Abstract
Allogeneic SCT for older patients remains challenging at least in part due to graft-versus-host disease (GVHD) and higher treatment-related mortality (TRM). We conducted a prospective pilot study primarily for older patients undergoing matched unrelated donor (MUD) SCT using a reduced-intensity (RIC) melphalan-based conditioning and post-transplant cyclophosphamide (PTCy)-based GVHD prophylaxis with tacrolimus and mycophenolate mofetil. Twenty-two patients (median age 64) underwent RIC MUD SCT for high-risk hematological malignancies including AML/MDS (73%), CML/MPD (18%), and other (10%). Two (9%) patients had early death; the rest (100%) engrafted. After a median follow-up of 17 months, 11 patients were alive and disease-free with an estimated 2-year progression-free (PFS) and overall (OS) survival of 48%. The cumulative incidences of grades 2-4 and 3-4 acute GVHD (aGVHD) at day +100 and 2-years post-SCT were 32% and 4%, and 59% and 24%, respectively. No cases of chronic GVHD (cGVHD) were noted. However, late acute GVHD was observed in 6 (27%) patients. In conclusion, RIC MUD SCT with melphalan-based conditioning and PTCy-based GVHD-based prophylaxis for older patients appears effective in controlling relapse. While cGVHD was not seen and early aGVHD appears controllable, a significant proportion developed late aGVHD responsible for higher TRM seen in these patients.
Keywords: matched unrelated donor transplant, post-transplant cyclophosphamide, graft-vs-host prevention, hematological malignancies, survival
INTRODUCTION
The incidence of hematological malignancies increases with advancing age.1 Allogeneic hematopoietic stem cell transplantation (SCT) is curative for many patients with hematological malignancies. Historically, SCT in older patient population has been more challenging due to higher transplant related mortality (TRM). Increasing age is associated with higher comorbidity index, lower performance status, and an intrinsically higher risk disease, all of which adversely impact the survival.2, 3 With advances in the understanding of disease biology, conditioning regimens, graft-vs-host disease (GVHD) prophylaxis, and supportive care, the elderly patients are increasingly being considered for SCT. Our group developed a reduced-intensity conditioning (RIC) regimen combining fludarabine and melphalan as a potential strategy to decrease TRM in older and/or unfit individuals.4, 5 Lower doses of melphalan (100mg/m2) appeared to be better tolerated in older individuals. Modified versions using this backbone – either by adding thiotepa or 2Gy total body irradiation (TBI) – have been used to facilitate engraftment in haploidentical transplants (haploSCT) using high-dose post-transplant cyclophosphamide (PTCy)-based GVHD prophylaxis.6, 7 PTCy has been shown to induce tolerance by eliminating rapidly proliferating alloreactive T-cells (compared to resting stem cells and memory T-cells) with proven efficacy in haploSCT.8, 9
After successful use in haploSCT, there is a growing interest in using PTCy-based GVHD prophylaxis in HLA-matched donor transplants, especially with unrelated donors, who have a higher incidence of GVHD. While PTCy alone as GVHD prophylaxis has been successfully used in younger patients undergoing MRD SCT,10,11 the use of single agent PTCy for GVHD prophylaxis was found to be inferior to conventional GVHD prophylaxis (tacrolimus, methotrexate and antithymocyte globulin, ATG) in older patients.12
Here we evaluated PTCy in combination with tacrolimus and MMF as GVHD prophylaxis predominantly for older patients with hematologic malignancies undergoing 10/10 matched unrelated donor (MUD) SCT.
METHODS
Patients and Clinical Trial Design
This was a prospective study conducted between June 2009 and September 2015 as a part of a 3-arm clinical trial (National Clinical Trial identifier NCT01010217). The MUD arm was added later on as a protocol modification and stopped accrual when the other two arms completed accrual. Results from the other 2 arms were recently reported.7 Patients with high-risk leukemia, including patients with active disease, were eligible for the study. High-risk disease was defined as AML in CR1 with intermediate-risk or high-risk features (requiring more than one cycle of induction therapy to achieve remission; a prior history of myelodysplastic syndrome (MDS) or myeloproliferative neoplasm; a French-American-British subtype of M6 or M7; adverse cytogenetics such as a complex karyotype or chromosomal abnormalities of −5, −7, 9q, 11q, 20q, 21q, 17, or +8; AML in CR2 or subsequent remission; MDS with an International Prognostic Scoring System score of intermediate-2 or higher or therapy-related MDS; or chronic myeloid leukemia (CML) refractory or resistant to at least two tyrosine kinase inhibitors or that progressed to an accelerated or blast-phase.
