Abstract
The present study compares 34 patients with thumb carpometacarpal osteoarthritis (37 thumbs) treated with the Elektra® prosthesis, with 18 patients (18 thumbs) treated with resection-suspension arthroplasty, with an overall mean follow-up period of 13.3 years. Evaluation with disability of arm and shoulder scores, pain via visual analogue scale and range of motion (radial and palmar abduction, and opposition) indicated no significant difference. However, the cohort with a surviving prosthesis showed significantly better subjective grip strength (p = 0.04). Complications occurred in 23 of the 37 thumbs in the prosthesis group compared with two in the resection-suspension arthroplasty patients. Seventeen prostheses required revision. At revision operations, we observed local signs of metallosis in 15 of 17 cases. The patients receiving resection-suspension arthroplasty were more satisfied with their treatment (p = 0.003). Therefore, we cannot recommend the implantation of Elektra® prosthesis and we speculate that the key problem of aseptic cup loosening is a result of the metal-on-metal bearing.
Level of evidence: III
Keywords: Arthroplasty, thumb carpometacarpal osteoarthritis, Elektra prosthesis, joint replacement, resection-suspension arthroplasty
Introduction
Trapeziectomy alone (Li et al., 2011; Yeoman et al., 2019) or with resection-suspension arthroplasty (RSA) is widely established in the treatment of osteoarthritis (OA) of the thumb carpometacarpal (CMC) joint, but there is no evidence that this technique is superior to other techniques (Vermeulen et al., 2011). A review of the literature reveals that a major drawback of this method is proximal migration of the thumb that can lead to a compromised pinch strength and disability (Ulrich-Vinther et al., 2008). Approaches towards total arthroplasty have been used in not only hip and knee surgery but also hand surgery, in an attempt to maintain thumb length and to achieve anatomical reconstruction of the thumb CMC joint, with early implants reported by Swanson (1972) and De La Caffiniere and Aucouturier (1979). Since then, several authors have reported their experiences with various thumb CMC prostheses. However, results show inconsistencies, especially in terms of complication or revision rates. The main drawback of replacement arthroplasty is aseptic cup loosening, cited as the most common reason for implant failure, leading to revision rates of 42%–51% (Hansen and Homilius, 2010; Hernández-Cortés et al., 2012; Kaszap et al., 2012; Klahn et al., 2012; Kollig et al., 2017). However, a number of good to excellent outcomes have also been published (Badia and Sambandam, 2006; Cootjans et al., 2017; Dehl et al., 2017; Krukhaug et al., 2014; Regnard, 2006; Vissers et al., 2019).
The major arguments for prosthesis implantation are better functional results in terms of range of motion, lower disability of arm and shoulder (DASH) scores, stronger grip, and faster and better pain relief. A few comparative studies contrasting thumb CMC prosthesis and RSA have been published, including two prospective studies and one retrospective study comparing the Elektra, ARPE, and Ivory prostheses with RSA, with a mean follow-up period of 1 to 4.5 years (Cebrian-Gomez et al., 2019; Robles-Molina et al., 2017; Ulrich-Vinther et al., 2008).
The aim of the present study was to conduct a long-term outcome comparison between patients who underwent Elektra prosthesis implantation and RSA.
Patients and methods
Between March 2004 and April 2006, implantation of the Elektra prosthesis and RSA represented the only two surgical treatment options available for primary thumb CMC OA at our centre. Radiographic stage 3–4 thumb CMC OA and failed non-surgical treatment were indications for surgery. Two Level 3 experienced hand surgeons performed both Elektra prosthesis implantation and RSA (Tang und Giddins, 2016). Allocation of treatment was performed on a voluntary basis. Patients received both verbal and written information about the two treatment options and could choose one of them. All patients who underwent either operation were assessed for the present retrospective cohort study. This trial was approved by the local ethical review board. The study followed the World Medical Association (WMA) Declaration of Helsinki. The lead author (FSM) enrolled the patients at follow-up appointments and all patients gave their informed oral consent to participate in the study.
