Abstract
Background
There is a renewed interest in lower field magnetic resonance imaging (MRI) systems for cardiovascular magnetic resonance (CMR), due to their favorable physical properties, reduced costs, and increased accessibility to patients with implants. We sought to assess the diagnostic capabilities of high-performance low-field (0.55 T) CMR imaging for quantification of right and left ventricular volumes and systolic function in both healthy subjects and patients referred for clinical CMR.
Methods
Sixty-five subjects underwent paired exams at 1.5 T using a clinical CMR scanner and using an identical CMR system modified to operate at 0.55 T. Volumetric coverage of the right ventricle (RV) and left ventricles (LV) was obtained using either a breath-held cine balanced steady-state free-precession acquisition or a motion-corrected free-breathing re-binned cine acquisition. Bland-Altman analysis was used to compare LV and RV end-systolic volume (ESV), end-diastolic volume (EDV), ejection fraction (EF), and LV mass. Diagnostic confidence was scored on a Likert-type ordinal scale by blinded readers.
Results
There were no significant differences in LV and RV EDV between the two scanners (e.g., LVEDV: p = 0.77, bias = 0.40 mL, correlation coefficient = 0.99; RVEDV: p = 0.17, bias = − 1.6 mL, correlation coefficient = 0.98), and regional wall motion abnormality scoring was similar (kappa 0.99). Blood-myocardium contrast-to-noise ratio (CNR) at 0.55 T was 48 ± 7% of the 1.5 T CNR, and contrast was sufficient for endocardial segmentation in all cases. Diagnostic confidence of images was scored as “good” to “excellent” for the two field strengths in the majority of studies.
Conclusion
A high-performance 0.55 T system offers good bSSFP CMR image quality, and quantification of biventricular volumes and systolic function that is comparable to 1.5 T in patients.
Trial registration
Keywords: Low-field MRI, Cardiovascular magnetic resonance, Cine function, Ventricular volumes
Background
The clinical adoption and use of cardiovascular magnetic resonance (CMR) has relied on accurate quantification of ventricular chamber size and systolic function [1–6]. CMR is typically performed using 1.5 T CMR systems and, less commonly, 3 T. However, lower field strengths (< 1 T) may offer advantages for CMR due to scaling of relaxation parameters (shorter T1, longer T2 and T2*) which are well-suited for gradient echo and balanced steady state free precession (bSSFP) contrast, lower specific absorption rate (SAR) to maximize flip angles, and improved magnetic field homogeneity throughout the thorax [7, 8]. Moreover, lower field CMR systems are inherently less expensive to manufacture and install, potentially increasing CMR accessibility in rural and austere environments.
We recently demonstrated a research 0.55 T CMR system for cardiac imaging, with maintained magnet design and gradient performance [9]. This system configuration is capable of technically demanding cardiac imaging. Given the fundamentality of cine measurements, which is clinically indicated in 92% of CMR exams [10], it is vital to maintain comparable diagnostic imaging quality at 0.55 T.
In this study, we implemented breath-held and free-breathing bSSFP cine acquisitions for 0.55 T. For clinical validation, we assessed whether the biventricular volumes, systolic function, and left ventricular (LV) mass acquired on a high-performance low-field (0.55 T) system would provide diagnostic data that were clinically comparable to those acquired on a standard 1.5 T clinical CMR scanner in patients referred for clinical CMR exams.
Methods
Ethics, consent and permissions
The study was approved by the local Institutional Review Board, and all subjects provided written informed consent (Clinicaltrials.gov NCT03331380, NCT03581318).
Image acquisition
Each subject was imaged on both a 1.5 T CMR system (MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) and a prototype CMR system modified to operate at 0.55 T (modified MAGNETOM Aera, Siemens Healthineers). The custom 0.55 T system maintained the gradient performance (maximum amplitude = 45mT/m and slew rate = 200mT/m/s) required for fast bSSFP imaging. Images were acquired using a 6-channel body array and 18-channel spine array tuned to operate at 0.55 T.
Both healthy subjects and patients referred for clinical CMR were studied. All subjects underwent cine imaging of the heart, using breath-held bSSFP techniques or, for patients who couldn’t hold their breath, a re-binned motion-corrected real-time cine sequence [5, 11, 12]. The same type of cine acquisition (breath-held vs. free breathing) was used on both 0.55 T and 1.5 T for each individual. We used volumetric coverage with a short-axis stack and standard three-, two-, and four-chamber long-axis views.
The reduced signal-to-noise ratio (SNR) from reduced magnetic polarization at 0.55 T was compensated with decreased bandwidth/longer repetition time (TR) and increased flip angles, which are amenable for bSSFP at lower field. Imaging parameters were selected to maximize SNR and contrast-to-noise ratio (CNR) without sacrificing spatiotemporal resolution, and breath-hold lengths <10s. Bloch equation simulations of myocardial signal, blood signal and blood-myocardium contrast for bSSFP acquisitions at 0.55 T were performed in Python 3.6 (https://github.com/hansenms/pybloch) using measured T1 and T2 values [9]. We simulated receiver bandwidths of 300 Hz/Px to 1100 Hz/Px and flip angles of 50° to 90°. Simulated signal and contrast at 0.55 T were scaled to our reference clinical 1.5 T cine acquisition protocol. Further optimization was performed in healthy subjects and imaging parameters were chosen according to the preference of local cardiologists.