A total of 21 patients were enrolled. One patient was eligible but was treated off protocol due to insurance reasons and was included in this analysis. All patients and donors provided written informed consent according to the Declaration of Helsinki.
The primary objective of this study was to determine the safety and non-relapse mortality (NRM). The study was monitored using the NRM incidence at day +100 as the primary safety endpoint. A Bayesian monitoring scheme was used with early stopping rules in which the trial would be stopped if the predicted NRM rate at day +100 was >25%. The secondary endpoints were the cumulative incidence of neutrophil and platelet recovery; the 1-year progression free (PFS) and overall survival (OS) rate; and the cumulative incidence (CI) of acute GVHD (aGVHD), chronic GVHD (cGVHD), and relapse. Late acute (LA)-GVHD was defined as persistent, recurrent, or new-onset aGVHD symptoms occurring >100 days post-SCT.13
For patients with AML/MDS, complete remission (CR) was defined as ≤5% bone marrow blasts with neutrophils ≥1×109/L and platelets ≥100×109/L with or without minimal residual disease. For CML, ≤5% marrow blasts with ≥1×109/L and platelets ≥100×109/L was defined as chronic phase (CP). Cytogenetic risk was classified according to the Southwestern Oncology Group (SWOG) risk category.14 Hematopoietic SCT comorbidity index (HCT-CI) was assessed as previously described.15 The day of neutrophil engraftment was defined as the first of three consecutive days with the neutrophils ≥0.5 x109/L. The time to PLT20 was defined as the first of the three consecutive days with platelets >20 x109/L in the absence of transfusion for at least seven preceding days.
Conditioning Regimens and Supportive Care
All patients received fludarabine (160 mg/m2 divided in 4 daily doses) and melphalan (100 mg/m2) as a single dose with either 2GyTBI (n=15, 68%) or thiotepa 5 mg/kg (n=7, 32%). Unmodified hematopoietic progenitor cells were obtained from 10/10 HLA-matched unrelated donors using either bone marrow (n=18, 86%) or peripheral blood (n=3, 14%) and infused on Day 0. All patients received PTCy (50 mg/kg/day) on Days +3 and +4 for GVHD prophylaxis, mycophenolate mofetil and tacrolimus starting on Day +5 until Day +100 and 6 months, respectively, in the absence of significant GVHD. All patients received standard supportive care including granulocyte colony–stimulating and antimicrobial prophylaxis as previously described.7
Statistical Analysis
The Kaplan-Meier method was used to estimate actuarial OS and PFS. NRM, relapse, and GVHD were estimated using the CI method to account for competing risks. Death was considered a competing risk for progression while disease progression or relapse death was considered competing risks for NRM. Death or disease progression prior to GVHD was considered competing risk for estimate of CI of GVHD. Analyses were performed primarily using STATA 14.0 (College Station, TX, USA).
RESULTS
Patient Characteristics
Clinical characteristics are described in Table 1. Median age at SCT was 64 years (range 35-74) with 18 (82%) patients being above the age 55 years. A majority (n=15, 68%) of the patients were males. Indication for SCT included AML/MDS (n=16, 73%), CML/MPD (n=4, 18%), and other (n=2, 10%). At the time of SCT, 7 (32%) patients had primary induction failure (PIF). Only 6 (27%) patients were in CR1, while 4 (18%) patients were in CR2 or second chronic phase (CP2) CML. For AML/MDS patients, cytogenetic risk category was categorized as good (n=2, 12%), intermediate (n=10, 62%), and poor-risk (n=4, 25%).
Table 1.
