Abstract
Introduction: Many patients continue to receive suboptimal services, inappropriate, unsafe, and costly care. Underutilization of research by health professionals is a common problem in the primary care setting. Although many theoretical frameworks can be used to help address such evidence-practice gaps, health care professionals may not be aware of the benefits of frameworks or of the most appropriate ones for their context and thus, may be faced with the challenge of selecting and using the most relevant one. Aim: The aim of this article was to describe the process used to adapt a knowledge translation framework to meet the local needs of health professionals working in one large primary care setting. Methods: The authors developed a 5-step approach for guideline implementation. This approach was informed by prior research and the authors’ experiences in supporting multidisciplinary teams of health care professionals during the implementation of evidence-based clinical guidelines into primary care practices. To ensure that the 5-step approach was practical and suitable for the context of guideline implementation by multidisciplinary teams in primary health care, the implementation team adapted the “knowledge-to-action” framework using a multistep process. Results: The implementation approach consisted of the following 5 steps: identification, context analysis, development of implementation plan, evaluation, and sustainability. All 5 steps were described alongside details about a national low back pain project. Discussion: This article describes a collaborative, grassroots process that addressed an identified need in one complex context by adapting a knowledge translation framework to meet the local needs of health professionals working in primary care settings. Existing implementation frameworks may be too complex or abstract for use in busy clinical contexts. The 5-step approach presented in this paper resulted in practical steps that are more readily understood by health care professionals and staff on “the ground.”
Keywords: implementation, evidence-based practice, quality improvement, knowledge translation, clinical practice guideline
Introduction
Health care systems still fail to guarantee that effective and cost-efficient care gets to all patients who need it.1 As a result, many patients continue to receive suboptimal services, inappropriate, unsafe, and costly care.1,2 Like with many areas in health care, underutilization of research by health professionals is a common problem in the primary care setting.1,3,4
As the volume of research evidence is growing at a rapid pace, health care professionals may be challenged to remain up to date with the latest findings from published research. Clinical practice guidelines, which include recommendations that are based on the best available evidence, aim to facilitate the uptake of evidence-based practices.5,6 Guidelines offer many potential benefits to health care professionals, patients, and health systems by supporting decision making and enhancing the efficiency and quality of health services, while reducing practice variations.7 However, guideline implementation is a complex process that can be hindered by a range of individual-, organizational-, and systems-level barriers.8-12 For instance, lack of awareness of, and negative attitudes toward existing guidelines, limited time to consult and appraise research and organizations that advocate for efficiency and productivity are among common barriers across practice areas and countries.13-15
Specific barriers likely impeding guideline implementation in primary health care practice in the Belgian context include health professionals’ lack of knowledge, skills, motivation in applying research in practice, and resistance to changing routine practice.16 In an attempt to overcome these barriers, ebpracticenet (a Belgian organisation responsible for guideline dissemination and implementation) mandated a team of implementation facilitators (composed of a postdoctoral researcher in medical education and implementation sciences [SP] and a social scientist [JC] experienced in change management in health care) to support the uptake of best practises in multidisciplinary groups managing conditions such as low back pain in the primary care setting. The implementation team chose to adopt a systematic, evidence-based and tailored approach to guide knowledge translation activities in primary care settings. The team aimed to (1) provide health care professionals with a theoretical foundation for the chosen implementation projects and (2) offer practical support to help multidisciplinary teams with the implementation of a low back pain guideline17 in primary health care. Low back pain is a leading cause of disability, affecting over 630 million people worldwide,18 resulting in considerable burden and high cost to society.19 The 1-year prevalence is estimated at over 40%, the lifetime prevalence at 70%, and the 1-year recurrence rate at over 30%, resulting in significant burden and high cost to society.20 Given that many health care professionals are involved in the diagnosis and treatment of low back pain, it is helpful to understand the steps involved in reducing evidence-practice gaps, as described in this article.
Knowledge translation (KT) is a process used to help reduce the evidence-practice gaps and enhance the uptake of research into practice.1 A recommended first step in KT or implementation initiatives is the selection of a conceptual framework or model that is grounded in robust theories of behavior change.