Typical parameters for the breath-held and free-breathing rebinned cine acquisitions are reported in Table 1. Breath-held acquisitions were reconstructed using a GRAPPA reconstruction, and free-breathing re-binned acquisitions were reconstructed using the L1-SPIRiT method previously described [13].
Table 1.
bSSFP cine imaging sequence parameters
| 0.55 T breath-held bSSFP cine | 1.5 T breath-held bSSFP cine | 0.55 T free breathing re-binned bSSFP cine | 1.5 T free breathing re-binned bSSFP cine | |
|---|---|---|---|---|
| Field of view (mm2) | 360 × 270 | 360 × 270 | 360 × 270 | 360 × 270 |
| Slice thickness (mm) | 8 | 8 | 8 | 8 |
| Matrix size | 256 × 192 | 256 × 140 | 192 × 108 | 192 × 119 |
| TE (ms) | 1.67 | 1.2 | 1.34 | 1.06 |
| TR (ms) | 4.1 | 2.79 | 3.24 | 2.52 |
| Acquired temporal resolution (ms) | 32 | 28 | N/A | N/A |
| Bandwidth (Hz/Px) | 350 | 1085 | 501 | 1085 |
| Parallel imaging acceleration factor | 2 | 2 | 3 | 4 |
| Seconds/slice | 9 | 8 | 18 | 16 |
| Calculated Phases | 30 | 30 | 26 | 30 |
| Flip angle (°) | 78 | 50 | 80 | 50 |
Sequence parameters for breath-held and free breathing re-binned cine acquisitions at 0.55 T and 1.5 T; bSSFP balanced steady statae free precession, TE echo time, TR repetition time
Image analysis
Manually assisted regions of interest were generated using suiteHEART software (NeoSoft, Pewaukee, Wisconsin, USA). For each paired dataset, the analysis of biventricular volumes was performed by the same operator (Level III CMR cardiologist with 18 years’ experience). Regional wall motion abnormality interpretation was performed using a 17-segment model, with interpretation blinded to clinical data. Segmentation and wall motion interpretation of matched subjects was separated by > 1 week to avoid memory bias.
Image quality analysis
SNR and CNR were measured in four healthy subjects imaged using the breath-held protocol at both 0.55 T and 1.5 T. SNR was measured using an SNR-scaled reconstruction [14], and CNR was calculated as CNR = SNRblood-SNRmyocardium. Relative SNR and CNR between 0.55 T and 1.5 T were compared to Bloch equation simulations. Blood-myocardium contrast index was calculated from the difference between the two tissue signal intensities, indexed to normal myocardium, and compared using matching regions-of interest at 0.55 T and 1.5 T in the healthy subject group.
Two independent readers (C.M. and S.M.S.; 18 and 11 years’ experience, respectively) assigned Likert-type ordinal scales to measure diagnostic confidence for each cine data set (1–5 scale in which 5 = excellent, 4 = good, 3 = adequate, 2 = fair, 1 = non-diagnostic). A total of 130 measurements were collected (65 cases × 2 readers). Data were deidentified and randomized, and scoring of paired data was separated by > 1 week. The diagnostic confidence rating was based upon 1) the ability to identify fine detailed structures such as chordae tendineae, trabeculation, and valve leaflets, 2) the interpretation of regional wall motion abnormalities, 3) the presence or absence of artifacts, and 4) general interpretability of images.
Statistical analysis
Descriptive data are reported as mean ± standard deviation (SD) with maximum and minimum values when appropriate or median with intraquartile range. Statistical analyses were performed using MedCalc Statistical Software version 12.7.7.0 (Ostend, Belgium). Bland-Altman [15] analyses, inter-study reproducibility (bias ±1.96SD), coefficient of variation between field strengths (SD/mean*100%), and correlation coefficient (r) were reported for quantitative comparisons of ventricular volumes, ejection fraction, stroke volume, and mass between the two CMR exams. The Wilcoxon test was used to compare paired quantitative measurements. Cohen’s kappa statistic was applied to compare regional wall motion scoring between 0.55 T and 1.5 T. Blinded diagnostic confidence interpretation scores were averaged, and Wilcoxon signed-rank sum test was performed to compare scored quality assessments between the two field strengths. Statistical significance was defined as a p value < 0.05.
Results
Patient characteristics
A total of 65 subjects (33 male, mean age 42.4 ± 15.5 years) underwent paired exams, with breath-held cine imaging used in 37 subjects and free-breathing re-binned cine imaging used in 28 subjects. Forty-four of the 65 subjects were clinically-referred patients and 21 subjects were healthy volunteers.
Twenty-seven of 44 (61.3%) patients were referred for assessment of cardiomyopathy, while 7/44 (15.9%) were referred for assessment of myocardial viability. The remaining patients were referred for indications such as valvular, congenital, aorta, or other assessment. Sixteen of 44 were referred for contrast-enhanced exams. Baseline patient characteristics are summarized in Table 2. The mean time between CMR exams was 10.0 ± 17.4 days.
Table 2.
