Abstract
Brain aging is accompanied by an accumulation of damaged proteins, which results from deterioration of cellular quality control mechanisms and decreased protein degradation. The ubiquitin-proteasome system (UPS) is the primary proteolytic mechanism responsible for targeted degradation. Recent work has established a critical role of the UPS in memory and synaptic plasticity, but the role of the UPS in age-related cognitive decline remains poorly understood. Here we measured markers of UPS function and related them to fear memory in rats. Our results show that age-related memory deficits are associated with reductions in phosphorylation of the Rpt6 proteasome regulatory subunit and corresponding increases in lysine-48 (K48)-linked ubiquitin tagging within the basolateral amygdala. Increases in K48 polyubiquitination were also observed in the medial prefrontal cortex and dorsal hippocampus. These data suggest that protein degradation is a critical component of age-related memory deficits. This extends our understanding of the relationship between the UPS, aging, and memory, which is an important step toward the prevention and treatment of deficits associated with normal cognitive aging and memory-related neurodegenerative diseases.
Keywords: Ubiquitin-proteasome system, protein degradation, trace fear conditioning, aging, amygdala, hippocampus, prefrontal cortex
1. Introduction
Even in the absence of brain disease, the gradual decline of cognitive ability with age is a growing problem. Age-related cognitive deficits exist on a continuum, wherein some individuals may develop pathologies such as Alzheimer’s or Parkinson’s disease, and other individuals do not display significant age-related deficits in cognitive ability (Rowe and Kahn, 1987). On the other hand, most individuals age somewhere between these two extremes of pathological and successful aging, in a pattern termed normal cognitive aging (Roberson et al., 2012). The proportion of the U.S. population aged over 65 years is projected to more than double by the year 2050, and one in every five individuals will be classified as aged (Ortman et al., 2014). Thus, the negative consequences of normal brain aging remain a major challenge to modern neuroscience, while the neurobiology underlying age-related cognitive decline remains largely unknown.
Aging is associated with a variety of biological changes at the cellular level which can be observed in gene expression, intracellular signaling, and metabolism. One critical component of the cellular aging process is a gradual decline in protein homeostasis, which is accompanied by an age-related decrease in function of the ubiquitin-proteasome system (UPS). The UPS is a major regulatory pathway that is responsible for the recognition and clearance of abnormal or damaged proteins, as well as regulation of short-lived proteins in response to intra- and extra-cellular signals (Jarome and Helmstetter, 2013). The UPS works through the attachment of polyubiquitin chains to target proteins, and these chains serve as a signal for degradation and proteolysis of the target by proteasomes. Ubiquitin ligases tag proteins for degradation by attaching polyubiquitin chains that identify them for processing by the 26S proteasome. The 26S complex consists of a 20S catalytic core and two 19S regulatory particles, which in turn contain subunits that either recognize appropriate polyubiquitinated targets or regulate the catalytic activity of the 20S core (Wang et al., 2005). Significantly, dysfunctions in the UPS are associated with neurodegenerative diseases such as Alzheimer’s (Oddo, 2008) and Parkinson’s disease (Betarbet et al., 2005). For example, one study demonstrated a decrease in proteasome activity in the hippocampus of Alzheimer’s disease brains compared to controls (Keller et al., 2000). To further illustrate, 20S proteasome enzymatic activity is reduced in the substantia nigra pars compacta in subjects with sporadic Parkinson’s disease (McNaught et al., 2003).
Our lab has argued for the importance of the UPS for the regulation of synaptic plasticity and memory. For example, UPS protein degradation is critical for the formation and stability of fear memories within the basolateral amygdala (BLA) (Jarome et al., 2011). Furthermore, memory formation for trace fear conditioning (TFC) requires UPS mediated protein degradation within the prelimbic (PL) subdivision of the medial prefrontal cortex (mPFC) (Reis et al., 2013). TFC is a variation on standard delay fear conditioning in which a stimulus free interval is inserted between the conditioned stimulus (CS) termination and onset of the unconditioned stimulus (UCS). TFC is also unique because it engages a brain network distinct from delay conditioning. For instance, TFC requires the dorsal hippocampus (DH) and mPFC, while delay conditioning with the same stimuli does not (Esclassan et al., 2009; Gilmartin and Helmstetter, 2010). Interestingly, while delay fear conditioning remains intact throughout the lifespan, aged rats show deficits in trace memory during testing (Moyer and Brown, 2006).
