Abstract
Background:
Irritability is a adverse effect of many antiseizure medications (ASMs), but there are no validated measures currently available to characterize this behavioral risk. We examined both child and parent/guardian versions of the Affective Reactivity Index (ARI), a validated measure developed for application in adolescent psychiatry, to determine its sensitivity to ASM-related irritability. We hypothesized irritability increases associated with levetiracetam (LEV) but not lamotrigine (LTG) or oxcarbazepine (OXC).
Method:
The ARI was administered to 71 child and parent/guardian pairs randomized to one of three common ASMs (LEV, LTG, OXC) used to treat new-onset focal (localization-related) epilepsy. Subjects were recruited as part of a prospective multicenter, randomized, open-label, parallel group design. The ARI was administered at baseline prior to treatment initiation and again at 3 months after ASM initiation.
Results:
There was a significant increase in ARI ratings for both child and parent/guardian ratings for LEV but not LTG or OXC when assessed 3 months after treatment initiation. When examined on the individual subject level using a criterion of at least a 3-point ARI increase, there was an increase associated with LEV for child ratings but not parent/guardian scores.
Conclusion:
Both child and parent/guardian versions of the ARI appear sensitive to medication-induced irritability associated with LEV on both the group and individual levels. The findings extend the applicability of ARI from characterizing the presence of clinical irritability as a psychiatric diagnostic feature to a more modifiable aspect of behavior change related to medication management and support its use in clinical trial applications.
Keywords: Antiseizure medications, Affective Reactivity Index, Irritability
1. Introduction
Irritability is common in many child/adolescent psychiatric conditions and is a diagnostic feature of many Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) categories including Mood Disorders, Oppositional Defiant Disorder, and Generalized Anxiety Disorder. Irritability is also a core characteristic of the new DSM-5 diagnostic category of Disruptive Mood Dysregulation Disorder [1]. Despite its importance in psychiatric diagnosis, irritability remains a difficult construct to formally operationalize. Multiple scales have been developed although they have been criticized because of their inclusion of items related to the effects of irritability expression including hostility or self-harm rather than assessing components of irritable/angry mood or behavioral outbursts [2].
The Affective Reactivity Index (ARI) [3] was developed to formally assess chronic irritability in both children and adults while providing good psychometric characteristics [2]. The ARI consists of questions of both irritable mood (e.g., easily annoyed by others) as well as temper outbursts (e.g., loses temper easily) but does not include items related to possible negative consequences of irritable mood such as aggression or hostility, which may also be related to other factors such as poor impulse control. Both parent- and self-report ARI versions are available for pediatric assessment in ages 6–17 years old.
The ARI has been successfully validated in patients with bipolar disorder, severe mood regulation, or anxiety [4] and applied to characterization of multidimensional psychiatric symptom patterns in children and adolescents [5]. However, its sensitivity to treatment-emergent behavioral adverse effects associated with medical therapy has not been established. Irritable mood is a risk associated with many antiseizure medications (ASMs), although it is unclear if tools used to assess irritability in psychiatric diagnoses are sensitive to medication-induced changes. Although irritability has been reported in most ASMs, adverse behavioral effects are more common in certain medications (e.g., levetiracetam (LEV), topiramate, phenobarbital) [6]. Of the common ASMs used to treat epilepsy, LEV is associated with irritability in 12.5% of treated adults based upon epileptologist characterization following a clinic visit [7]. A meta-analysis of behavioral adverse effects of LEV in children described an increased relative risk of behavioral adverse effects (2.18 compared with placebo), in which irritability was the most common [8]. Given the absence of a validated measure to assess irritability and also the variability in how irritability is defined, estimates of irritability incidence across ASMs are poorly characterized.