Characteristics of patients and healthy volunteers
| Characteristic | All subjects (n = 65) |
|---|---|
| Age (years) | |
| Mean ± standard deviation | 42.4 ± 15.5 |
| Minimum, Maximum | 18.8, 70.5 |
| Left ventricular ejection fraction (%) on 1.5 T | |
| Mean ± standard deviation | 55.3 ± 8.7 |
| Indication for scan - n(%) | |
| Healthy subjects | 21(32.3) |
| Nonischemic cardiomyopathy | 27 (41.5) |
| Viability | 7 (10.8) |
| Valve/shunt | 6(9.2) |
| Other | 4 (6.2) |
| Referred for contrast enhanced exam | 16 (24.6) |
Characteristics of patient age, ejection fraction and indication for clinically-referred CMR for patients and healthy volunteers
Image quality
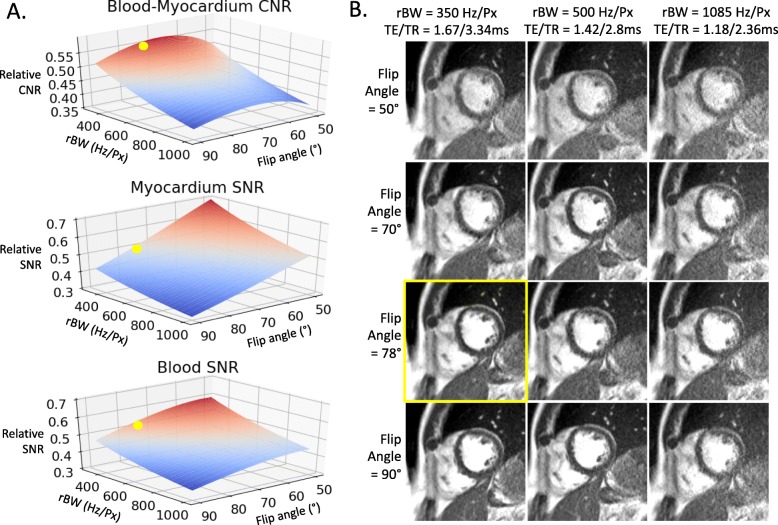
Figure 1a provides Bloch equation simulations of 0.55 T SNR and blood-myocardium CNR, scaled to simulated 1.5 T SNR and CNR with our clinical cine protocol. These simulations predicted that 0.55 T CNR would be most similar to 1.5 T with flip angle = 68°. Figure 1b provides representative images in a healthy subject for a range of parameters (flip angle, receiver bandwidth, TR and TE). For our 0.55 T breath-held cine imaging, we selected a receiver bandwidth of 350 Hz/Px, and a flip angle of 78°, which we preferred over the simulated optimum of 68°.
Fig. 1.
bSSFP parameter optimization for 0.55 T. (a) Simulations and (b) healthy subject imaging demonstrating parameter optimization for bSSFP cine imaging at 0.55 T by varying flip angle and receiver bandwidth (rBW). Simulated SNR and CNR are scaled relative to simulated 1.5 T SNR and CNR for our standard cine protocol. The yellow dots in (a) and yellow frame in (b) demonstrate the selected parameter combination
Additional file 1 provides a side-by-side comparison of image quality for matched parameters at both field strengths. At 0.55 T, SNR and CNR more closely match 1.5 T using the optimized protocol with higher flip angle and reduced receiver bandwidth. Notably, by using the 0.55 T protocol for imaging at 1.5 T, artifacts were introduced by the long-TR optimized for 0.55 T, and a 78° was infeasible at 1.5 T due to SAR restrictions.
Additional file 1. Side-by-side comparison of 0.55 T and 1.5 T breath-held bSSFP cine protocols applied using 0.55 T and 1.5 T MRI systems. A clear SNR improvement is observed using the optimized protocol at 0.55 T. At 1.5 T, image artifacts are introduced using the long-TR 0.55 T protocol and a 78 flip angle was unattainable. rBW = receiver bandwidth.
Bloch equation simulations of our breath-held bSSFP protocols at 1.5 T and 0.55 T predicted that myocardial SNR at 0.55 T would be 50% of 1.5 T, blood SNR at 0.55 T would be 53% of 1.5 T, and 0.55 T CNR would be 55% of 1.5 T. SNR and CNR were measured in four healthy volunteers imaged at both 0.55 T and 1.5 T. After scaling SNR for differences in voxel size between 0.55 T and 1.5 T protocols, relative SNR between the two field strengths was measured to be 43 ± 6% in myocardium, 58 ± 6% in blood, and relative CNR was 48 ± 7%. Difference between measured and simulated relative SNR and CNR is attributed to the SNR-penalty associated with the coil g-factor for GRAPPA reconstruction at 0.55 T. The blood-myocardium contrast index, which was calculated from the absolute signal intensity difference normalized to the myocardium, was higher at 0.55 T (2.4 ± 0.81 at 0.55 T vs 1.98 ± 0.34 at 1.5 T, p = 0.0004), due to the application of a higher flip angle at 0.55 T causing signal suppression in the myocardium. Blood-myocardium contrast was sufficient for endocardial segmentation in all cases.
