SUMMARY
BACKGROUND:
Cigarette smoking contributes to tuberculosis (TB) epidemiology. However, limited evidence exists on how smoking impacts TB treatment outcomes such as treatment loss to follow-up and culture conversion.
METHODS:
This meta-analysis assessed current evidence of the impact of active cigarette smoking on TB treatment outcomes. PubMed, Scopus, Embase, and the Cochrane Library were searched for English-language articles published from database inception through 2017. Articles addressing active pulmonary TB and cigarette smoking were identified and data abstracted. Smokers were defined as those who smoked every day or some days at the time of interview/diagnosis. Non-smokers did not smoke at the time of interview/ diagnosis. Unfavorable outcomes included any outcome other than cure or completion of TB treatment. Three different data sets were examined: 8 articles addressing unfavorable treatment outcomes, 9 analyzing only treatment loss to follow-up, and 5 addressing delayed smear or culture conversion. Studies that had <20 subjects or that addressed only populations with comorbidities were excluded.
RESULTS:
We identified 1030 studies; 21 studies fulfilled the inclusion/exclusion criteria. Smokers had greater odds of unfavorable outcomes (pooled odds ratio [pOR] 1.23, 95%CI 1.14–1.33), delayed smear or culture conversion (pOR 1.55, 95%CI 1.04–2.07), and treatment loss to follow-up (pOR 1.35, 95%CI 1.21–1.50).
CONCLUSION:
Cigarette smoking is associated with negative treatment results and delayed conversion to negative smear or culture, suggesting smoking is an important factor for consideration in TB elimination efforts.
Keywords: tobacco, conversion, loss to follow-up, Koch bacillus, tuberculosis
RÉSUMÉ
CONTEXTE:
La consommation de cigarettes contribue à l’épidémiologie de la tuberculose (TB). Mais il existe peu de données rdatives à l’impact du fait de fumer sur le résultat du traitement de la TB comme les pertes de vue et la conversion de culture.
MÉTHODE:
Cette méta analyse a évalué les données actuelles relatives à l’impact du tabagisme actif sur les résultats du traitement de la TB. Les articles en langue anglaise publiés du début de la base de données jusqu’en 2017 ont été recherchés sur PubMed, Scopus, Embase et la Cochrane Library. Les articles abordant la TB pulmonaire active et la consommation de cigarettes ont été identifiés et les données résumées. Les fumeurs ont été définis comme ceux qui fumaient chaque jour ou certains jours au moment de l’entretienldu diagnostic. Les non-fumeurs ne fumaient pas au moment de l’entretienldu diagnostic. Les résultats défavorables ont indus tous les résultats autres que la guérison ou l’achévement du traitement de TB. Trois ensembles de données différents ont été examinés : 8 articles abordant les résultats defavorables du traitement, 9 analysant seulement les pertes de vue et 5 abordant le retard de la conversion du frottis ou de la culture. Les études comprenant moins de 20 sujets ou consacrées seulement à des populations ayant des comorbidités ont été exclues.
RÉSULTATS:
Nous avons identifié 1030 études ; 21 études ont rempli les critères d’inclusionlexclusion. Les fumeurs ont eu des risques plus élevés de résultats défavorables (odds ratio combinés [pOR] 1,23; IC95% 1,14–1,33), retard de conversion du frottis ou de la culture (pOR 1,55; IC95% 1,04–2,07), et pertes de vue (pOR 1,35; IC95% 1,21–1,50).
CONCLUSION:
Le fait de fumer des cigarettes est associé à un résultat négatif du traitement et à un retard de conversion du frottis ou de la culture, suggérant qu’il s’agit d’un facteur important dont il faut tenir compte dans les activités d’étimination de la TB.
RESUMEN
MARCO DE REFERENCIA:
El consumo de cigarrillos es uno de los factores que modifican las características epidemiológicas de la tuberculosis (TB). Sin embargo, se cuenta con poca evidencia sobre la influencia del tabaquismo en los desenlaces terapéuticos de la enfermedad como Ia pérdida durante el seguimiento y la conversión dd cultivo.