Patients with rheumatoid arthritis, any history of trauma (e.g. Bennett’s fracture-dislocation or Rolando fracture), concomitant scapho-trapezio-trapezoid OA, or any previous thumb CMC surgery were excluded, whereas bilateral surgical treatment was not defined as an exclusion criterion. Date of birth, sex, and side of the operation were recorded. Furthermore, a follow-up period of a minimum of 12 years was a prerequisite for inclusion in the study cohort. A Kaplan–Meier plot was generated separately, highlighting all cases of implant failures in the prosthesis cohort.
Surgical technique
Both procedures described below were performed under regional brachial plexus anaesthesia by a senior hand surgeon assisted by a resident, using a tourniquet.
For the total arthroplasty, we used the Elektra prosthesis (Small Bone Innovations Inc., Morrisville, Pennsylvania, USA; formerly Fixano, Péronnas, France). This prosthesis, introduced in 1996, is an unconstrained, uncemented ball-and-socket prosthesis consisting of three components: a hydroxyapatite-coated titanium stem available in four sizes, a chrome–cobalt steel cup comprising a hydroxyapatite-coated thread for fixation into the trapezium, and a chrome–cobalt steel head on a neck with four sizes (Regnard, 2006). For prosthesis implantation, the technique described by Regnard (2006) was applied in our institution. Post-interventional care included 3 weeks immobilization in a splint, followed by 6 weeks of hand therapy.
The same surgical approach was used for RSA, but the thumb CMC joint and also the scaphotrapeziotrapezoid joint were exposed by sharp dissection. The trapezium was fragmented with an osteotome and carefully removed. After identifying the extensor carpi radialis longus tendon, we dissected and mobilized this tendon, split it into equal halves, and divided one-half 4 cm proximal to its insertion. Then, the extensor carpi radialis longus tendon strip was passed through a previously drilled hole (3.5 mm diameter), orientated from the ulnar edge of the joint surface distally to the dorsal base of the thumb metacarpal (MC). This ligament reconstruction was secured using a Micro Mitek bone anchor (Johnson and Johnson, USA) and protected with a K-wire placed through the first and second MC. Postoperatively, the thumb was immobilized in a splint for 6 weeks, after which the K-wire was removed, and the patients underwent extensive hand therapy for another 6 weeks.
Follow-up
Clinical follow-up included at least eight appointments (2 weeks, 4 weeks, 6 weeks, 12 weeks, 26 weeks, 1 year, 2 years, and minimum 12 years post-intervention and additionally, at any time symptoms occurred). Radiographs were taken in two planes (anteroposterior and lateral) at each visit. For the prosthesis cohort, these radiographs were evaluated according to the following criteria: dislocation/subluxation of the prosthesis, implant loosening (radiolucent areas around the prosthesis components), cup tilting (deviating axis of more than 20° compared with the intraoperative radiograph), and adjacent joint OA. The RSA cohort was radiographically assessed with respect to thumb length, adduction deformity, and adjacent joint OA. At least 12 years postoperatively, we conducted a detailed clinical examination of the patients who had a surviving Elektra prosthesis or had had RSA, assessing the patients’ individual symptoms and abilities using the DASH score. We also intended to ascertain whether surgery affected subjective grip strength. Therefore, we recorded a high or low loss of strength with the value −2 or −1, a low or high gain of strength with the value +1 or +2, and no noticeable change with the value 0. The range of thumb radial and palmar abduction was measured using a goniometer. We determined the distance (in centimetres) between the thumb tip and the fifth metacarpophalangeal joint while the patients opposed their thumbs maximally. Patient satisfaction was investigated by asking the patients if they would undergo the performed surgical intervention again. Pain was assessed using a visual analogue scale (VAS) ranging from 0 (no pain) to 100 (worst imaginable pain). All complications were noted, and in cases of implant failure and consecutive revision surgery, the in-vivo survival time of the prosthesis was calculated. The clinical assessments and radiograph evaluation were performed by an independent, experienced examiner who did not participate in the surgery.
Statistical methods
Descriptive statistics are presented as mean standard deviation or as median and range (interquartile range) in case of non-normal data. Comparative testing was done using the unpaired t-test, Mann–Whitney U-test, and Fisher’s exact test. The prosthesis survival curve was plotted as a Kaplan–Meier curve. A value of p < 0.05 was considered significant.
