipei City | Compared with weeks before an Asian Dust Storm event, the rate of clinic visits during weeks after the dust storms increased by 2.54% (95% CI: 2.43, 2.66) for preschool children (≤ 6 years of age) and 5.03% (95% CI: 4.87, 5.20) for schoolchildren. | |
| Carlsen et al., 2016 | Umea Vasterbotten (Sweden) | Children 11 to 12 years old | Cross-sectional | N = 95 | PM10, PM2.5, O3, NO2 and NOx were measured at the local monitoring stations. | FENO was assessed on each participant 2x/week. Questionnaire about respiratory health, use of asthma medication, and rhinitis was filled out by parents. |
In multi-pollutant models, an interquartile range increase in 24hr PM10 was associated with increases in FENO between 6.9 ppb (95% CI: 0.0–14) and 7.3 ppb (95% CI: 0.4–14.9), suggesting exposure related sub-clinical airway inflammation in healthy children. |
| Wantanabe et. Al 2016 | Matsue (Japan) | Children 8 to 9 years old | Panel study design | N = 339 | A LIDAR was used to monitor the concentration of sand dust particles (SPM), PM2.5, SO2, NO2, and O3. | PEF measured daily by each child throughout the course of the study with the exception of Saturdays, Sundays, and public holidays in the morning using a peak flow meter | An increment of 0.018 km(−1) in sand dust particles was significantly associated with a decrease in PEF (−3.62 L/min; 95% CI, −4.66 to −2.59). An increase of 14.0 μg/m(3) in SPM and 10.7 μg/m(3) in PM2.5 led to a significant decrease of −2.16 L/min (−2.88 to −1.43) and −2.58 L/min (−3.59 to −1.57), respectively, in PEF. |
| Neisi et al. 2017 | Ahvaz (Iran) | Healthy elementary school children 9 to 13 years old | Cross-sectional | N = 105 | Dusty versus normal days were defined using visibility, wind speed, and hourly PM10 concentration | FENO concentration measured using a NObreath® analyzer. FVC was measured by a spirometer | PM2.5 and PM10 were higher during dusty days than normal days. Mean FENO values were significantly higher during dusty days (20.3 ppb) than normal days (14.23 ppb) (p< 0.05). Mean FVC values were also significantly lower during dusty days versus normal (p < 0.05). |
Abbreviations used: FENO: Fraction of exhaled nitrogen oxide; FEV: Forced Expiratory Volume; FVC: Forced Vital Capacity; ISAAC: International Study of Asthma and Allergies in Childhood; LIDAR: light detection and ranging system; NO2: Nitrogen dioxide; NOx: Nitrogen oxide; O3: Ozone; PEF: Peak Expiratory Flow; PM10: any particle measuring less than 10 μm in diameter; PM2.5: any particle measuring less than 2.5 μm in diameter; SO2: Sulphur dioxide; SPM: Suspended particulate matter; 95% CI: 95% confidence interval
Children are highly susceptible to the impacts of air pollutants, as their lungs and immune systems continue to develop throughout childhood, and particle deposition has been shown to be higher in young children and asthmatics (Chalupa et al. 2004; Ginsberg et al. 2005; Ostro et al. 2009). In addition to acute adverse effects of exposure to PM, such as asthma exacerbations and respiratory distress, children may be at risk of long-term effects of exposure to ambient dust-borne contaminants, such as deficits in lung growth, airway inflammation and new onset asthma (Chen et al. 2015). Given that early life insults to the lung may elevate the risk of long-term disease (Stocks and Sonnappa 2013; Stocks et al. 2013), such increases in ambient PM due to the drying of the Sea may influence long-term lung health. However, relatively few studies have assessed the impact of ambient dust exposures on children’s health and to our knowledge, all have focused on acute or relatively short-term health effects. Despite the limited studies, these studies support a role for wind-blown dust exposures in potential impacts on respiratory symptoms, asthma, and lung inflammation.
A growing number of studies from dust-storm prone regions around the world have begun to provide evidence of acute effects of wind-blown dust on children’s health (Table 2). Among children in Greece, increases in PM10 likely due to desert dust transported from the Sahara, were associated with a 2.5% increase in emergency hospital admissions for pediatric asthma (Samoli et al. 2011). A study of Japanese children found similar associations between asthma hospitalizations and heavy dust events within the previous week (Kanatani et al. 2010). Asian dust storm events have been associated with significant increases in respiratory clinic visits among preschool and school children in Taiwan (Chien et al. 2012). Similarly, Asian dust storms were associated with reduced pulmonary function among asthmatic children in Korea (Hong et al. 2010) and more recently, with decreased pulmonary function in healthy schoolchildren in Japan (Watanabe et al. 2017). Other recent studies have explored the relation between coarse PM10 exposure and fractional exhaled nitric oxide (FeNO), a biomarker of sub-clinical airway inflammation, among healthy school children. A Swedish study assessed children biweekly over two months and found that short-term changes in coarse PM were associated with significant increases in FeNO, even after accounting for other ambient air pollutants (Carlsen et al. 2016). Among Iranian children, researchers found that, compared to normal days, dusty days were related to significant increases in FeNO and decreases in lung function (Neisi et al. 2017). These studies provide evidence of the acute health effects of wind-blown dust exposures among children, which, with repeated insults, have the potential to influence respiratory health over time.
