Abstract
Objectives:
To study the frequency of suicidal ideation and its association with clinical and neurobiological correlates among cognitively intact autosomal dominant Alzheimer’s disease (ADAD) at-risk individuals.
Methods/Design:
In a cross-sectional study of 183 ADAD at-risk individuals (91 mutation carriers and 92 noncarriers), we compared the frequency of suicidal ideation among carriers and noncarriers. Linear mixed-effects models with family-level random effects evaluated the relationships between geriatric depression scale (GDS), neuropsychiatric inventory-questionnaire (NPI-Q), and suicidal ideation scores among all ADAD at-risk individuals. An interaction term was added to the regression models to evaluate the interactions of suicidal ideation and mutation status on neuropsychiatric symptoms.
Results:
Twenty-six (14.20%) ADAD at-risk individuals (13 [14.28%] carriers and 13 [14.13%] noncarriers) had suicidal ideation. The frequency of suicidal ideation did not differ between carriers and noncarriers. Suicidal ideation was associated with higher GDS among all ADAD at-risk individuals. When stratified into mutation carrier status, noncarriers with suicidal ideation had higher GDS than carriers. There was no statistically significant association between suicidal ideation and NPI-Q among ADAD at-risk individuals. Awareness of mutation status, neuropsychological performances, and cerebrospinal fluid AD biomarkers were not associated with suicidal ideation among carriers and noncarriers.
Conclusions:
Suicidal ideation is common among cognitively intact ADAD at-risk individuals. While ADAD at-risk individuals with suicidal ideation have greater depressive symptoms, noncarriers with suicidal ideation have higher GDS scores than carriers. Interestingly, awareness of the mutation status was not associated with suicidal ideation in our study. Early identification of suicidal thoughts can facilitate timely interventions to prevent suicidal behaviours.
Keywords: autosomal dominant Alzheimer’s disease, dominantly inherited Alzheimer’ network, neuropsychiatric symptoms, suicidal ideation
1 |. INTRODUCTION
Autosomal dominant Alzheimer’s disease (ADAD) represents less than 1% of all cases of Alzheimer’s disease. As several mutations associated with ADAD are known, ADAD is considered an important model to investigate and potentially better understand other more common forms of Alzheimer’s disease.1 Mutations associated with ADAD include mutations in presenilin 1 (PSEN1), presenilin 2 (PSEN2), or mutations/duplication of amyloid precursor protein (APP). Mutation carriers typically develop dementia at an early age (~30–50 years), and their offspring have a 50/50 chance to inherit the mutation. Thereby having a parent with ADAD renders the offspring at risk for ADAD.
Individuals at risk of inheriting the genetic mutation are facing the anxiety of possibly being a mutation carrier.2 Moreover, they may witness their loved ones suffer from ADAD.3 Hence, these individuals may be vulnerable to harbouring suicidal thoughts. According to the studies surveying individuals who were likely to seek presymptomatic testing for APOE genotype (associated with most prevalent sporadic form of AD), 6.6% to 11.6% of participants endorsed that they would seriously consider suicide if found to be at high risk for AD.4 Studies in individuals at risk for Huntington’s disease (HD), an autosomal dominant neurodegenerative disease, indicate an increased prevalence of suicidal ideation.5,6 However, the frequency of suicidal ideation among ADAD at-risk individuals is unclear. Elucidating the frequency and neurobiological characteristics of suicidal ideation among individuals at risk for ADAD is of paramount importance, as an early identification of suicidal thoughts may facilitate a timely intervention to prevent suicidal behaviours.
While several risk factors for suicide have been identified,7 major mental illness such as depression and low scores on tests of intelligence is among most strongly replicated risk factors for suicide.8,9 Depression has separately been found to increase suicide risk in patients with dementia.10 HD mutation carriers with suicidal ideation are often more depressed, anxious, and aggressive and have more frequent past attempts of suicide than mutation carriers without suicidal ideation.6 Surprisingly, a recent study reported that depressive symptoms were less common in cognitively intact ADAD mutation carriers than in noncarriers, whereas mildly symptomatic mutation carriers had more depression-related symptoms than noncarriers.11 The latter was in line with an earlier study in 33 women who did not know their Alzheimer’s disease–related PSEN1 genotype. Despite not demented, PSEN1 mutation carriers in this study scored worse than noncarriers in cognitive tests (mini-mental state examination [MMSE] and trail making test), thereby indicating mild symptomatology. PSEN1 mutation carriers in this study scored higher in the Beck depression inventory and thereby had higher levels of depression than PSEN1 mutation noncarriers.12 However, an investigation of individuals who endorsed that they would consider suicide when found to have “high risk” APOE allele did not demonstrate any specific characteristics in suicide endorsers compared with nonendorsers, with the exception of feelings of nonsupport. Thereby, further investigations are required to determine if depression or other neurobiological characteristics are associated with suicidal ideation among individuals at risk for ADAD.
Accumulating evidence has yielded support to the concept of neurobiological vulnerability to suicidal behaviour.13,14 The involvement of genetic factors has been demonstrated by family, twin, and adoption studies.15,16 When compared with nonsuicidal patients with similar mental disorders, individuals with a history of suicidal acts have been found to have altered functioning of the serotonergic system and hypothalamic-pituitary-adrenal axis17 with potential dysregulations in dopamine and norepinephrine systems.18–20 Several neuropsychological deficits have been demonstrated in patients with suicidal behaviour21,22 including higher attention to specific negative emotional stimuli, impaired decision-making, reduced verbal fluency, and decreased problem-solving abilities. In this study, we investigate whether and which clinical and biological factors contribute to suicidal behaviours and suicide ideation in individuals at risk for ADAD.