In the present study we examined whether age-related deficits in TFC memory are associated with changes in plasticity-related protein degradation processes, particularly in regard to the UPS. To these ends, we quantified the relative phosphorylation of Rpt6 and accumulation of lysine-48 (K48)-linked polyubiquitinated proteins. Rpt6 is an ATPase subunit in the 19S regulatory particle of the proteasome, and is phosphorylated at Serine-120 (S120) by kinase Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) (Djakovic et al., 2009). Additionally, CaMKII-dependent phosphorylation of Rpt6 at S120 regulates synaptic strength in hippocampal neurons (Djakovic et al., 2012). Research from our lab demonstrates that CaMKII regulates Rpt6 phosphorylation and proteasome activity during the formation of a long-term fear memory (Jarome et al., 2013), while inhibition of CaMKII prevents retrieval-induced increases in proteasome activity and Rpt6 phosphorylation in the amygdala (Jarome et al., 2016). We also quantified K48 polyubiquitination. There are several lysine sites on ubiquitin in which polyubiquitin chains can form, but lysine-48 (K48) linkage is only associated with the proteasome. Thus, K48 polyubiquitin chains are degradation specific and serve as an indicator of ubiquitin tagging and protein turnover. Additionally, K48 polyubiquitination increases following memory retrieval (Jarome et al., 2016; Jarome et al., 2013). Further research demonstrates that K48 specifically increases in the cytoplasmic and nuclear components of the cell during context fear conditioning consolidation, while it increases in the synaptic region during reconsolidation (Orsi et al., 2019). In order to understand if protein degradation processes are an important component of age-related memory impairments, we need more information about how such age-related alterations relate to these established mechanisms. In the present study we sought to determine if age-related changes in UPS function within key brain areas known to be important for memory would reflect predicted behavioral impairments. If UPS function is specifically compromised as a function of age within one or more of the brain regions previously implicated in the retrieval of fear memory, we would expect to see a decrement in behavioral performance as a result.
2. Methods
2.1. Animals and housing conditions
Subjects were male Fisher 344 (F344) rats obtained from the National Institute on Aging (NIA) colony at Charles River (Raleigh, NC) at the ages of 3-, 15-, and 22-mo old at the time of delivery. Rats were individually housed with ad libitum access to water and rat chow. The animal colony was maintained at a 14:10-h light–dark cycle with all experiments occurring under the light portion of the cycle. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Milwaukee and conducted within the ethical guidelines of the National Institutes of Health (NIH)
2.2. Conditioning Apparatus
Fear conditioning was conducted in a set of four Plexiglas and stainless steel chambers within sound-attenuating boxes, as described previously (Ferrara et al., 2017). Briefly, the floor included 18 stainless steel bars connected to a shock generator (Coulbourn Instruments). Each chamber had a speaker to allow delivery of white noise cues, overhead illumination with a 7.5W bulb, and ventilation fans to provide a constant background noise (55 dB). The chambers were cleaned with 5% ammonium hydroxide solution between sets of rats (Context A). A set of similar chambers (Context B) served as a shifted context for auditory CS testing. There were several distinct features associated with Context B, including textured Plexiglas flooring, infrared illumination, and 5% acetic acid cleaning solution.
2.3. Trace Fear Conditioning Procedures
All animals were handled for three days prior to behavioral manipulation. This consisted of transport to the behavior room and gentle restraint in a towel. TFC training was conducted in Context A, while auditory CS testing (retrieval) was conducted in Context B. All animals were trained in TFC on Day 1 with 10 CS-UCS pairings after a 2 min baseline (BL). The CS was a 10 s white noise cue (72 dB) and the UCS was a 1 s electric footshock (1 mA). The CS and UCS were separated by a 30 s trace interval (TI), and CS-UCS pairings were separated by a variable intertrial interval (ITI) of 5.25 ± 0.5 min. One day following conditioning, rats received a long-term memory test consisting of two 15 s CS presentations following a 2 min BL period. The two CSs were separated by an ITI of 175 s, and the second CS was followed by a 2 min post-CS period. This ITI and post-CS period were combined for behavioral analysis and are referred to as the stimulus free period (SFP) during retrieval testing (Gilmartin et al., 2012; Kwapis et al., 2011).
2.4. Behavioral Scoring
Freezing behavior is defined as the cessation of all movement excluding respiration (Fanselow, 1980) and was automatically scored in real-time with FreezeScan 1.0 detection software (Clever Sys, Inc.) calibrated to a trained human observer.
2.5. Crude Synaptosomal Membrane Fractionation
Animals were sacrificed with an overdose of isoflurane 90 minutes following memory testing. Brains were rapidly removed and flash frozen on dry ice. Using a rat brain matrix (Harvard Apparatus) on dry ice, the BLA, mPFC and DH were dissected from the brains. Synaptosomal membrane fractions were obtained using methods previously described (Jarome et al., 2011) with minor alterations noted below. Tissue samples were homogenized in TEVP buffer with 320 mM Sucrose and centrifuged at 1000 x g for 10-minutes at 4°C. The supernatant was collected and spun at 10,000 x g for 10-minutes at 4°C. The resulting pellet containing the synaptosomal fraction was resuspended in phospho-homogenization buffer (50 mM Tris-HCl, 6 mM sodium deoxycholate, 150 mM NaCl, 1mM NaF, two mini EDTA-free complete protease inhibitor tablets (Roche), 0.1% SDS, 1 mM sodium orthovanadate) and measured using a 660nm protein assay (Pierce).