We report ARI sensitivity to drug-related irritability in children with newly diagnosed focal (localization-related) epilepsy who were randomized to one of three common ASMs used to treat pediatric localization-related epilepsy [i.e., LEV, lamotrigine (LTG), oxcarbazepine (OXC)]. The ARI was administered to children and their parent/guardian prior to treatment initiation and 3 months after starting ASM therapy. We hypothesized that if the ARI is sensitive to drug-induced irritability, then increased ARI scores from both child and parent/guardian ratings should be present in children randomized to LEV. No ARI treatment increase was hypothesized for either OXC or LTG.
2. Methods
2.1. Clinical trial
The ARI was administered as part of a Phase IV clinical trial funded by the Patient Centered Outcomes Research Institute (PCORI) to establish potential cognitive and behavioral effects of three common ASMs used to treat new-onset focal (localization-related) epilepsy in children (Cognitive ASM Outcomes in Pediatric Localization-Related Epilepsy (COPE), Clinical Trials ID: NCT01891890). The COPE was a prospective multicenter, randomized, open-label, parallel group study. The protocol was approved by the Institutional Review Boards at all participating sites. The study started in August 2013 and was terminated in October 2015 because of poor recruitment. There were 10 clinical sites that enrolled study patients.
2.2. Subjects
Potential subjects were identified during their initial pediatric neurology visit, typically in new-onset seizure clinics. Inclusion criteria include age between 6 years, 0 months and 12 years, 11 months at the time of enrollment with a new diagnosis of focal (localization-related) epilepsy with or without secondary generalization according to the International League Against Epilepsy (ILAE) criteria. The child’s parent/guardian provided informed consent, and assent was obtained from children according to each site’s institutional guidelines. All participants were ASM naïve.
A total of 75 children consented to participation. One subject declined participation after being randomized because of displeasure regarding the randomized treatment. Three subjects did not have ASM properly coded prior to study closure resulting in 71 evaluable subjects receiving ASM monotherapy.
2.3. Study design
Patients were randomized in a 1:1:1 fashion to LEV, LTG, or OXC. These ASMs followed uniform titration rates (Table 1). At the time of the 3-month follow-up testing, LTG subjects were taking 7.0 mg/kg of tablets or chewable tablets daily in 2 equally divided doses. Oxcarbazepine subjects received 25 mg/kg of tablets or liquid daily in 2 equally divided doses. Levetiracetam subjects received 30 mg/kg of tablets or liquid daily in 2 equally divided doses. Antiseizure medications were not provided by the study; they were obtained by providing the family with a prescription to fill at their local pharmacies using available insurance and copayments.
Table 1.
Titration schedule.
| LEV | LTG | OXC | |
|---|---|---|---|
| Week | Dose | Dose | Dose |
| 1 | 10 mg/kg | 0. 3 mg/kg | 5 mg/kg |
| 2 | 20 mg/kg | 0. 3 mg/kg | 15 mg/kg |
| 3 | 30 mg/kg | 0. 6 mg/kg | 25 mg/kg |
| 4 | 30 mg/kg | 0. 6 mg/kg | 25 mg/kg |
| 5 | 30 mg/kg | 1. 2 mg/kg | 25 mg/kg |
| 6 | 30 mg/kg | 1. 8 mg/kg | 25 mg/kg |
| 7 | 30 mg/kg | 2. 4 mg/kg | 25 mg/kg |
| 8 | 30 mg/kg | 3. 0 mg/kg | 25 mg/kg |
| 9 | 30 mg/kg | 4. 5 mg/kg | 25 mg/kg |
| 10 | 30 mg/kg | 4. 5 mg/kg | 25 mg/kg |
| 11 | 30 mg/kg | 7. 0 mg/kg | 25 mg/kg |
| 12 | 30 mg/kg | 7. 0 mg/kg | 25 mg/kg |
| Target | 30 mg/kg | 7. 0 mg/kg | 25 mg/kg |
Although COPE was designed as a 6-month trial, we analyzed only baseline and 3-month timepoints to maximize the sample size for analysis due to missing data at 6 months (n = 9) because of early project termination. In addition, we included only participants in which ratings from both children and parent/guardian were completed so that scores would represent the same subject cohort.