Implementation researchers have argued that the use of theoretical frameworks is helpful for increasing the likelihood of successful guideline implementation and in turn, reducing the research-practice gap.21-28 Theoretical frameworks can be used to (1) describe or guide the process of guideline implementation, (2) identify the problem (evidence-practice gap) to be addressed, (3) identify determinants of change (ie, related barriers and enablers), (4) develop tailored KT interventions to address evidence-practice gaps, and (5) evaluate the impact of the KT interventions.23,29-32
While there is increasing interest in the use of theoretical frameworks in implementation research, few implementation projects have made use (or good use) of these.21,31-38 The sheer number of frameworks has led to concerns among researchers about the challenges associated with selecting the most suitable framework for a given implementation project.23,27,39-41 The choice of framework is typically guided by the project purpose.42 Guideline implementers and health care professionals working in primary care may not be aware of the benefits of frameworks or of the most appropriate ones for their context and thus, may be faced with the challenge of selecting and using the most relevant one.42
The aim of this article was to describe the process used to adapt a KT framework to meet the local needs of health professionals working in one large primary care setting.
Methods
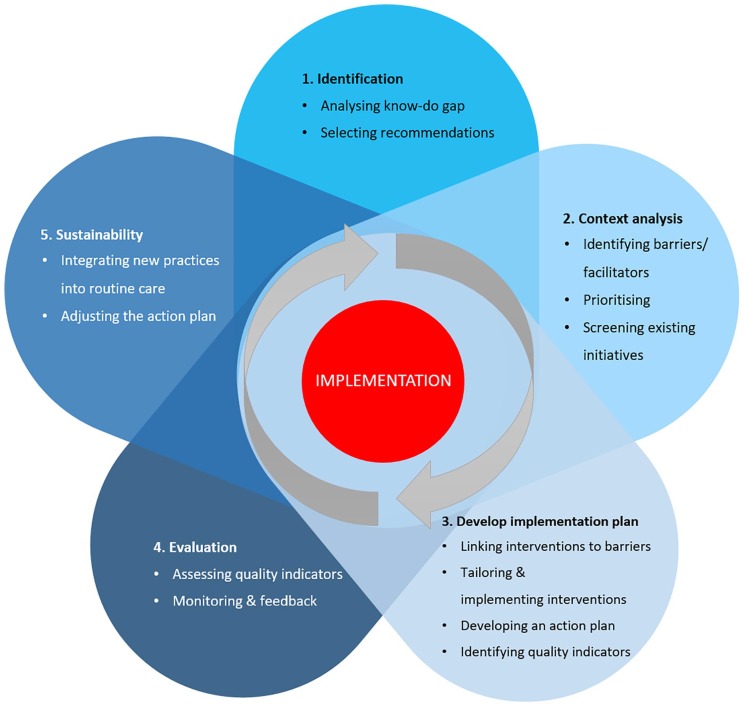
The authors developed a 5-step approach for guideline implementation (see Figure 1). This approach was informed by prior research43,44 and the authors’ experiences in supporting multidisciplinary teams of health care professionals during the implementation of evidence-based clinical guidelines into primary care practices. We describe the steps next.
Figure 1.
A 5-step approach for guideline implementation.