MÉTODO:
En el presente metanàlisis se evaluó la evidencia existente sobre el efecto del tabaquismo activo en los desenlaces del tratamiento antituberculoso. Se realizó una búsqueda en las bases de datos PubMed, Scopus, Embase y la biblioteca Cochrane de artículos en inglés publicados desde el inicio de las rnismas hasta el 2017. Se escogieron los artículos que abordaban la TB pulmonar activa y el consumo de cigarrillos y se extrajeron los datos. Se definió como fumador la persona que fumaba todos los días o algunos días en el momento de la entrevista o del diagnóstic. Los no fumadores, no fumaban en el momento de la entrevista o del diagnóstico. Los desenlaces desfavorables incluyeron todo desenlace diferente de la curación o la compleción del tratamiento antituberculoso. Se analizaron tres conjuntos de datos, a saber: ocho artículos que examinaban los desenlaces desfavorables, nueve que abordaban solo la pérdida durante el seguimiento y 15 artículos que examinaban el retraso de conversión de la baciloscopia o el cultivo. Se excluyeron los estudios que contaban con menos de 20 participantes o que solo analizaban poblaciones con enfermedades concomitantes.
RESULTADOS:
Se encontraron 1030 estudios; 21 satisfacían los criterios de inclusión y exclusión. En los fumadores era mayor la probabilidad de un desenlace desfavorable (OR combinado [ORp] 1,23; IC95% 1,14–1,33), un retraso en la conversión de la baciloscopia o el cultivo (ORp 1,55; IC95% 1,04–2,07) y la pérdida durante el seguimiento del tratamiento (ORc 1,35; IC95% 1,21–1,50).
CONCLUSIÓN:
El consumo de cigarrillos se asoció con resultados desfavorables del tratamiento y retrasos en la conversión a una baciloscopia o un cultivo negativos, lo cual indica que el tabaquismo es un aspecto importante que debe tenerse en cuenta en las iniciativas de eliminación de la TB.
IN 2017, 1.6 MILLION PEOPLE died of tuberculosis (TB) worldwide.1 One potential factor promoting TB disease progression is smoking.2 High rates of smoking and of TB tend to occur in the same countries.2 About 80% of worldwide smokers live in low- and middle-income countries (LMICs), where the majority of TB deaths also occur.1,3
The 2014 US Surgeon General’s Report on smoking concluded that there was sufficient evidence to infer a causal relationship between smoking and increased risk of TB disease and mortality. Smokers have a TB disease risk approximately twice that of their non-smoking counterparts.4 Over 20% of the global TB cases are associated with smoking.4 Smoking might also impact the likelihood of progression to active disease although it is still unclear if the association between smoking and TB disease is due to increased infection risk or reactivation to active disease.4
This interplay is further supported by host pathogen mechanisms. In the process of infection, alveolar macrophages are the first line of defense against TB, engulfing inhaled bacteria.5 However, macrophage count is low in the alveoli, and macrophages must congregate and travel from other alveoli to the infection site.5 A 2016 study showed that smoking increases macrophage lysosomal debris, preventing them from migrating effectively to infection sites and removing bacteria before they enter cells.5
Despite strong evidence linking smoking and TB disease, evidence on the impact of smoking on TB treatment outcomes is still limited. These poor TB treatment outcomes might include acquired drug resistance, death, and disability from TB and might lead to TB transmission in the community.6 Drug resistance is of particular concern due to a paucity of new antibiotics for drug-resistant TB (DR-TB) treatment, requiring longer, and resulting in more toxic treatment regimens and lower success rates.6 In the 2016 Sustainable Development Goals (SDGs) Report, the World Health Organization (WHO), aimed to reduce TB incidence by 80% and TB death by 90% by 2030 while simultaneously reducing mortality due to non-communicable disease (largely impacted by tobacco use) by a third.7 If smoking leads to poorer TB outcomes, achieving these goals may be impossible without addressing smoking cessation in addition to other smoking-associated risk factors.
We aimed to determine the current evidence on the impact of smoking on TB treatment outcomes. We attempted to fill in the gaps from previous meta-analyses and provide a pooled effect size for the impact of smoking on unfavorable TB treatment results, such as treatment loss to follow-up (LTFU), and delayed smear or culture conversion.