There were 21 subjects randomized to LEV, with 13 child/parent pairs completing the 3-month evaluation. One LEV parent/guardian did not complete baseline assessment, one LEV parent/guardian LEV subject did not complete the 3-month assessment, and 6 additional LEV children did not complete the 3-month assessment despite parent/guardian data were completed for 3 participants. Consequently, there were 5 child/parent participants remaining in the study but with incomplete ARI datasets for analysis. There were 24 subjects randomized to LTG with 21 child/parent pairs completing the 3-month evaluation. There were 26 subjects randomized to OXC with 22 child/parent pairs completing the 3-month evaluation (Fig. 1).
Fig. 1.
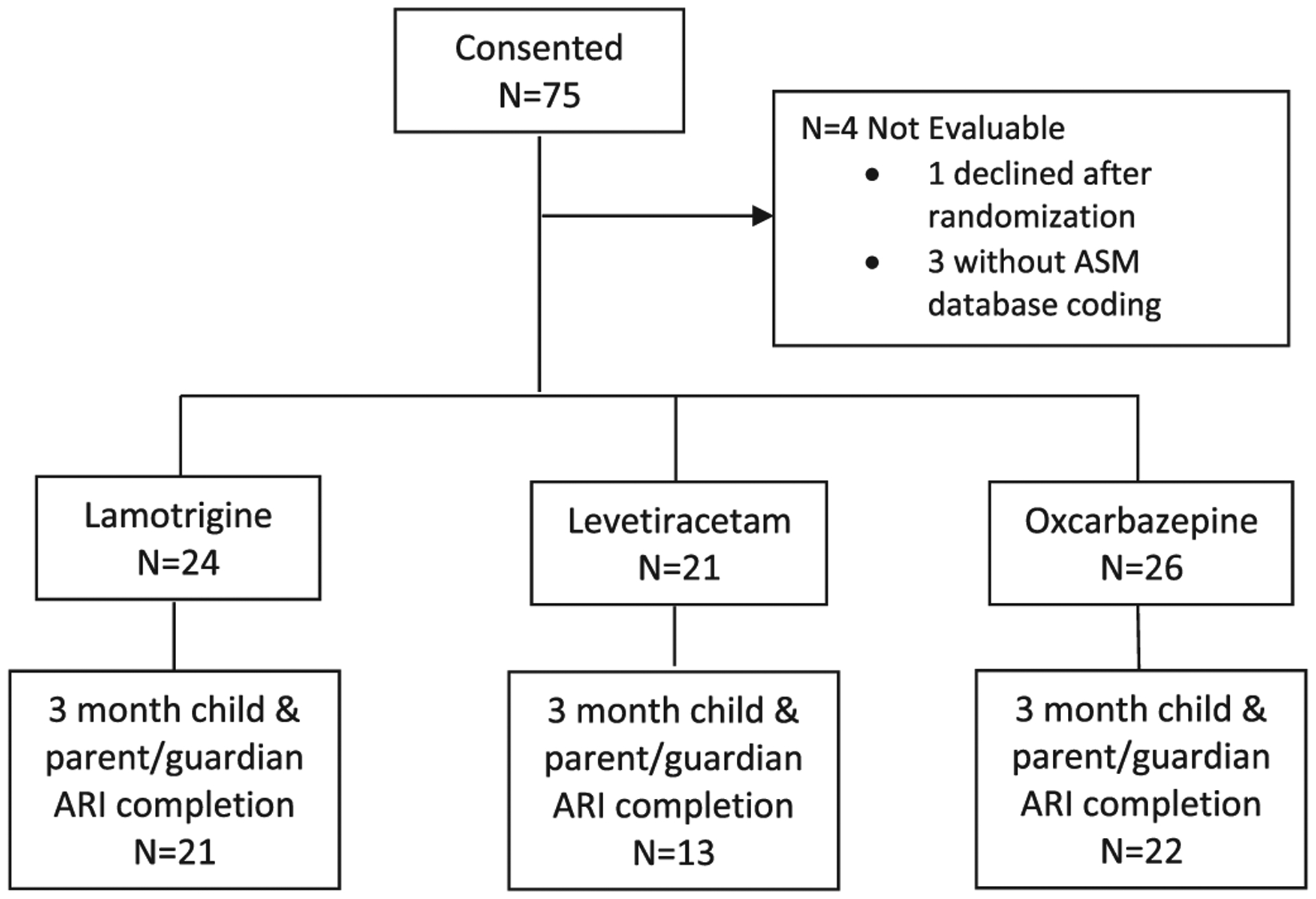
Flow diagram of subject assessment and 3-month assessment follow-up.