2.6. Western Blot Method
Following synaptosomal preparation, protein levels were normalized and were loaded into a 7.5% SDS/PAGE gel and then transferred to PVDF membranes using a Turbo Transfer System (BioRad). Membranes were incubated in 5% milk in Tris-buffered saline (TBS) + 0.1% Tween-20 (blocking buffer) for 1 h before being incubated in primary antibody solutions for phosphorylated Rpt6-Serine120 (pRpt6; ProSci, 1:850), total Rpt6 (tRpt6; Enzo Life Sciences, 1:825), K48 polyubiquitin (Cell Signaling, 1:500), or actin (Cell Signaling, 1:1,000) and 3% bovine serum albumen in TBS + 0.1% Tween-20 overnight at 4°C. Membranes were then incubated in the appropriate secondary antibody (1:20,000) in blocking buffer for 60 min. Following a final wash, membranes were prepped in a chemiluminescence solution for 3 min. Images were captured and densitometry performed using NIH Genesys. The phosphorylated Rpt6-Serine120 rabbit polyclonal antibody was generated commercially (ProSci) against a synthetic peptide [NH2-CALRND(pS)YTLHK-OH] as described previously (Djakovic et al., 2012; Jarome et al., 2013).
2.7. Data Analysis
A two-way repeated-measures ANOVA was used to compare mean percent time freezing during training and a one-way ANOVA was used to examine the change from baseline freezing during the SFP and CS periods during the retrieval test session. These were followed by Fisher’s least significant differences (LSD) post hoc tests. One-way ANOVAs followed by Fisher’s LSD post hoc tests were also used to compare mean optical density across groups for proteins of interest using western blots. Normalized western blot samples are expressed as a percentage of TFC-trained 3-mo old animals. Statistical outliers were screened according to the methods outlined in Field (2005). The data presented in this paper excludes, in total, one outlier from the 22-mo old condition. This 22-mo old animal was an outlier on two measures, BLA-pRpt6 (Z = 2.345) and BLA-K48 (Z = 2.078). Thus, this one animal was removed from all subsequent analyses, resulting in final sample sizes of n = 10, 9, and 9 (3-, 15- and 22-mo old animals, respectively). Statistical significance was defined as p < 0.05. Data are presented as mean ± standard error of the mean (SEM).
3. Results
3.1. Aging Results in Deficits in Trace Fear Conditioning
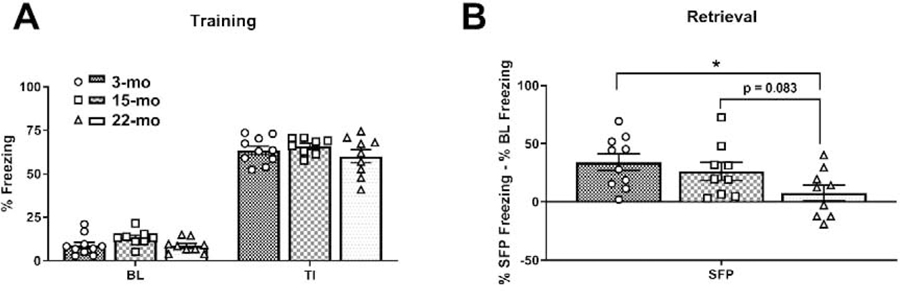
Previous work has demonstrated that aged rats have deficits in trace fear conditioning (Moyer and Brown, 2006). In the present study, we aimed to replicate and extend these results. We first found that 3-, 15-, and 22-mo old F344 rats perform equivalently during training (Fig. 1A) showing normal reactions to footshock. Specifically, we found a significant main effect of time (F(1, 25) = 1059, p < 0.0001), a trend towards a main effect of age (F(2, 25) = 2.824, p = 0.0784) and no significant interaction (F(2, 25) = 0.3279, p = 0.7235). To test the long-term retention of fear memory, animals received a retrieval session in a shifted context 24 hr after training. We quantified behavioral performance at retrieval by calculating the difference between each animal’s baseline freezing at retrieval and the mean of that animal’s SFP freezing (i.e. the ITI and post-CS period, 295 seconds total) to better account for individual differences in baseline values (Lattal, 1999). Here we found a significant overall effect of age (F(2, 25) = 3.615, p = 0.0418). Importantly, in post hoc comparisons the 3- and 15-mo old animals froze more during the SFP than 22-mo old animals (p = 0.0142 and p = 0.0831, respectively; Fig. 1B). SFP freezing in TFC-trained rats reflects a conditional response to the offset itself and thus can be considered a conditional response to the CS (Gilmartin et al., 2012). However, as a secondary measure also analyzed the CS freezing as a difference from the baseline freezing (mean percent CS freezing – mean percent BL freezing), but we did not observe any significant differences (F(2, 25) = 0.5827, p = 0.5658). Together, these results suggest that aging results in a deficit in long term memory for TFC, specifically when one looks at the SFP period.
Figure 1. Aging results in deficits in trace fear conditioning memory retrieval.
(A) During training, all animals (3-mo [n = 10], 15-mo old rats [n = 9] and 22-mo old rats [n = 9]) froze equivalently at BL and during the TIs. (B) However, during retrieval testing 24 hr later, significant age-related deficits in long term memory were noted. Specifically, the SFP period, 22-mo old rats froze significantly less than 3-mo old rats and tended to freeze less than 15-mo old rats. Data are presented as mean ± SEM. * denotes p < 0.05 in a Fisher’s LSD post hoc test.