An additional concern related to children’s health is the emerging evidence that suggests that wind-blown PM also may be related to allergies, atopic conditions and eye diseases. Recent publications have reported that dust may promote allergic disease by acting as an allergic adjuvant, and showed that dust storms may enhance allergic symptoms among allergen-sensitized individuals, independently of increases in other ambient allergens (Mimura et al. 2014; Kanatani, 2016). In addition to respiratory endpoints, there is suggestive evidence that dust storms may be associated with allergic and atopic conditions. Higher incidence of conjunctivitis in young children was observed among those with exposure to dust storm events in Taiwan (Chien et al. 2014). Asian dust storm particles have also been linked to skin irritation and have been found to induce pro-inflammatory and immunomodulatory changes in cultured epidermal cells (Choi et al. 2011; Otani et al. 2011; Onishi et al. 2015).
Finally, wind-blown dust exposure and dust events may be important exposure pathways for microbes, fungi and viruses that can be carried on dust particles (Yamaguchi et al. 2016; Maki et al. 2017; Behzad et al 2018). Wind-blown dust has been shown to carry various microbes and pathogens, which can cause respiratory illness when inhaled (Behzad et al 2018). A recent study that monitored airborne bacteria during Asian dust storm events found that dust events can transport large amounts of bacterial cells and that airborne bacterial abundance was more than 10-fold higher on severe dust days, than on less dusty days (Yamaguchi et al. 2014). In the Imperial Valley, annual incident cases of Valley Fever in California increased by approximately 70% from 2009 to 2012 and while there are likely a number of factors, elevated dust levels, along with hotter, drier conditions have likely contributed to Valley Fever incidence (Park et al. 2005; Ampel 2010). Increasing amounts of PM from the shrunken Salton Sea basin could carry additional pathogens to communities, increasing the potential for infections and respiratory illness after dust events. Together, these studies begin to indicate possible long-term health significance of increasing dust exposures among children and highlight the need for longitudinal studies of the long-term impacts of these exposures.
The Aral Sea: children’s health after lakebed desiccation
The changes observed at the Aral Sea provide an example of the possible public health consequences of lakebed desiccation. The diversion of water for irrigation from the Aral Sea basin resulted in exposure of 36,000 square kilometer of former seabed over the course of 40 years (Micklin 1998) and created one of the highest dust deposition rates globally (O’Hara et al. 2000). However, the few studies of wind-blown dust and children’s respiratory health in this region have been largely inconclusive. One study observed that school-aged children living near the Aral Sea had twice the reported recent wheeze symptoms along with lower lung function compared to an age- and sex-matched control group living farther away from the sea (Kunii et al. 2003). A separate study of lung function among children (age 7–9) around the Aral Sea suggested that while exposure to dust did not fully explain the variations in lung function among different communities, high levels of dust exposure during the summer may have an adverse effect on measured lung function (Bennion et al. 2007). Others also found an association between proximity to the Aral Sea and levels of renal tubular dysfunction in children, measured via urinary markers, which could indicate consequences for long-term renal health and hypertension risk (Kaneko et al. 2003). The case of the Aral Sea highlights the need for longitudinal studies of wind-blown dust exposures that can establish baseline data about health and begin to more clearly elucidate the effects of long-term wind-blown PM exposures on children’s health.