Selection of a Theoretical Framework
To familiarize themselves with the literature, SP and JC reviewed and considered all articles mentioned in the article by Nilsen et al32 on implementation theories, models, and frameworks. Moreover, articles that were found in databases or were recommended by senior researchers in the field were reviewed. Additionally, discussions with researchers at international conferences helped gaining a better insight into the variety of available theoretical frameworks. Overall, many different frameworks and models exist (ie, process models, determinant frameworks, classic theories, implementation theories and evaluation frameworks), each one serving a different purpose.32,45 For example, process models outline the steps used to move evidence into practice whereas determinant frameworks are helpful for identifying and understanding the factors that are likely to support or inhibit behavior change.32
The 5-step approach displayed in Figure 1 is adapted from the well-known “knowledge-to-action (KTA)” framework.43,44 Given the mandate of ebpracticenet, the KTA framework was selected because it is user friendly, clear, frequently used in implementation research, and aims to guide the whole process of moving research into practice.32,44,46,47 Other well-known advantages of the KTA framework include collaboration between stakeholders, consideration of the local context, and the iterative and cyclical nature of the KT process.43,48-52
A process framework was in line with what the implementation team of ebpracticenet needed at this stage. The KTA framework contains two principal components: A knowledge creation funnel and an action cycle. The action cycle refers to the implementation process and consists of 7 stages: identifying a problem in practice or a gap in knowledge and identifying, reviewing, and selecting the knowledge to be implemented to address the gap; adapting or customizing the knowledge to the local context; evaluating the determinants of the knowledge use; selecting, tailoring and implementing interventions to address the knowledge or practice gap; monitoring the knowledge use in practice; evaluating the outcomes or impact of using the new knowledge; and determining strategies for ensuring that the new knowledge is sustained.43
Development of the 5-Step Approach for Guideline Implementation
To ensure that the 5-step approach (Figure 1) was practical and suitable for the context of guideline implementation by multidisciplinary teams in primary health care, the implementation team adapted the KTA framework using a multistep process. SP and JC had multiple discussions about the adaptations that were necessary to accommodate the local context. They reached consensus about the following aspects.
First, the 7 stages of the KTA framework were reduced to 5 steps with clear and succinct names: identification, context analysis, development of implementation plan, evaluation, and sustainability. This was done in order to make the names of the steps as comprehensible and practical as possible.
Second, SP and JC included a subset of embedded tasks for each of the 5 steps, as shown in Figure 1, to make the 5-step approach practical and user-friendly. All tasks were based on the KTA framework, except for the following tasks that were added based on an exploratory literature search and the implementation facilitators’ professional experiences: “selecting recommendations”10 (in step 1), “screening existing initiatives” (in step 2), and “integrating new practice in routine care”53 (in step 5).
Third, additional details were provided for steps that were deemed too abstract or too generic by the implementation facilitators, such as Tables 2 and 3, which were adopted from other research.53,54
Table 2.
Levels of Potential Barriers and Facilitators for Implementation: Adaptation of Existing Classification.54
| Level | Examples | What Are (Potential) Barriers? | What Are Facilitators? |
|---|---|---|---|
| Clinical guideline/pathway | Advantages in practice, feasibility, credibility, accessibility, attractiveness | ||
| Individual professional | Awareness, knowledge, attitude, motivation to change, behavioral routines | ||
| Patient | Knowledge, skills, attitude, compliance | ||
| Social context | Opinion of colleagues, culture of the network, collaboration, leadership | ||
| Organizational context | Organization of care processes, staff, capacities, resources, structures | ||
| Economic and political context | Financial arrangements, regulations, policies |
Table 3.
Steps to Support Implementing Interventions Based on a Model for Inducing Change in Professional Behavior.53
| Orientation | 1. Promote awareness of change 2. Stimulate interest and involvement |
| Insight | 3. Create understanding 4. Develop insight into own routines |
| Acceptance | 5. Develop positive attitude to change 6. Create positive intentions/decision to change |
Moreover, a few practical hands-on tools were included to facilitate some of the steps as SP and JC found the description in the original KTA framework too limited. Table 1, which was based on other academic work, was added.10,55
Table 1.
A Tool to Facilitate Selection of Recommendations.10
| Guideline recommendation | Are the consequences of nonadherence serious? | Is there a large amount of nonadherence or inequitable adherence? | Is the recommended practice feasible in the targeted settings? | Is implementing the recommendation a priority? |
|---|---|---|---|---|
Finally, a group of end users provided feedback on the perceived usefulness of the 5-step approach during a formal meeting facilitated by SP and JC. Participants included a general practitioner with experience in implementation research (MV) and several health professionals involved in the low back pain project (see description of the stakeholder group below in step 1 “Identification”).