Patient and donor demographics and allograft characteristics
| Characteristic | Value |
|---|---|
| Age in years, median (range) | 64 (35-74) |
| Gender, N (%) | |
| Female | 7 (32) |
| Male | 15 (68) |
| Conditioning, N (%) | |
| Flu/Mel100/TBI | 15 (68) |
| Flu/Mel100/Thiotepa | 7 (32) |
| Diagnosis N (%) | |
| AML / MDS | 16 (73) |
| CML / MPD | 4 (18) |
| CLL | 1 (5) |
| Aplastic Anemia | 1 (5) |
| Disease Status, N (%) | |
| PIF | 7 (32) |
| CR1 | 6 (27) |
| CR2 / CP2 | 4 (18) |
| Other | 5 (23) |
| Cytogenetic Risk, N (%) | |
| Good | 2 (9) |
| Intermediate | 10 (45) |
| Bad | 5 (23) |
| Unknown | 5 (23) |
| Cell Source, N (%) | |
| Peripheral blood | 3 (14) |
| Bone marrow | 19 (86) |
| HCT-CI, median (range) | 2 (0-8) |
Legends: Flu – fludarabine; Mel – melphalan; TBI – total body irradiation; AML – acute myeloid leukemia; MDS – myelodysplastic syndrome; CML – chronic myeloid leukemia; MPD – myeloproliferative disorder; CLL – chronic lymphocytic leukemia; PIF – primary induction failure; CR1 – first complete remission; CR2 – second complete remission; Cytogenetics according to Southwestern Oncology Group cytogenetics risk category; HCT-CI – hematopoietic stem cell transplant comorbidity index
Engraftment and Hematopoietic recovery
Two (9%) patients had early death; the rest of patients (n=20, 100%) engrafted with 1 (5%) experiencing delayed engraftment. Among the 20 patients who engrafted, the median time to neutrophil and platelet recovery was 20 (range 10-25) and 28 (range 16-111) respectively.
Acute and Chronic GVHD and Non-Relapse Mortality
The cumulative incidence of grade 2-4 and 3-4 aGVHD at day +100 was 32% and 4% respectively (Table 2 and Figure 1). The cumulative incidence of aGVHD grade 2-4 and 3-4 at 2-years was 59% and 24%, respectively. Interestingly, no cases of cGVHD were noted throughout the study period.
Table 2.
Transplant and GVHD related outcomes
| Transplant Outcomes | Percentage (range) |
|---|---|
| Outcomes, percent (95% CI) | |
| 2-year OS | 48 (22-69) |
| 2-year PFS | 48 (23-69) |
| 2-year RI | 9 (2-35) |
| 2-year NRM | 43 (24-76) |
| Acute GVHD maximum grade, percent (95% CI) | |
| Grade 2-4 | 32 (14-51) |
| Grade 3-4 | 4 (2-35) |
| 2-year cumulative incidence of acute GVHD, percent (95% CI) | |
| Grade 2-4 | 59 (42-84) |
| Grade 3-4 | 24 (11-51) |
| Chronic GVHD, N (%) | |
| de novo | 0 (0) |
| Relapsing | 0 (0) |
| Progressive | 0 (0) |
Legend: OS – overall survival; PFS – progression free survival; RI – relapse incidence; NRM – non-relapse mortality; GVHD – graft-vs-host disease
Figure 1.