Here, in a cross-sectional observation of cognitively intact individuals with a positive family history of ADAD from the dominantly inherited Alzheimer’s network (DIAN) cohort, we investigate the frequency and the clinical and neurobiological correlates of suicidal ideation among cognitively intact ADAD at-risk individuals. We also stratify the individuals based on their mutation carrier status to test our hypothesis that the neuropsychiatric factors linked to suicide ideation are different between ADAD mutation carriers and noncarriers.
2 |. METHODS
2.1 |. Study participants
Data analysed in this study were obtained from the DIAN (DIAN Data Freeze 11, February 2017). DIAN is an international registry of individuals at risk for developing ADAD. It contains over 330 individuals—both symptomatic and asymptomatic ADAD mutation carriers and their noncarrier siblings (who serve as a genetically similar control sample) are included. The participants are not required to know their mutation status.23
We selected cognitively intact ADAD at-risk individuals, defined by clinical dementia rating 024 and MMSE ≥24.25 Data from each individual’s first visit with the following assessments were analysed: (a) suicide question from the Uniform Data Set (UDS) B6, (b) 15-item geriatric depression scale (GDS), (c) informant-based neuropsychiatric inventory-questionnaire (NPI-Q), (d) awareness of mutation status, (e) neuropsychological assessments, and (f) biological factors (genetic and cerebrospinal fluid [CSF]). The history of previous suicide attempts and family history of completed suicides were not available.
2.2 |. Ethical approvals and patient consents
The DIAN study was approved by the Institutional Review Boards of all of the participating institutions. Informed written consent was obtained from all participants at each site.
2.3 |. Neuropsychiatric assessments
In the section of the suicide question from the UDS B6, each participant was asked if he or she feels that life is not worth living, has expressed a wish to die, or has talked about committing suicide in the past or currently. The individual was classified as having current or past suicide ideation if all answers were yes. The GDS assesses the recent mood of the participants.26 Higher GDS scores suggest greater depressive symptoms. The NPI-Q measures the presence and severity of behavioural disturbances, and higher NPI-Q scores suggest greater neuropsychiatric symptoms (NPS).27 The 12 domains (NPS) of NPI-Q include agitation, anxiety, apathy, appetite changes, delusions, depression, disinhibition, abnormal elevated mood, hallucinations, irritability, repetitive motor behaviours, and sleep behaviour changes. The data of the awareness of mutation status and neuropsychiatric assessments were collected during the same study visit.
2.4 |. Neuropsychological assessments
The UDS Neuropsychological Test Battery was administered in the DIAN study as previously described.28 Scores from the following cognitive assessments were analysed in this study: Wechsler memory scale—revised logical memory (story A only) and digit span (forward and backward); category fluency (animals and vegetables); trail making test, parts A and B; digit symbol from the Wechsler adult intelligence scale–revised;
2.5 |. Genetic assessments
DNA sequencing of APP, PSEN1, and PSEN2 genes was performed by the DIAN Genetics Core investigators to determine the presence of family-specific disease-causing mutation as previously described.11
2.6 |. CSF assessments
CSF Aβ1–42, total tau, and ptau181 were analysed by the DIAN Bio-marker Core at the Washington University. The CSF levels of Aβ1–42, total tau, and ptau181 were measured by immunoassay using the Luminex bead-based multiplexed xMAP technology (INNO-BIA AlzBio3™, Innogenetics, Ghent, Belgium) as previously described.29
2.7 |. Statistical analysis
Statistical analyses were performed with the statistical software R, version 3.3.2 (R Development Core Team 2015). Descriptive statistics and frequency distributions of baseline demographics, clinical, and genetic characteristics were summarized for all ADAD at-risk individuals with and without suicidal ideation, using family-level random-effects models for both continuous and categorical measurements. The study population was then stratified into mutation carriers and noncarriers, and the baseline characteristics were summarized for each group using similar family-level random-effects models.
The frequency of suicidal ideation among all ADAD at-risk individuals was calculated. Logistic mixed-effects models compared the frequency of suicidal ideation among mutation carriers and noncarriers while accounting for clustering with families through a family-level random effect. An interaction term was then added to the model to evaluate the interactions of mutation status and awareness of mutation status on the frequency of suicide ideation.
Linear mixed-effects models with family-level random effects evaluated the differences in GDS and NPI-Q scores between suicidal and nonsuicidal individuals among all cognitively intact ADAD at-risk individuals. If a significant interaction was identified between mutation status and suicidal ideation, we then evaluated the mutation carrier and noncarrier groups separately using the same model. We modelled GDS/NPI-Q as a function of suicidal ideation and covariates. Below, the model is written in pseudo-R format (R Development CoreTeam 2015), where GDSij/NPI-Qij denotes the GDS or the NPI-Q score for the jth person from the ith family, Suicideij indicates if this person demonstrates suicidal ideation, and Xij represents fixed-effect covariates for age, gender, education, APOE ε4 status, and family mutation type (APP, PSEN1, and PSEN2):
where Fi represents a random effect for all individuals from family i and εij is the residual error assumed independent and normally distributed for all individuals.
The family-level random-effect term accounts for the correlations between individuals within the same family. Although correlations between family members might vary with the relationship type, because of the fairly small sizes of the families, this was modelled with a single random effect.
