Abstract
As part of EPA's commitment to reducing animal testing, the Office of Pesticide Programs (OPP) created the Hazard and Science Policy Council (HASPOC). This group considers requests for waiving animal study requirements for human health risk assessments and makes recommendations based on a weight-of-the-evidence approach. Since its inception in 2012, the HASPOC has evaluated over one thousand requests to waive animal studies required by default for pesticide evaluation. Here, the number of studies waived, and the types of studies represented were analyzed to determine the impact of the HASPOC decisions in terms of animal and monetary savings. Overall, the waiving of studies by HASPOC resulted in over 200 thousand animals saved. There were also savings of over $300 million in study costs and over $6 million in study review costs as well as less time spent in study processing and review by EPA staff. Thus, the HASPOC has built significant efficiencies into the risk assessment process while continuing to protect human health.
Keywords: Risk assessment, Pesticides, HASPOC, 3Rs
1. Introduction
The mission of the Environmental Protection Agency's (EPA) Office of Pesticide Programs (OPP) within the Office of Chemical Safety and Pollution Prevention (OCSPP) is to protect humans and the environment from potential risks associated from exposure to pesticide chemicals. The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) is the federal statute that governs the registration, distribution, sale, and use of pesticides in the United States. Under 40 Code of Federal Regulations (CFR) Part 158, EPA-OPP requires substantial toxicology and exposure testing for human health and the environment to support registration of food and non-food use pesticides. The total cost of toxicology and exposure testing ranges from $200–300 million for a conventional, food-use pesticide. To assess the potential hazard of a chemical for human health risk assessment, toxicological studies in laboratory animals are generally used to provide information on a wide range of adverse health outcomes, routes of exposure, exposure durations, species, and lifestages. The human health toxicology testing costs $8–16 million and uses 5000–7000 animals.
Federal regulations give EPA substantial discretion to make registration decisions based on what the Agency believes are the most relevant and important data for each action. The actual data and studies required may be modified on an individual basis to fully characterize the use and properties of specific pesticide products under review. The Agency may waive data requirements or request additional data beyond the default studies. Alternative methods and approaches can also be considered.
In 2007, the National Research Council (NRC) released its report, Toxicity Testing in the Twenty-first Century: A Vision and a Strategy, which provides a new paradigm for toxicology testing that is less reliant on in vivo testing in laboratory animals, but instead uses human-relevant cells and tissues, understanding pathways to toxicity, and development of computational and predictive modeling. Shortly after the NRC's 2007 report, EPA-OPP developed its own “Strategic Vision for Adopting 21st Century Science Methodologies” specific to pesticide testing that provides the framework for implementing the NRC's new vision for toxicology testing. This strategic vision describes several key areas including development of a broader suite of computer-aided methods to better predict potential hazards and exposures and to focus testing on likely risks of concern; improved approaches to more traditional toxicity tests to minimize the number of animals used while expanding the amount of information obtained; and improved understanding of toxicity pathways to allow development of non-animal tests that better predict how exposures relate to adverse effects.
In 2013, EPA-OPP developed its document entitled Guiding Principles for Data Requirements (USEPA-OPP, 2013a) (herein called the guiding principles document). The purpose of the guiding principles document is to provide consistency in the identification of data needs, promote and optimize full use of existing knowledge, and focus on the critical data needed for risk assessment. The guiding principles document notes that EPA-OPP needs to “… ensure there is sufficient information to reliably support registration decisions that are protective of public health and the environment while avoiding the generation and evaluation of data that does not materially influence the scientific certainty of a regulatory decision …. avoid unnecessary use of time and resources, data generation costs, and animal testing.”
At EPA-OPP, the Hazard and Science Policy Council (HASPOC) is tasked with considering requests for waiving most guideline mammalian toxicity studies for use in human health risk assessment, with the exception of the acute six-pack (acute lethality and irritation/sensitization studies), which is the purview of a different committee within OPP. HASPOC follows a specific approach outlined in guidance developed for waiving repeat dose studies (USEPA-OPP, 2013b). This guidance entitled Part 158 Toxicology Data Requirements: Guidance for Neurotoxicity Battery, Subchronic Inhalation, Subchronic Dermal and Immunotoxicity Studies describes a weight-of-the-evidence (WOE) approach to waiving four study types that considers physical-chemical properties, the exposure and toxicology profile, and the pesticidal and mammalian mode of action. HASPOC applies this same general WOE approach when considering the need for multiple other guideline studies including, but not limited to, developmental and reproduction studies, as well as chronic and subchronic oral studies. This paper describes EPA-OPP's systematic WOE approach, as applied by HASPOC, in considering the need for guideline animal studies for pesticide regulatory purposes.