METHODS
Article selection
The initial literature search was conducted between 22 September 2016 and 4 October 2016 of articles written in English or with English translations. Articles from database inception up to the time of the initial literature search were included. Articles were retrieved from four databases: EMBASE, Cochrane Library, PubMed, and Scopus using a structured and/or search strategy. Title screen, abstract screen, and full-text reviews were conducted to examine articles for relevance to both the exposure and outcome. Literature reviews, previous meta-analyses, editorials, and position statements identified through the initial search were also examined for relevant citations. An updated literature search was conducted on 25 August 2017 to identify newly published articles that matched the inclusion/exclusion criteria. No trial registries or unpublished studies were assessed. Reported summary measures of effect were used in the analysis. Included articles were reviewed by two secondary reviewers (SM and IA) to ensure that exposure definitions used in the included articles aligned with the inclusion criteria.
Inclusion criteria
Articles of all study designs with an appropriate control that addressed current cigarette smoking and the TB treatment outcomes of interest with a measure of effect were included. Articles that did not provide a confidence interval were not included in the final analysis. In studies with overlapping data, the study providing a more complete analysis with more information on subject selection, statistical methods, and controlled confounders was included in the final analysis. If both studies were comparable, the more recent publication was included.
Exposure
Current cigarette smoking included the use of cigarettes and bidis. Bidis are tobacco flakes rolled in paper or tendu leaves and then smoked. They are commonly used in India, Bangladesh, and other South Asian countries, especially among populations of lower socio-economi1; status.8 Analysis was limited to combustible forms of tobacco. Active smoking was defined by the personal use of cigarettes, rather than exposure to environmental/secondhand smoke. Current smokers were defined as those who smoked every day or some days at the time of the interview or diagnosis. Current non-smokers were those who may or may not have smoked in the past but did not smoke at the time of the interview or diagnosis. This grouping of former and never smokers in the same category may increase bias toward the null, causing the pooled effect measures to be more conservative. The exposure definitions were used as a quality control measure to decrease variability between studies.
Outcome
Categories of interest were treatment LTFU, delayed smear or culture conversion, and unfavorable outcomes as a combined category. Combined unfavorable outcomes included treatment failure, transfer to different treatment facilities, loss to follow-up, and death. Eight papers presented data as a combined unfavorable outcomes category that included all outcomes other than cure or completion; we therefore used this combined outcome as an outcome of interest. Nine articles were analyzed for treatment LTFU (including the results of Solliman et al.9) and five were analyzed for delayed smear or culture conversion. Treatment LTFU was defined as treatment interruption or treatment stop after initiation. Delayed smear or culture conversion was defined as prolonged culture or sputum smear positivity ≥2 months after treatment initiation. TB status, both at diagnosis and at the end of treatment, was determined through clinical and laboratory diagnostics including sputum smear, culture, clinical examination, and chest radiograph.
Exclusion criteria
Articles in all languages were included during the literature search but were examined for English translations after collection. Foreign language publications without English translation were excluded. Articles that addressed only populations with comorbidities, such as diabetes, HIV/AIDS (human immunodeficiency virus/acquired immune-deficiency syndrome), or renal disease were excluded because we aimed to identify the relationship between smoking and treatment outcomes in the general population. Furthermore, articles addressing only multidrug- and extensively drug-resistant TB (MDRIXDR-TB) were excluded, as treatment outcomes are poorer in these patients and dependent on many other factors. Case studies with fewer than 20 subjects were also excluded to prevent sample bias. Review articles and editorials were excluded.
Data abstraction
Articles underwent title and abstract screen performed by one reviewer (EW). Included articles then underwent further abstraction using a standardized data collection tool, with full-data abstraction conducted by one reviewer (EW). A subset of included studies (66%) were additionally reviewed and abstracted for key effect measures by two secondary reviewers (IA and SM) to ensure abstraction consistency. Information collected included study variables, such as sample size, exposure and definitions, LTFU, and study design, and patient variables such as diagnostic methods, and comorbidities. Studies were categorized into three groups based on type of outcome: unfavorable outcomes, treatment LTFU, and delayed smear or culture conversion.
Statistical analysis
Statistical analysis was conducted using STAT A v14 .2 (StataCorp, College Station, TX, USA) with STATA packages for meta-analysis using a fixed-model approach at an alpha level of 5%. Analysis was stratified by outcome type. No analysis contained fewer than three studies, and adjusted effect measures from multivariable analysis were used whenever available.