An interaction term was added to the regression models to evaluate the interaction between suicidal ideation and mutation status on GDS/NPI-Q:
We compared the Akaike’s information criterion (AIC) of the model with the interaction term with a reduced model without the interaction term to infer whether the interaction model demonstrates a better fit to the data. We further evaluated the associations of suicidal ideation with individual subcomponents of the NPI-Q using similar linear mixed-effects models.
In a secondary analysis, we used similar linear mixed-effects models with family-level random effects to evaluate the differences in cognitive scores between suicidal and nonsuicidal individuals among all cognitively intact ADAD at-risk individuals.
Results are reported as (β,SE), which refer to the slope estimates from the regression models and their standard errors. Bonferroni corrections were performed to correct the aforementioned analyses for multiple comparisons for all neuropsychiatric and neuropsychological outcomes.
3 |. RESULTS
3.1 |. Baseline demographics and sample characteristics
One hundred eighty-three (n = 183) cognitively intact ADAD at-risk individuals were included in the study. Ninety-one (49.73%) were mutation carriers and 92 (50.27%) were noncarriers. There was no statistically significant difference in the age, gender, education, APOE ε4 status, parental age of onset, estimated age of onset, and mutation type among all cognitively intact ADAD at-risk individuals with or without suicidal ideation (Table 1).
TABLE 1.
Baseline demographics and sample characteristics of cognitive-intact ADAD mutation and nonmutation carriers with and without suicidal ideation
| Total ADAD at-Risk Individuals (n = 183) | ADAD Mutation Carriers (n = 91) | ADAD Nonmutation Carriers (n = 92) | ||||
|---|---|---|---|---|---|---|
| Suicide Ideation (n = 26) | No Suicide Ideation (n = 157) | Suicide Ideation (n = 13) | No Suicide Ideation (n = 78) | Suicide Ideation (n = 13) | No Suicide Ideation (n = 79) | |
| Age in years, mean, (SD) | 41.48 (8.68) | 39.76 (10.32) | 41.54 (8.05) | 37.00 (9.36) | 41.62 (9.59) | 42.48 (10.55) |
| Male, n (%) | 11 (42.30) | 60 (38.21) | 6 (46.15) | 30 (38.46) | 5 (38.46) | 30 (37.97) |
| Education in years, mean, (SD) | 13.77 (2.48) | 14.96 (2.54) | 12.92 (2.32) | 14.97 (2.62)* | 14.62 (2.43) | 14.94 (2.48) |
| APOE ε4 carriers, n (%) | 7 (26.92) | 42 (26.75) | 3 (23.07) | 21 (26.92) | 4 (30.76) | 21 (26.58) |
| Parental age of onset in years, mean (SD) | 47.69 (6.19) | 46.39 (6.55) | 48.92 (4.82) | 46.63 (6.62) | 46.46 (7.31) | 46.15 (6.52) |
| EYO, years, mean (SD) | −7.86 (9.45) | −7.73 (9.65) | −9.35 (8.59) | −10.02 (8.17) | −6.37 (10.36) | −5.46 (10.48) |
| Mutation Type | ||||||
| APP, n (%) | 8 (30.76) | 41 (26.11) | 4 (30.76) | 16 (20.51) | 4 (30.76) | 25 (31.64) |
| PS1, n (%) | 18 (69.23) | 101 (64.33) | 9 (69.23) | 55 (70.51) | 9 (69.23) | 46 (58.22) |
| PS2, n (%) | 0 (0.00) | 15 (9.55) | 0 (0.00) | 7 (8.97) | 0 (0.00) | 8 (10.12) |
| Aware of mutation status, n (%) | 7 (26.92) | 40 (25.47) | 3 (23.07) | 23 (29.48) | 4 (30.76) | 17 (21.51) |
| CSF Aβ1–42, pg/mL, mean (SD)a | 394.66 (105.75) | 416.54 (138.46) | 378.92 (116.97) | 360.33 (143.66) | 423.52 (82.92) | 466.18 (113.40) |
| CSF p-tau181p, pg/mL, mean, (SD)a | 42.26 (30.31) | 39.73 (24.63) | 50.28 (34.93) | 49.45 (29.82) | 27.57 (9.96) | 31.01 (14.13) |
| CSF t-tau, pg/mL, mean (SD)a | 67.36 (28.79) | 73.66 (56.19) | 71.77 (34.11) | 91.21 (73.37) | 59.28 (14.32) | 58.16 (27.00) |
Note. P values were assessed using family-level random-effects models for the continuous variables and categorical variables, taking into account the analysis of multiple family members within the families. A logistic mixed-effect model compared the frequency of suicidal ideation among mutation and nonmutation carriers, accounting for family-level random effect.
Abbreviations: ADAD, autosomal dominant Alzheimer’s disease; CSF, cerebrospinal fluid; EYO, estimated years of onset; GDS, geriatric depression scale.
27 mutation carriers and 26 nonmutation carriers did not have CSF data for the visit.
P < .05.
Mutation carriers with suicidal ideation had significantly lower education compared with those without suicidal ideation (12.92 vs 14.97 years; slope = −2.00, SE = 0.77, P = .011) (Table 1). There is no statistically significant difference in age, gender, APOE ε4 status, parental age of onset, estimated age of onset, and mutation type among mutation carriers with or without suicidal ideation. Among mutation noncarriers, those with suicidal ideation compared with those without suicidal ideation did not differ significantly in age, gender, education and APOE ε4 status, parental age of onset, estimated age of onset, or mutation type (Table 1).