A population at risk: health and vulnerability in the Imperial Valley
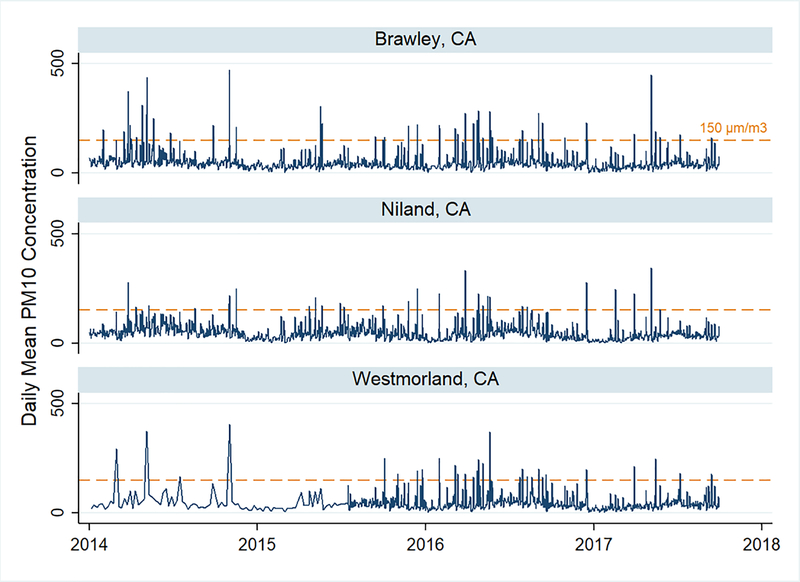
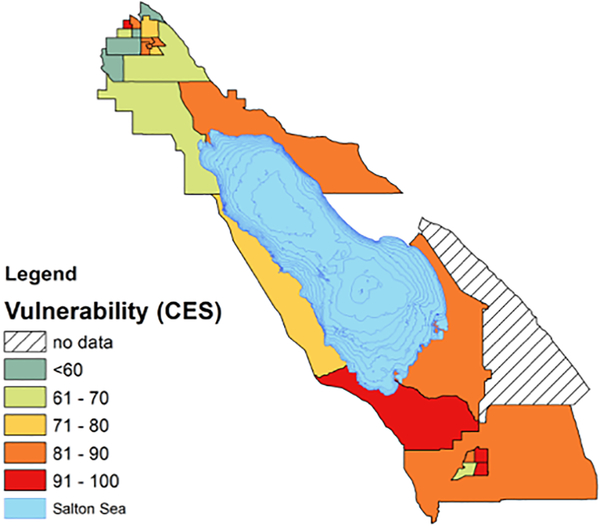
The agricultural southeastern border region of California already faces frequent local dust storms that can create high levels of dust due to industrial and agricultural activities and the desert environment around the Imperial Valley. Recorded peak daily concentrations of PM10 at levels nearly 10 times the state and federal limits (CARB 2012) (Figure 3). Imperial County is primarily Mexican/Mexican-American (~83%), with some of the highest rates of unemployment and poverty in the nation (Bureau 2014). An estimated 10,000 children (one-third of residents) live in the towns nearest to the southern edge of the Sea. According to the statewide tool, CalEnviroScreen, the majority of the census tracts in this region are among some of the most vulnerable in the state to pollution, as measured by socioeconomic (education, housing, linguistic isolation, poverty and unemployment) and health indicators (asthma, cardiovascular disease and low birth weight) (OEHHA 2018) (Figure 4). In this region, approximately 23,000 residents, around 1 in 5, have been diagnosed with asthma (Lipsett et al. 2009; CEHTP 2017). Currently, emergency departments in Imperial County treat three times more pediatric asthma visits than elsewhere in California (Marshall 2017). Evidence from a cross-border pilot study of asthma incidence found the prevalence of physician-diagnosed asthma among 13–14 year olds was more than 4 times higher in the Imperial Valley cities compared to those just across the border in Mexico (26.5% versus 5.8%) (Lipsett et al. 2009). Given the demographic and ethnic similarities of these two sites, these findings support the hypothesis that there are likely important environmental factors impacting asthma prevalence in the Imperial Valley on the US side of the border.
Figure 3.
Particulate matter levels <10 μm in diameter (PM10) as measured in μg/m3 by Imperial County Air Pollution Control District monitors in Imperial County, CA 2014–2017. The dashed line (150 μg/m3) represents the 24-hour average standard for PM10.
Figure 4.
Map of census tracts surrounding the Salton Sea, colored by CalEnviroscreen population characteristics, which represent biological traits, health status and community indicators that can increase vulnerability to pollution. CES population vulnerability percentile is calculated by assigning percentile scores for multiple population characteristics, to individual census tracts in California. Nearly all census tracts in Imperial County scored rank among the top 20% of census tracts most vulnerable to pollution.
Looking ahead: Water scarcity, dust and health
Across the southwestern US, increased production of wind-blown dust and environmental exposures to such non-combustion related sources of PM are a growing health threat, due to long-standing drought conditions and increasingly limited water resources. As more inland lakes in the US and globally face risk of drying due to diverted water flow, drought and overuse, including the Great Salt Lake (Utah, US), Lake Mead (Arizona, US), Lake Chad (Chad/Cameroon, Nigeria), Lake Urmia (Iran) and the Dead Sea (Jordan/Israel/Palestine), exposure to wind-blown sediments will become an increasingly important emerging health risk. Furthermore, dust events are becoming more frequent, with the number of dust storms more than doubling from the 1990s to 2000s (Tong et al. 2017). In arid regions, such as the Imperial Valley, the mobilization, transport and deposition of wind-blown dust can contribute to significant ecological, economic and health issues (Griffin et al. 2001). Nonetheless, epidemiological studies on this emerging exposure remain limited and the health impacts of non-combustion related PM on communities, especially children and other vulnerable populations, are largely unknown (De Sario et al. 2013; Ma et al. 2016).