3.2. Aging is Associated with Changes in Protein Degradation Processes
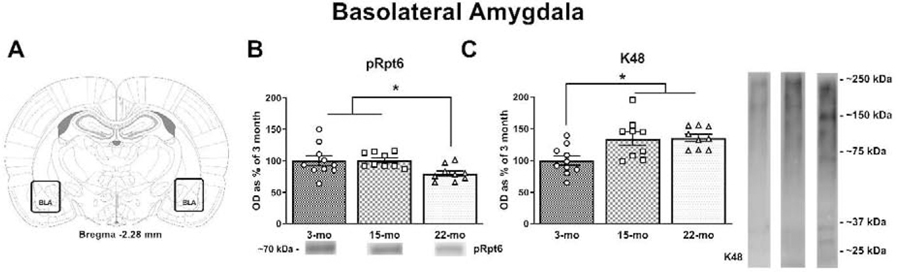
Next we identified how memory-driven UPS signaling changes with age in several brain regions critical for TFC, including the BLA, mPFC and DH. Prior work shows that the acquisition or retrieval of fear memory increases UPS-related signaling at amygdala synapses (Jarome et al., 2011; Orsi et al., 2019). Through western blot analysis of BLA tissue (Fig. 2A), we quantified the phosphorylation of proteasome regulatory subunit Rpt6 at Serine-120, a site known to be critical for the regulation of increases in proteasome activity and activity-dependent changes in synaptic strength (Djakovic et al., 2012). We found that age was associated with a significant difference in phosphorylated Rpt6 (pRpt6) protein expression (Fig. 2B; F(2, 25) = 4.024, p = 0.0305). More specifically, 3-mo old animals and 15-mo old animals displayed higher levels of pRpt6 compared to 22-mo old animals (p = 0.0218 and p = 0.0199, respectively). However, tRpt6 did not differ across age groups (F(2, 25) = 0.7646, p = 0.4761) supporting the lack of an age-related difference in the total number of proteasomes present. We also performed western blots for a lysine-48 (K48) polyubiquitin tag that targets proteins for degradation by the proteasome (Jarome et al., 2016; Jarome et al., 2013). We found that K48 protein levels were significantly altered by age (Fig. 2C; F(2, 25) = 6.599, p = 0.0050). Specifically, both 15- and 22-mo old rats had significantly higher K48 protein levels compared to 3-mo old rats (p = 0.0052 and p = 0.0040, respectively). Finally, actin levels displayed no significant differences (F(2, 25) = 0.8937, p = 0.4218). Altogether these data suggest that activity-dependent protein degradation processes are impaired in the BLA, which is leading to an accumulation of ubiquitin-tagged proteins.
Figure 2. Aging is associated with changes in markers of protein degradation in the amygdala.
(A) Tissue was collected from the BLA (adapted from Paxinos & Watson, 2007). (B) 22-mo old rats displayed deficits in pRpt6 protein levels compared to 3- and 15-mo old animals. (C) 15- and 22-mo old rats also displayed significant increases in K48 compared to 3-mo old animals. Data are presented as mean ± SEM. * denotes p < 0.05 in a Fisher’s LSD post hoc test.
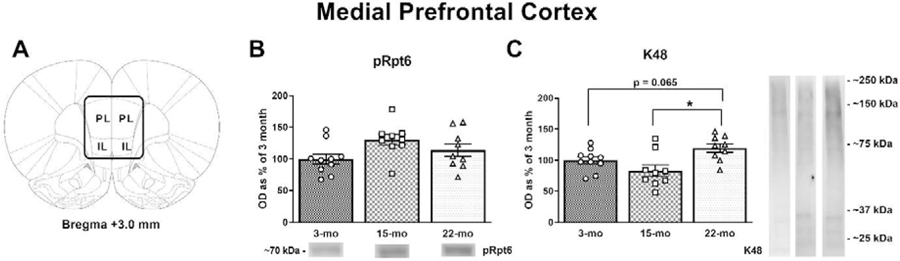
While plasticity in the BLA appears to be generally important for aversive learning, neurons in the mPFC and DH are specifically required when a trace interval intervenes between the CS and UCS (Esclassan et al., 2009; Gilmartin and Helmstetter, 2010). Research from our lab has also demonstrated a functional role for prefrontal UPS-mediated degradation in the consolidation of a TFC memory (Reis et al., 2013). Aging has also affects mPFC neuronal excitability and hinders the extinction of a TFC memory (Kaczorowski et al., 2012). Overall, these results suggest that the mPFC may likely show age-related declines in UPS activity; thus, we quantified the same proteins (as in the BLA) in tissue samples collected from the mPFC (Fig. 3A). We found no significant effect of pRpt6 (Fig. 3B; F(2, 25) = 2.951, p = 0.0707) and a tendency towards an effect of tRpt6 (F(2, 25) = 0.0709, p = 0.9318). We also analyzed K48 protein levels in the mPFC (Fig. 3C; F(2, 25) = 6.123, p = 0.0068) and found that 22-mo old animals display a tendency towards higher levels of K48 polyubiquitination compared to 3-mo old rats (p = 0.0654) and significantly higher levels compared to 15-mo old rats (p = 0.0018). Actin, on the other hand, displayed no significant differences (F(2, 25) = 1.27, p = 0.2984).