2.4. Affective Reactivity Index
The ARI contains 6 items assessing specific behaviors (e.g., easily annoyed by others, loses temper easily) that are rated on a 3-point scale (0 = not true, 1 = somewhat true, 2 = definitely true) with possible scores ranging from 0 to 12. There is an additional question that does not contribute to the overall score that asks to what degree irritability contributes to general impairment. Because behavior over the last 6 months is rated using standard instructions, wording was changed for follow-up visits to characterize interval change.
Child and parent/guardian versions of the questionnaire exist, with only slight wording changes to accommodate differences in who is being evaluated. For the parent/guardian version, questions are answered directly by the informant. For the child version, questions are read to the child. We analyzed child and parent/guardian ratings for the total ARI score separately, which formed our primary analyses. Although we did not anticipate finding significant effects for the single overall irritability question due to low variability associated with a single score, this measure was also analyzed. A similar statistical approach was used throughout by subjecting data to a 3 (ASM) × 2 (treatment) mixed design Analysis of Variance (ANOVA) with post hoc analyses. A chi-square analysis was also conducted to examine differences in the proportion of children who had a change in irritability scores across ASMs.
3. Results
3.1. Patient characteristics
Average ages (years) at enrollment were as follows: LTG (8.9, Standard Deviation (SD) = 2.1), OXC (8.9, SD = 1.9), and LEV (9.0, SD = 2.4). There were 9 females and 12 males randomized to LEV, 15 females and 9 males randomized to LTG, and 14 females and 12 males randomized to OXC. Although females scored slightly higher than males for both child [3.0 (SD = 2.5) vs. 2.4 (SD = 2.4)] and parent/guardian [2.8 (SD = 3.3) vs. 2.1 (SD = 3.3)] ARI versions, these differences were not statistically significant.
3.2. Child ARI ratings
Irritability symptoms were endorsed by children (Table 2). The mixed design ANOVA for child ARI identified an ASM × treatment interaction (F = 3.80, p = .029, partial η2 = 0.125). There was no significant main effect for treatment alone (F = 2.21, p = .14, partial η2 = 0.04), although a trend for the main effect of ASM was present (F = 2.42, p = .10, partial η2 = 0.084).
Table 2.
Child ARI scores.
| Baseline | Month 3 | |
|---|---|---|
| Total ARI score | ||
| Levetiracetam (n = 13) | 2.8 (2.4) | 4.7 (3.1) |
| Lamotrigine (n = 21) | 3.3 (2.6) | 2.8 (2.6) |
| Oxcarbazepine (n = 22) | 2.1 (2.3) | 2.2 (1.6) |
| Impairment due to irritability | ||
| Levetiracetam (n = 13) | 0.62 (0.77) | 0.62 (0.87) |
| Lamotrigine (n = 21) | 0.62 (0.81) | 0.71 (0.84) |
| Oxcarbazepine (n = 22) | 0.36 (0.73) | 0.32 (0.57) |
Simple main effect follow-up analyses for each ASM identified a trend for increased ARI scores following LEV initiation (F = 4.15, p = .064, partial η2 = 0.257). There were no effects for either LTG (F = 0.98, p = .334, partial η2 = 0.047) or OXC (F = 0.04, p = .845, partial η2 = 0.002).