Pooled effect estimates and forest plots were calculated using study reported summary effect measures and confidence intervals (Cis). Risk ratios (RRs), when reported, were used as an estimated odds ratio (OR). Tests of study heterogeneity and P. were also conducted.10 Egger’s tests and funnel plots were used to assess for possible publication bias. Influence plots were created to identify studies that may be impacting the pooled result more than other studies and determine the consistency across studies grouped together.11
RESULTS
From the four databases used, 1030 references were identified; 261 duplicates were discarded. Articles were excluded based upon the predetermined inclusion and exclusion criteria. Four additional articles were identified through citations in reviews and editorials. The secondary literature search yielded no additional articles for inclusion. After abstract, title, data and reference list review, 21 references were eligible for inclusion. No randomized controlled trials were available due to the type of exposure and ethical considerations.
Unfavorable treatment outcomes
A total of 8 studies (1 case control and 7 cohort studies) were included in the analysis examining the impact of cigarette smoking on unfavorable treatment outcomes.9,12–18 Two of these studies provided RRs, which were used to estimate an OR for the pooled analysis. No significant heterogeneity was present between these eight studies (I2. 48.2%). A fixed-effects model was thus used to calculate the pooled effect estimate. In current smokers, the odds of unfavorable outcomes were 23% higher (pooled OR [pOR] 1.23, 95%0 1.14–1.33) than those of current non-smokers (Figure).
Figure.
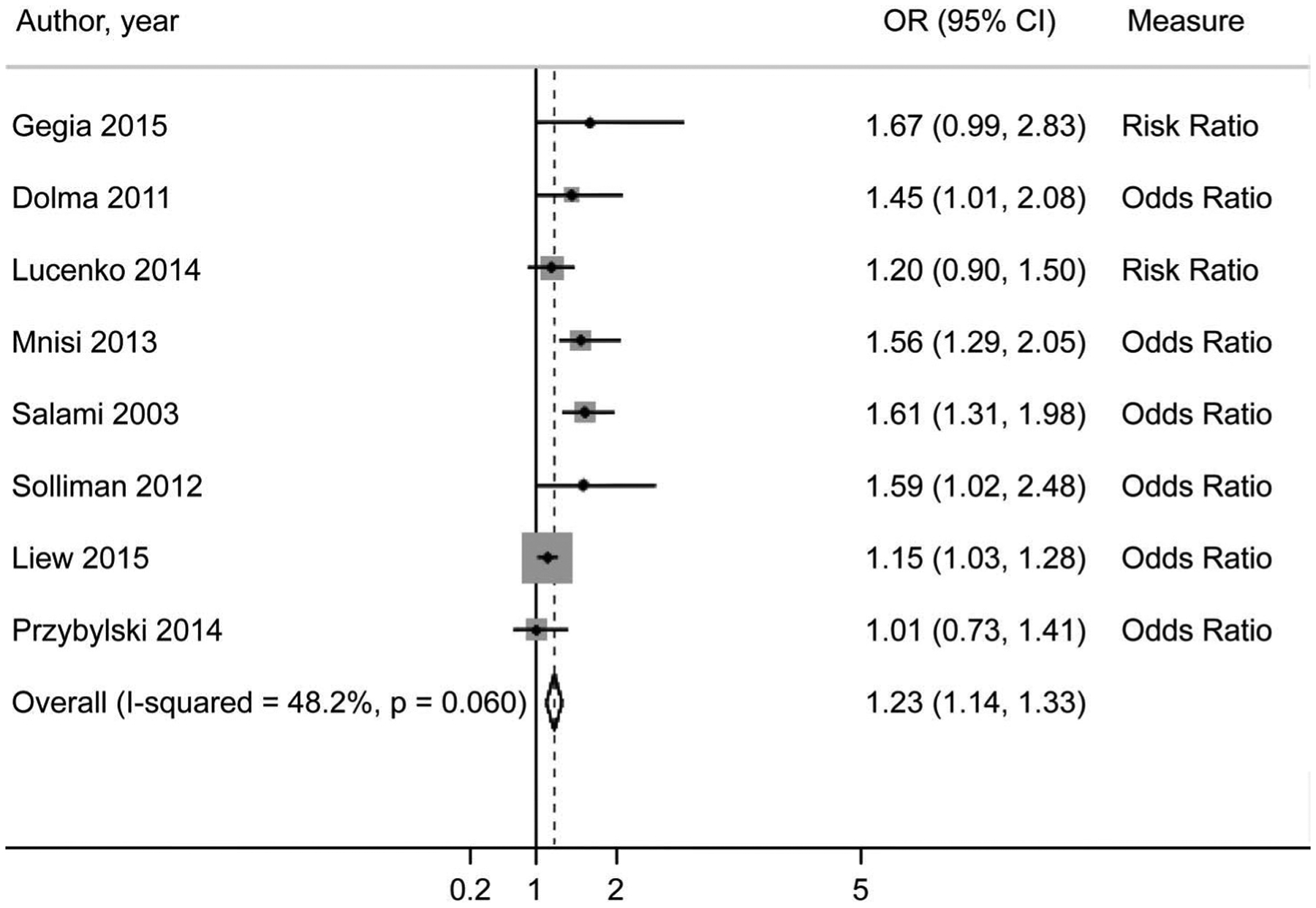
Forest plot of pooled effect estimate (OR) of current smokers and unfavorable treatment outcomes.9, 12–18 OR= odds ratio; Cl =confidence interval.