1. Identification
The first step consisted of forming a team of relevant stakeholders (eg, health care professionals, relevant organizations and agencies, policymakers, patient groups, and KT experts) who could work collaboratively to facilitate the entire implementation process and ensure that the results would be applicable and relevant to the local context.56 The stakeholder in the national implementation project on low back pain included physiotherapists, general practitioners, sport physicians, psychologists, a pain therapist, neuro surgeons, an orthopedic surgeon, an anesthesiologist, government staff, professional associations, and a patient representative. This group of 30 stakeholders was involved in all steps of Figure 1 to make sure that the implementation project was relevant and suitable to the local context.
Analyzing Know-Do Gap
Before the team could work toward improving the quality of care, we needed to measure the extent of the gap between best evidence and current practice in low back pain.43,57 This was done by collecting data from over 350 general practitioners’ electronic health records and a national health insurance database.
Selecting Recommendations
Given that implementing an entire guideline at once likely not feasible, the aim of this step was to prioritize the guideline recommendations with the greatest potential for benefit. Table 1 helped facilitate this selection process, while considering the know-do gap. For each guideline recommendation, the various criteria represented in Table 1 were scored as 1 (no), 2 (probably not), 3 (uncertain), 4 (probably), or 5 (yes).10 Each stakeholder individually scored the low back pain guideline individually and sent his/her scores to SP and JC who summarized all responses. This summary formed the starting point for a group discussion with all relevant stakeholders. The discussion ended with a list of key recommendations that became the target of the KT interventions. An example of such a key recommendation was “consider an exercise programme (specific exercises or a combination of approaches) for people with low back pain with or without radicular pain. Take patient’s specific needs, capabilities and preferences into account when choosing the type of exercise programme.”
2. Context Analysis
Identifying Barriers and Facilitators
Models and frameworks designed to identify barriers and facilitators share a number of similarities.32,58,59 Grol and Wensing54 categorized barriers and facilitators in 6 levels: innovation, individual professional, patient, social context, organizational context, and economic and political context. Table 2 shows examples for each of these levels. The 2 implementation facilitators of ebpracticenet replaced “innovation” with “clinical guideline/pathway” as it made more sense in their local context. In the process facilitated by SP and JC, the low back pain project stakeholders were asked to individually identify barriers and facilitators for the key recommendations that were selected in the previous step. It was important that an adequate number of stakeholders be involved to make sure that as many barriers and facilitators as possible were identified. The individual lists of barriers and facilitators were sent to SP and JC who summarized these into organized themes. This summary formed the starting point for a group discussion with all stakeholders involved in the low back pain project.
Prioritizing
All barriers and facilitators were ranked based on the degree of “know-do gap,” “perceived importance,” and “modifiability.”10 This ranking was completed during a group discussion with all involved stakeholders. The group discussion ended with a few key barriers that the team agreed to focus on during the subsequent steps of the implementation process. An example of such a key barrier was “many patients with low back pain prefer a passive treatment, such as surgery, rather than an exercise programme but they are not aware of the advantages and disadvantages of both approaches.”
Screening Existing Initiatives
Before selecting an appropriate implementation strategy to address a specific barrier, it was important to inquire about existing or completed initiatives within primary care in Belgium targeting such barrier. Stakeholders of the low back pain project contacted colleagues and other individuals who were responsible for other initiatives. Lessons were learned from these initiatives and the new implementation strategies were built on and strengthened existing projects.
3. Develop Implementation Plan
Linking Interventions to Barriers
Careful thought and considerable judgement must go into selecting implementation strategies or interventions, as well as tailoring strategies to previously identified barriers while considering the characteristics of the practice environment.55 As few unique strategies can successfully address a range of barriers and change practice, different combinations of strategies were considered.8 During the low back pain project, evidence of the effectiveness of existing international implementation strategies53,60,61 was consulted but this was considered along with the information gained in steps 1 and 2. We also took guidance on selecting and reporting implementation strategies62-64 into account. The following interventions were chosen for the low back pain project:
Professional development for health professionals consisting of an e-learning module about the content of the low back pain guideline which was individually completed online by health professionals in preparation of multidisciplinary face-to-face sessions, containing 20 participants with a mix of general practitioners, physiotherapists and psychologists. These 2-hour sessions (approximately 30 in total) focused briefly on the content of the guideline while allowing sufficient time to discuss patient cases and multidisciplinary communication and collaboration.