In the US, the distributions and types of rural pollution are typically not well characterized, nor are the population-level health effects of such types of pollution (Michael et al. 2010). The intersection of rural populations with race and class often exacerbates environmental injustices. The industrialization of rural areas for agricultural or energy production, coupled with the transfer of resources from rural to urban communities can harm rural populations for urban beneficiaries (Kelly-Reif and Wing 2016). Rural low-income and people of color often lack the financial and social resources to mitigate their exposures, such as moving households to less polluted areas, using air conditioners instead of opening windows, or influencing local and state government to reduce pollution exposures (Thu 2001). Pressure to control the effects of rural environmental degradation is lessened because of the lack of political power and the direct benefits to the urban majority. Rural areas, like the Imperial Valley, tend to bear increasingly heavy cumulative economic and community health impacts of environmental changes, but are often overlooked by academics and policy-makers. A scientific framework to monitor pollutants and health outcomes in rural places through partnerships between researchers and rural community members can promote broader goals of economic, racial and social justice (Kelly-Reif and Wing 2016; English et al. 2017). In Imperial Valley, community-based organizations are bringing increasing attention to local public health issues and advancing efforts to address local concerns. As increasing levels of wind-blown PM and dust events are likely to burden agricultural and rural regions, community-engaged techniques to better understand the impacts of this emerging exposure on acute and chronic health are important for future epidemiological research.
The disappearance of the Salton Sea is just one example of how the intersection between competing water demands and a changing climate coupled with short-term planning and limited community engagement can have extensive and unforeseen public health implications. In 2017, fourteen years after the water transfer settlement, the California Natural Resources Agency released a 10-year plan to address the drying shoreline and loss of habitat. Despite this effort and growing awareness of this issue, the proposed $383 million patchwork of shallow ponds and wetlands along the receding shorelines are estimated to cover less than half of the nearly 100 square miles anticipated to be exposed over the next decade. The plan is poised to leave local populations at risk of increasing wind-blown PM exposures, which could have potentially devastating implications for human health. Improved understanding of the composition and toxicity of contaminated airborne particles from drying lakes, like the Salton Sea, as well as the geographic scope of the dust emissions, is critical to understanding and addressing the potential environmental health impacts in the region. Resources to rapidly deploy dust management measures and meaningful collaboration across agencies, government and the community residents may facilitate development of both mitigation and adaptive measure to the changing environment. Participatory forms of decision making and governance with respect to disaster and environmental health, can support multi-stakeholder dialogue, empower communities in social action and generate collective knowledge to build resiliency and improve public health (Berke et al. 2018; Cox et al. 2014; Wing et al. 2008). The shrinking of the Salton Sea illustrates a public health and environmental justice crisis that requires action by to protect public health and community well-being.
Acknowledgments
Funding sources and ethical considerations
Funding for this study was provided by NIEHS R01 (1R01ES029598-01), the NIEHS Southern California Environmental Health Sciences Center Research Program (5P30ES007048-20), and a pilot grant from the Keck School of Medicine Dean’s Pilot Program. The funding agencies that supported this work had no role in the planning, design, or execution of this study, nor any role in data analysis or manuscript preparation. The authors have no competing personal or financial interests.
References
- Abuduwaili J, Liu D, Wu G. 2010. Saline dust storms and their ecological impacts in arid regions. 2:144–150, 10.3724/SP.J.1227.2010.00144. [DOI] [Google Scholar]
- Akinbami LJ, Simon AE, Rossen LM. 2016. Changing trends in asthma prevalence among children. Pediatrics 137:1–1, 10.1542/peds.2015-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampel NM. 2010. What’s behind the increasing rates of coccidioidomycosis in Arizona and California? Current infectious disease reports 12:211–216, 10.1007/s11908-010-0094-3. [DOI] [PubMed] [Google Scholar]
- Barnum DA, Bradley T, Cohen M, Wilcox B, Yanega G. 2017. State of the Salton Sea—a science and monitoring meeting of scientists for the Salton Sea. (Open-File Report). 2017–1005. Reston, VA, 10.3133/ofr20171005. [DOI] [Google Scholar]
- Bennion P, Hubbard R, O’Hara S, Wiggs G, Wegerdt J, Lewis S, et al. 2007. The impact of airborne dust on respiratory health in children living in the Aral Sea region. Int J Epidemiol 36:1103–1110, PM ID: 17911152, 10.1093/ije/dym195. [DOI] [PubMed] [Google Scholar]
- Behzad H, Mineta K, Gojobori T. Global ramifications of dust and sandstorm microbiota. Genome biology and evolution. 2018. June 29;10(8):1970–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke P, Quiring S, Olivera D, Horney J. 2018. Addressing Challenges to Building Resilience Through Interdisciplinary Research and Engagement. Risk Anal, 10.1111/risa.13202 [DOI] [PubMed] [Google Scholar]
- Bhan N, Kawachi I, Glymour MM, Subramanian SV. 2015. Time trends in racial and ethnic disparities in asthma prevalence in the United States from the behavioral risk factor surveillance system (BRFSS) study (1999–2011). American Journal of Public Health 105:1269–1275, 10.2105/AJPH.2014.302172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breck J, Eisenhardt L, Mueller S, Tran A. 2018. Prioritizing Cost-Effective Dust Mitigation at the Salton Sea. Santa Barbara, CA: https://www.bren.ucsb.edu/research/2018Group_Projects/documents/Salton_Seafarers_Final_Report_redacted.pdf [Google Scholar]
- Bruehler G, de Peyster A. 1999. Selenium and other trace metals in pelicans dying at the Salton Sea. Bull Environ Contam Toxicol 63:590–597 [DOI] [PubMed] [Google Scholar]
- Cakmak S, Dales R, Kauri LM, Mahmud M, Van Ryswyk K, Vanos J, et al. 2014. Metal composition of fine particulate air pollution, and acute changes in cardiorespiratory physiology. Environmental Pollution 189:208–214, 10.1016/j.envpol.2014.03.004. [DOI] [PubMed] [Google Scholar]
- CARB (California Air Resources Board). 2012. Biennial report on air quality trends and emission control programs.