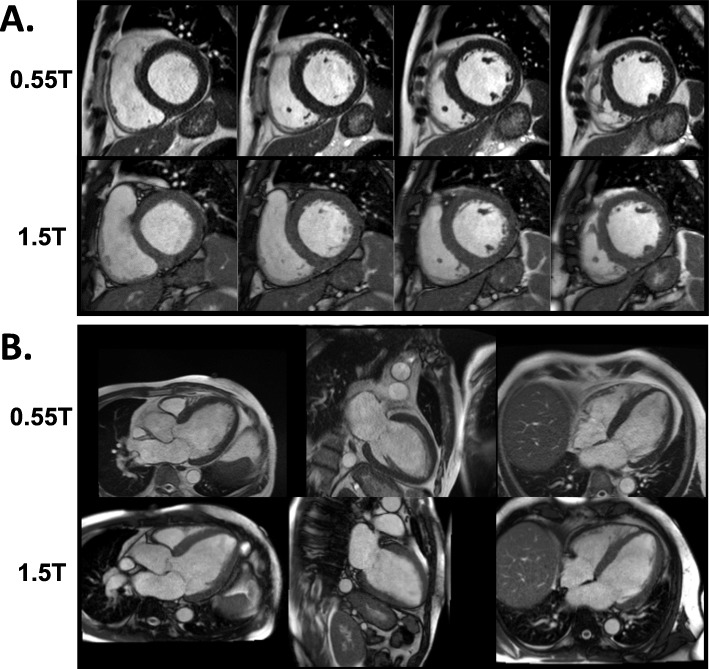
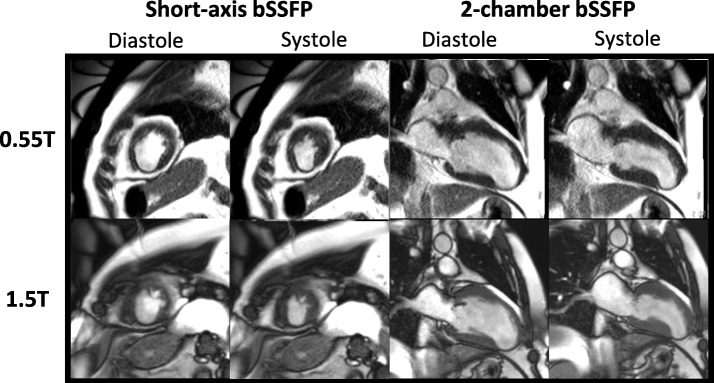
Figure 2 illustrates the image quality for a paired 0.55 T and 1.5 T breath-held study in a patient with a severe cardiomyopathy. Additional file 2 illustrates the image quality for a paired free-breathing study in a patient with sickle cell disease and a large pericardial effusion. The L1-SPIRiT reconstruction used for the free-breathing acquisition results in similar image quality between 0.55 T and 1.5 T.
Fig. 2.
Image quality of 0.55 T and 1.5 T breath-held cine. Examples of 0.55 T and 1.5 T breath-held cine bSSFP in (a) short axis and (b) long axis slices from a patient with a nonischemic cardiomyopathy
Additional file 2. Comparator example short axis breath-held bSSFP cines from a patient with chronic myocardial infarction and apical aneurysm acquired at 1.5 T.
Ventricular chamber assessment
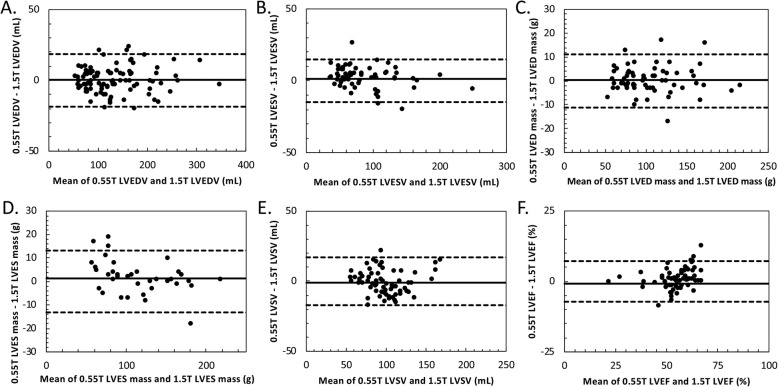
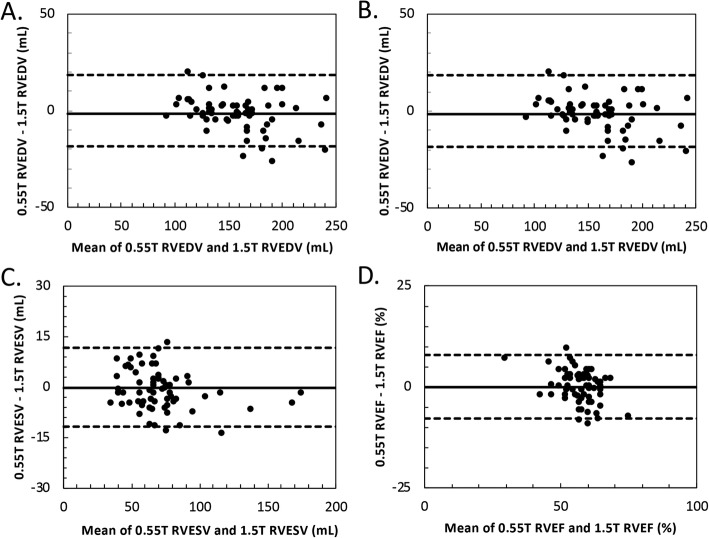
Quantitative comparison of ventricular chamber volumes showed excellent correspondence between the 0.55 T images and standard 1.5 T images. Table 3 summarizes the main ventricular findings for each field strength. All measured LV and right ventricular (RV) parameters were comparable between the two field strengths (p = not significant (NS), see Table 3). Measurements of LV and RV volumes, ejection fraction (EF) and LV mass were highly reproducible (Figs. 3 and 4). For example, interstudy reproducibility (bias ±1.96xSD) of LV end-diastolic mass between 0.55 T and 1.5 T was 0.4 ± 11.2 g and LV end-diastolic volume (EDV) was 0.4 ± 18.6 mL. Table 4 summarizes the interstudy reproducibility, coefficient of variation, and correlation coefficient for measurements compared between 0.55 T and 1.5 T. Results were similar for breath-held and free-breathing acquisitions, and separate Bland-Altman plots for the two acquisition types are provided in Additional file 4.