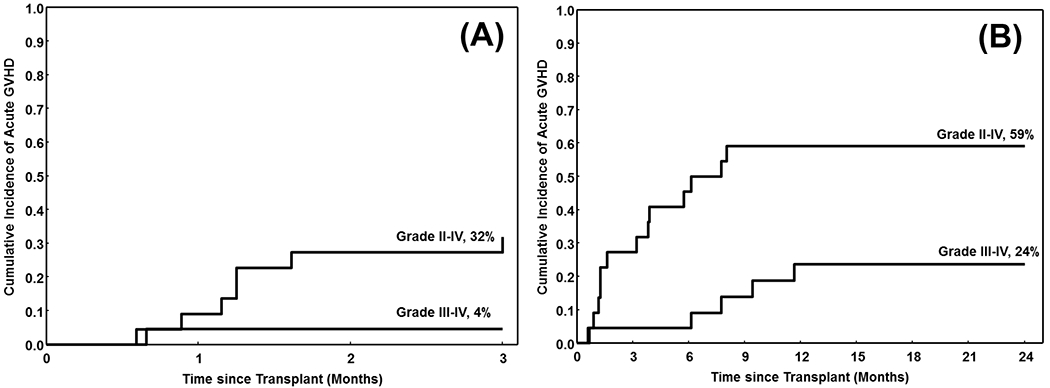
The cumulative incidence of acute graft-vs-host disease (aGVHD) (A) Grades 2-4 and 3-4 occurring within 3 months of transplant, and (B) Grades 2-4 and 3-4 aGVHD occurring within 2 years of transplant
Six (27%) patients developed late acute GVHD – 3 during or after steroid taper (2 had skin- and 1 had GI GVHD), 2 after tacrolimus taper (both had GI GVHD), and 1 after MMF taper (skin GVHD). Three (50%) of these 6 patients died (one because of GVHD, one because of interstitial pneumonitis and one from an undetermined cause). Overall, non-relapse mortality (NRM) at 2-years was 43% [95% confidence interval (CI) 24-76].
Relapse and Survival
At the end of the study period, 11 of 22 patients were alive and in remission with a median follow-up of 17 months (range 6-38). The 2-year overall (OS) and progression-free survival (PFS) for the cohort was 48%, with a 2-year relapse incidence (RI) of only 9% (Figure 2).
Figure 2.
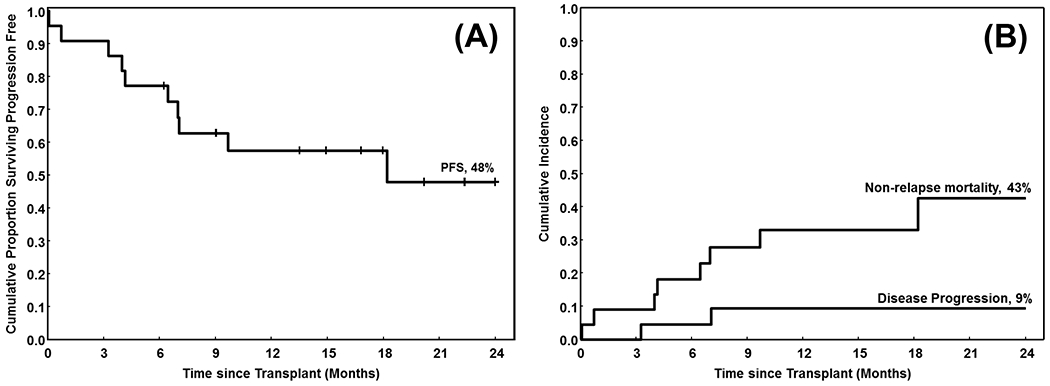
The cumulative proportion of patients (A) surviving progression free (progression-free survival, PFS), and (B) surviving (overall survival, OS). The X-axis shows time from stem cell transplant (in months)
A total of 11 deaths were noted in this cohort (Table 3). The most common cause of death was infections (n=7, 64%) with recurrent/persistent disease being the second most common (n=2, 18% at last follow-up). The exact cause of death could not be determined in one patient.
Table 3.
Mortality after Transplantation
| Primary Cause of Death | N (%) |
|---|---|
| Persistence/recurrence of the disease | 2 (18) |
| Acute GVHD | 1 (9) |
| Infections | 7 (64) |
| Unknown | 1 (9) |
| Total | 11 (100) |
Legend: GVHD – graft-versus-host disease
DISCUSSION
Allogeneic SCT for older patients remains a challenge, at least in part due to higher incidence of GVHD. Hence, control of GVHD remains even a greater priority for older individuals. PTCy alone has been evaluated in MRD SCT and found to be safe and effective.10, 11, 16 Other studies have shown that outcomes of younger (median age approximately 40 years) patients undergoing MUD SCT, using PTCy in combination of other immunosuppressant such as cyclosporine, tacrolimus, MMF to be as good as those with conventional GVHD prophylaxis.17, 18 Taken together, these studies suggest that, for younger patients, PTCy alone may be sufficient, especially in the setting of MRD SCT. In contrast, our group previously studied PTCy alone for MUD SCT in a group of older patients (median age 62 years). In a matched cohort analysis, PTCy alone cohort had higher TRM and incidence of GVHD with an overall inferior outcome compared to the conventional GVHD prophylaxis (tacrolimus, methotrexate, ATG).8 Recently, we showed the feasibility and efficacy of PTCy-based GVHD prophylaxis in older patients receiving haploidentical donor SCT.19 This raised the question whether PTCy in combination with tacrolimus and MMF would be more effective in preventing GVHD and decrease TRM in older patients receiving a MUD SCT.