- Cantor A 2016. The public trust doctrine and critical legal geographies of water in California. Geoforum 72:49–57, 10.1016/j.geoforum.2016.01.007. [DOI] [Google Scholar]
- Carlsen HK, Boman P, Björ B, Olin AC, Forsberg B. 2016. Coarse fraction particle matter and exhaled nitric oxide in non-asthmatic children. International Journal of Environmental Research and Public Health 13:1–11, 10.3390/ijerph13060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case H, Delgado A, Nguyen T, Osugi D, Barnum D, Decker D, et al. 2013. Salton Sea ecosystem monitoring and assessment plan. Sacramento, CA, 10.3133/ofr20131133. [DOI] [Google Scholar]
- CEHTP (California Environmental Health Tracking Program). 2017. Asthma Data-Imperial County. Richmond, CA. [Google Scholar]
- Chalupa DC, Morrow PE, Oberdorster G, Utell MJ, Frampton MW. 2004. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect 112:879–882, 10.1289/ehp.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Eckel SP, Breton CV, Gilliland FD. 2015. Chronic effects of air pollution on respiratory health in southern California children: Findings from the Southern California children’s health study. Journal of Thoracic Disease 7:46–58, 10.3978/j.issn.2072-1439.2014.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien LC, Yang CH, Yu HL. 2012. Estimated effects of Asian dust storms on spatiotemporal distributions of clinic visits for respiratory diseases in Taipei children (Taiwan). Environ Health Perspect 120:1215–1220, 10.1289/ehp.1104417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien LC, Lien YJ, Yang CH, Yu HL. 2014. Acute increase of children’s conjunctivitis clinic visits by Asian dust storms exposure - a spatiotemporal study in Taipei, Taiwan. PLoS One 9:e109175, 10.1371/journal.pone.0109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Shin DW, Kim W, Doh SJ, Lee SH, Noh M. 2011. Asian dust storm particles induce a broad toxicological transcriptional program in human epidermal keratinocytes. Toxicology letters 200:92–99, 10.1016/j.toxlet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Cohen MJ, Hyun KH. 2006. Hazard: The future of the Salton Sea with no restoration project.
- Cox R, Hamlen M. 2014. Community Disaster Resilience and the Rural Resilience Index. Am. Behav. Sci. 59, 220–237., 10.1177/0002764214550297 [DOI] [Google Scholar]
- Crooks JL, Cascio WE, Percy MS, Reyes J, Neas LM, Hilborn ED. 2016. The association between dust storms and daily non-accidental mortality in the United States, 1993? 2005. Environ Health Perspect 124:1735–1743, 10.1289/EHP216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sario M, Katsouyanni K, Michelozzi P. 2013. Climate change, extreme weather events, air pollution and respiratory health in Europe. European Respiratory Journal 42:826–843, 10.1183/09031936.00074712. [DOI] [PubMed] [Google Scholar]
- De Vlaming V, DiGiorgio C, Fong S, Deanovic LA, De La Paz Carpio-Obeso M, Miller JL, et al. 2004. Irrigation runoff insecticide pollution of rivers in the Imperial Valley, California (USA). Environmental Pollution 132:213–229, 10.106/j.envpol.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Eccles LA. 1979. Pesticide residues in agricultural drains, southeastern desert area, California. (Water-Resources Investigations Report). 79–16, 10.3133/wri7916. [DOI] [Google Scholar]
- English PB, Olmedo L, Bejarano E, Lugo H, Murillo E, Seto E, et al. 2017. The Imperial County community air monitoring network: A model for community-based environmental monitoring for public health action. Environ Health Perspect 125:074501, 10.1289/EHP1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formation Environmental LLC, Air Sciences Inc, PlanTierra. 2018. Salton Sea Air Quality Mitigation Program: 2016/2017 Annual Report and Emissions Estimates. Imperial Irrigation District; Imperial, CA: https://www.iid.com/home/showdocument?id=17055 [Google Scholar]
- Frie AL, Dingle JH, Ying SC, Bahreini R. 2017. The effect of a receding saline lake (the Salton Sea) on airborne particulate matter composition. Environmental science & technology 51:8283–8292, 10.1021/acs.est.7b01773. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Kummarapurugu ST, Tong H, Soukup JM, Dailey LA, Boykin E, et al. 2014. Biological effects of desert dust in respiratory epithelial cells and a murine model. Inhalation Toxicology 26:299–309, 10.3109/08958378.2014.888109. [DOI] [PubMed] [Google Scholar]
- Gill TE. 1996. Eolian sediments generated by anthropogenic disturbance of playas: Human impacts on the geomorphic system and geomorphic impacts on the human system. Geomorphology 17:207–228, 10.1016/0169-555X(95)00104-D. [DOI] [Google Scholar]