We next analyzed the single question asking about impairment due to irritability. There were no statistically significant effects for the ASM × treatment interaction (F = 0.11, p = .893, partial η2 = 0.004), nor main effects for treatment (F = 0.015, p = .902, partial η2 = 0.000) or ASM (F = 1.88, p = .163, partial η2 = 0.066). As such, no post hoc analyses were performed.
3.3. Parent/guardian ARI ratings
Parents also endorsed irritability symptoms (Table 3). Analysis of the parent/guardian ratings yielded a trend for an ASM × treatment interaction (F = 2.42, p = .098, partial η2 = 0.084). There was no main effect of treatment (F = 1.58 p = .215, partial η2 = 0.029) although there was a main effect for ASM (F = 3.22, p = .048, partial η2 = 0.108). Similar to child report, the group taking OXC had lower rated symptoms of irritability overall.
Table 3.
Parent/guardian ARI scores.
| Baseline | Month 3 | |
|---|---|---|
| Total ARI score | ||
| Levetiracetam (n = 13) | 3.3 (3.6) | 4.8 (3.0) |
| Lamotrigine (n = 21) | 2.9 (3.6) | 2.5 (2.9) |
| Oxcarbazepine (n = 22) | 1.5 (2.6) | 1.7 (2.4) |
| Impairment due to irritability | ||
| Levetiracetam (n = 13) | 0.46 (0.88) | 1.1 (0.64) |
| Lamotrigine (n = 21) | 0.29 (0.64) | 0.38 (0.67) |
| Oxcarbazepine (n = 22) | 0.23 (0.53) | 0.32 (0.48) |
Follow-up analyses of treatment for each ASM independently revealed a treatment effect for LEV (F = 6.03, p = .030, partial η2 = 0.335). There were no effects for LTG (F = 0.40, p = .535, partial η2 = 0.02) or OXC (F = 0.36, p = .554, partial η2 = 0.02).
Analysis of the single question asking about overall disruption from irritability yielded a treatment effect (F = 7.50.9, p = .008, partial η2 = 0.124), an effect for ASM (F = 4.10, p = .022, partial η2 = 0.134), and a trend for treatment × ASM interaction (F = 2.70, p = .076, partial η2 = 0.093). In contrast to child ratings, parent ratings of disruption increased from 0 to 3 months across all three ASMs. Similar to child ratings, parents’ rating of overall disruption was lowest for OXC. Follow-up analyses for main effects of each ASM indicated a significant treatment effect for LEV (F = 5.33, p = .040, partial η2 = 0.308) but not for LTG (F = 0.388, p = .540, partial η2 = 0.019) or OXC (F = 0.66, p = .427, partial η2 = 0.030).
3.4. Individual subject response
We sought to characterize the ARI scores at the individual subject levels. We selected a change score of 3 or more as reflecting a meaningful increase in irritability based upon the literature in which a child-reported cutpoint of 3 or greater was used for diagnostic classification analyses [5]. Even though a cutpoint of 4 or greater was used for parent/guardian ratings for diagnostic classification [5], we used change scores of 3+ for parent/guardian classification since change scores of 4+ were infrequent.
There was a differential effect of ASM across treatments (χ2 = 6.9, p = .003) for child ratings, with a higher frequency of ARI increases associated with LEV using a 3-point criterion (see Table 4). This criterion did not reflect any ASM differences when examining individual patient/guardian ARI change scores.
Table 4.
