Abstract
Purpose
To describe how a referral center for cardiac electrophysiology (EP) rapidly changed to comply with the ongoing COVID-19 healthcare emergency.
Methods
We present retrospective data about the modification of daily activities at our EP unit, following the pandemic outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Italy. In particular, in the context of a pre-existing “hub-and-spoke” network, we describe how procedure types and volumes have changed in the last 3 months.
Results
Since our institution was selected as a COVID-19 referral center, the entire in-hospital activity was reorganized to assist more than 1000 COVID-positive cases. Only urgent EP procedures, including ventricular tachycardia ablation and extraction of infected devices, were both maintained and optimized to meet the needs of external hospitals. In addition, most of the non-urgent EP procedures were postponed. Finally, following prompt internal reorganization, both outpatient clinics and on-call services underwent significant modification, by integrating telemedicine support whenever applicable.
Conclusion
We presented the fast reorganization of an EP referral center during the ongoing COVID-19 healthcare emergency. Our hub-and-spoke model may be useful for other centers, aiming at a cost-effective management of resources in the context of a global crisis.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Electrophysiology, Arrhythmology, Hub and spoke
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia is a newly recognized illness that, following the first outbreak in China, rapidly spreads around the world [1].The World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) as a public health emergency of international concern on March 11, 2020. The clinical spectrum of COVID-19 in Italy ranged from mild to critical cases, with lethality variability across regions, being higher in Northern Italy, in a possible association with atmospheric pollution [2]. While public health is promoting any measure to limit the transmission of the disease, in-hospital daily clinical activity is called for a fast reorganization to adapt to the healthcare emergency [3]. As a general directive, units uncapable of respiratory intensive care, including cardiac electrophysiology (EP), are often forced either to significantly reduce volume loads or completely interrupt their activity. However, redirection of resources is widely variable, depending on both COVID-19 local case load and specific activities qualifying certain hospitals as referral centers in a pre-existing “hub-and-spoke” network. Our EP unit plays as a referral center for a number of procedures, including ventricular tachycardia (VT) ablation or cardiac implantable electronic devices (CIED) extraction; activity was rapidly reorganized to favor the minimal risk-to-benefit ratio. We describe the COVID-19 outbreak impact on daily clinical practice at a referral EP center.
COVID-19 and EP
Arrhythmic manifestations
Scientific reports have documented relevant associations of the COVID-19 infection with cardiac arrhythmias: (1) palpitations or documented arrhythmias occurred in 7–17% of the COVID-19 patients [4, 5]; furthermore, their prevalence is even higher among the ICU patients, where up to 44% of cases are involved [5]; (2) arrhythmia type and prognostic significance may be considerably variable, as described in association with COVID-19 infection [6]; based on our in-hospital experience, the prevalence of atrial fibrillation (AF) among COVID-19 patients with pneumonia is approximately 5%; furthermore, in the setting of troponin elevation, new-onset ventricular arrhythmias should raise suspicion for underlying myocarditis [7–9]; no major bradyarrhythmias have been reported; however, self-limiting phases of junctional rhythm has been described in a patient with Takotsubo-like presentation [10]; (3) as in other febrile conditions, the risk of life-threatening arrhythmic events may be increased in patients with Brugada syndrome [11]; (4) strict QT interval monitoring is required for many of the medications currently under investigation for the treatment of COVID-19; in particular, hydroxychloroquine and antiretroviral medications [3, 12] are prone to cause QT prolongation and subsequent proarrhythmic effects; thus, class III antiarrhythmic drugs like amiodarone should be carefully used in these patients; and (5) finally EP should be aware of the potential risk factors associated with an increased cardiovascular mortality among COVID-19 patients, including age, male gender, hypertension, and treatment with RAAS inhibitors [13].
In-hospital precautions
As per hospital policy, every EP at our institution wears personal protective equipment (PPE) before visiting patients with either documented or suspected COVID-19 infection. In line with the current guidelines indications [3], PPE includes a face mask, protective eyewear, gown, and gloves.
COVID-19-positive patients were admitted to single-bed rooms and put on droplet and contact precautions. Given the shortage of N95 masks, a common surgical mask with protective eyewear is used for routine daily practice and low-risk contacts. However, considering the local epidemiology, all patients, independently of their symptoms, underwent pharyngeal swab both on admission and before any transfer to other in-hospital units. As a general directive, relatives were not allowed to access hospital wards and were encouraged to keep only email or phone contact with the caregivers.
EP laboratories
In case of known COVID-19 positivity, a dedicated laboratory has been provided. Additional precautions were used inside and outside the EP lab.
The staff pays particular attention to wearing complete PPE and to having equipment in the room at the start of any procedure. Waste materials, together with all equipped masks, cuffs, and gowns, are stored in appropriate bins immediately removed after the procedure.
In suspected or confirmed COVID-19-positive cases, EP procedures were scheduled at the end of the day as extensive disinfection/cleaning would be required post-procedure.