Figure 3. Aging is associated with increases in K48 in the medial prefrontal cortex.
(A) Tissue was collected from the mPFC (adapted from Paxinos & Watson, 2007). (B) No significant differences in pRpt6 were noted. (C) 22-mo old animals displayed a tendency towards increased levels of K48 compared to 3-mo old animals, significant increases in K48 compared to 15-mo old animals. Data are presented as mean ± SEM. * denotes p < 0.05 in a Fisher’s LSD post hoc test.
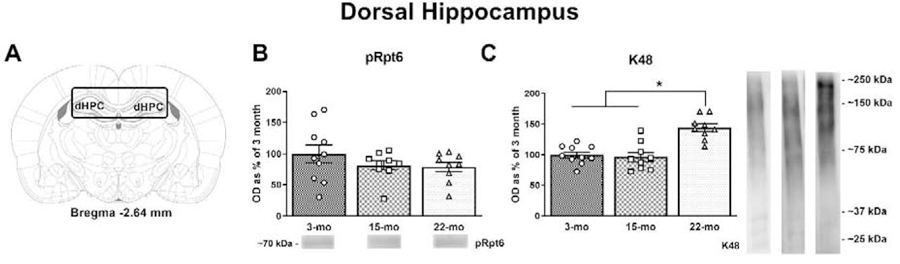
The DH is another brain region that is critical for learning the association between a CS and a UCS when a trace interval is interposed between the two stimuli; for instance, lesions of the DH impair trace eyeblink conditioning (Weiss et al., 1999). In another study, increases in DH CA1 pyramidal cell firing to the CS and UCS were diminished in aged animals that were unable to learn trace eyeblink conditioning, while these aged, learning-impaired animals also showed alterations in the coordinated firing of all excitatory and inhibitory pyramidal cell types within CA1 ensembles (McEchron et al., 2001). Intra-DH CA1 infusions of a proteasome inhibitor also blocks long-term memory formation following inhibitory avoidance training (Lopez-Salon et al., 2001). Therefore, we also used western blots to analyze tissue from the DH (Fig. 4A) for markers of UPS activity in young and aged animals. No differences were noted in pRpt6 (Fig. 4B; F(2, 25) = 1.164, p = 0.3285), while a trend towards a difference in tRpt6 was noted (F(2, 25) = 3.02, p = 0.0669). K48, on the other hand, did differ as a function of age (Fig. 4C; F(2, 25) = 17.72, p < 0.0001), and post-hoc analyses revealed that 22-mo old animals had significantly higher protein levels compared to 3- and 15- old animals (p’s < 0.0001). Actin, again, did not differ across age groups (F(2, 25) = 2.248, p = 0.1265).
Figure 4. Aging is associated with increases in K48 in the dorsal hippocampus.
(A) Tissue was collected from the DH (adapted from Paxinos & Watson 2007). (B) No differences were observed in pRpt6. (C) 22-mo old rats displayed significant increases in K48/Actin compared to 3- and 15-mo old rats. Data are presented as mean ± SEM. * denotes p < 0.05 in a Fisher’s LSD post hoc test.
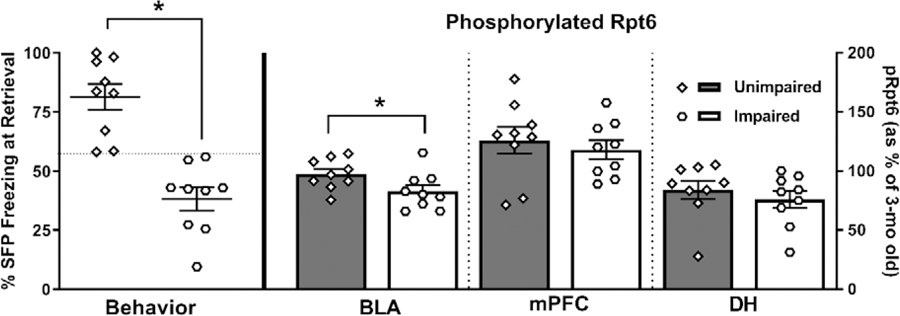
To better understand the role of phosphorylation of Rpt6 in TFC memory retrieval, we divided 15-mo and 22-mo old animals into ‘impaired’ and ‘unimpaired’ subgroups, as is commonly done in the field of aging and memory (Gallagher et al., 2015; Gazzaley et al., 2005; Le Jeune et al., 1996). We performed a median split using the percent time spent freezing during the SFP period at retrieval testing (57.08%) and classified those above the median as unimpaired and those below as impaired. We then analyzed pRpt6 protein expression in each brain region in relation to the presence of behavioral impairment (Fig. 5). Not surprisingly, during the SFP of the retrieval session, impaired animals froze significantly less than unimpaired animals (t(16) = 5.842, p < 0.0001). Importantly, in the BLA, impaired animals had significantly lower pRpt6 protein levels (t(16) = 2.167, p = 0.0457). We did not find any significant differences in the mPFC (t(16) = 0.576, p = 0.5727) or DH (t(16) = 0.7723, p = 0.4512) using this analysis.
Figure 5. Memory impaired rats display reductions in pRpt6 within the basolateral amygdala.