The mapped funnel plot is generally symmetrical, indicating minimal publication bias. This is further supported by a non-significant Egger’s test result (P = 0.078). The influence plots indicate that Liew et al. has a strong effect on the pooled results, and that omission of this study would yield a stronger pooled effect measure.17 This study has a low effect measure with a tight confidence interval, which are used to weight studies in the pooled analysis. Because of this weighting, the inclusion of this study, which reported a lower effect measure than the other studies, would drive the results toward the null.11,17
Delayed smear or culture conversion
A total of 5 studies (1 case control and 4 cohort studies) were included in the pooled analysis for the impact of active cigarette smoking on delayed smear or culture conversion.19–23 All included studies provided ORs. There was no significant heterogeneity present between the included studies. The fixed-effect model was thus used to estimate the pooled effect measure. In current smokers, the odds of delayed smear/culture conversion were 55% higher (pOR 1.55, 95%CI 1.04–2.07) than those of current non-smokers.
The mapped funnel plot to estimate the publication bias was asymmetrical, indicating published studies skew toward a positive association between smoking and delayed conversion, which suggests that the association found in the literature may be higher than in an operational setting. This was further supported by a significant Egger’s test result (P = 0.007). Furthermore, the mapped influence plot indicates that the Feng study has strong effect and decreases the pooled estimate.20
Treatment loss to follow-up
Nine studies (three case controls and six cohort studies) were included in the analysis of the impact between smoking and treatment LTfU.9,24–31 No significant difference between study heterogeneity was detected in the included studies. A fixed model was used. In current smokers, the odds of treatment LTFU were 35% higher (pOR 1.35, 95%CI 1.21–1.50) than those of current non-smokers.
The mapped funnel plot indicated that there was publication bias in the positive direction; this was further supported by a significant Egger’s test result (P = 0.002). The influence plot indicated that no individual study was driving the pooled effect measures for these results. Additional figures showing these results are available upon request.
DISCUSSION
In this meta-analysis, we found that active smoking was significantly associated with combined unfavorable treatment results, treatment LTFU, and delayed sputum smear or culture conversion. Our analysis showed that smoking increased the odds of unfavorable treatment outcomes overall; there was minimal publication bias.
Our results suggest that active smokers are more likely to have delayed smear or culture conversion, indicating a longer period of potential infectiousness, which could be predictive of prolonged treatment duration. Although prolonged treatment duration was not examined in this study, Atif et al. showed that smoking may be a risk factor for prolonged intensive phase treatment.32 Smoking increases the presence of particles in macrophage lysosomes, leading to reduced migration and TB granuloma breakdown.33 TB patients who smoke have reduced macrophage phagocytic capacity, suggesting that their macrophages are less capable of engulfing foreign bacteria than non-smoking TB patients and may travel more slowly than the macrophages of non-smokers.5,34 Our finding of increased odds of delayed smear or culture conversion may be explained in part by these mechanisms.
Smokers also seem more likely to be lost to treatment follow-up based on our findings. This may be due to socio-economic barriers and poor general health. The majority of the smokers live in LMICs, where healthcare access can be limited.3 Socio-economic status is an additional risk factor that needs to be considered when discussing TB.6 Smokers tend to have lower socio-economic status, suggesting potential additional financial barriers for smokers which may impact TB treatment outcome.6,7 Smoking also decreases overall general health and increases risk for other diseases such as chronic obstructive pulmonary disease (COPD), cardiovascular disease, diabetes, and rheumatoid arthritis, potentially decreasing the ability of smokers to travel.4,35 A limitation of our analysis is the inability to examine and control for these confounding variables (e.g., chronic alcoholism) at an individual level.36 These factors could only be considered if reported by the study. However, we attempted to mitigate this limitation by using multivariable adjusted results wherever possible.