2. Materials and methods
EPA-OPP established the Hazard and Science Policy Council (HASPOC) to serve as the central forum for evaluation of human health hazard data waivers. In addition, the HASPOC provides input in developing related overarching risk assessment guidance, and also makes recommendations on the implementation, harmonization, and consistent use of multiple data sources and policies in human health risk assessment. The members of the HASPOC are selected from OPP with representation from across the office focusing on the divisions that conduct human health hazard and risk assessments. These include the Health Effects Division, the Antimicrobials Division, and the Biopesticides and Pollution Prevention Division. Members have appropriate experience in risk assessment and the underlying technical disciplines which support it, as well as experience in supporting regulatory decision-making. The committee is primarily comprised of senior toxicologists and exposure scientists across the relevant divisions.
HASPOC's primary responsibility is the consideration of data waivers and the policies associated with evaluating them. EPA data requirements are listed under Section 40 of the Code of Federal Regulations Part 158 (40 CFR Part 158). This section includes data requirements for conventional, biochemical and antimicrobial pesticides. The regulations do allow for flexibility in these data requirements under Part 158.30, which states that EPA has the authority to establish or modify data needs for individual pesticide chemicals. OPP has a long history of practicing flexibility in implementing Part 158 data requirements. Hence, the data required may be modified on a case-by-case basis to fully characterize the use, properties, characteristics, or effects of specific pesticide products under review.
Part 158.45 specifically allows for the waiver of data requirements; the Agency can waive data requirements it finds are not necessary, while still ensuring that sufficient data are available to make a determination required by the applicable statutory standards. Conversely, there may be occasions where the core guideline requirements may not be sufficient to permit EPA to evaluate the potential of a product to cause unreasonable adverse effects to humans or the environment. Under those circumstances, as determined by the Agency, EPA may require the submission of additional data or information beyond that specified in 40 CFR Part 158. Part 158.75 allows for EPA to request additional data (or alternative approaches) identified as important to the risk management decision.
2.1. Weight of evidence (WOE) approach
When considering data waivers, the HASPOC relies on two OPP guidance documents: (1) Guiding Principles for Data Requirements and (2) Part 158 Toxicology Data Requirements: Guidance for Neurotoxicity Battery, Subchronic Inhalation, Subchronic Dermal and Immunotoxicity Studies (USEPA-OPP, 2013a; USEPA-OPP, 2013b). These documents provide scientific and policy principles associated with evaluating data waivers using a WOE framework. The Guiding Principles document describes the importance of only requiring data that inform regulatory decision-making and avoiding unnecessary use of time and resources, data generation costs, and animal testing. The Part 158 Toxicology Data Requirements document describes a WOE approach that considers hazard considerations when evaluating data waiver requests. If a waiver cannot be granted, this document also provides guidance on retaining a database uncertainty factor until the study is conducted and/or other information is used to fill the data gap. If a waiver is granted, a database uncertainty factor is not needed. While this guidance document is specific to only a subset of toxicology studies, HASPOC still has flexibility to waive other guideline and non-guideline studies such as developmental, reproductive, developmental neurotoxicity, chronic/carcinogenicity toxicity, and comparative thyroid assays (CTAs).
As noted above, the HASPOC committee is comprised of both toxicologists and exposure scientists, as the decision-making process considers both hazard and exposure, and is a risk-based approach. The WOE approach for waivers includes specific considerations such as: (1) physical/chemical properties; (2) use and exposure patterns; (3) hazard characterization such as the toxicity profile, information on mode of action or adverse outcome pathways, and other pesticides in the class; and (4) risk assessment implications. Examples of application of this WOE approach are described in Table 1. Since this approach is a risk-based approach, both exposure and toxicity information are considered. The non-dietary and dietary assessments represent risk after considering the hazard and exposure of the active ingredient.
Table 1.