3.2 |. Frequency of suicide ideation
Among the total cognitively intact ADAD at-risk individuals, 26 (14.20%) had suicidal ideation. When stratified into mutation carrier status, 13 (14.28%) mutation carriers and 13 (14.13%) noncarriers had suicidal ideation (Table 1). There was no statistically significant difference in the frequency of suicidal ideation between mutation carriers and noncarriers (slope = 0.05, SE = 0.45, P = 0.91).
3.3 |. Awareness of mutation status
The frequency of awareness of mutation status did not differ significantly among all cognitively intact ADAD at-risk individuals with or without suicidal ideation (7 [26.92%] vs 40 [25.47%]; slope = 0.03, SE = 0.58, P = .95). There was no significant interaction between mutation status and awareness of mutation status on the frequency of suicidal ideation (slope = 0.54, SE = 0.71, P = .44), suggesting no significant difference in the association between suicidal ideation and awareness of mutation status among mutation carriers and noncarriers.
3.4 |. Neuropsychiatric symptoms
Using linear mixed-effects models, suicide ideation was associated with higher GDS scores among all cognitively intact ADAD at-risk individuals (slope = 2.54, SE = 0.36, P ≤ .001, 95% CI, 1.79–3.17) (Table 2). A significant interaction between the variables of suicidal ideation and mutation status on GDS indicated that GDS was higher among mutation noncarriers with suicidal ideation compared with mutation carriers (slope = −2.78, SE = 0.69, P ≤ .001). By calculating AIC, the model with the interaction term fitted better than the model without the interaction (AIC was 706.89 with the interaction vs 721.42 without, P ≤ .001). When stratified into mutation carrier status, mutation carriers with suicidal ideation had higher GDS scores than those who were not suicidal (slope = 1.13, SE = 0.50, P = .026, 95% CI, 0.183–2.096). Among noncarriers, those who were suicidal had higher GDS scores (slope = 3.95, SE = 0.49, adjusted P ≤ .001, 95% CI, 2.91–4.86).
TABLE 2.
Associations between neuropsychiatric symptoms and suicidal ideation, among ADAD mutation and nonmutation carriers
| Total ADAD at-Risk Individuals (n = 183) | ||||
|---|---|---|---|---|
| Slope | Standard Error | Confidence Interval | Unadjusted P value | |
| GDS | 2.54 | 0.36 | 1.79–3.17 | <.001 |
| NPI-Q | 0.64 | 0.31 | 0.04–1.26 | .042 |
| ADAD Mutation Carriers (n = 91) | ||||
| GDS | 1.13 | 0.50 | 0.183–2.096 | .026 |
| ADAD Nonmutation Carriers (n = 92) | ||||
| GDS | 3.95 | 0.49 | 2.91–4.86 | <.001 |
Note. P values were assessed using linear mixed-effect models with family-level random effects, corrected for age, gender, APOE, education, and family mutation type, taking into account the analysis of multiple family members within the families. As no significant interaction between suicidal ideation and mutation status on NPI-Q scores was found, no further analysis was performed on the mutation and nonmutation carrier groups.
Abbreviations: ADAD, autosomal dominant Alzheimer’s disease; GDS, geriatric depression scale; NPI-Q, neuropsychiatric inventory-questionnaire.
While we found that suicidal ideation was associated with higher NPI-Q scores among all cognitively intact ADAD at-risk individuals (slope = 0.64, SE = 0.31, P = .042, 95% CI, 0.04–1.26) (Table 2), this result was not statistically significant after Bonferroni correction for multiple comparisons. Exploratory analysis of the NPI-Q subcomponent suggested that suicidal ideation is associated with severity of depressive symptoms among all cognitively intact ADAD at-risk individuals (slope = 0.29, SE = 0.11, P = .016, 95% CI, 0.08–0.50) (Figure 1). There was no significant interaction between suicidal ideation and mutation status on NPI-Q scores, suggesting no significant difference in NPI-Q between mutation carriers and noncarriers with or without suicidal ideation.
FIGURE 1.