Our study suggests a link between smoking and poor TB outcomes. Because of this, one could hypothesize that smoking cessation could be potentially associated with improved outcomes, but further study is needed. El Sony et al. has attempted to determine if smoking cessation was an effective and feasible intervention to decrease both smoking and TB. They found that patients enrolled in a tobacco cessation intervention had higher TB cure and treatment completion rates and were more likely to quit smoking.37 Our results also suggest the potential benefits of addressing smoking in TB control and elimination programs. Integration of tobacco control and TB treatment programs could target both epidemics simultaneously. In addition to informing TB patients of the risks of negative treatment outcomes associated with smoking, TB treatment support could include programmatic tools to promote tobacco cessation. For example, phone support services for smoking cessation could be integrated with text-based TB treatment reminders.38,39
Limitations of this meta-analysis include the observational nature of the data and poor quality of existing studies, publication bias, and lack of concise and standard definitions. Many studies also failed to address confounding variables, such as extent of disease, bacillary load, type of TB treatment and drug resistance, and reported only TB status without identifying the diagnostic method. In addition, inclusion of failure to complete TB treatment in the overall negative outcomes may also confound the results, as poor adherence to treatment or treatment LTFU is likely related to multiple causes, one of which could be smoking. Poor study quality may impact effect measures of LTFU, leading to skewed individual study results, as the LTFU may be differential. This was addressed by weighting using reported confidence intervals when calculating pooled effect estimates, and influence plots were examined to identify highly influential articles.
While TB outcomes are often standardized due to definitions established by the World Health Organization, smoking exposure was inconsistently reported across studies.40 A dose-response relationship could thus not be established between smoking and negative TB treatment outcomes. Due to the inconsistency of exposure definitions, the definitions abstracted from included articles were examined by secondary reviewers to ensure that interpretation of study exposures was consistent.
We found that smoking is associated with poorer overall treatment outcomes, including lower likelihood of the combined outcome of treatment completion or cure, and delayed smear or culture conversion. Poor treatment outcomes could have an effect on the potential for community TB transmission and acquired drug resistance,22,41 as delayed conversion can prolong infectiousness and increase transmission.22 This outcome does not influence acquired drug resistance directly, but can be a marker for inadequate treatment, including due to treatment LTFU or incorrect ingestion of drugs, which can in turn lead to acquired drug resistance. Failure to convert or to complete therapy successfully can also be related to extensive disease burden and death during TB treatment.41
New studies targeting outcomes such as progression from latent infection to active TB disease, relapse, disease severity, acquired resistance, transmission to others, and treatment success are needed. In addition, active smoking is not the only type of smoke exposure that impacts health. Research is also required to better understand the impact of secondhand smoke on TB treatment and outcomes. Further research in this area can provide a more complete picture of how the smoking and TB epidemics intersect, and help identify strategies for intervention.
CONCLUSION
Although the impact of smoking on TB has been partially addressed through observational studies, analysis and interpretation of these are difficult due to differences in controlling for confounders and the lack of standardized exposure definitions. Our meta-analysis indicates that smoking is associated with treatment LTFU, other pooled unfavorable outcomes, and delayed sputum or culture conversion. These results indicate that smoking is an important factor for consideration in TB elimination efforts, especially in areas with high rates of both smoking and TB.
Acknowledgements
The authors would like to thank J Genkinger for her statistical expertise and H Belcher for her mentorship.
This project was conducted as part of the James A Ferguson Emerging Infectious Disease Research Initiatives for Student Enhancement (RISE) Fellowship Program funded by the Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA). Columbia University Mailman School of Public Health (New York, NY, USA) funded a stipend for the corresponding author. This work was supported by the Cooperative Agreement (no 5USOMN000025) funded by the CDC and by an appointment to the Research Participation Program at CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the US Department of Health and Human Services, US Government. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Declaration of Interests:
EYW reports a student stipend from Columbia University Mailman School of Public Health, grants and an appointment in the Research Participation Program from CDC during the conduct of the study. The remaining authors have no conflicts of interest to disclose.