Examples of application of the WOE approach in HASPOC's decision-making process – considerations for a subchronic inhalation study waiver.
| Pesticide | Chemical A | Chemical B | |
|---|---|---|---|
| WOE considerations | Toxicity Profile | Low acute inhalation toxicity (no pulmonary effects up to limit concentration) | Acute inhalation toxicity studies showed pulmonary lesions |
| Physical/chemical properties | Low vapor pressure and Henry's Law constant-not expected to volatilize | Low vapor pressure and Henry's Law constant-not expected to volatilize | |
| Use and exposure patterns | Potential for short- and intermediate-term occupational inhalation exposure | Potential for short- and intermediate-term occupational and short-term residential inhalation exposure | |
| Margins of Exposure (MOEs) | Inhalation MOEs: 13,000–25,000,000 (> 10X the level of concern [LOC] of 100) using an inhalation point of departure (POD) selected from an oral study | Inhalation MOEs: 130–28,000 (4 scenarios below 10X the LOC) using an inhalation POD selected from an oral study | |
| Information from chemical class or structurally-related pesticides | No structurally related pesticides with inhalation information identified | No structurally related pesticides with inhalation information identified | |
| Risk assessment implications | An inhalation study is not expected to provide PODs to change the overall risk picture | An inhalation study may provide more sensitive PODs for risk assessment than the oral studies | |
| HASPOC Recommendation | Inhalation study can be waived | Inhalation study is needed |
In the absence of a study, the HASPOC will use the WOE approach noted above. When considering exposure and risk implications, the HASPOC relies on a threshold for requiring data. Exposure and risk estimates are presented that are reflective of the use patterns most likely to results in risks of concern for the chemical under review. For non-dietary exposures, Margins of Exposure (MOEs) are used to express the risk estimate. The MOE is the ratio of the toxicity point of departure (POD, mg/kg/day) to the estimated exposure (mg/kg/day) based on the intended use of the pesticide. The MOE is then compared to a regulatory level of concern (LOC) which is a combined uncertainty factor used for risk assessment/regulatory purposes. For a complete database, the LOC is generally 100 and consists of 10X factors for interspecies and intraspecies extrapolation. For HASPOC waiver considerations, the threshold for MOEs is typically 10X times the LOC. The higher threshold that HASPOC uses for the purpose of granting a waiver is intended to account for the additional uncertainty associated with not having a specific toxicity study (e.g., use of an oral study for inhalation risk assessment which requires route-to-route extrapolation). In addition to MOEs (used to express non-dietary risk estimates), other risk metrics (such as cancer risk) are used as necessary.
Animal study waiver requests can be submitted to the HASPOC by the registrant or by the OPP review team at any point it is deemed applicable, either during the submission of a new pesticide registration package or during Registration Review (15-year review cycle for pesticides already in the market). However, most of the study waiver requests received by HASPOC are initiated by the OPP review teams. Also, OPP review teams are responsible for the waiver request presentation to HASPOC irrespective of who submits the request. Registrants are not present during the HASPOC deliberations.
As noted above, waiver submissions may occur at different stages of risk assessment for any chemical. Furthermore, some chemicals may come to HASPOC several times for various studies as the exposure and hazard may change over time as information is gathered. Therefore, it is important to note that the number of waivers presented here is not a 1:1 ratio with chemicals. For example, a particular chemical may come to HASPOC for evaluation of multiple waivers including subchronic inhalation, immunotoxicity, and acute neurotoxicity. Alternatively, a chemical may come to HASPOC at the beginning of Registration Review, and the HASPOC may recommend for the requirement of a subchronic inhalation study based on the WOE at that time. However, after gaining additional information (e.g., specific use information), the agency may be able to refine the exposure scenarios such that the original WOE is no longer supported and the HASPOC would then recommend to waive the subchronic inhalation study.
When making decisions, the HASPOC relies primarily on the information presented by the lead scientist for the chemical or policy under review, and any associated supporting materials, in combination with deliberations of its members. HASPOC decisions are made using a majority rule approach, and achieving consensus is optimal. Decisions and recommendations of the HASPOC are based on WOE considerations, as described in Table 1. Recommendations are captured in a final memorandum produced on a particular topic or for a particular chemical and are publicly available in registration dockets.
2.2. Calculations
Since 2012, the number and type of data waivers granted for each chemical has been tracked. This allows for the calculation of savings with respect to animals as well as cost both in terms of the regulated community and EPA contractor review costs.