When 15- and 22-mo old rats were classified as impaired or unimpaired, not only did impaired animals display significantly less freezing during the SFP period of memory retrieval testing, but they also display significant reductions in pRpt6 in the BLA, but not in the mPFC or DH. Data are presented as mean ± SEM. * denotes p < 0.05 in a Student’s t-test.
4. Discussion
Here we provide new data supporting the idea that age-related memory deficits are associated with changes in brain protein degradation processes. This work is particularly novel and important because little research within the field of aging has focused on the amygdala as a potentially critical brain region underlying age-related memory deficits. We found that aging resulted in significant deficits in TFC memory retrieval and in changes in the expression of proteins associated with the UPS. Phosphorylation of Rpt6 at S120 is required for CaMKIIα-dependent stimulation of the proteasome (Djakovic et al., 2012; Djakovic et al., 2009), and we found that normal aging is associated with decreases in pRpt6 in the BLA. We also observed an increase in K48 linked protein in aged animals in the BLA, mPFC, and DH. This increase in polyubiquitinated protein suggests that there is an accumulation of tagged protein, potentially as a result of deficits in proteasome activity (Kim et al., 2011). Taken together these data suggest that aging results in deficits in UPS function which may be closely related to TFC memory retrieval impairments.
In the current study we chose to focus on TFC memory retrieval. Rats were sacrificed 90 min following retrieval since this time point shows maximal activation of the UPS after auditory fear memory retrieval (Jarome et al., 2011). However, within the dorsal hippocampus, previous studies have shown that maximal pRpt6 protein levels are sometimes observed within 0 to 30 min following context exposure (Cullen et al., 2017), and maximal polyubiquitination was observed 60 min following the retrieval of a context fear memory (Lee et al., 2008). Thus, our selection of a 90 min timepoint may have missed the maximal age-related differences in protein degradation processes in some brain regions. Another potential limitation in the current study is that we are unable to determine whether these differences are dependent on the act of memory retrieval per se, or if they reflect stable age-related baseline changes in UPS function. However, in one study of the aging brain, no significant differences were noted in baseline 20S proteasome activity within the hippocampus of young and old rats (Giannini et al., 2013). On the other hand in another study the basal level of polyubiquitinated proteins in the hippocampus was increased with age (Zeier et al., 2011). Thus, additional research would be necessary to determine baseline levels of Rpt6 phosphorylation across several brain regions.
Another caveat is that it is difficult to delineate whether aged rats fail to initially learn TFC or if they fail to consolidate the memory. During training, equivalent freezing across groups during the TI period seems to indicate that the animals learned something about the relationship between the CS and the trace interval and respond normally to shock. TI freezing at training can be compared to SFP freezing at retrieval. However, 24 hr after training when animals were tested for the long-term retention of that fear memory, aged animals froze less during the SFP period than their younger counterparts. SFP freezing is commonly used to measure learning of TFC, as it represents CS-induced fear to the trace interval and encompasses the time at which the TFC-trained animal would have received the footshock during training (Blum et al., 2006; Detert et al., 2008; Kwapis et al., 2011; Quinn et al., 2002; Yoon and Otto, 2007). Finally, the present study does not provide insight into sex-specific differences in protein degradation processes. Given the gender differences in age-related neurodegenerative conditions such as Alzheimer’s disease (Mielke et al., 2014) it is important that future studies directly consider sex as a biological variable.
While we observed a significant reduction in pRpt6 in the BLA of aged animals with a corresponding increase in K48 in the same region, we did not observe reductions in pRpt6 in the mPFC or DH (where increases in K48 were also noted). Although we analyzed the biochemistry of the BLA, mPFC and DH independently, it is important to keep in mind that these brain regions are likely acting as a circuit. Differences in pRpt6 in the BLA may have functional influences on K48 in other regions via a larger circuit interaction is consistent with a growing body of research that is characterizing TFC-related circuits. For example, we have previously demonstrated that functional disconnection of the PL with the BLA impairs the acquisition of TFC (Gilmartin et al., 2012). TFC memory formation is also prevented when the mPFC is silenced specifically during the TI using optogenetic inhibition (Gilmartin et al., 2013). However, circuit-related issues in aging remain relatively unexplored. This is an important area for future research.