References
- 1.World Health Organization. Tuberculosis fact sheet. Geneva, Switzerland: WHO, 2018. http://www.who.int/en/news-room/fact-sheets/detail/tuberculosis. Accessed November 2019. [Google Scholar]
- 2.Amere GA, Nayak P, Salindri AD, Venkat Narayan KM, Magee MJ. Contribution of smoking to tuberculosis incidence and mortality in high tuberculosis burden countries. Am J Epidemiol2018. 187(9): 1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Tobacco fact sheet. Geneva, Switzerland: WHO, 2018. http://www.who.int/mediacentrelfactsheetslfs339/en/. Accessed November 2019. [Google Scholar]
- 4.US Department of Health and Human Services. The health consequences of smoking-50 years of progress: a report of the Surgeon General. Atlanta, GA: USA. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 5.Berg RD, Levitte S, O’Sullivan MP, et al. Lysosomal disorders drive susceptibility to tuberculosis by compromising macrophage migration. Cell 2016; 165(1): 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slama K, Chiang CY, Enarson DA, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis 2007; 11(10): 1049–1061. [PubMed] [Google Scholar]
- 7.World Health Organization. World Health Statistics, 2016: monitoring health for the SDGs, Sustainable Development Goals. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 8.Rahman M, Sakamoto J, Fukui T. Bidi smoking and oral cancer: a meta-analysis. Int J Cancer 2003; 106(4): 600–604. [DOI] [PubMed] [Google Scholar]
- 9.Solliman MA, Hassali MA, Al-Haddad MS, et al. Treatment outcome of new smear-positive pulmonary tuberculosis patients in north east Libya. Lat Am J Pharm 2012; 31(4): 567–573. [Google Scholar]
- 10.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011; 342: d549. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JA, Bradburn MJ, Egger M. Meta-analysis in Stata In: Egger M, Smith GD, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. London, UK: BMJ Publishing Group, 2001: pp 347–369. [Google Scholar]
- 12.Gegia M, Magee MJ, Kempker RR, et al. Tobacco smoking and tuberculosis treatment outcomes: a prospective cohort study in Georgia. Bull World Health Organ 2015; 93(6): 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolma KG, Adhikari L, Mohapatra PK, Mahanta J. Determinants for the retreatment groups of pulmonary tuberculosis patients treated in a DOTS programme in Sikkim, India. Indian J Tuberc 2011; 58(4): 178–188. [PubMed] [Google Scholar]
- 14.Lucenko I, Riekstina V, Perevoscikovs J, et al. Treatment outcomes among drug-susceptible tuberculosis patients in Latvia, 2006–2010. Public Health Action 2014; 4(Suppl 2): S54–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mnisi T, Turnbo J. Govender I. Factors associated with pulmonary tuberculosis outcomes among inmates in Potchefstroom Prison in North West Province. S Mr J Infect Dis 2015; 28(2): 96–101. [Google Scholar]
- 16.Salami AK, Oluboyo PO. Management outcome of pulmonary tuberculosis: a nine year review in Ilorin. West Afr J Med 2003; 22(2): 114–119. [DOI] [PubMed] [Google Scholar]
- 17.Liew SM, Khoo EM, HoB K, et al. Tuberculosis in Malaysia: predictors of treatment outcomes in a national registry. Int J Tuberc Lung Dis 2015; 19(7): 764–771. [DOI] [PubMed] [Google Scholar]
- 18.Przybylski G, Dabrowska A, Trzcinska H. Alcoholism and other soda-demographic risk factors for adverse TB-drug reactions and unsuccessful tuberculosis treatment-data from ten years’ observation at the Regional Centre of Pulmonology, Bydgoszcz, Poland. Med Sci Manit 2014; 20:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pefura-Yone EW, Kengne AP, Kuaban C. Non-conversion of sputum culture among patients with smear positive pulmonary tuberculosis in Cameroon: a prospective cohort study. BMC Infect Dis 2014; 14: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng JY, Huang SF, Ting WY, et al. Gender differences in treatment outcomes of tuberculosis patients in Taiwan: a prospective observational study. Clin Microbial Infect 2012; 18(9): E331–E337. [DOI] [PubMed] [Google Scholar]
- 21.Maciel EL, Brioschi AP, Peres RL, et al. Smoking and 2-month culture conversion during anti-tuberculosis treatment. Int J Tuberc Lung Dis 2013; 17(2): 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijenbandring de Boer R, Oliveira e Souza Filho JB, Cobelens F, et al. Delayed culture conversion due to cigarette smoking in active pulmonary tuberculosis patients. Tuberculosis (Edinb) 2014; 94(1): 87–91. [DOI] [PubMed] [Google Scholar]