In this cohort, we treated predominantly older individuals with median age 64 (up to age of 74 years) with a uniform melphalan-based conditioning regimen consisting of reduced doses of melphalan.7 Only a quarter of the patients were in CR1 and a high proportion of AML/MDS patients had a high-risk disease by cytogenetics. Despite that, we noted a 2-year predicted PFS and OS of 48%, suggesting that outcomes may be better than using PTCy alone for GVHD prevention in older individuals.12 Encouraging was a very low incidence of relapse; however, TRM, primarily related pulmonary complications (mainly infections), remained high. We recently reported fluid overload/retention as an independent adverse prognostic factor.20 It is unclear whether the higher susceptibility to fluid overload in older population played a role in the higher TRM seen in this cohort especially considering that increased hydration is applied during cyclophosphamide administration. Larger study population is needed for a comprehensive assessment of predictors of NRM, including fluid overload, in this context. In addition, while the cumulative incidence of grade 2–4 and 3–4 aGVHD at day 100 was low, we observed a higher incidence of late aGVHD, including severe aGVHD, after discontinuation of immunosuppression, arguably negatively impacting TRM at a later time point.
Our group previously performed a similar study using PTCy as single agent for GVHD prophylaxis and RIC conditioning in older patients.12 Using PTCy alone, the CI of grade 2–4 aGVHD, 3–4 aGVHD, and cGVHD was 58%, 22%, and 18%, respectively.12 Survival appeared to be inferior in this group compared with a matched cohort of patients treated with conventional GVHD prophylaxis driven by higher TRM related to higher incidence of GVHD12. The addition of tacrolimus and MMF in the current cohort compares favorably with these data, suggesting that the combination of tacrolimus/MMF added to PTCy may significantly reduce the incidence of acute and mostly chronic GVHD and possibly improve survival. Although very low incidence of cGVHD was noted, a number of patients appeared to develop late acute GVHD. In addition, in a large EBMT retrospective analysis that employed RIC conditioning with conventional GVHD prophylaxis (cyclosporine A with methotrexate, steroids, or MMF) noted a 2-year Rl, NRM, and leukemia free survival of 25%, 42%, and 34% respectively in older (age ≥50 years) patients, which is similar to results noted in our study.21 These outcomes are also comparable to the results from a large meta-analysis of elderly AML patients undergoing allogeneic SCT.22 In that study, the estimated 2-year PFS was 47% suggesting that RIC followed by PTCy/tacrolimus/MMF-based GVHD prophylaxis may be at least as effective as conventional GVHD prophylaxis in older patients, although a head-to-head comparison is warranted.
In conclusion, our results suggest that PTCy in combination with tacrolimus and mycophenolate is a more effective strategy compared to PTCy alone to prevent GVHD for older patients with hematological malignancies undergoing RIC MUD SCT. Prospective studies are needed to clarify the best approach GVHD prophylaxis in older individuals.