Results
Patient demographics and are shown in Table 1. Initially, we enrolled 34 patients in the Elektra prosthesis cohort and 18 patients in the RSA cohort, but five patients of each group were lost to follow-up. In the prosthesis group, two patients died during the required minimum follow-up period of 12 years. Three patients had moved away from the area of our clinic and could not be reached for an invitation to a clinical examination. In case of the RSA group, five patients were excluded because during the prerequisite minimum follow-up period of 12 years, two patients died and two moved away, and one patient was excluded owing to his severe multimorbidity, which made any clinical examination impossible. Overall mean follow-up period was 13.3 years, 13.1 years (range 12.2–14.3) in the Elektra prosthesis cohort and 13.6 years (12.9–14.7) in the RSA cohort.
Table 1.
Demographics of patients undergoing different operations.
| Demographics | Elektra prosthesis | Resection-suspension arthroplasty |
|---|---|---|
| Patients/thumbs (number) | 29/32 | 13/13 |
| Right/left thumbs (number) | 13/19 | 7/6 |
| Women/men (number) | 26/3 | 12/1 |
| Mean age at time of operation (range) | 54 years (36–71) | 58 years (51–64) |
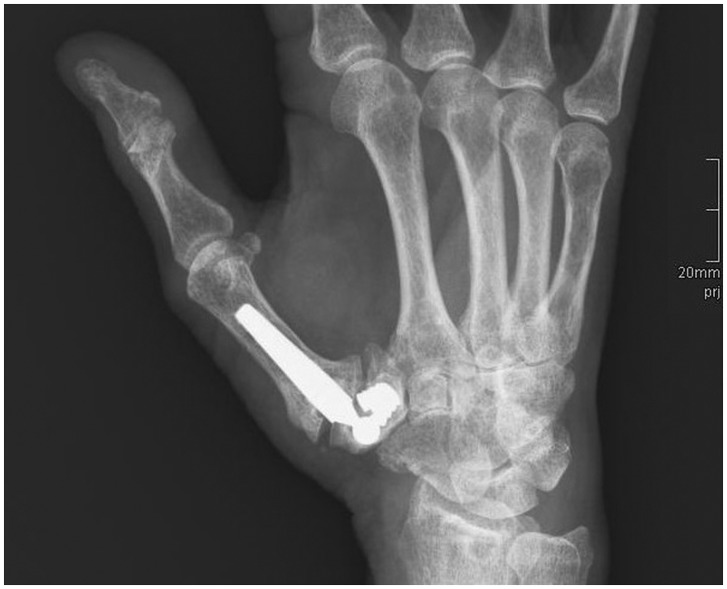
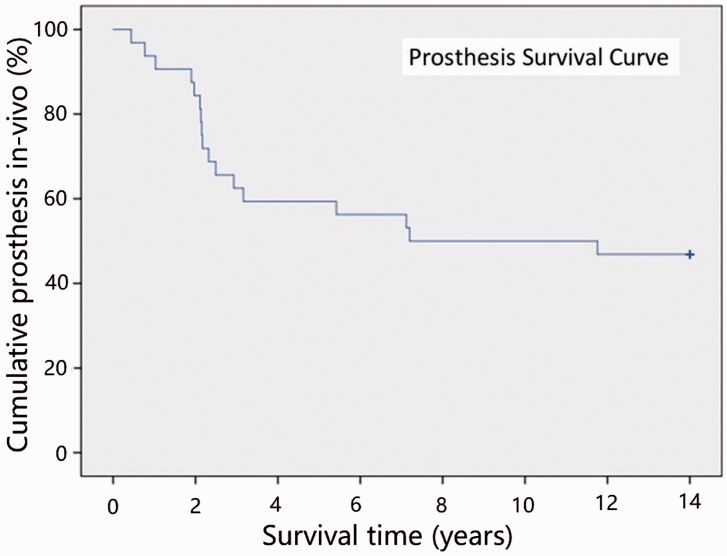
In the Elektra prosthesis group, we detected 23 major complications (72% complication rate) in 22 patients: 17 cases of aseptic cup loosening (Figure 1), four cases of cup tilting, one case of dislocation, and one case of allergic reaction. In 17 patients (53%), we had to perform a revision surgery, including removal of the prosthesis and conversion to RSA. While performing revision surgery, local signs of metallosis were observed in 15 of these 17 cases. The Elektra prosthesis survival curve was generated by using the in-vivo survival time of each prosthesis in a Kaplan–Meier plot (Figure 2). In the RSA group, two major complications occurred: one case of proximal migration of the thumb and one case of neo-joint instability or hypermobility. Thus, the Elektra prosthesis shows a significantly higher complication rate (p = 0.001).