Table 3.
Ventricular volume measurements at 0.55 T and 1.5 T
| 0.55 T cine | 1.5 T cine | P value | |
|---|---|---|---|
| LVEDV (mL) | 171.0 (144.8–224.5) | 173.0 (144.8–222.5) | 0.77 |
| LVESV (mL) | 73.2 (60.2–105.0) | 70.7 (56.9–108.3) | 0.13 |
| LVED mass (g) | 100.0 (79.5–127.8) | 100 (78.8–128.5) | 0.72 |
| LVES mass (g) | 103.0 (82.7–138.3) | 103.0 (81.3–134.5) | 0.08 |
| LVSV (mL) | 96.8 (83.1–110.5) | 97.5 (82.6–113.0) | 0.28 |
| LVEF (%) | 55.8 (52.2–59.6) | 56.0 (51.7–61.1) | 0.07 |
| RVEDV (mL) | 158.0 (134.0–173.3) | 160.0 (133.8–185.3) | 0.17 |
| RVESV (mL) | 67.8 (54.8–76.4) | 67.5 (56.6–77.2) | 0.10 |
| RVSV (mL) | 91.2 (78.0–101.3) | 92.2 (75.0–104.5) | 0.97 |
| RVEF (%) | 57.0 (54.0–62.0) | 58.0 (54.0–61.0) | 0.93 |
Comparison of LV and RV end-diastole volume, end-systolic volume, end-diastolic mass, end-systolic mass, stroke volume and ejection fraction calculated by breath-held or free-breathing re-binned cine at both 0.55 T and 1.5 T field strengths; EDV end diastolic volume, EF ejection fraction, ESV end systolic volume, LV left ventricular, RV right ventricular
Fig. 3.
Bland-Altman comparisons of left ventricular measurements at 0.55 T and 1.5 T. Bland Altman comparisons of (a) LVEDV, (b) LVESV, (c) LVED mass, (d) LVES mass, (e) LV stroke volume (SV), and (f) LVEF measured using both breath-held and free-breathing cine protocols. LV measurements are highly reproducibly between 0.55 T and 1.5 T
Fig. 4.
Bland-Altman comparisons of RV measurements at 0.55 T and 1.5 T. Bland Altman comparisons of (a) RVEDV, (b) RVESV, (c) RVSV, and (d) RVEF measured using measured using both breath-held and free-breathing cine protocols. RV measurements are highly reproducible between the 0.55 T and 1.5 T scanners
Table 4.
Interstudy bias, interstudy variability, and correlation coefficient
| Inter study reproducibility (bias ± 1.96xSD) between field strengths | coefficient of variation | Correlation coefficient | ||
|---|---|---|---|---|
| LVEDV | All | 0.4 ± 18.6 mL (− 18.4 mL to 18.8 mL) | 3.3% | 0.99 |
| Breath-held | 0.0 ± 20.6 mL (−20.6 mL to 20.6 mL) | 4.5% | 0.98 | |
| Free-breathing | 0.9 ± 15.9 mL (−15.0 mL to 16.9 mL) | 2.3% | 0.99 | |
| LVESV | All | 1.3 ± 14.8 mL (−13.5 mL to 16.2 mL) | 5.3% | 0.98 |
| Breath-held | 1.3 ± 18.2 mL (−16.9 mL to 19.5 mL) | 6.4% | 0.98 | |
| Free-breathing | 1.4 ± 9.0 mL (−7.7 mL to 10.4 mL) | 3.7% | 0.99 | |
| LVED Mass | All | 0.4 ± 11.2 g (−10.8 g to 11.5 g) | 2.9% | 0.99 |
| Breath-held | 0.1 ± 12.9 g (−12.8 g to 12.9 g) | 3.2% | 0.99 | |
| Free-breathing | 0.7 ± 8.6 g (−7.9 g to 9.3 g) | 2.5% | 0.99 | |
| LVES Mass | All | 1.3 ± 13.2 g (−11.8 g to 14.6 g) | 3.0% | 0.99 |
| Breath-held | 2.2 ± 14.9 g (−12.7 g to 17.1 g) | 3.6% | 0.99 | |
| Free-breathing | 0.2 ± 10.4 g (−10.2 g to 10.5 g) | 2.3% | 0.99 | |
| LVSV | All | −1.0 ± 17.1 mL (−18.0 mL to 16.1 mL) | 5.1% | 0.95 |
| Breath-held | −1.2 ± 19.4 mL (− 10.6 mL to 18.2 mL) | 6.2% | 0.89 | |
| Free-breathing | -0.7 ± 13.8 mL (−14.4 mL to 13.1 mL) | 3.6% | 0.98 | |
| LVEF | All | −0.8 ± 7.2% (−8.0 to 6.4%) | 5.8% | 0.91 |
| Breath-held | −0.9 ± 8.9%(−9.8 to 8%) | 6.3% | 0.91 | |
| Free-breathing | −0.6 ± 4.15% (−4.8 to 3.5%) | 5.1% | 0.88 | |
| RVEDV | All | −1.6 ± 18.5 mL (−20 mL to 16.9 mL) | 2.9% | 0.98 |
| Breath-held | -1.6 ± 16.0 mL (−17.6 mL to 14.4 mL) | 2.6% | 0.95 | |
| Free-breathing | −1.5 ± 21.7 mL (−23.1 mL to 20.2 mL) | 3.3% | 0.98 | |
| RVESV | All | −1.2 ± 11.7 mL (− 12.9 mL to 10.5 mL) | 5.4% | 0.97 |