Proteolytic signaling pathways are critical in synapse development, synaptic plasticity, and the maintenance of neuronal health (for reviews see Bingol and Sheng, 2011; Jarome and Helmstetter, 2013). Once activated, the UPS can potentially regulate a large number of downstream signaling pathways and synaptic plasticity processes. For instance, the transcription factor cAMP response element binding protein (CREB) is a downstream effector of proteasome activity (Ehlers, 2003). Overall, Ehlers (2003) demonstrated that activity regulates postsynaptic composition and signaling through the UPS, which may serve as a link between synaptic activity, protein turnover, and the reorganization of synapses. The results of the present study suggest that plasticity processes tied to the UPS change with age. UPS activity and protein clearance is impaired in the amygdala as observed through decreased phosphorylation of Rpt6 and increased K48 polyubiquitination. This aggregation of proteins in aged rats is further observed in the mPFC and DH. Importantly, when animals are classified as impaired or unimpaired based on behavioral performance, pRpt6 protein levels are correlated with the degree of impairment. One possible mechanism that may explain this correlation is a decrease in CaMKII activation in learning-impaired animals, as we have previously shown that CaMKII regulates the phosphorylation of Rpt6 and promotes memory destabilization following retrieval (Jarome et al., 2016). We have also shown that CaMKII regulates pRpt6 during the formation of a long-term memory (Jarome et al., 2013). Additionally, there is a growing body of evidence for a role for N-methyl-D-aspartate receptor (NMDA) receptor in this CaMKII-UPS pathway (Jarome et al., 2013; Jarome et al., 2011). Further, NMDA dysfunction has been linked to age-related oxidative stress. For instance, this age-related, oxidative stress-linked NDMA receptor hypoactivation has been observed in the hippocampus (Kumar and Foster, 2013), mPFC (Guidi et al., 2015), and amygdala (Zhan et al., 2018). Altogether, these changes in UPS-associated proteins may be responsible for age-related deficits in TFC memory, and these proteins could potentially serve as novel targets for the treatment and prevention of age-related cognitive decline.
5. Conclusions
In short, the current study provides new evidence that normal aging is associated with deficits in brain protein degradation processes. After confirming that aging results in significant deficits in TFC memory (see Moyer and Brown, 2006) we found that activity-dependent protein degradation processes are impaired, most notably in the BLA. Across several brain regions (BLA, mPFC, and DH) we also observed increases in accumulated proteins tagged for degradation with K48 polyubiquitination. Taken together, these data provide compelling evidence that aging results in changes in quality-control protein degradation processes, specifically with regard to the UPS. Future research aimed at investigating methods of rescuing proteasome activity to reinstate normal learning and memory in aged animals will have important implications for the treatment and prevention of cognitive decline and neurodegenerative pathologies such as Alzheimer’s disease.
Older rats show clear memory impairment when tested with trace fear conditioning.
In aged animals the activity-driven enhancement of UPS mediated protein degradation normally seen during memory retrieval was significantly attenuated in some brain areas.
The degree of behavioral impairment in middle aged and old rats was related to the degree of UPS dysfunction in the basolateral amygdala.
Acknowledgments
This work was supported by NIH grants AG042814 to J.R.M. and AG053854 and MH112141 to F.J.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare no competing financial interests.
BD, SP, JM and FH designed the study. BD, SP and PC collected the data. BD and FH analyzed the data.
BD, JM and FH wrote the manuscript.
References
- Betarbet R, Sherer TB, Greenamyre JT, 2005. Ubiquitin–proteasome system and Parkinson’s diseases. Experimental neurology 191, S17–S27. [DOI] [PubMed] [Google Scholar]
- Bingol B, Sheng M, 2011. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron 69(1), 22–32. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK, 2006. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport 17(3), 341–344. [DOI] [PubMed] [Google Scholar]
- Cullen PK, Ferrara NC, Pullins SE, Helmstetter FJ, 2017. Context memory formation requires activity-dependent protein degradation in the hippocampus. Learning & Memory 24(11), 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detert JA, Kampa ND, Moyer JR Jr, 2008. Differential effects of training intertrial interval on acquisition of trace and long-delay fear conditioning in rats. Behavioral neuroscience 122(6), 1318. [DOI] [PubMed] [Google Scholar]
- Djakovic SN, Marquez-Lona EM, Jakawich SK, Wright R, Chu C, Sutton MA, Patrick GN, 2012. Phosphorylation of Rpt6 regulates synaptic strength in hippocampal neurons. Journal of Neuroscience 32(15), 5126–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic SN, Schwarz LA, Barylko B, DeMartino GN, Patrick GN, 2009. Regulation of the proteasome by neuronal activity and CAMKII. Journal of Biological Chemistry, jbc. M109. 021956. [DOI] [PMC free article] [PubMed]
- Ehlers MD, 2003. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nature neuroscience 6(3), 231. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR, 2009. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus 19(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, 1980. Conditional and unconditional components of post-shock freezing. The Pavlovian journal of biological science 15(4), 177–182. [DOI] [PubMed] [Google Scholar]
- Ferrara NC, Cullen PK, Pullins SP, Rotondo EK, Helmstetter FJ, 2017. Input from the medial geniculate nucleus modulates amygdala encoding of fear memory discrimination. Learning & Memory 24(9), 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A, 2005. Discovering statistics using SPSS. Sage publications. [Google Scholar]
- Gallagher M, Burwell R, Burchinal M, 2015. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behavioral neuroscience 129(4), 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’esposito M, 2005. Top-down suppression deficit underlies working memory impairment in normal aging. Nature neuroscience 8(10), 1298. [DOI] [PubMed] [Google Scholar]