Frequency of interval ARI increase (3+) following ASM initiation. There is a significant effect (p = .03) for Child ARI ratings that is not present for parent/guardian scores.
| Child ARI | LEV (N = 13) | LTG (N = 21) | OXC (N = 22) |
|---|---|---|---|
| Increase | 5(38%) | 1 (5%) | 3 (14%) |
| No increase | 8 (62%) | 20 (95%) | 19(86%) |
| Parent/guardian ARI | LEV (n = 13) | LTG (n = 21) | OXC (n = 22) |
| Increase | 2 (15%) | 3 (14%) | 1 (5%) |
| No increase | 11 (85%) | 18 (86%) | 21 (95%) |
4. Discussion
This report demonstrates ARI sensitivity to differential ASM-induced irritability associated with LEV, but not with LTG or OXC. The ARI also appears to be an appropriate measure to characterize irritability as a treatment-emergent adverse effect of ASM initiation at the individual subject level. These findings extend the applicability of ARI from characterizing the presence of clinical irritability as a psychiatric diagnostic feature to a more modifiable aspect of behavior change related to medication management and support its use in clinical trial applications. The average baseline ARI scores for both children (2.7, SD = 2.4) and parent/guardians (2.4, SD = 3.3) were higher at baseline than previously reported values for healthy control children (1.2, SD = 1.8) or their parent/guardians (0.4, 0.8) [5], although the children in that report were significantly older (12.9 years, SD = 2.7) than our subjects with new-onset epilepsy (8.8 years, SD = 2.0).
We hypothesized that although there are risks of behavioral side effects of ASMs in the treatment of pediatric epilepsy, there would be increases in ARI scores for LEV not seen in comparator ASMs of LTG and OXC given LEV’s higher risk of irritability. According to the Food and Drug Administration (FDA) package insert, children on LEV had increased rates of hostility compared with placebo (11.9% vs. 6.2%) with almost 11% reporting behavioral symptoms requiring drug discontinuation or dose reduction. These reported levels of irritability rely on spontaneous reports and provide the rationale for anticipating a specific LEV effect to demonstrate the sensitivity of ARI to drug-induced changes.
Despite some minor differences in level of statistical significance that likely reflect small sample size, the same overall pattern of ratings was present for both child and parent/guardian ARI versions. Although both child and parent/guardian versions of the ARI reflected increased irritability associated with LEV, a slightly different pattern was observed when asked a single general question of whether irritability has caused any problems overall. Although children are able to recognize increased irritability associated with LEV, they do not perceive this as having an overall effect in terms of their interactions. In contrast, not only do parent/guardians describe the increased irritability, they also note how increased irritability is associated with creating problems as a result of this behavioral change.
4.1. Limitations
Although this study did not address whether ARI would return to pretreatment levels if LEV were discontinued, standard clinical practice is to switch from LEV to a different ASM when intolerable irritability associated with LEV initiation develops. The sample sizes in the study are too small to generate reliable incidences of irritability associated with LEV, and there are presently no data of which we are aware to characterize what magnitude of ARI should be considered clinically meaningful. Although LEV was predicted to be associated with higher ARI scores, there were multiple statistical comparisons made without control for Type I error rate. Another limitation is associated with closing the study because of poor recruitment, a problem in conducting pediatric clinical trials that is well recognized [9]. Because early study termination resulted in closure of the Coordinating Center, we were unable to send out appropriate queries to identify the randomized ASM for the 3 subjects in whom drug randomization was not entered at the time of study entry. Similarly, we are unable to identify reasons for failure to complete the ARI at follow-up, which occurred more frequently with LEV than other ASMs. However, incomplete ARI data were present for either child or parent/guardian in 5 LEV subjects despite their continued study participation through the 3-month study visit, so it seems unlikely that adverse effects were contributing to differential study withdrawal. Nevertheless, if irritability were a reason for study withdrawal, then treatment-emergent irritability prevalence would be underestimated. However, even if this assumption is not correct, the sample size of this report is insufficient to estimate treatment-emergent irritability risks.