| Breath-held | −0.5 ± 11.7 mL (− 12.2 mL to 11.2 mL) | 5.1% | 0.95 | |
| Free-breathing | −2.2 ± 11.7 mL (−13.9 mL to 9.5 mL) | 5.8% | 0.98 | |
| RVSV | All | −0.2 ± 19.7 mL (−19.9 mL to 19.5 mL) | 5.7% | 0.92 |
| Breath-held | 0.1 ± 17.7 mL (−18.3 mL to 17.1 mL) | 5.4% | 0.86 | |
| Free-breathing | 0.3 ± 22.4 mL (−22.1 mL to 22.7 mL) | 6.0% | 0.94 | |
| RVEF | All | −0.1 ± 7.7% (−8.0 to 7.8%) | 4.0% | 0.82 |
| Breath-held | −0.6 ± 7.4% (−7.5 to 7.3%) | 3.9% | 0.97 | |
| Free-breathing | 0.0 ± 8.6% (−8.6 to 8.6%) | 4.2% | 0.69 | |
Interstudy bias, interstudy variability, and correlation coefficient between 0.55 T and 1.5 T for quantitative ventricular volume and systolic function measurements. Coefficient of variation was calculated from the standard deviation between 0.55 T and 1.5 T measurements, divided by the mean of the two measurements
Identification of regional wall motion abnormalities
Regional wall motion abnormalities were identified in nine subjects with a total of 72 abnormal segments. Sector-wise comparison of the extent of regional wall motion abnormalities revealed a close correlation between the 0.55 T and 1.5 T in the identification of abnormalities (kappa 0.99). Figure 5 illustrates the appearance of a thinned chronic infarction and apical aneurysm on 0.55 T and 1.5 T scanners. Additional file 3 demonstrates example cine imaging movie of the wall motion abnormality on both CMR systems. This patient had an aortic bioprosthetic valve from a prior surgery, and the artifact is modestly improved using 0.55 T.
Fig. 5.
Example wall motion abnormality at 0.55 T and 1.5 T. Breath-held cine images from 0.55 T (top row) and 1.5 T (bottom row) are provided for a patient with a chronic myocardial infarction and apical aneurysm resulting in regional wall motion abnormality. Videos of wall motion abnormality are provided in Additional file 3
Additional file 3. Example short axis breath-held bSSFP cines from a patient with chronic myocardial infarction and apical aneurysm acquired at 0.55 T
Diagnostic confidence scores
The overall diagnostic confidence scores were slightly higher for the 1.5 T field strength; mean scores of 4.79 ± 0.54 at 0.55 T vs 4.88 ± 0.32 at 1.5 T, p = 0.0039; however, the scores of both field strengths were predominantly within the good to excellent quality categories (Fig. 6).
Fig. 6.
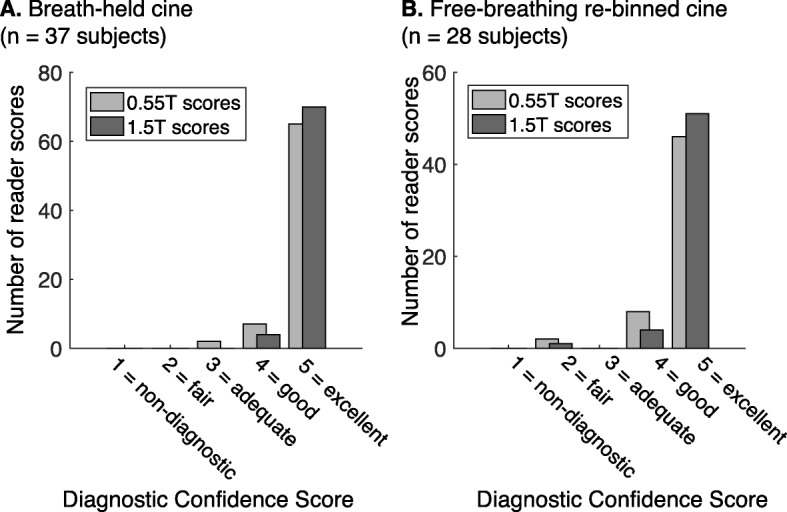
Diagnostic Confidence scoring results. Histogram of scores of diagnostic confidence from two blinded expert readers for (a) breath-held cine and (b) free-breathing re-binned cine. The majority of the scores fall into the excellent category. A total of 130 measurements were collected (65 subjects × 2 readers)
Discussion
This study demonstrates the cine image quality available from a high-performance 0.55 T CMR system. We found that cine imaging of the RV and LV at low field provides diagnostic imaging comparable to that acquired on a standard clinical 1.5 T CMR scanner. The interstudy comparisons revealed close agreement in volumetric assessment and high diagnostic confidence for 0.55 T. While other studies have performed preliminary investigations of cine imaging on healthy subjects at 0.35 T [7, 8], this is the first study to evaluate a cohort of subjects with disease. The performance of diagnostic cardiac imaging at lower field could have profound impacts on the cost, and therefore accessibility, of CMR.