A 1-page leaflet, poster, and short video about key messages for patients, such as “keep moving because this is healthy and it strengthens your back.” This information was disseminated via multiple channels (eg, general practitioners).
Key messages for general practitioners were added in the clinical decision support system of their electronic health record in order to assist them during consultations with patients suffering low back pain.
All interventions took place in all parts of Belgium, which meant that all information was available in French and Dutch.
Though the low back pain project focused mainly on primary care, the team also aimed to work in partnership with additional implementation initiatives in the hospital settings, such as an e-learning module and poster, containing key messages of the low back pain guideline, for all staff at the emergency department of a main hospital in Belgium.
Tailoring and Implementing the Interventions
It was essential to adapt the interventions to the target group and local context. The steps in Table 3 helped the implementation of the interventions.
Developing an Action Plan
To increase the likelihood of successful change, it was important to develop a concrete action plan with details about who is doing what, how, and when.65 Although all stakeholders were involved in the entire implementation process, they were essential in the development of this plan. The plan also facilitated the follow-up of all actions. It was useful to pilot test the implementation plan on a small scale and then scale up gradually.53
Identifying Quality Indicators
Which quality indicators or outcome measures are relevant to evaluate depends on the selected interventions. The lists by Flottorp et al10 as well as Proctor et al66 provided an array of possible quality indicators: structural, process, and outcome indicators (eg, system or organizational level, practice level, and individual level, including health care provider and patient health outcomes).55,67 For the development of high-quality indicators regarding low back pain, rigorous and evidence-based development methods68 were used. First, a systematic literature review was performed to identify indicators used internationally. Second, a Delphi study containing 2 rounds was conducted. In the first round, experts were asked to score the list of indicators, which resulted from the literature review, based on their relevance. The 64 participating experts could also suggest additional indicators. In the second round, experts were able to adjust their score based on the average score of the whole expert group. Afterward, 12 experts discussed in a focus group which indicators were realistic and measurable. A subsequent consensus meeting with 14 individuals resulted in a final list of 17 quality indicators. An example of such indicators is “number of patients with low back pain referred to physiotherapist per 1000 inhabitants.”
4. Evaluation
Assessing Quality Indicators
Operationalization of quality indicators will depend on various elements such as feasibility and the type of quality indicator. The low back pain data for this evaluation phase as well as step 5 “sustainability” are forthcoming.
Monitoring and Feedback
The stakeholders of the low back pain project will develop a plan about “what and when” to monitor or evaluate, as well as what type of feedback is useful for the stakeholders. Ideally, the monitoring process should be ongoing and iterative in order to adjust the strategies and action plan.
5. Sustainability
Integrating New Practices Into Routine Care
It is suggested that changes are integrated into routine care and embedded into the organization in order to make the practice change as sustainable as possible.53 Execution of this phase will depend on factors such as the local context and the type of KT strategies. The strategy concerning the clinical decision support system in general practitioners’ electronic health record is already integrated into routine care, which is likely to enhance the chances of sustainable change. Other relevant actions to increase sustainability will be discussed with the team of stakeholders.
Adjusting the Action Plan
The action plan will be adjusted based on monitoring and evaluation of outcomes. It is also possible that new barriers and facilitators will be identified over time. This underscores the cyclical nature of the implementation process.
Discussion
Many theoretical frameworks can be used to support the translation and uptake of research findings into health care practices.32 However, health care professionals may not be aware of, and ready to use frameworks for specific knowledge translation initiatives.42 This article describes a collaborative, grassroots process that addressed this issue by adapting a KT framework to meet the local needs of health professionals working in primary care settings. Existing implementation frameworks may be too complex or abstract for use in busy clinical contexts, which is in line with health care professionals’ perception that guidelines are too complex to be useful.16 Therefore, the 5-step approach presented in this article resulted in hands-on, user-friendly, practical steps that are more readily understood by health care professionals and staff on “the ground.” The 5-step process can support (multidisciplinary) teams with guideline implementation projects at different levels (individual, regional, or national). Although the 5-step approach for guideline implementation was developed for the primary care setting, it may also be adapted for use in other settings.