As with all open-label studies, our results are subject to the criticism that participants were aware of treatment risks and which may have contributed to subjective behavioral reporting. However, a similar pattern was observed with both children and parent/guardian ratings, and we think it is less likely that children would have the same information risk influencing ARI ratings as adults. The strengths of this report include randomized ASM assignment and a treatment population of children with new-onset epilepsy who were treatment naïve.
4.2. Conclusions
Both child and parent/guardian versions of the ARI appear sensitive to medication-induced irritability associated with LEV on both the group and individual level. These findings extend the applicability of ARI from characterizing the presence of clinical irritability as a psychiatric diagnostic feature to assessing more modifiable behavior changes related to medication management. Thus, both child and parent/guardian ARI versions appear to be appropriate instruments for clinical trial application to characterize treatment-emergent irritability risks.
Declarations of interest
Dr. Loring reports no competing interests. Dr. Meador reports research support from the NIH and Sunovion Pharmaceuticals and travel support from Eisai. The Epilepsy Study Consortium pays Dr. Meador’s university for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher-Smith Laboratories, UCB, and Vivus Pharmaceuticals. Dr. Shinnar reports no competing interests. Dr. Gaillard reports no competing interests. Dr. Wheless reports being a consultant to Eisai, Supernus, Shire, Mallinckrodt, Aquestive, Greenwich, INSYS, Inc., Neuralis, Zogenix, and BioMarin pharmaceuticals. Dr. Kessler reports no competing interests. Dr. Conry reports no competing interests. Dr. Berl reports no competing interests. Dr. Burns reports no competing interests. Dr. Glauser reports being a consultant to UCB and Supernus. Dr. Kinkead reports no competing interests. Dr. Cnaan reports no competing interest.
Funding
This study was supported by PCORI contract 527. There was also support at Children's National Hospital site from the Intellectual and Developmental Disabilities Research Center (U54 HD090257).
References
- [1].Brotman MA, Kircanski K, Leibenluft E. Irritability in children and adolescents. Annu Rev Clin Psychol 2017;13(1):317–41. 10.1146/annurev-clinpsy-032816-044941 [DOI] [PubMed] [Google Scholar]
- [2].Mulraney MA, Melvin GA, Tonge BJ. Psychometric properties of the affective reactivity index in Australian adults and adolescents. Psychol Assess 2014;26(1):148–55 10.1037/a0034891. [DOI] [PubMed] [Google Scholar]
- [3].Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, et al. The affective reactivity index: a concise irritability scale for clinical and research settings. J Child Psychol Psychiatry 2012;53(11):1109–17. 10.1111/j.1469-7610.2012.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stoddard J, Stringaris A, Brotman MA, Montville D, Pine DS, Leibenluft E. Irritability in child and adolescent anxiety disorders. Depress Anxiety 2014;31(7):566–73 10.1002/da.22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kircanski K, Zhang S, Stringaris A, Wiggins JL, Towbin KE, Pine DS, et al. Empirically derived patterns of psychiatric symptoms in youth: a latent profile analysis. J Affect Disord 2017;216:109–16 10.1016/j.jad.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev 2007;17(4):413–25. [DOI] [PubMed] [Google Scholar]
- [7].Chen B, Choi H, Hirsch LJ, Katz A, Legge A, Buchsbaum R, et al. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017; 76:24–31. doi: 10.1016/j.yebeh.2017.08.039. [DOI] [PubMed] [Google Scholar]
- [8].Halma E, de Louw AJ, Klinkenberg S, Aldenkamp AP, DM IJ, Majoie M. Behavioral side-effects of levetiracetam in children with epilepsy: a systematic review. Seizure 2014;23(9):685–91 10.1016/j.seizure.2014.06.004. [DOI] [PubMed] [Google Scholar]
- [9].Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004;364 (9436):803–11 10.1016/s0140-6736(04)16942-0. . [DOI] [PubMed] [Google Scholar]