Compared with historic low-field CMR systems, we expect this system to perform better for CMR because it is a closed-bore design, pairing a modern homogeneous magnet, contemporary radiofrequency (RF) chain, and fast gradient architecture with a lower field. CMR hardware performance is important for bSSFP cine imaging. bSSFP became a workhorse sequence for CMR after 1999, when high-performance gradient system were ubiquitous [16]. Gradient speed is required for rapid gradient switching during bSSFP imaging, and field homogeneity is required to limit banding and other artifacts. Most modern commercial low field systems are not suitable for CMR exams, because they are designed with compromised gradient performance or use a permanent magnet design with unsatisfactory field homogeneity. Our system combines contemporary hardware at a lower field strength of 0.55 T and other CMR studies have also used a high-performance 0.35 T system [7, 8, 17]. We modified an existing 1.5 T system to operate at lower field and chose 0.55 T to reduce device heating (interventional metallic devices and implanted CIEDs), while maintaining reasonable bSSFP image quality based on simulations.
Variability between paired exams can be introduced through physiological differences between days, in addition to differences in coils, scan parameters, noise characteristics and epicardial fat appearance. This study compared imaging protocols optimized for blood-myocardium contrast at each field strength, rather than matched protocols for “best-to-best” comparison. The interstudy coefficients of variation between CMR systems of biventricular volumes, LV mass and biventricular ejection fraction ranged from 2.3 to 6.4%, and was similar to previously reported values of interstudy variability on repeated measures on the same system, interstudy variability between field strengths (1.5 T and 3 T), and variability between observers [1, 4, 6, 18–21]. For example, Grothues et al. [18] report coefficients of variation between 3.7–6.2% when comparing repeated CMR LV measurements in a mixed group of subjects including normal subjects and patients with pathology. In our study, the bias was largest for the RV volumes.
The decrease in SNR at 0.55 T was expected but did not prohibit volumetric quantification or good diagnostic confidence in the interpretation of the studies, which was equivalent between field strengths. Image acquisition time and breath-hold length was equivalent between the two protocols. At 0.55 T, specific absorption ratio (SAR) limitations are virtually nonexistent enabling higher flip angles, and field homogeneity increases linearly (in Hz) with field strength, allowing increased TR without bSSFP banding artifacts at lower field. T1 is shorter and T2 is modestly longer at lower field strength, which compensates for some SNR loss. SNR could be further improved using more efficient data sampling (e.g., spiral or echo planar imaging (EPI)) or using advanced reconstruction techniques [8]. The epicardial fat appearance was different at 0.55 T because fat and water are in the same passband for TR = 4.1 ms, reducing the dark interface between fat and water observed at 1.5 T and 3.0 T.
Limitations of this study include the potential physiological variability introduced by time between exams, and the limited scope of comparison of only RV and LV cine function. The coil geometry of the prototype receiver arrays retuned for 0.55 T prohibited high acceleration factors using GRAPPA reconstruction, and receiver coils could be optimized in the future to improve image quality, SNR, and acceleration factor. Future work will assess other vital CMR measurements, including late-gadolinium enhancement, black blood imaging, and phase-contrast flow, on this high-performance low field CMR system.
Conclusion
Our study demonstrates that using a high-performance 0.55 T CMR system with optimized bSSFP parameters, the fundamental assessment LV mass, biventricular volumes, and systolic function can be performed with high diagnostic confidence comparable to the current clinical standard in both healthy subjects and clinical patients.
Supplementary information
Additional file 4. Bland Altman comparisons of LVEDV, LVESV, LVEDM, LVESM, LVSV, LVEF, RVEDV, RVESV, RVSV, and RVEF separated for breath-held and free-breathing cine acquisitions.
Acknowledgements
We thank the Siemens Healthcare team for their assistance in CMR system modification to 0.55 T. We thank Dr. Robert Lederman for his valuable input and for his assistance with the research protocol.
Abbreviations
- bSSFP
Balanced steady-state free precession
- CMR
Cardiovascular magnetic resonance
- CNR
Contrast to noise ratio
- LV
Left ventricle/left ventricular
- LVEDS
Left ventricular end-systolic volume
- LVEDS
Left ventricular end-systolic volume
- LVEDM
Left ventricular end-diastolic mass
- LVESM
Left ventricular end-systolic mass
- LVEF
Left ventricular ejection fraction
- LVSV
Left ventricular stroke volume
- MRI
Magnetic resonance imaging
- MI
Myocardial infarction
- rBW
Receiver bandwidth
- RF
Radiofrequency
- TR
Repetition time
- RV
Right ventricle/right ventricular
- RVEDV
Right ventricular end-diastolic volume
- RVESV
Right ventricular end-systolic volume
- RVEF
Right ventricular ejection fraction
- RVSV
Right ventricular stroke volume
- SAR
Specific absorption ratio
- SI
Signal intensity
- SNR
Signal-to-noise ratio
Authors’ contributions
WPB, AC, MYC, and SMS conceived of the study, design, coordination of the study, and drafting of the manuscript. AC, PK, and HX were involved in sequence programming and optimization, and image reconstruction. SLT, MYC, SMS, WPB, JLH, ML, and CM were involved in patient recruitment and enrollment. CM, DM, SMS, PK, HX, WPB, AC, and MYC were involved in the acquisition and interpretation of data. All authors were involved in the final editing of the manuscript and approve its content. The authors read and approved the final manuscript.