Figure 1.
Radiograph showing failure of an Elektra prosthesis (aseptic cup loosening).
Figure 2.
Kaplan–Meier survival curve for the Elektra prosthesis.
Clinical outcomes for the surviving Elektra prosthesis cases and RSA patients are summarized in Table 2. Subjective grip strength was significantly better in the prosthesis group. The patient satisfaction estimates corroborate the findings, as only 44% of the prosthesis group patients, in comparison with 92% in the RSA group, would undergo the procedure again. Thus, RSA patients are significantly more satisfied with their procedure (p = 0.003).
Table 2.
Clinical outcomes for the surviving prosthesis and resection-suspension arthroplasty groups at long-term follow-up.
| Evaluation | DASH score (mean (SD)) | VAS score (median (IQR)) | Radial abduction (mean (SD)) | Palmar abduction (mean (SD)) | Opposition (median (IQR)) | Subjective grip strength (median (IQR)) |
|---|---|---|---|---|---|---|
| Elektra prosthesis | 23 (26) | 0 (40) | 56° (12°) | 50° (17°) | 0 cm (0) | 0 (0) |
| Resection-suspension arthroplasty | 37 (26) | 0 (20) | 51° (8°) | 57° (5°) | 0 cm (1) | −1 (1) |
| p-valuea | 0.08 | 0.62 | 0.32 | 0.08 | 0.65 | 0.04 |
Unpaired t-test was used for DASH scores, palmar, and radial abduction. Mann–Whitney U-test was used for VAS scores, opposition, and subjective grip strength. Bold indicates statistically significant p-values.
Discussion
The present retrospective cohort study comparing the Elektra prosthesis and RSA shows that the main difference between these two treatment options for thumb CMC OA is found in the complication rates. In 72% of the prosthesis patients, at least radiographic implant failure was observed and in 53% implant removal and conversion to RSA had to be performed. In contrast to the Elektra group patients, no RSA group patient had to be surgically revised. The revision rate in the prosthesis group can be compared with the results reported by Hernández-Cortés et al. (2012) (47% loosening rate). Other studies concerning the Elektra prosthesis reported lower revision rates ranging from 0%–24%; however, the relatively shorter mean follow-up periods (1–4.5 years) render a direct comparison with our findings difficult (Klahn et al., 2012; Regnard, 2006; Ulrich-Vinther et al., 2008). Nevertheless, these authors hypothesized that the cup design and the biomechanical characteristics of its bony fixation are the reasons for the high loosening rates.
Hansen and Snerum (2008) also discussed the bone quality to be the main problem in achieving a stable cup fixation. Moreover, other authors have reported that mediocre bone quality was a statistically significant factor for surgical revision of the Maia® prosthesis in their study (Bricout and Rezzouk, 2016), thus supporting the above hypothesis. In addition to these arguments, we speculate that the metal-on-metal articulation is an important reason underlying the aseptic cup loosening of Elektra prosthesis. This type of bearing is rarely used in current joint replacements because of potential complications, such as massive metallosis, leading to pseudotumours, implant loosening, elevated blood metal levels, and even neurological symptoms (Grote et al., 2018). A number of studies reporting low implant failure rates in none metal-on-metal type prostheses support our hypothesis. The Arpe® system and Maia® system show revision rates of 5%–9% and 4%–11%, respectively; both prostheses use a metal-on-polyethylene bearing (Bricout and Rezzouk, 2016; Cootjans et al., 2017; Robles-Molina et al., 2017; Toffoli and Teissier, 2017).