There are many reasons for failure to implement clinical practice guidelines, including the guideline itself (eg, ambiguity, inconsistency, and incompleteness).69 Consequently, it is essential that guideline implementers collaborate with guideline developers to increase guideline implemenentability.69 Still, there may be other barriers, such as health professionals skepticism toward the scientific evidence underlying guideline recommendations, patient characteristics (eg, the patient’s lack of compliance with recommended care) and financial barriers (eg, treatment not reimbursed by health insurance).16 Therefore, clinical decision making should consider recent evidence-based clinical practice guideline recommendations in combination with patient’s preferences and values, and health care professionals’ clinical expertise within the local context of primary care.70
Although guideline implementation is very complex and there are many causes for failure, some problems may be avoided by an understanding of existing barriers prior to implementing the KT intervention. The 5-step approach described in this article can increase professionals’ awareness of these issues. Depending on the health care professionals’ needs, however, additional models or frameworks can be considered for a particular purpose, such as identifying barriers or evaluating outcomes of implementation efforts.42 Nevertheless, this article provides a practical starting point for health care professionals who want to improve guideline implementation in primary care settings.
Acknowledgments
The authors would like to express their gratitude to all stakeholders involved in the national low back pain project.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Sanne Peters  https://orcid.org/0000-0001-6235-1752
https://orcid.org/0000-0001-6235-1752
Bart Depreitere  https://orcid.org/0000-0002-7458-0648
https://orcid.org/0000-0002-7458-0648
References
- 1. Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? 1998. Milbank Q. 2005;83:843-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glasziou P, Straus S, Brownlee S, et al. Evidence for underuse of effective medical services around the world. Lancet. 2017;390:169-177. [DOI] [PubMed] [Google Scholar]
- 4. Villar-Alvarez F, Moreno-Zabaleta R, Mira-Solves JJ, et al. Do not do in COPD: consensus statement on overuse. Int J Chron Obstruct Pulmon Dis. 2018;13:451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alonso-Coello P, Irfan A, Sola I, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010;19:e58. [DOI] [PubMed] [Google Scholar]
- 6. Lenzer J, Hoffman JR, Furberg CD, Ioannidis JP; Guideline Panel Review Working Group. Ensuring the integrity of clinical practice guidelines: a tool for protecting patients. BMJ. 2013;347:f5535. [DOI] [PubMed] [Google Scholar]
- 7. Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mickan S, Burls A, Glasziou P. Patterns of “leakage” in the utilisation of clinical guidelines: a systematic review. Postgrad Med J. 2011;87:670-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci. 2013;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagliardi AR. “More bang for the buck”: exploring optimal approaches for guideline implementation through interviews with international developers. BMC Health Serv Res. 2012;12:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagliardi AR, Alhabib S; Members of Guidelines International Network Implementation Working Group. Trends in guideline implementation: a scoping systematic review. Implement Sci. 2015;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grimmer-Somers K, Lekkas P, Nyland L, Young A, Kumar S. Perspectives on research evidence and clinical practice: a survey of Australian physiotherapists. Physiother Res Int. 2007;12:147-161. [DOI] [PubMed] [Google Scholar]
- 14. Sharek PJ, Mullican C, Lavanderos A, et al. Best practice implementation: lessons learned from 20 partnerships. Jt Comm J Qual Patient Saf. 2007;33(12 suppl):16-26. [DOI] [PubMed] [Google Scholar]
- 15. Cortoos PJ, De Witte K, Peetermans WE, Simoens S, Laekeman G. Opposing expectations and suboptimal use of a local antibiotic hospital guideline: a qualitative study. J Antimicrob Chemother. 2008;62:189-195. [DOI] [PubMed] [Google Scholar]
- 16. Hannes K, Goedhuys J, Aertgeerts B. Obstacles to implementing evidence-based practice in Belgium: a context-specific qualitative evidence synthesis including findings from different health care disciplines. Acta Clin Belg. 2012;67:99-107. [DOI] [PubMed] [Google Scholar]
- 17. Van Wambeke P, Desomer A, Ailliet L, et al. Low back pain and radicular pain: assessment and management—summary. https://kce.fgov.be/sites/default/files/atoms/files/KCE_287C_Low_back_pain_Summary.pdf. Accessed March 17, 2020.