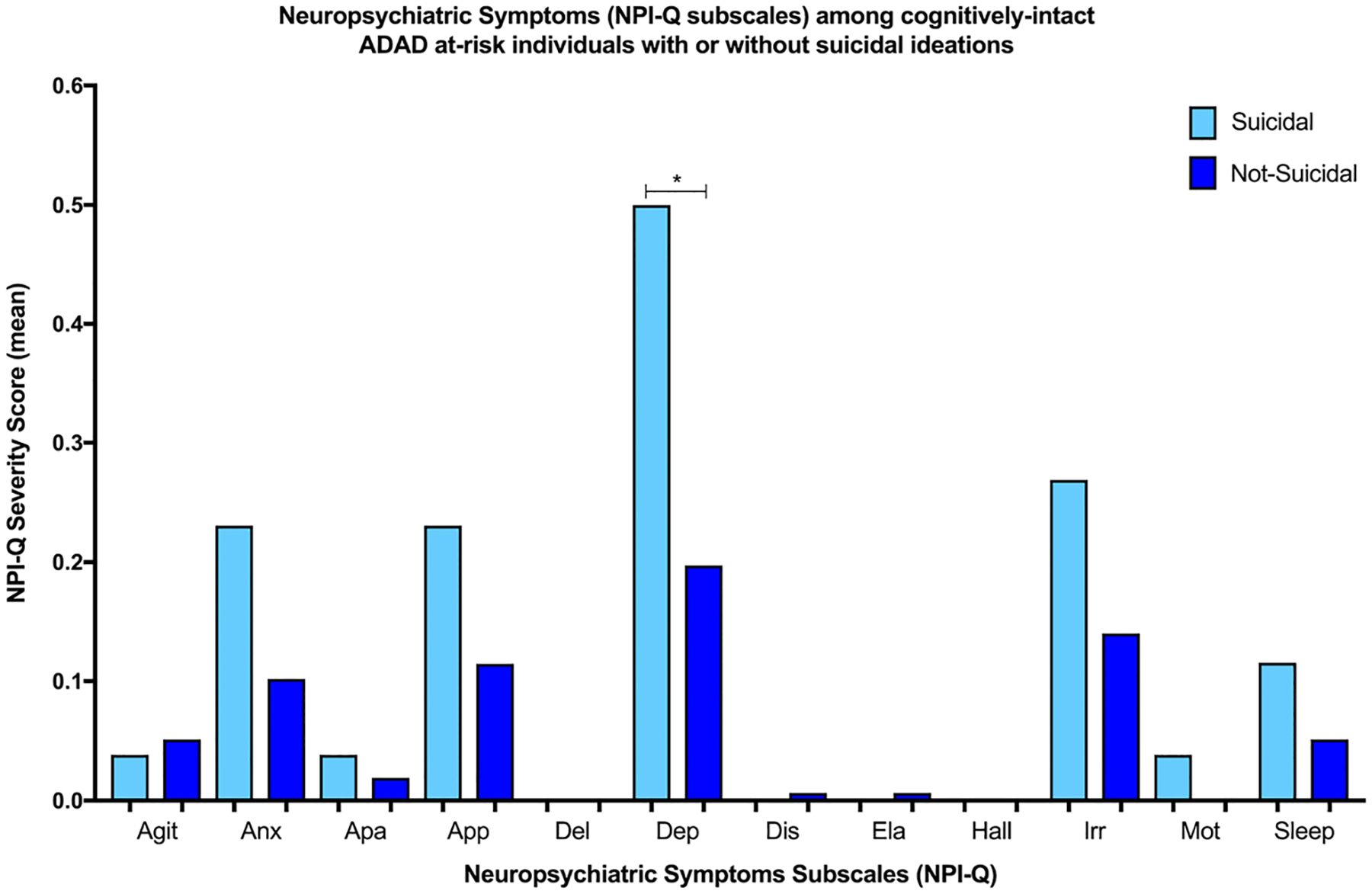
Neuropsychiatric symptoms (NPI-Q subscales) among cognitively intact ADAD at-risk individuals with or without suicidal ideations.The figure shows the differences in NPI-Q subscale mean severity scores among cognitively intact ADAD at-risk individuals, with and without suicidal ideations.*P < .05.Agit, agitation; Anx, anxiety; Apa, apathy; App, appetite changes; Del, delusions; Dep, depression; Dis, disinhibition; Ela, abnormal elevated mood; Hall, hallucinations; Irr, irritability; Mot, repetitive motor behaviours; Sleep, sleep behaviour changes
3.5 |. Neuropsychological performances
There was no statistically significant difference in cognitive scores among all cognitively intact ADAD at-risk individuals, with or without suicidal ideation (Table 3). There was no significant interaction between suicidal ideation and mutation status on cognitive scores, suggesting no significant difference in cognitive scores between mutation carriers and noncarriers with or without suicidal ideation.
TABLE 3.
Means and standard deviations of neuropsychological assessments among asymptomatic ADAD at-risk individuals, with or without suicidal ideation
| Total ADAD at-Risk Individuals (n = 183) | |||
|---|---|---|---|
| Suicide Ideation (n = 26) Mean (SD) | No Suicide Ideation (n = 157) Mean (SD) | P value | |
| MMSE | 28.85 (1.40) | 29.30 (0.98) | .148 |
| Boston naming test | 27.92 (1.80) | 28.01 (1.98) | .963 |
| WAIS-R digit symbol | 58.58 (11.56) | 62.66 (12.73) | .508 |
| Digit span forward | 9.04 (2.18) | 9.25 (1.80) | .738 |
| Digit span backward | 7.46 (1.98) | 7.65 (2.16) | .936 |
| Trail A | 21.15 (6.38) | 22.47 (6.90) | .148 |
| Trail B | 56.73 (19.39) | 56.45 (24.17) | .377 |
| Letters fluency | 44.88 (10.30) | 43.10 (11.44) | .325 |
| Animal | 22.62 (5.44) | 23.09 (5.24) | .912 |
| Vegetable | 14.96 (4.09) | 15.62 (4.24) | .579 |
| Logical memory | 14.50 (4.48) | 15.32 (3.82) | .606 |
| Words immediate | 5.27 (1.80) | 5.71 (1.95) | .572 |
| Words delayed | 2.62 (2.08) | 2.55 (2.02) | .482 |
Note. P values were assessed using linear mixed-effect models with family-level random effects, corrected for age, gender, APOE, education, and family mutation type, taking into account the analysis of multiple family members within the families.
Abbreviations: ADAD, autosomal dominant Alzheimer’s disease; MMSE, mini-mental state examination; WAIS-R, Wechsler adult intelligence scale–revised.
3.6 |. CSF AD biomarkers
There was no statistically significant difference in the CSF AD biomarkers (Aβ1–42, p-tau181, and t-tau) among all cognitively intact at-risk individuals, mutation carriers, or noncarriers with or without suicidal ideation (Table 1).