Footnotes
Financial Disclosures: The authors have no conflict of interest to declare for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians 2017; 67(1): 7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Sun Yq, Xu Lp, Zhang Xh, Liu Dh, Chen H, Wang Y et al. A retrospective comparison of BU-fludarabine and BU-CY regimens in elderly patients or in patients with comorbidities who received unmanipulated haploidentical hematopoietic SCT. Bone Marrow Transplant 2015; 50(4): 601–603. doi: 10.1038/bmt.2014.303 [DOI] [PubMed] [Google Scholar]
- 3.Ciurea SO, Rodrigues M, Giralt S, de Lima M. Aging, acute myelogenous leukemia, and allogeneic transplantation: do they belong in the same sentence? Clin Lymphoma Myeloma 2009; 9(4): 289–297. doi: 10.3816/CLM.2009.n.057 [DOI] [PubMed] [Google Scholar]
- 4.Oran B, Giralt S, Saliba R, Hosing C, Popat U, Khouri I et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant 2007; 13(4): 454–462. doi: 10.1016/j.bbmt.2006.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popat U, de Lima MJ, Saliba RM, Anderlini P, Andersson BS, Alousi AM et al. Long-term outcome of reduced-intensity allogeneic hematopoietic SCT in patients with AML in CR. Bone Marrow Transplant 2012; 47(2): 212–216. doi: 10.1038/bmt.2011.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciurea SO, Saliba R, Rondon G, Pesoa S, Cano P, Fernandez-Vina M et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant 2010; 45(3): 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaballa S, Ge I, El Fakih R, Brammer JE, Kongtim P, Tomuleasa C et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer 2016; 122(21): 3316–3326. doi: 10.1002/cncr.30180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanakry CG, Ganguly S, Zahurak M, Bolaños-Meade J, Thoburn C, Perkins B et al. Aldehyde Dehydrogenase Expression Drives Human Regulatory T Cell Resistance to Posttransplantation Cyclophosphamide. Science Translational Medicine 2013; 5(211): 211ra157–211ra157. doi: 10.1126/scitranslmed.3006960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol 2016; 13(1): 10–24. doi: 10.1038/nrclinonc.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol 2014; 32(31): 3497–3505. doi: 10.1200/JCO.2013.54.0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanakry CG, Tsai HL, Bolanos-Meade J, Smith BD, Gojo I, Kanakry JA et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 2014; 124(25): 3817–3827. doi: 10.1182/blood-2014-07-587477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alousi AM, Brammer JE, Saliba RM, Andersson B, Popat U, Hosing C et al. Phase II Trial of Graft-versus-Host Disease Prophylaxis with Post-Transplantation Cyclophosphamide after Reduced-Intensity Busulfan/Fludarabine Conditioning for Hematological Malignancies. Biol Blood Marrow Transplant 2015; 21(5): 906–912. doi: 10.1016/j.bbmt.2015.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtan SG, Khera N, Levine JE, Chai X, Storer B, Liu HD et al. Late acute graft-versus-host disease: a prospective analysis of clinical outcomes and circulating angiogenic factors. Blood 2016; 128(19): 2350–2358. doi: 10.1182/blood-2015-09-669846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood 2000; 96(13): 4075–4083. [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106(8): 2912–2919. doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010; 115(16): 3224–3230. doi: 10.1182/blood-2009-11-251595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess BT, Gao F, DiPersio JF, Westervelt P, Vij R, Uy GL et al. Use of Post-Transplant Cyclophosphamide (PTCy) with Mycophenolate Mofetil and Tacrolimus in HLA Matched Allogeneic Hematopoietic Cell Transplant Is Safe and Associated with Acceptable Transplant Outcomes. Blood 2015; 126(23): 1950–1950. [Google Scholar]
- 18.Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016; 127(11): 1502–1508. doi: 10.1182/blood-2015-10-672071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciurea SO, Shah MV, Saliba RM, Gaballa S, Kongtim P, Rondon G et al. Haploidentical Transplantation for Older Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant 2017. (Article in Press). doi: 10.1016/j.bbmt.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rondón G, Saliba RM, Chen J, Ledesma C, Alousi AM, Oran B et al. Impact of Fluid Overload as New Toxicity Category on Hematopoietic Stem Cell Transplantation Outcomes. Biology of Blood and Marrow Transplantation 2017; 23(12): 2166–2171. doi: 10.1016/j.bbmt.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringdén O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al. Reduced Intensity Conditioning Compared With Myeloablative Conditioning Using Unrelated Donor Transplants in Patients With Acute Myeloid Leukemia. Journal of Clinical Oncology 2009; 27(27): 4570–4577. doi: 10.1200/JCO.2008.20.9692 [DOI] [PubMed] [Google Scholar]
- 22.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biology of Blood and Marrow Transplantation 2016; 22(4): 651–657. doi: 10.1016/j.bbmt.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]