To calculate the number of animals saved, the number of animals per study, as specified in the OCSPP study guidelines (USEPA, 2019), was multiplied by the number of waivers granted by study type. For non-guideline studies, the average number of animals used in submitted studies was used. To calculate the study cost savings to the registrant, the average study cost as reported by EPA's Biological and Economic Analysis Division in 2018 (USEPA-OPP, 2018) was multiplied by the number of waivers granted by study type. To calculate contractor cost savings to EPA, the flat rate charged to EPA for the creation of data evaluation records (DERs) for each study was multiplied by the number of waivers granted by study type. This assumes that every study not waived would be conducted and a DER created by the contractor. These estimates are considered conservative and likely underestimate total study costs and animal savings because they do not take into account the costs and animals associated with preliminary studies often conducted prior to the full guideline studies or costs associated with EPA secondary review of DERs.
3. Results
3.1. Compilation and analysis of HASPOC study waiver requests
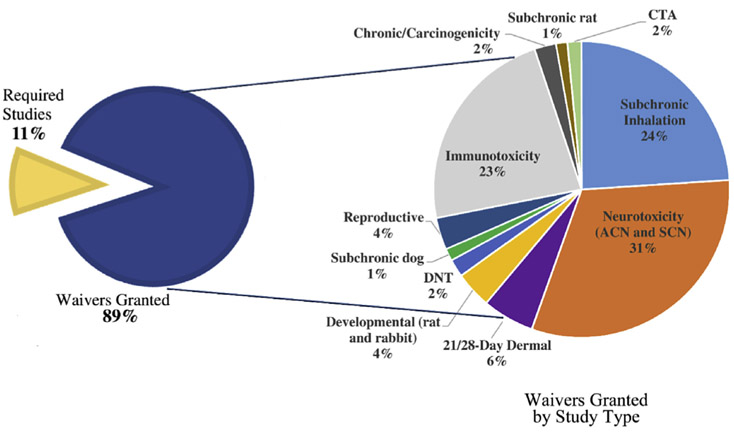
Between 2012 and May 2018, HASPOC received 1095 requests to waive animal studies for pesticides. These studies were either a standard requirement by law for the registration of pesticides as warranted by the 40 CFR Part 158, or conditionally required based on the toxicity and/or exposure profile of a chemical (such as the subchronic inhalation toxicity study and the comparative thyroid assay [CTA]). Of the 1095 requests received, HASPOC recommended that the study requirement be waived in 89% of the cases, with only 11% of the cases resulting in the study requirement remaining in place (Fig. 1). The most common reasons for waiver requests being denied included MOEs that were below 10X the LOC and insufficient information to address potential lifestage susceptibility (for example, if a chemical only has information on adult animals, a developmental toxicity study is less likely to be waived). Most of the waivers granted were for the neurotoxicity battery [Acute neurotoxicity (ACN) and subchronic neurotoxicity (SCN)] (31%), subchronic inhalation (24%) and immunotoxicity (23%) studies (Fig. 1). These four studies are also the most commonly requested to be waived, with the neurotoxicity battery studies alone comprising 330 of the waiver requests presented to HASPOC (Appendix 1).
Fig. 1. Decisions for animal study waiver requests presented to HASPOC.
Left: percentage of waivers granted or denied (i.e. required studies) by HASPOC using the WOE approach. Right: breakdown of waivers granted by study type.
3.2. Animal savings based on waived studies
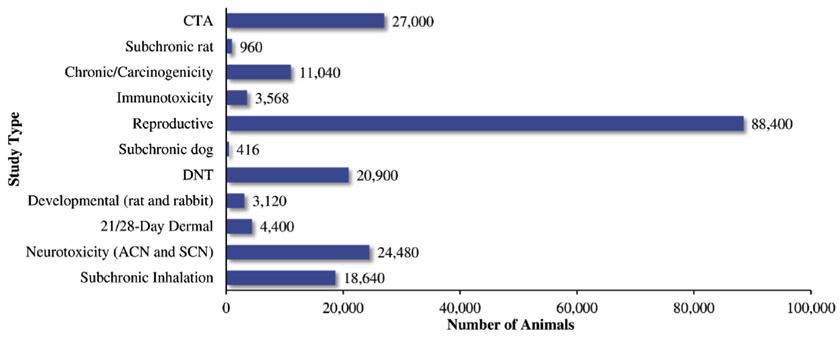
Between 2012 and May 2018, recommendations from HASPOC to waive the requirement to perform animal studies to assess pesticide toxicity have saved the lives of an estimated 202,924 research animals, including rats, rabbits, mice and dogs. The most commonly waived studies ( neurotoxicity battery) saved an estimated 24,800 animals (Fig. 2). Interestingly, although the reproductive toxicity studies accounted for only 4% of all studies waived (Fig. 1), this study type utilizes the largest number of animals per study (Appendix 1) and as such, the most animals were saved by waiving the reproductive toxicity studies (Fig. 2).