4 |. DISCUSSION
Our study showed that 14.2% of cognitively intact ADAD at-risk individuals harbored suicidal thoughts regardless of their genetic mutation status. While suicidal ideation was associated with greater depressive symptoms in all cognitively intact ADAD at-risk individuals, mutation noncarriers with suicidal ideation had higher GDS than mutation carriers with suicidal ideation. Awareness of mutation status, neuropsychological performances, and levels of CSF AD biomarkers were not associated with suicidal ideation among mutation carriers and noncarriers.
The frequency of suicidal ideation among cognitively intact ADAD at-risk individuals is clinically significant, given that the prevalence of suicidal ideation in the general population is 9.2% according to a cross-national study30 and has been shown to be much lower in certain populations.31 We further found that suicidal ideation is equally common among mutation carriers and noncarriers. With the availability of genetic counselling, individuals within ADAD families may undergo genetic testing, especially if clinical trials are available.32 While the knowledge of genetic mutation carrier status may alleviate the uncertainty and anxiety of developing AD and allow planning for the future,33 some individuals may react adversely to having a genetic mutation as they are destined to develop AD years later, hence possibly leading to suicidal thoughts.34,35 We also hypothesized that noncarriers may develop suicidal ideation because of a variety of reasons: anxiety of potentially being a carrier, burden of being a caregiver for their loved ones, seeing their loved ones suffer, and losing them because of AD. Our results suggest that suicidal thoughts may not be related to AD pathophysiology, given a lack of association between suicidal ideation and CSF AD biomarkers. Hence, our findings indicate that being an individual at risk for ADAD, independent of the mutation carrier status, is a vulnerability factor for suicide ideation.
In our study, ADAD mutation carriers and noncarriers with suicidal ideation have significantly higher GDS scores than those without suicidal ideation. Although our findings are consistent with the literature whereby depression is a well-known risk factor for suicidal ideation,36 we found that ADAD mutation noncarriers with suicidal ideation have greater depressive symptoms than mutation carriers with suicidal ideation. This finding is important for clinicians as noncarriers might not undergo neuropsychiatric assessments or screening for suicidal thoughts during routine clinic consults.6 Greater depressive symptoms in noncarriers with suicidal ideation may be partly due to guilt and partly due to the burden of being in the family with ADAD having the insight into what their family members with the genetic mutation will go through.37 Hence, during the evaluation of ADAD at-risk individuals, both mutation carriers and noncarriers, especially those with greater depressive symptoms, should be monitored closely for suicidal ideation.
Importantly, being aware of the mutation status did not influence the frequency of suicidal ideation regardless of the mutation carrier/noncarrier status. One might assume that the knowledge of not having inherited the mutation would alleviate the anxiety of possibly developing AD, hence decreasing suicidal thoughts, while the confirmation of having inherited genetic mutation would increase the vulnerability to suicidal thoughts.37 However, our findings suggest that the knowledge of mutation status may not influence the risk for suicidal ideation. That may indicate the presence of additional yet unclear risk factors for suicidal ideation in the ADAD at-risk families, warranting further investigations.
We could not identify any specific impairment in the psychometric performances comparing suicidal and nonsuicidal individuals. This is partly in line with the literature. Indeed, it has been found that deficits in decision-making, category verbal fluency, and interference Stroop test are associated with suicidal behaviours but only in patients with mood disorders.22,38 However, differences in cognitive control have been found in suicide attempters compared with suicide ideators in young39 and elderly40 populations. Neurocognitive alterations represent relevant vulnerability factors and potential endophenotypes of suicidal behaviour and should thus be further investigated in nonsymptomatic AD and demented populations.
There are limitations in our study. First, the single suicide question encompasses three components, which assess different levels of suicidal ideation. This may reduce the specificity in detecting the specific suicidal thought that may lead to an attempt. Second, the suicide question includes both present and previous suicide ideation. Having had suicidal ideation in the past may not relate to current NPS. Third, GDS is originally designed to detect depressive symptoms among elderly individuals and may thus not be suitable for the younger participants in the DIAN cohort. Fourth, as our study is cross sectional and the data of suicide attempts are not available, the nature of the suicidal thoughts and whether they lead to a suicide attempt cannot be concluded from our study. Fifth, while the inclusion of a comparator group, ideally of family members of individuals with sporadic AD, will be ideal to control for stress and caregiver burden, these data are unavailable for this study. Hence, this may confound the interpretation of our results. However, in addition to ADAD mutation carriers, we have included their noncarrier siblings as a comparator group. The noncarrier siblings, who also serve as a genetically similar control sample, may enable the control for stress and caregiver burden. Lastly, it may be considered more logical to model suicidal ideation as the outcome and GDS or NPI-Q as predictor variables, rather than the reverse as we have done here. Our choice is made for practical reasons: The data set is of modest size, and linear mixed-effects models are far more stable than logistic mixed-effects models. We are unable to obtain estimates for all our models of interest when using logistic models. All our results should be interpreted as associative, and inference of cause-effect must be explored in studies explicitly designed for such a goal.
Suicide prevention requires a multifaceted approach, especially focusing on mental health conditions. A review of suicide prevention strategies suggests that educating the clinician in identifying and treating depression and restricting access to lethal methods can reduce suicide rates.41 Furthermore, a qualitative study in HD shows that partners, relatives, and health care professionals play an important role in supporting HD patients with suicidal ideation in their coping strategies.42 Therefore, identification and intervention to address the complex issues of neurobiological markers and dementia will help clinicians mitigate the risk of suicide in patients with AD. We suggest that, in future studies, a more comprehensive suicide questionnaire, such as the Columbia suicide severity rating scale43 that measures a broader spectrum and levels of suicidal thoughts, should be used. In addition, a prospective study will be important to better understand the trajectory of suicidal thoughts in cognitively intact ADAD at-risk individuals, so as to determine which risk factors are more associated with suicide attempts.