- Gill TE, Gillette DA, Niemeyer T, Winn RT. 2002. Elemental geochemistry of wind-erodible playa sediments, Owens Lake, California. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 189:209–213, 10.1016/S0168-583X(01)01044-8. [DOI] [Google Scholar]
- Ginsberg GL, Foos BP, Firestone MP. 2005. Review and analysis of inhalation dosimetry methods for application to children’s risk assessment. J Toxicol Environ Health A 68:573–615, 10.1080/15287390590921793. [DOI] [PubMed] [Google Scholar]
- Gomez SR, Parker RA, Dosman JA, McDuffie HH. 1992. Respiratory health effects of alkali dust in residents near desiccated old wives lake. Arch Environ Health 47:364–369, 10.1080/00039896.1992.9938376. [DOI] [PubMed] [Google Scholar]
- Griffin DW, Kellogg CA, Shinn EA. 2001. Dust in the wind: Long-range transport of dust in the atmosphere and its implications for global public and ecosystem health. Global Change and Human Health 2:20–33, 10.1023/A:1011910224374. [DOI] [Google Scholar]
- Gupta P, Singh S, Kumar S, Choudhary M, Singh V. Effect of dust aerosol in patients with asthma. Journal of Asthma. 2012. March 1;49(2):134–8. [DOI] [PubMed] [Google Scholar]
- Hart CM, González MR, Simpson EP, Hurlbert SH. 1998. Salinity and fish effects on Salton Sea microecosystems: Zooplankton and nekton. Hydrobiologia 381:129–152, 10.1023/A:1003231708756. [DOI] [Google Scholar]
- Bayram Hasan, Bogan Mustafa, Kul Seval, Oktay Murat M., Muge Akpinar-Elci Al B. 2015. Effects of desert dust storms and meteorological variables on emergency room visits and hospitalization due to COPD in southeast Turkey In: American Thoracic Society 2015 International Conference, Vol. 191: American Journal of Respiratory and Critical Care Medicine. [Google Scholar]
- Hong Y-C, Pan X-C, Kim S-Y, Park K, Park E-J, Jin X, et al. 2010. Asian dust storm and pulmonary function of school children in Seoul. Science of The Total Environment 408:754–759, 10.106/j.scitotenv.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Kanatani KT, Ito I, Al-Delaimy WK, Adachi Y, Mathews WC, & Ramsdell JW (2010). Desert dust exposure is associated with increased risk of asthma hospitalization in children. American Journal of Respiratory and Critical Care Medicine, 182(12), 1475–1481, 10.1164/rccm.201002-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani KT, Hamazaki K, Inadera H, Sugimoto N, Shimizu A, Noma H, Onishi K, Takahashi Y, Itazawa T, Egawa M and Sato K, 2016. Effect of desert dust exposure on allergic symptoms: a natural experiment in Japan. Annals of Allergy, Asthma & Immunology, 116(5): 425–430, 10.106/j.anai.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Chiba M, Hashizume M, Kunii O, Sasaki S, Shimoda T, et al. 2003. Renal tubular dysfunction in children living in the Aral Sea. Arch Dis Child 88:966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Reif K, Wing S. 2016. Urban-rural exploitation: An underappreciated dimension of environmental injustice. Journal of Rural Studies 47:350–358, 10.1016/j.jrurstud.2016.03.010. [DOI] [Google Scholar]
- Kelly FJ, Fussell JC. 2012. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmospheric Environment 60:504–526, 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- King J, Etyemezian V, Sweeney M, Buck BJ, Nikolich G. 2011. Dust emission variability at the Salton Sea, California, USA. Aeolian Research 3:67–79, 10.1016/j.aeolia.2011.03.005. [DOI] [Google Scholar]
- Kunii O, Hashizume M, Chiba M, Sasaki S, Shimoda T, Caypil W, et al. 2003. Respiratory symptoms and pulmonary function among school-age children in the Aral Sea region. Arch Environ Health 58:676–682, 10.3200/AEOH.58.11.676-682. [DOI] [PubMed] [Google Scholar]
- LeBlanc LA, Kuivila KM. 2008. Occurrence, distribution and transport of pesticides into the Salton Sea basin, California, 2001–2002. Hydrobiologia 604:151–172, 10.1007/s10750-008-9316-1. [DOI] [Google Scholar]
- Lipsett M, Smorodinsky S, English P, Copan L. 2009. Basta border asthma & allergies study: Final report. Richmond, CA. [Google Scholar]