To be noted, 10% of the procedures performed in last month were on COVID-19-positive patients, including electrical cardioversions (ECV), CIED implants, and endomyocardial biopsies (EMB). Whenever applicable, simple EP maneuvers like ECV or implantable loop recorder (ILR) procedures were performed at the bedside instead of the lab. There were no cases of reported infection among healthcare providers. A limited number of our EP physicians (2/14) were forced to quarantine following contact with COVID-19-positive patients prior to the disease manifestations. However, they had no symptoms, and pharyngeal swab was repeated twice in both cases with negative results.
Hub EP unit activity at the time of COVID-19
General hospital reorganization
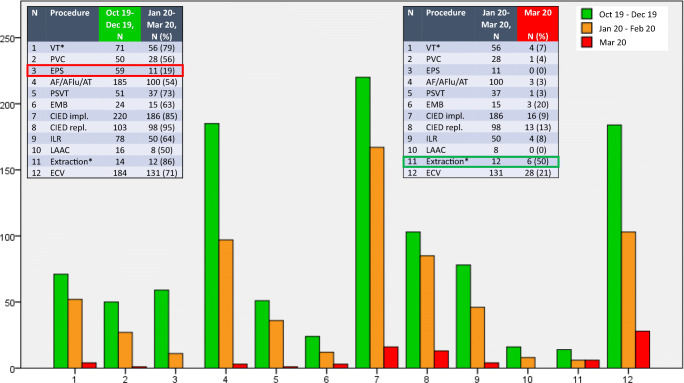
San Raffaele Scientific Institute was chosen as referral center for the COVID-19 infection, and all in-hospital activities significantly changed during the last month. Some units were temporary closed. Other units, as the EP one, underwent significant reduction in both number of beds (gradual reduction from 35 to 10, − 71% in less than 2 months) and procedure volume. In particular, as shown in Fig. 1, the maximal reduction of the procedural load is observed during the last month, since emergency status was declared at our hospital, following more than 1000 COVID-19-positive cases admitted. Importantly, anesthesiologists were called to dedicate most of their time to the ICU patients with pneumonia.
Fig. 1.
Procedure volume reduction in a hub EP center at the time of COVID-19 pandemia. Comparison between our EP unit activity during the last trimester of 2019 (green) and first trimester of 2020. The first trimester of 2020 was in turn subdivided into “moderate restriction” (from January to February, orange) and “massive restriction” of activity following the healthcare emergency outbreak at our institution (March 2020, red). For each procedure (1–12, as shown in both tables), bar height refers to absolute counts. Most important restrictions in last trimester regarded non-urgent procedures, like EPS (red box), while relative increase was observed in urgent device extractions for infective endocarditis (green box), as expected at a referral center.
*VT ablation and lead/device extraction are referral procedures at our EP unit as a hub center.
AF/AFlu/AT, atrial fibrillation, flutter or tachycardia ablation; CIED, cardiac implantable electronic devices, including pacemakers, ICDs, and CRT; ECV, electrical cardioversion; EMB, endomyocardial biopsy; EP, electrophysiology; EPS, electrophysiological study; ILR, implantable loop recorder; LAAC, left atrial appendage closure; PSVT, paroxysmal supraventricular tachycardia ablation, including nodal and accessory pathway-related reentry tachycardias; PVC, premature ventricular complexes ablation; VT, ventricular tachycardia ablation
Hub-and-spoke networking
Influx of patients from spoke hospitals was limited to cases requiring VT ablation [14, 15] or CIED extraction [16], as the main hub-qualifying procedures.
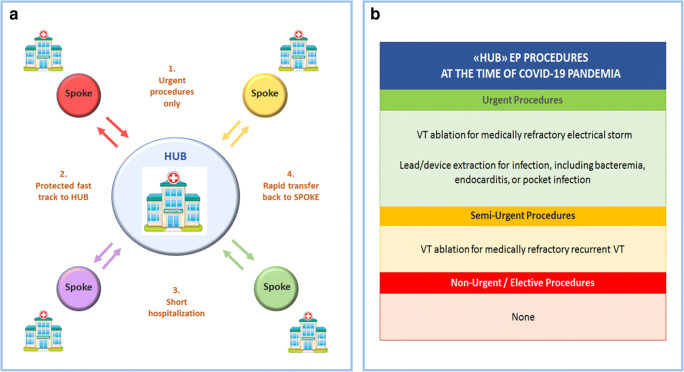
In particular, to guarantee a safe and appropriate referral of patients from spoke centers to the hub, four “golden rules” were observed (Fig. 2, panel A): (1) only urgent indications were accepted; these measures are in total compliance with the current HRS/ACC/AHA guidelines recommendations [3], although they were already ongoing much before their publication (Fig. 2, panel B); (2) fast-track service by ambulance was utilized to minimize transportation time and personnel exposure. On arrival to EP hub ward, medical staff would observe standard precautions as for COVID-19-positive patients, which included the use of PPE and minimizing contact to only the required; even in the absence of symptoms, pharyngeal swab was always obtained on admission; (3) following the procedure, in-hospital stay of patients was kept to a minimum, to avoid any risk of bidirectional infection; and (4) whenever prolonged hospitalization was needed, as soon as clinically stable, the patient was transferred back to the spoke center until discharge to home.