Although patient satisfaction is a relatively subjective parameter, our findings suggest that this outcome correlates with the complication rate. Our study shows significantly higher subjective grip strength in the group with a surviving prosthesis than in RSA group and a strong tendency towards a lower DASH score. These benefits of prosthesis may result from a more anatomical reconstruction of the thumb CMC joint. Certain comparative studies confirm these findings and even demonstrate statistically better results in nearly every functional outcome measure (Cebrian-Gomez et al., 2019; Robles-Molina et al., 2017; Ulrich-Vinther et al., 2008). Nevertheless, the considerable difference in the follow-up period (1–4.5 years vs. more than 13 years in our study design) renders a direct comparison problematic. Considering that 5% of 55-year-old women, who have a statistical remaining lifetime of more than 25 years, already suffer from thumb base OA, we emphasize that sustainability is a crucial factor in surgery (Jonsson, 2017).
Our study has several limitations. First, we included a number of subjective parameters in our assessment. Owing to a lack of preoperative data, we included subjective grip strength as a parameter, although it may be difficult to estimate present grip strength in comparison with that more than 12 years ago. The small sample sizes, especially in the RSA cohort, and a lack of randomized sampling are the other drawbacks of the study.
In conclusion, we cannot recommend Elektra prosthesis implantation for thumb CMC arthrosis because a significantly higher grip strength cannot outweigh such a high complication and revision rates due to aseptic cup loosening. Nevertheless, if a prosthesis system could overcome the hurdle of aseptic cup loosening, total thumb CMC arthroplasty could become a reasonable therapeutic option for thumb base surgery. Therefore, randomized studies with a comparable long-term follow-up period are needed to verify the sustainability of such a CMC prosthesis.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Study was approved by ethical committee: Ethikkommission des Landes Oberösterreich (EK Nr: 1094/2018).
References
- Badia A, Sambandam SN. Total joint arthroplasty in the treatment of advanced stages of thumb carpometacarpal joint osteoarthritis. J Hand Surg Am. 2006, 31: 1605–14. [DOI] [PubMed] [Google Scholar]
- Bricout M, Rezzouk J. Complications and failures of the trapeziometacarpal Maia® prosthesis. A series of 156 cases. Hand Surg Rehabil. 2016, 35: 190–8. [DOI] [PubMed] [Google Scholar]
- Cebrian-Gomez R, Lizaur-Utrilla A, Sebastia-Forcada E, Lopez-Prats FA. Outcomes of cementless joint prosthesis versus tendon interposition for trapeziometacarpal osteoarthritis. A prospective study. J Hand Surg Eur. 2019, 44: 151–8. [DOI] [PubMed] [Google Scholar]
- Cootjans K, Vanhaecke J, Dezillie M, Barth J, Pottel H, Stockmans F. Joint survival analysis and clinical outcome of total joint arthroplasties with the ARPE implant in the treatment of trapeziometacarpal osteoarthritis with a minimal follow-up of 5 years. J Hand Surg Am. 2017, 42: 630–8. [DOI] [PubMed] [Google Scholar]
- Dehl M, Chelli M, Lippmann S, Benaissa S, Rotari V, Moughabghab M. Results of 115 Rubis II reverse thumb carpometacarpal joint prostheses with a mean follow-up of 10 years. J Hand Surg Eur. 2017, 42: 592–8. [DOI] [PubMed] [Google Scholar]
- De La Caffiniere JY, Aucouturier P. Trapezio-metacarpal arthroplasty by total prosthesis. Hand. 1979, 11: 41–6. [DOI] [PubMed] [Google Scholar]