- Ma Y, Xiao B, Liu C, Zhao Y, Zheng X. 2016. Association between ambient air pollution and emergency room visits for respiratory diseases in spring dust storm season in Lanzhou, China. International Journal of Environmental Research and Public Health 13:613–613, 10.3390/ijerph13060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T, Kurosaki Y, Onishi K, Lee KC, Pointing SB, Jugder D, et al. 2017. Variations in the structure of airborne bacterial communities in Tsogt-Ovoo of Gobi desert area during dust events. Air quality, atmosphere, & health 10:249–260, 10.1007/s11869-016-0430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallone S, Stafoggia M, Faustini A, Gobbi GP, Marconi A, Forastiere F. 2011. Saharan dust and associations between particulate matter and daily mortality in Rome, Italy. Environ Health Perspect 119:1409–1414, 10.12989/eph.1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JR. 2017. Why emergency physicians should care about the Salton Sea. Western Journal of Emergency Medicine 18:1008, 10.5811/westjem.2017.8.36034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael H, Evan F, Joel H. 2010. Pollution sources and mortality rates across rural-urban areas in the United States. The Journal of Rural Health 26:383–391, 10.1111/j.1748-0361.2010.00305.x. [DOI] [PubMed] [Google Scholar]
- Micklin P 1998. International and regional responses to the Aral crisis: An overview of efforts and accomplishments. Post-Soviet Geography and Economics 39:399–416. [Google Scholar]
- Mimura T, Yamagami S, Fujishima H, Noma H, Kamei Y, Goto M, et al. 2014. Sensitization to Asian dust and allergic rhino-conjunctivitis. Environ Res 132:220–225, 10.1016/j.envres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Neisi A, Vosoughi M, Idani E, Goudarzi G, Takdastan A, Babaei AA, et al. 2017. Comparison of normal and dusty day impacts on fractional exhaled nitric oxide and lung function in healthy children in Ahvaz, Iran. Environmental Science and Pollution Research 24:12360–12371, 10.1007/s11356-017-8853-4. [DOI] [PubMed] [Google Scholar]
- Neophytou AM, Yiallouros P, Coull BA, Kleanthous S, Pavlou P, Pashiardis S, Dockery DW, Koutrakis P, Laden F. Particulate matter concentrations during desert dust outbreaks and daily mortality in Nicosia, Cyprus . Journal of Exposure Science and Environmental Epidemiology. 2013. May;23(3):275, https://doi.org/10.1038.jes.2013.10. [DOI] [PubMed] [Google Scholar]
- O’Hara SL, Wiggs GFS, Mamedov B, Davidson G, Hubbard RB. 2000. Exposure to airborne dust contaminated with pesticide in the Aral Sea region. The Lancet 355:627–628, 10.1016/S0140-6736(99)04753-4. [DOI] [PubMed] [Google Scholar]
- OEHHA, Office of Environmental Health Hazard Assessment. 2018. California communities environmental health screening tool, version 3.0 (Calenviroscreen 3.0). Sacramento, CA. [Google Scholar]
- Onishi K, Otani S, Yoshida A, Mu H, Kurozawa Y. 2015. Adverse health effects of Asian dust particles and heavy metals in Japan. Asia-Pacific journal of public health 27:Np1719–1726, 10.1177/1010539511428667. [DOI] [PubMed] [Google Scholar]
- Ostro B, Roth L, Malig B, Marty M. 2009. The effects of fine particle components on respiratory hospital admissions in children. Environ Health Perspect 117:475–480, 10.1289/ehp.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Onishi K, Mu H, Kurozawa Y. 2011. The effect of Asian dust events on the daily symptoms in Yonago, Japan: A pilot study on healthy subjects. Arch Environ Occup Health 66:43–46, 10.1080/19338244.2010.506499. [DOI] [PubMed] [Google Scholar]
- Park BJ, Sigel K, Vaz V, Komatsu K, McRill C, Phelan M, et al. 2005. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998–2001. The Journal of infectious diseases 191:1981–1987, 10.1086/430092. [DOI] [PubMed] [Google Scholar]
- Perez L, Tobías A, Querol X, Pey J, Alastuey A, Díaz J, et al. 2012. Saharan dust, particulate matter and cause-specific mortality: A case-crossover study in Barcelona (Spain). Environment International 48:150–155, 10.1016/j.envinto.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Pu B, Ginoux P. 2017. Projection of American dustiness in the late 21st century due to climate change. Scientific Reports 7:5553, 10.1038/s41598-017-05431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JS, Flocchini RG, Cahill TA, Ruth RS, Salgado DP. 1994. Local meteorological, transport, and source aerosol characteristics of late autumn Owens Lake (dry) dust storms. Atmospheric Environment 28:1699–1706. [Google Scholar]
- Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. 2011. Acute effects of air pollution on pediatric asthma exacerbation: Evidence of association and effect modification. Environ Res 111:418–424, 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Sapozhnikova Y, Bawardi O, Schlenk D. 2004. Pesticides and PCBs in sediments and fish from the Salton Sea, California, USA. Chemosphere 55:797–809, 10.1016/j.chemosphere.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Setmire JG, Wolfe JC, Stroud RK. 1990. Reconnaissance investigation of water quality, bottom sediment, and biota associated with irrigation drainage in the Salton Sea area, California, 1986–87. (Water-Resources Investigations Report). 89–4102. [Google Scholar]