- 18. Hartvigsen J, Hancock MJ, Kongsted A, et al. ; Lancet Low Back Pain Series Working Group. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356-2367. [DOI] [PubMed] [Google Scholar]
- 19. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389:736-747. [DOI] [PubMed] [Google Scholar]
- 21. Davies P, Walker AE, Grimshaw JM. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement Sci. 2010;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58:107-112. [DOI] [PubMed] [Google Scholar]
- 23. Bhattacharyya O, Reeves S, Garfinkel S, Zwarenstein M. Designing theoretically-informed implementation interventions: fine in theory, but evidence of effectiveness in practice is needed. Implement Sci. 2006;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ceccato NE, Ferris LE, Manuel D, Grimshaw JM. Adopting health behavior change theory throughout the clinical practice guideline process. J Contin Educ Health Prof. 2007;27:201-207. [DOI] [PubMed] [Google Scholar]
- 25. Rycroft-Malone J, Bucknall T. Using theory and frameworks to facilitate the implementation of evidence into practice. Worldviews Evid Based Nurs. 2010;7:57-58. [DOI] [PubMed] [Google Scholar]
- 26. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(8 suppl 2):II46-II54. [DOI] [PubMed] [Google Scholar]
- 27. Martinez RG, Lewis CC, Weiner BJ. Instrumentation issues in implementation science. Implement Sci. 2014;9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brehaut JC, Eva KW. Building theories of knowledge translation interventions: use the entire menu of constructs. Implement Sci. 2012;7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tavender EJ, Bosch M, Gruen RL, et al. Developing a targeted, theory-informed implementation intervention using two theoretical frameworks to address health professional and organisational factors: a case study to improve the management of mild traumatic brain injury in the emergency department. Implement Sci. 2015;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perez R, Brehaut JC, Taljaard M, Stiell IG, Clement CM, Grimshaw J. Theory of planned behaviour can help understand processes underlying the use of two emergency medicine diagnostic imaging rules. Implement Sci. 2014;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang L, Bernhardsson S, Vernooij RW, et al. ; Members of the Guidelines International Network Implementation Working Group. Use of theory to plan or evaluate guideline implementation among physicians: a scoping review. Implement Sci. 2017;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8:1-72. [DOI] [PubMed] [Google Scholar]
- 34. Davis D, Evans M, Jadad A, et al. The case for knowledge translation: shortening the journey from evidence to effect. BMJ. 2003;327:33-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Colquhoun HL, Letts LJ, Law MC, MacDermid JC, Missiuna CA. A scoping review of the use of theory in studies of knowledge translation. Can J Occup Ther. 2010;77:270-279. [DOI] [PubMed] [Google Scholar]
- 36. Painter JE, Borba CP, Hynes M, Mays D, Glanz K. The use of theory in health behavior research from 2000 to 2005: a systematic review. Ann Behav Med. 2008;35:358-362. [DOI] [PubMed] [Google Scholar]
- 37. Prestwich A, Sniehotta FF, Whittington C, Dombrowski SU, Rogers L, Michie S. Does theory influence the effectiveness of health behavior interventions? Meta-analysis. Health Psychol. 2014;33:465-74. [DOI] [PubMed] [Google Scholar]
- 38. Michie S. Designing and implementing behaviour change interventions to improve population health. J Health Serv Res Policy. 2008;13(suppl 3):64-69. [DOI] [PubMed] [Google Scholar]
- 39. Godin G, Belanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitchell SA, Fisher CA, Hastings CE, Silverman LB, Wallen GR. A thematic analysis of theoretical models for translational science in nursing: mapping the field. Nurs Outlook. 2010;58:287-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michie S, Johnston M, Abraham C, et al. ; “Psychological Theory” Group. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care. 2005;14:26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davis R, Campbell R, Hildon Z, Hobbs L, Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychol Rev. 2015;9:323-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26:13-24. [DOI] [PubMed] [Google Scholar]