Funding
Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (Z1A-HL006213, Z1A-HL006220).
Competing interests
The authors are investigators on a US Government Cooperative Research and Development Agreement (CRADA) with Siemens Healthcare. Siemens participated in the modification of the CMR system from 1.5 T to 0.55 T.
Dr. Bandettini is principal investigator of a site involved in a multi-center trial sponsored by Bayer. The trial is unrelated to the current work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12968-020-00618-y.
References
- 1.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21(16):1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 2.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2(4):271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 3.Bellenger NG, Grothues F, Smith GC, Pennell DJ. Quantification of right and left ventricular function by cardiovascular magnetic resonance. Herz. 2000;25(4):392–399. doi: 10.1007/s000590050031. [DOI] [PubMed] [Google Scholar]
- 4.Blalock SE, Banka P, Geva T, Powell AJ, Zhou J, Prakash A. Interstudy variability in cardiac magnetic resonance imaging measurements of ventricular volume, mass, and ejection fraction in repaired tetralogy of Fallot: a prospective observational study. J Magn Reson Imaging. 2013;38(4):829–835. doi: 10.1002/jmri.24050. [DOI] [PubMed] [Google Scholar]
- 5.Cross R, Olivieri L, O'Brien K, Kellman P, Xue H, Hansen M. Improved workflow for quantification of left ventricular volumes and mass using free-breathing motion corrected cine imaging. J Cardiovasc Magn Reson. 2016;18:10. doi: 10.1186/s12968-016-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39(3):750–755. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 7.Rashid S, Han F, Gao Y, Sung K, Cao M, Yang Y, et al. Cardiac balanced steady-state free precession MRI at 0.35 T: a comparison study with 1.5 T. Quant Imaging Med Surg. 2018;8(7):627–636. doi: 10.21037/qims.2018.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonetti OP, Ahmad R. Low-field cardiac magnetic resonance imaging: a compelling case for cardiac magnetic resonance's future. Circ Cardiovasc Imaging. 2017;10(6):e005446. doi: 10.1161/CIRCIMAGING.117.005446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell-Washburn AE, Ramasawmy R, Restivo MC, Bhattacharya I, Basar B, Herzka DA, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology. 2019;293:190452. doi: 10.1148/radiol.2019190452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global Cardiovascular Magnetic Resonance Registry I. Kwong RY, Petersen SE, Schulz-Menger J, Arai AE, Bingham SE, et al. The global cardiovascular magnetic resonance registry (GCMR) of the society for cardiovascular magnetic resonance (SCMR): its goals, rationale, data infrastructure, and current developments. J Cardiovasc Magn Reson. 2017;19(1):23. doi: 10.1186/s12968-016-0321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue H, Kellman P, Larocca G, Arai AE, Hansen MS. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. J Cardiovasc Magn Reson. 2013;15:102. doi: 10.1186/1532-429X-15-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellman P, Chefd'hotel C, Lorenz CH, Mancini C, Arai AE, McVeigh ER. High spatial and temporal resolution cardiac cine MRI from retrospective reconstruction of data acquired in real time using motion correction and resorting. Magn Reson Med. 2009;62(6):1557–1564. doi: 10.1002/mrm.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue H, Inati S, Sørensen TS, Kellman P, Hansen MS. Distributed MRI reconstruction using gadgetron-based cloud computing. Magn Reson Med. 2015;73(3):1015-25. [DOI] [PMC free article] [PubMed]
- 14.Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54(6):1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 16.Bundy J, Simonetti O, Laub G, Finn JP. Segmented trueFISP cine imaging of the heart. Proc Int Soc Magn Reson Med. 1999.
- 17.Varghese J, Crabtree C, Craft J, Liu Y, Jin N, Ahmad R, et al. Cine and flow imaging at 0.35 T and comparison to 3T. SCMR. 2019:P238..
- 18.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. doi: 10.1016/S0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 19.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147(2):218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Maroules CD, McColl R, Khera A, Peshock RM. Interstudy reproducibility of SSFP cine magnetic resonance: impact of magnetic field strength and parallel imaging. J Magn Reson Imaging. 2008;27(5):1139–1145. doi: 10.1002/jmri.21343. [DOI] [PubMed] [Google Scholar]
- 21.Luijnenburg SE, Robbers-Visser D, Moelker A, Vliegen HW, Mulder BJ, Helbing WA. Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging. Int J Cardiovasc Imaging. 2010;26(1):57–64. doi: 10.1007/s10554-009-9501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 4. Bland Altman comparisons of LVEDV, LVESV, LVEDM, LVESM, LVSV, LVEF, RVEDV, RVESV, RVSV, and RVEF separated for breath-held and free-breathing cine acquisitions.