- Stafoggia M, Zauli-Sajani S, Pey J, Samoli E, Alessandrini E, Basagaña X, et al. 2015. Desert dust outbreaks in southern Europe: Contribution to daily PM10 concentrations and short-term associations with mortality and hospital admissions. Environ Health Perspect 124:413–420, https://doi.org/10.1289.ehp.1409164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks J, Hislop A, Sonnappa S. 2013. Early lung development: Lifelong effect on respiratory health and disease. The Lancet Respiratory Medicine 1:728–742, 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- Stocks J, Sonnappa S. 2013. Early life influences on the development of chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 7:161–173, 10.1177/1753465813479428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WW, Wong TW, Wong AH, Hui DS. 2012. Effect of dust storm events on daily emergency admissions for respiratory diseases. Respirology (Carlton, Vic) 17:143–148, 10.1111/j.1440-1843.2011.02056.x. [DOI] [PubMed] [Google Scholar]
- Thalib L, & Al-Taiar A (2012). Dust storms and the risk of asthma admissions to hospitals in Kuwait. Science of the Total Environment, 433, 347–351, 10.106/j.scitotenv.2012.06.082. [DOI] [PubMed] [Google Scholar]
- Thu TM. 2001. Agriculture, the environment, and sources of state ideology and power. Culture & Agriculture 23:1–7. [Google Scholar]
- Tobias A, Perez L, Diaz J, Linares C, Pey J, Alastruey A, et al. 2011. Short-term effects of particulate matter on total mortality during Saharan dust outbreaks: A case-crossover analysis in Madrid (Spain). Sci Total Environ 412–413:386–389, 10.1016/j.scitotenv.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Tompson AFB. 2016. Born from a flood: The Salton Sea and its story of survival. Journal of Earth Science 27:89–97. [Google Scholar]
- Tong DQ, Wang JXL, Gill TE, Lei H, Wang B. 2017. Intensified dust storm activity and valley fever infection in the southwestern United States. Geophysical Research Letters 44:4304–4312, 10.1002/2017GL073524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl RA, Henry RN. 2002. Characteristics and contaminants of the Salton Sea sediments. Hydrobiologia 473:47–54. [Google Scholar]
- Wang W, Delgado-Moreno L, Conkle JL, Anderson M, Amrhein C, Ye Q, et al. 2012. Characterization of sediment contamination patterns by hydrophobic pesticides to preserve ecosystem functions of drainage lakes. Journal of Soils and Sediments 12:1407–1418. [Google Scholar]
- Watanabe M, Kurai J, Sano H, & Shimizu E (2015a). Effect of exposure to an Asian dust storm on fractional exhaled nitric oxide in adult asthma patients in Western Japan. The Journal of Medical Investigation, 62(3.4), 233–237, 10.2152/jmi.62.233. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Noma H, Kurai J, Sano H, Iwata K, Hantan D, Tohda Y and Shimizu E, 2017. Association of Short-Term Exposure to Ambient Fine Particulate Matter with Skin Symptoms in Schoolchildren: A Panel Study in a Rural Area of Western Japan. International Journal of Environmental Research and Public Health, 14(3): 299, 10.3390/ijerph14030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs GFS, O’Hara SL, Wegerdt J, Van Der Meer J, Small I, Hubbard R. 2003. The dynamics and characteristics of aeolian dust in dryland central Asia: Possible impacts on human exposure and respiratory health in the Aral Sea basin. Geographical Journal 169:142–157. [Google Scholar]
- Wing S, Horton R, Muhammad N, Grant G, Tajik M, Thu K. 2008. Integrating epidemiology, education, and organizing for environmental justice: community health effects of industrial hog operations. Am. J. Public Health 98, 1390–1397., 10.2105/AJPH.2007.110486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu EG, Bui C, Lamerdin C, Schlenk D. 2016. Spatial and temporal assessment of environmental contaminants in water, sediments and fish of the Salton Sea and its two primary tributaries, California, USA, from 2002 to 2012. Science of the Total Environment 559:130–140, 10.1016/j.scitotenv.2016.03.144 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Park J, Kodama M, Ichijo T, Baba T, Nasu M. 2014. Changes in the airborne bacterial community in outdoor environments following Asian dust events. Microbes and environments 29:82–88, 10.1264/jsme2.ME13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Baba T, Ichijo T, Himezawa Y, Enoki K, Saraya M, et al. 2016. Abundance and community structure of bacteria on Asian dust particles collected in Beijing, China, during the Asian dust season. Biological & Pharmaceutical Bulletin 39:68–77, 10.1248/bpb.b15-00573. [DOI] [PubMed] [Google Scholar]