Fig. 2.
Number of animals saved based on the type of studies recommended to be waived by HASPOC.
3.3. Study cost savings to the regulated community based on waived studies
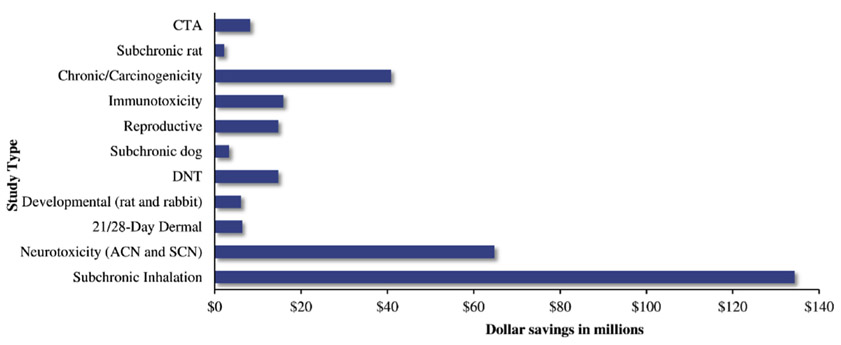
Between 2012 and May 2018, recommendations from HASPOC to waive animal study requirements have saved an estimated $317,067,300 in study costs to the regulated community. This estimate is based on the average cost of a main study for each study type and does not include the cost savings for potential preliminary studies that may be performed to determine adequate dosing, or particle size, as is often the case with inhalation studies. As shown in Fig. 3, the largest study cost savings were from waiving the subchronic inhalation study. This was due to the subchronic inhalation study being the third most expensive study to perform (average $587,500 per study, Appendix 1) and the subchronic inhalation study being one of the most commonly waived studies (24% of all waivers granted, Fig. 1). Not surprisingly, a large contribution to study cost savings also came from the neurotoxicity studies (the most commonly waived studies, Fig. 1) and the chronic/carcinogenicity studies (the most expensive study to perform at $1.8 million per study, Appendix 1).
Fig. 3. Savings to the regulated community in study costs.
Monetary amounts are in US dollars.
3.4. Contractor cost savings to EPA based on waived studies
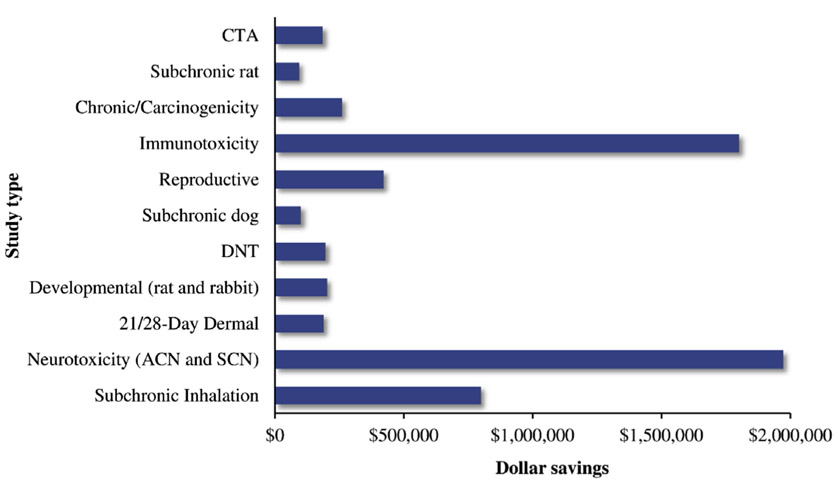
Due to the large volume of studies submitted for pesticide registration, EPA often employs contractors to create first drafts of data evaluation records (DERs) for each individual study, which are then reviewed by EPA scientists and incorporated into risk assessments. Contractors charge a flat rate to EPA for drafting DERs, based on the study type. Through May 2018, the waiving of study requirements by HASPOC saved EPA an estimated $6,215,014 in contractor costs for drafting DERs. The largest savings in contractor costs were from waiving the neurotoxicity, immunotoxicity and subchronic inhalation studies (Fig. 4).
Fig. 4. Savings to EPA in contractor costs.
Monetary amounts are in US dollars.