- Grote CW, Cowan PC, Anderson DW, Templeton KJ. Pseudotumor from metal-on-metal total hip arthroplasty causing unilateral leg edema. Case presentation and literature review. BioRes Open Access. 2018, 7: 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Homilius M. Failed total carpometacarpal joint prosthesis of the thumb. Results after resection arthroplasty. Scand J Plast Reconstr Surg Hand Surg. 2010, 44: 171–4. [DOI] [PubMed] [Google Scholar]
- Hansen TB, Snerum L. Elektra trapeziometacarpal prosthesis for treatment of osteoarthrosis of the basal joint of the thumb. Scand J Plast Reconstr Surg Hand Surg. 2008, 42: 316–9. [DOI] [PubMed] [Google Scholar]
- Hernández-Cortés P, Pajares-López M, Robles-Molina MJ, Gómez-Sánchez R, Toledo-Romero MA, De Torres-Urrea J. Two-year outcomes of Elektra prosthesis for trapeziometacarpal osteoarthritis. A longitudinal cohort study. J Hand Surg Eur. 2012, 37: 130–7. [DOI] [PubMed] [Google Scholar]
- Jonsson H. Age related prevalence of hand osteoarthritis diagnosed by photography (HOASCORE). BMC Musculoskelet Disord. 2017, 18: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszap B, Daecke W, Jung M. High frequency failure of the Moje thumb carpometacarpal joint arthroplasty. J Hand Surg Eur. 2012, 37: 610–6. [DOI] [PubMed] [Google Scholar]
- Klahn A, Nygaard M, Gvozdenovic R, Boeckstyns MEH. Elektra prosthesis for trapeziometacarpal osteoarthritis. A follow-up of 39 consecutive cases. J Hand Surg Eur. 2012, 37: 605–9. [DOI] [PubMed] [Google Scholar]
- Kollig E, Weber W, Bieler D, Franke A. Failure of an uncemented thumb carpometacarpal joint ceramic prosthesis. J Hand Surg Eur. 2017, 42: 599–604. [DOI] [PubMed] [Google Scholar]
- Krukhaug Y, Lie SA, Havelin LI, Furnes O, Hove LM, Hallan G. The results of 479 thumb carpometacarpal joint replacements reported in the Norwegian Arthroplasty Register. J Hand Surg Eur. 2014, 39: 819–25. [DOI] [PubMed] [Google Scholar]
- Li YK, White C, Ignacy TA, Thoma A. Comparison of trapeziectomy and trapeziectomy with ligament reconstruction and tendon interposition. A systematic literature review. Plast Reconstr Surg. 2011, 128: 199–207. [DOI] [PubMed] [Google Scholar]
- Regnard PJ. Electra trapezio metacarpal prosthesis. Results of the first 100 cases. J Hand Surg Br. 2006, 31: 621–8. [DOI] [PubMed] [Google Scholar]
- Robles-Molina MJ, López-Caba F, Gómez-Sánchez RC, Cárdenas-Grande E, Pajares-López M, Hernández-Cortés P. Trapeziectomy with ligament reconstruction and tendon interposition versus a trapeziometacarpal prosthesis for the treatment of thumb basal joint osteoarthritis. Orthopedics. 2017, 40: e681–6. [DOI] [PubMed] [Google Scholar]
- Swanson AB. Disabling arthritis at the base of the thumb. Treatment by resection of the trapezium and flexible (silicone) implant arthroplasty. J Bone Joint Surg Am. 1972, 54: 456–71. [PubMed] [Google Scholar]
- Tang JB, Giddins G. Why and how to report surgeons' levels of expertise. J Hand Surg Eur. 2016, 41: 365–6. [DOI] [PubMed] [Google Scholar]
- Toffoli A, Teissier J. MAÏA trapeziometacarpal joint arthroplasty. Clinical and radiological outcomes of 80 patients with more than 6 years of follow-up. J Hand Surg Am. 2017, 42: 838.e1–8. [DOI] [PubMed] [Google Scholar]
- Ulrich-Vinther M, Puggaard H, Lange B. Prospective 1-year follow-up study comparing joint prosthesis with tendon interposition arthroplasty in treatment of trapeziometacarpal osteoarthritis. J Hand Surg Am. 2008, 33: 1369–77. [DOI] [PubMed] [Google Scholar]
- Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis. A systematic review. J Hand Surg Am. 2011, 36: 157–69. [DOI] [PubMed] [Google Scholar]
- Vissers G, Goorens CK, Vanmierlo B, et al. Ivory arthroplasty for trapeziometacarpal osteoarthritis. 10-year follow-up. J Hand Surg Eur. 2019, 44: 138–45. [DOI] [PubMed] [Google Scholar]
- Yeoman TFM, Stone O, Jenkins PJ, McEachan JE. The long-term outcome of simple trapeziectomy. J Hand Surg Eur. 2019, 44: 146–50. [DOI] [PubMed] [Google Scholar]