- 23.Abal AT, Jayakrishnan B, Parwer S, El Shamy A, Abahussain E, Sharma PN. Effect of cigarette smoking on sputum smear conversion in adults with active pulmonary tuberculosis. Respir Med 2005; 99(4): 415–420. [DOI] [PubMed] [Google Scholar]
- 24.Rathee D, Arora P, Meena M, et al. Comparative study of clinico-bacterio-radiological profile and treatment outcome of smokers and nonsmokers suffering from pulmonary tuberculosis. Lung India 2016; 33(5): 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinidiyapathirage J, Senaratne W, Wickremasinghe R. Prevalence and predictors of default with tuberculosis treatment in Sri Lanka. Southeast Asian J Trap Med Public Health 2008; 39(6): 1076–1082. [PubMed] [Google Scholar]
- 26.Ahmad SR, Velhal GD. Study of treatment interruption of new sputum smear positive TB cases under DOTS strategy. lnt J Med Sci Public Health 2014; 3(8): 977–981. [Google Scholar]
- 27.Tachfouti N, Slama K, Berraho M, et al. Determinants of tuberculosis treatment default in Morocco: results from a national cohort study. Pan Afr Med J 2013; 14: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slama K, Tachfouti N, Obtel M, Nejjari C. Factors associated with treatment default by tuberculosis patients in Fez, Morocco. East Mediterr Health J 2013; 19(8): 687–693. [PubMed] [Google Scholar]
- 29.Roy N, Basu M, Das S, Mandai A, Dutt D, Dasgupta S. Risk factors associated with default among tuberculosis patients in Darjeeling district of West Bengal, India. J Family Med Prim Care 2015; 4(3): 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santha T, Garg R, Frieden TR, et al. Risk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur District, South India, 2000. Int J Tuberc Lung Dis 2002; 6(9): 780–788. [PubMed] [Google Scholar]
- 31.Chang KC, Leung CC, Tam CM. Risk factors for defaulting from anti-tuberculosis treatment under directly observed treatment in Hong Kong. lnt J Tuberc Lung Dis 2004; 8(12): 1492–1498. [PubMed] [Google Scholar]
- 32.Atif M, Sulaiman SA, Shafie AA, Babar ZU. Duration of treatment in pulmonary tuberculosis: are international guidelines on the management of tuberculosis missing something? Public Health 2015; 129(6): 777–782. [DOI] [PubMed] [Google Scholar]
- 33.Russell DG, Barry CE 3rd, Flynn JL. Tuberculosis: what we don’t know can, and does, hurt us. Science 2010; 328(5980): 852–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aryanpur M, Mortaz E, Masjedi MR, et al. Reduced phagocytic capacity of blood monocyte/macrophages in tuberculosis patients is further reduced by smoking. Iran J Allergy Asthma Immunol2016; 15(3): 174–182. [PubMed] [Google Scholar]
- 35.National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. Health effects of cigarette smoking. Atlanta, GA, USA: Centers for Disease Control and Prevention, 2018. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm. Accessed November 2019. [Google Scholar]
- 36.Volkmann T, Moonan PK, Miramontes R, Oeltmann JE. Excess alcohol use and death among tuberculosis patients in the United States, 1997–2012. J Tuberc Res 2016; 4(1): 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Sony A, Slama K, Salieh M, et al. Feasibility of brief tobacco cessation advice for tuberculosis patients: a study from Sudan. Int J Tuberc Lung Dis 2007; 11(2): 150–155. [PubMed] [Google Scholar]
- 38.World Health Organization. Developing and improving national toll-free tobacco quit line services. Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 39.World Health Organization. Handbook for the use of digital technologies to support tuberculosis medication adherence. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 40.World Health Organization. Definitions and reporting framework for tuberculosis- 2013 revision (updated December 2014). Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 41.Djouma FN, Noubom M, Ateudjieu J, Donfack H. Delay in sputum smear conversion and outcomes of smear-positive tuberculosis patients: a retrospective cohort study in Bafoussam, Cameroon. BMC Infect Dis 2015; 15: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]