4. Discussion and conclusions
The HASPOC's WOE approach, together with the multidisciplinary makeup of the council, allows for the integration of available toxicity and exposure information for a pesticide to make data need determinations that are scientifically sound and protective of human health. When looking at the large percentage of studies waived (89%) by HASPOC, it is worth noting that specific considerations for each study type may result in certain study waiver requests being presented and waived more or less often than others. For example, waivers are most commonly requested and granted for the neurotoxicity studies (31%, Fig. 1). This may be because information from the chemical class and the available chemical-specific toxicity database may be sufficient to determine that neurotoxicity studies would not provide the most sensitive endpoint for risk assessment. Furthermore, even for pesticides that are known neurotoxicants, such as the organophosphates, clinical signs of neurotoxicity may not be the most sensitive effect, so a special study such as the comparative cholinesterase assay may be recommended instead of the neurotoxicity studies. In contrast, few waivers are requested and granted (4%) for the developmental and reproductive studies because OPP often relies on these studies to assess potential life stage susceptibilities. However, even these studies may be waived in specific situations, such as when there is little potential for exposure (e.g. in the case of pesticides manufactured with tamper-proof packaging).
With close to 1000 study waivers granted and an estimate of over 200,000 research animals saved, the HASPOC plays an important role in reducing the number of animals used for pesticide toxicity testing. The HASPOC has also contributed to replacing animal studies by recommending, for example, that a pharmacokinetic study be performed in lieu of a toxicity study in several cases. Furthermore, the HASPOC routinely considers ways to refine animal testing by recommending that studies of shorter duration or with a single gender be performed, whenever feasible.
The waiving of animal toxicity study requirements can also be quantified in its monetary value. In fact, the millions of dollars saved to both the regulated industry and to EPA are staggering. Although the dollar savings to EPA were measured only in contractor costs for the drafting of study DERs, there are many additional steps in which resources may be saved by the agency but for which a specific monetary value is difficult to ascertain. For example, when an animal study is not performed, a few of the additional tasks for which EPA saves time and employee hours may include: reviewing proposed study protocols, meeting with registrants when questions/issues arise for a particular study, processing of submitted studies, reviewing draft DERs, and incorporating study information into the risk assessment. Thus, the true cost savings to EPA are likely substantially underestimated here.
In conclusion, the establishment of the HASPOC has allowed OPP to increase harmonization across different divisions, and consistently integrate toxicity, exposure and other information in determining data needs for pesticides. The HASPOC has also built efficiencies into the risk assessment process while protecting human health, since less studies submitted means less resources spent and a better focus on the most important issues identified for each risk assessment. Furthermore, with EPA's renewed interest in implementing the 3Rs of animal testing (reduce, replace and refine), the HASPOC is at the forefront of accomplishing scientific and regulatory goals for this implementation in the pesticide field.
Supplementary Material
Acknowledgments
Funding
This work was conducted in the course of the authors’ employment at the United States Environmental Protection Agency.
Abbreviations:
- HASPOC
Hazard and Science Policy Council
- OPP
Office of Pesticide Programs
- OCSPP
Office of Chemical Safety and Pollution Prevention
- FIFRA
Federal Insecticide Fungicide and Rodenticide Act
- NRC
National Research Council
- CFR
Code of Federal Regulations
- WOE
Weight of Evidence
- CTA
Comparative Thyroid Assay
- NGO
non-governmental organizations
- NAM
New Approach Methodology
- MOE
Margin of Exposure
- LOC
Level of Concern
- POD
Point of Departure
- DER
Data Evaluation Record
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yrtph.2019.104481.
References
- USEPA-OPP, 2013a. In: Guiding Principles for Data Requirements, . https://www.epa.gov/sites/production/files/2016-01/documents/data-require-guide-principle.pdf.
- USEPA-OPP, 2013b. In: Part158 Toxicology Data Requirements: Guidance for Neurotoxicity Battery, Subchronic Inhalation, Subchronic Dermal and Immunotoxicity Studies, . https://www.epa.gov/sites/production/files/2014-02/documents/part158-tox-data-requirement.pdf.
- USEPA-OPP, 2018. In: Cost Estimates of Studies Required for Pesticide Registration, . https://www.epa.gov/pesticide-registration/cost-estimates-studies-required-pesticide-registration.
- USEPA, 2019. In: Health Effects Test Guidelines, . https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-870-health-effects-test-guidelines.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.