5 |. CONCLUSION
Our findings suggest that suicidal ideation is common among both mutation carriers and noncarriers in ADAD at-risk families. Both ADAD mutation carriers and noncarriers should be monitored closely so that timely interventions can be offered to alleviate any suicidal ideation to prevent a suicide attempt.
Key points.
14.2% of ADAD at-risk persons have suicidal thoughts regardless of their mutation status.
Suicidal ideation is associated with greater depressive symptoms in all asymptomatic ADAD at-risk individuals.
Nonmutation carriers with suicidal thoughts have greater GDS than mutation carriers with suicidal thoughts.
No neurobiological correlates of suicidal ideation among cognitively intact ADAD at-risk individuals.
ACKNOWLEDGEMENTS
Data collection and sharing for this project were supported by The Dominantly Inherited Alzheimer’s Network (DIAN, U19AG032438) funded by the National Institute on Aging (NIA), the German Center for Neurodegenerative Diseases (DZNE), and Raul Carrea Institute for Neurological Research (FLENI) and partially supported by the Research and Development Grants for Dementia from Japan Agency for Medical Research and Development, AMED, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI). This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications. We acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study.
SOURCE OF FUNDING
This study is funded in part by CIHR through the ELSI committee of the CCNA. Kok Pin Ng is supported by the National Medical Research Council, Research Training Fellowship, Singapore.
Footnotes
CONFLICT OF INTEREST
SG is an investigator in DIAN-TU. The other authors report no disclosure.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Dominantly Inherited Alzheimer Network. Restrictions apply to the availability of these data, which were used under license for this study.
REFERENCES
- 1.Bateman RJ, Aisen PS, De Strooper B, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3(1):1 10.1186/alzrt59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olney N, Teng E, Zhou Y, et al. Neuropsychiatric symptoms in early autosomal dominant and late onset alzheimer’s disease. Neurol. 2016;86(16). http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L72251254%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=00283878&id=doi:&atitle=Neuropsychiatric+symptoms+in+early+autosomal+dominant+and+late+onset+alzheimer%27s+disease&stitl [Google Scholar]
- 3.Pickett T, Altmaier E, Paulsen JS. Caregiver burden in Huntington’s disease. Rehabil Psychol. 2007;52(3):311–318. 10.1037/0090-5550.52.3.311 [DOI] [Google Scholar]
- 4.Hunt KS, Lindor RA, Caselli RJ, et al. Predictive testing for Alzheimer’s disease: suicidal ideation among healthy participants. Alzheimers Dement. 2014;10(4):P610 10.1016/j.jalz.2014.05.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulsen JS, Ferneyhough Hoth K, Nehl C, Stierman L, Group THS. Critical periods of suicide risk in Huntington’s disease. Am J Psychiatry. 2005;162(4):725–731. 10.1176/appi.ajp.162.4.725 [DOI] [PubMed] [Google Scholar]
- 6.Hubers AAM, van Duijn E, Roos RAC, et al. Suicidal ideation in a European Huntington’s disease population. J Affect Disord. 2013;151 (1):248–258. 10.1016/j.jad.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56(7):617–626. 10.1001/archpsyc.56.7.617 [DOI] [PubMed] [Google Scholar]
- 8.Brown GK, Beck AT, Steer RA, Grisham JR. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68(3):371–377. 10.1037//0022-006X.68.3.371 [DOI] [PubMed] [Google Scholar]
- 9.Batty GD, Kivimäki M, Bell S, et al. Psychosocial characteristics as potential predictors of suicide in adults: an overview of the evidence with new results from prospective cohort studies/692/699/631/477/2811 review-article. Transl Psychiatry. 2018;8(1):22 10.1038/s41398-017-0072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyfried LS, Kales HC, Ignacio RV, Conwell Y, Valenstein M. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7 (6):567–573. 10.1016/j.jalz.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringman JM, Liang LJ, Zhou Y, et al. Early behavioural changes in familial Alzheimer’s disease in the dominantly inherited Alzheimer network. Brain. 2015;138(4):1036–1045. 10.1093/brain/awv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringman JM. Female preclinical presenilin-1 mutation carriers unaware of their genetic status have higher levels of depression than their nonmutation carrying kin. J Neurol Neurosurg Psychiatry. 2004;75 (3):500–502. 10.1136/jnnp.2002.005025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. 2014;1(1):63–72. 10.1016/S2215-0366(14)70220-2 [DOI] [PubMed] [Google Scholar]
- 14.Roy B, Dwivedi Y. Understanding epigenetic architecture of suicide neurobiology: a critical perspective. Neurosci Biobehav Rev. 2017;72:10–27. 10.1016/j.neubiorev.2016.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brent DA, Oquendo M, Birmaher B, et al. Familial pathways to earlyonset suicide attempt: risk for suicidal behavior in offspring of mood-disordered suicide attempters. Arch Gen Psychiatry. 2002;59 (9):801–807. 10.1001/archpsyc.59.9.801 [DOI] [PubMed] [Google Scholar]