- 44. Field B, Booth A, Ilott I, Gerrish K. Using the Knowledge to Action Framework in practice: a citation analysis and systematic review. Implement Sci. 2014;9:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med. 2012;43:337-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Milat AJ, Li B. Narrative review of frameworks for translating research evidence into policy and practice. Public Health Res Pract. 2017;27:2711704. [DOI] [PubMed] [Google Scholar]
- 47. Birken SA, Powell BJ, Shea CM, et al. Criteria for selecting implementation science theories and frameworks: results from an international survey. Implement Sci. 2017;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grimshaw JM, Eccles MP, Walker AE, Thomas RE. Changing physicians’ behavior: what works and thoughts on getting more things to work. J Contin Educ Health Prof. 2002;22:237-243. [DOI] [PubMed] [Google Scholar]
- 49. Lavis JN, Robertson D, Woodside JM, McLeod CB, Abelson J; Knowledge Transfer Study Group. How can research organizations more effectively transfer research knowledge to decision makers? Milbank Q. 2003;81:221-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oborn E, Barrett M, Racko G. Knowledge translation in health care: a review of the literature. Cambridge Judge Business School Working Paper Series 2010. https://www.jbs.cam.ac.uk/fileadmin/user_upload/research/workingpapers/wp1005.pdf. Accessed March 17, 2020.
- 51. Thomas A, Menon A, Boruff J, Rodriguez AM, Ahmed S. Applications of social constructivist learning theories in knowledge translation for healthcare professionals: a scoping review. Implement Sci. 2014;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Graham I, Tetroe J. The knowledge to action framework. In: Rycroft-Malone J, Bucknell T, eds. Models and Frameworks for Implementing Evidence-Based Practice: Linking Evidence to Action. Oxford, England: Wiley-Blackwell; 2010:207-222. [Google Scholar]
- 53. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225-1230. [DOI] [PubMed] [Google Scholar]
- 54. Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust. 2004;180(6 suppl):S57-S60. [DOI] [PubMed] [Google Scholar]
- 55. Registered Nurses’ Association of Ontario. Toolkit: Imple-mentation of Best Practice Guidelines. 2nd ed. Toronto, Ontario, Canada: Registered Nurses’ Association of Ontario; 2012. [Google Scholar]
- 56. McCormack B, Rycroft-Malone J, Decorby K, et al. A realist review of interventions and strategies to promote evidence-informed healthcare: a focus on change agency. Implement Sci. 2013;8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182:E73-E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Atkins L, Francis J, Islam R, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leeman J, Birken SA, Powell BJ, Rohweder C, Shea CM. Beyond “implementation strategies”: classifying the full range of strategies used in implementation science and practice. Implement Sci. 2017;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies—a synthesis of systematic review findings. J Eval Clin Pract. 2008;14:888-897. [DOI] [PubMed] [Google Scholar]
- 62. Bartholomew LK, Parcel GS, Kok G, Gottlieb NH. Intervention Mapping: A Process for Designing Theory- and Evidence-Based Health Promotion Programs. San Francisco, CA: McGraw-Hill; 2001. [Google Scholar]
- 63. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Powell BJ, Fernandez ME, Williams NJ, et al. Enhancing the impact of implementation strategies in healthcare: a research agenda. Front Public Health. 2019;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Munce S, Kastner M, Cramm H, et al. Applying the knowledge to action framework to plan a strategy for implementing breast cancer screening guidelines: an interprofessional perspective. J Cancer Educ. 2013;28:481-487. [DOI] [PubMed] [Google Scholar]
- 66. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ. 2015;350:g7818. [DOI] [PubMed] [Google Scholar]
- 68. Kotter T, Blozik E, Scherer M. Methods for the guideline-based development of quality indicators—a systematic review. Implement Sci. 2012;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shiffman RN, Dixon J, Brandt C, et al. The GuideLine Implementability Appraisal (GLIA): development of an instrument to identify obstacles to guideline implementation. BMC Med Inform Decis Mak. 2005;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72. [DOI] [PMC free article] [PubMed] [Google Scholar]