- Giannini C, Kloß A, Gohlke S, Mishto M, Nicholson TP, Sheppard PW, Kloetzel P-M, Dahlmann B, 2013. Poly-Ub-substrate-degradative activity of 26S proteasome is not impaired in the aging rat brain. PLoS One 8(5), e64042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ, 2010. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning & Memory 17(6), 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ, 2012. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiology of learning and memory 97(4), 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Miyawaki H, Helmstetter FJ, Diba K, 2013. Prefrontal activity links nonoverlapping events in memory. Journal of Neuroscience 33(26), 10910–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi M, Kumar A, Foster TC, 2015. Impaired attention and synaptic senescence of the prefrontal cortex involves redox regulation of NMDA receptors. 35(9), 3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Ferrara NC, Kwapis JL, Helmstetter FJ, 2016. CaMKII regulates proteasome phosphorylation and activity and promotes memory destabilization following retrieval. Neurobiology of learning and memory 128, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Helmstetter FJ, 2013. The ubiquitin–proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiology of learning and memory 105, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Ruenzel W, Helmstetter FJ, 2013. CaMKII, but not protein kinase A, regulates Rpt6 phosphorylation and proteasome activity during the formation of long-term memories. Frontiers in behavioral neuroscience 7, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ, 2011. Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS One 6(9), e24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Davis SJ, Moyer JR Jr, 2012. Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiology of aging 33(8), 1744–1757. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR, 2000. Impaired proteasome function in Alzheimer’s disease. Journal of neurochemistry 75(1), 436–439. [DOI] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, 2011. Systematic and quantitative assessment of the ubiquitin-modified proteome. Molecular cell 44(2), 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC, 2013. Linking redox regulation of NMDAR synaptic function to cognitive decline during aging. Journal of Neuroscience 33(40), 15710–15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Schiff JC, Helmstetter FJ, 2011. Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learning & memory 18(11), 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, 1999. Trial and intertrial durations in Pavlovian conditioning: issues of learning and performance. Journal of Experimental Psychology: Animal Behavior Processes 25(4), 433. [DOI] [PubMed] [Google Scholar]
- Le Jeune H, Cecyre D, Rowe W, Meaney M, Quirion R, 1996. Ionotropic glutamate receptor subtypes in the aged memory-impaired and unimpaired Long–Evans rat. Neuroscience 74(2), 349–363. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Choi J-H, Lee N, Lee H-R, Kim J-I, Yu N-K, Choi S-L, Lee S-H, Kim H, Kaang B-K, 2008. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319(5867), 1253–1256. [DOI] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, e Souza TM, Izquierdo I, Pasquini JM, Medina JH, 2001. The ubiquitin–proteasome cascade is required for mammalian long-term memory formation. European Journal of Neuroscience 14(11), 1820–1826. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Weible AP, Disterhoft JF, 2001. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. Journal of neurophysiology 86(4), 1839–1857. [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Belizaire R, Isacson O, Jenner P, Olanow CW, 2003. Altered proteasomal function in sporadic Parkinson’s disease. Experimental neurology 179(1), 38–46. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA, 2014. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clinical epidemiology 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR Jr, Brown TH, 2006. Impaired trace and contextual fear conditioning in aged rats. Behavioral neuroscience 120(3), 612. [DOI] [PubMed] [Google Scholar]
- Oddo S, 2008. The ubiquitin-proteasome system in Alzheimer’s disease. Journal of cellular and molecular medicine 12(2), 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi SA, Devulapalli RK, Nelsen JL, McFadden T, Surineni R, Jarome TJ, 2019. Distinct subcellular changes in proteasome activity and linkage-specific protein polyubiquitination in the amygdala during the consolidation and reconsolidation of a fear memory. Neurobiology of learning and memory 157, 1–11. [DOI] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA, Hogan H, 2014. An aging nation: the older population in the United States. United States Census Bureau, Economics and Statistics Administration, US Department of Commerce. [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS, 2002. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus 12(4), 495–504. [DOI] [PubMed] [Google Scholar]
- Reis DS, Jarome TJ, Helmstetter FJ, 2013. Memory formation for trace fear conditioning requires ubiquitin-proteasome mediated protein degradation in the prefrontal cortex. Frontiers in behavioral neuroscience 7, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, DeFazio RA, Barnes CA, Alexander GE, Bizon JL, Bowers D, Foster TC, Glisky EL, Levin BE, Ryan L, 2012. Challenges and opportunities for characterizing cognitive aging across species. Frontiers in aging neuroscience 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL, 1987. Human aging: usual and successful. Science 237(4811), 143–149. [DOI] [PubMed] [Google Scholar]
- Wang Q, Young P, Walters KJ, 2005. Structure of S5a bound to monoubiquitin provides a model for polyubiquitin recognition. Journal of molecular biology 348(3), 727–739. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF, 1999. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behavioural brain research 99(2), 123–132. [DOI] [PubMed] [Google Scholar]
- Yoon T, Otto T, 2007. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiology of learning and memory 87(4), 464–475. [DOI] [PubMed] [Google Scholar]
- Zeier Z, Madorsky I, Xu Y, Ogle WO, Notterpek L, Foster TC, 2011. Gene expression in the hippocampus: regionally specific effects of aging and caloric restriction. Mechanisms of ageing and development 132(1–2), 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J-Q, Zheng L-L, Chen H-B, Yu B, Wang W, Wang T, Ruan B, Pan B-X, Chen J-R, Li X-F, 2018. Hydrogen sulfide reverses aging-associated amygdalar synaptic plasticity and fear memory deficits in rats. Frontiers in neuroscience 12. [DOI] [PMC free article] [PubMed] [Google Scholar]