- 16.Turecki G, Ernst C, Jollant F, Labonté B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35(1):14–23. 10.1016/j.tins.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 17.Chatzittofis A, Nordström P, Hellström C, Arver S, Åsberg M, Jokinen J. CSF 5-HIAA, cortisol and DHEAS levels in suicide attempters. Eur Neuropsychopharmacol. 2013;23(10):1280–1287. 10.1016/j.euroneuro.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Courtet P, Jollant F, Castelnau D, Buresi C, Malafosse A. Suicidal behavior: relationship between phenotype and serotonergic genotype. Am J Med Genet - Semin Med Genet. 2005;133 C(1):25–33. 10.1002/ajmg.c.30043 [DOI] [PubMed] [Google Scholar]
- 19.Carballo JJ, Akamnonu CP, Oquendo MA. Neurobiology of suicidal behavior. An integration of biological and clinical findings. Arch Suicide Res. 2008;12(2):93–110. 10.1080/13811110701857004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33(26):2609–2614. 10.1016/0024-3205(83)90344-2 [DOI] [PubMed] [Google Scholar]
- 21.Jollant F, Lawrence NL, Olié E, Guillaume S, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry. 2011;12(5):319–339. 10.3109/15622975.2011.556200 [DOI] [PubMed] [Google Scholar]
- 22.Richard-Devantoy S, Berlim MT, Jollant F. A meta-analysis of neuropsychological markers of vulnerability to suicidal behavior in mood disorders. Psychol Med. 2014;44(8):1663–1673. 10.1017/S0033291713002304 [DOI] [PubMed] [Google Scholar]
- 23.Moulder KL, Snider BJ, Mills SL, et al. Dominantly inherited alzheimer network: facilitating research and clinical trials. Alzheimer’s Res Ther. 2013;5(5):48 10.1186/alzrt213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurol. 1993;43(11):2412–2414. 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 26.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4(3):173–178. 10.1177/089198879100400310 [DOI] [PubMed] [Google Scholar]
- 27.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a Brief Clinical Form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. 10.1176/appi.neuropsych.12.2.233 [DOI] [PubMed] [Google Scholar]
- 28.Storandt M, Balota DA, Aschenbrenner AJ, Morris JC. Clinical and psychological characteristics of the initial cohort of the dominantly inherited Alzheimer network (DIAN). Neuropsychology. 2014;28 (1):19–29. 10.1037/neu0000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagan AM, Xiong C, Jasielec MS, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6(226):226ra30–226ra30. 10.1126/scitranslmed.3007901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nock MK, Borges G, Bromet EJ, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192(2):98–105. 10.1192/bjp.bp.107.040113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X-L, Zhong B-L, Xiang Y-T, et al. Prevalence of suicidal ideation and suicide attempts in the general population of China: A meta-analysis. Int J Psychiatry Med. 2015;49(4):296–308. 10.1177/0091217415589306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grill JD, Bateman RJ, Buckles V, et al. A survey of attitudes toward clinical trials and genetic disclosure in autosomal dominant Alzheimer’s disease. Alzheimers Res Ther. 2015;7(1):50 10.1186/s13195-015-0135-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dufrasne S, Roy M, Galvez M, Rosenblatt DS. Experience over fifteen years with a protocol for predictive testing for Huntington disease. Mol Genet Metab. 2011;102(4):494–504. 10.1016/j.ymgme.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 34.Bird TD. Outrageous fortune: the risk of suicide in genetic testing for Huntington disease. Am J Hum Genet. 1999;64(5):1289–1292. 10.1086/302388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almqvist EW, Bloch M, Brinkman R, Craufurd D, Hayden MR. A worldwide assessment of the frequency of suicide, suicide attempts, or psychiatric hospitalization after predictive testing for Huntington disease. Am J Hum Genet. 1999;64(5):1293–1304. 10.1086/302374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Draper B, MacCuspie-Moore C, Brodaty H. Suicidal ideation and the “wish to die” in dementia patients: the role of depression. Age Ageing. 1998;27(4):503–507. [DOI] [PubMed] [Google Scholar]
- 37.Hallowell N, Arden-Jones A, Eeles R, et al. Guilt, blame and responsibility: Men’s understanding of their role in the transmission of BRCA1/2 mutations within their family. Sociol Health Illn. 2006;28(7):969–988. 10.1111/j.1467-9566.2006.00515.x [DOI] [PubMed] [Google Scholar]
- 38.Richard-Devantoy S, Olié E, Guillaume S, Courtet P. Decision-making in unipolar or bipolar suicide attempters. J Affect Disord. 2016;190:128–136. 10.1016/j.jad.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 39.Stewart JG, Glenn CR, Esposito EC, Cha CB, Nock MK, Auerbach RP. Cognitive control deficits differentiate adolescent suicide ideators from attempters. J Clin Psychiatry. 2017;78(6):e614–e621. 10.4088/JCP.16m10647 [DOI] [PubMed] [Google Scholar]
- 40.Richard-Devantoy S, Szanto K, Butters MA, Kalkus J, Dombrovski AY. Cognitive inhibition in older high-lethality suicide attempters. Int J Geriatr Psychiatry. 2015;30(3):274–283. 10.1002/gps.4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann JJ, Haas A, Mehlum L, Phillips M. Suicide prevention strategies. JAMA. 2005;294(16):2064–2074. 10.1001/jama.294.16.2064 [DOI] [PubMed] [Google Scholar]
- 42.Hubers AAM, Hamming A, Giltay EJ, et al. Suicidality in Huntington’s disease: a qualitative study on coping styles and support strategies. J Huntingtons Dis. 2016;5(2):185–198. 10.3233/JHD-160188 [DOI] [PubMed] [Google Scholar]
- 43.Posner K, Brown GK, Stanley B, et al. The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]