Fig. 2.
New configuration of our EP unit as a referral center at the time of COVID-19 pandemia. Left panel (A): Four “golden rules” for safe and appropriate referral of patients to our EP unit in a hub-and-spoke model. Right panel (B): Referral procedures performed at our center, with an approved indication at the time of COVID-19 pandemia. Classification of procedures and class of recommendations (green, indicated; yellow, borderline indication; red, non-indicated) are supported by the recently published HRS/AHA/ACC guidelines [3]
EP procedures
As for the local patients, only urgent and non-posticipable EP procedures were performed as well (Table 1), including transcatheter ablation, with indications restricted to patients with symptomatic drug-refractory VT recurrences causing appropriate implantable cardioverter defibrillator (ICD) shocks or electrical storms. However, extracorporeal membrane oxygenator (ECMO)-supported procedures were kept to a minimum: this strategy allowed for sparing both ICU beds and anesthesiology personnel, as a maximal priority at a COVID-19 referral center. Conversely, ablation of all supraventricular arrhythmias, including AF, atrial flutter, and tachycardia, as well as paroxysmal supraventricular tachycardias, was no more performed, unless in life-threatening conditions (i.e., pre-excited AF) or in patients suffering from multiple drug-refractory inappropriate shocks. Ablation of premature ventricular complexes underwent significant restriction, while diagnostic invasive electrophysiological studies (EPS) were never performed during the last month in the absence of an urgent indication (Fig. 1, Table 1).
Table 1.
Indications to EP procedures at the time of COVID-19 pandemia
| Urgent procedures—always performed | |
|
➢ Ablation of drug-refractory electrical storms ➢ Ablation of life-threatening drug-refractory SVA ➢ Ablation of WPW syndrome or pre-excited AF with syncope or cardiac arrest ➢ Secondary prevention ICD implant ➢ PM implant in symptomatic AVB (3rd degree or Mobitz II) or SND with long pauses ➢ CIED replacement in end-of-life status in patients PM-dependent or with appropriate ICD shocks ➢ Lead/device revision for malfunctioning in patients PM-dependent or with appropriate ICD shocks ➢ Lead/device extraction for infections (endocarditis, sepsis, pocket infection) ➢ EMB for fulminant myocarditis ➢ ECV for life-threatening or symptomatic drug-refractory SVA ➢ TEE for urgent ECV | |
| Semi-urgent procedures―performed in selected cases | |
|
➢ Ablation of drug-refractory recurrent VT ➢ Ablation of PVC and SVA in drug-refractory symptomatic patients ➢ Primary prevention CIED implant in high-risk patients ➢ CIED replacement in end-of-life status | |
| Non-urgent procedures—never performed | |
|
➢ Ablation of PVC and SVA in stable patients ➢ PVS for risk stratification ➢ Primary prevention CIED implant in stable patients ➢ CIED replacement with > 6 weeks of battery remaining ➢ Extraction of non-infected leads/devices in a good functional status ➢ ECV for stable and well-tolerated arrhythmias ➢ LAA closure in patients who can be on oral anticoagulants ➢ TEE for routine assessment of valves/LAA closure devices, or for non-urgent ECV ➢ ILR implant* ➢ EMB for non-fulminant myocarditis* ➢ Tilt-table testing ➢ MRI exams |
EP procedures performed at our institution are shown. Following the COVID-19 pandemic outbreak, indications were rapidly modified to comply with the ongoing healthcare emergency
*Isolated exceptions occurred, as described in detail in the main text
AF atrial fibrillation; AVB atrioventricular block; CIED cardiac implantable electronic devices; ECV electrical cardioversion; EMB endomyocardial biopsy; ICD implantable cardioverter defibrillator; ILR implantable loop recorder; LAA left atrial appendage; MRI magnetic resonance imaging; PM pacemaker; PVC premature ventricular complexes; PVS programmed ventricular stimulation; SND sinus node disease; SVA supraventricular arrhythmias; TEE transesophageal echocardiogram; VT ventricular tachycardia; WPW Wolff-Parkinson-White
CIED implant
Implant of CIED, including pacemakers, ICDs, and cardiac resynchronization therapy (CRT) devices, was restricted to urgent cases only. In particular, all secondary prevention implants were performed as previously, while primary prevention indications were limited to cases with clear guideline-based indications and additional alarm sings. For instance, we performed ICD implant in a patient with dilated cardiomyopathy, diffuse areas of late gadolinium enhancement, and recurrent episodes of non-sustained ventricular tachycardia with lypothimia. Also, we performed CRT-D implant in a patient with left bundle branch block, following repeated in-hospital admissions for decompensated heart failure. On the other hand, non-urgent primary prevention implants were all postponed. Of course, device replacement following end-on-life battery status was regularly performed, with usual priority given to pacemaker (PM)-dependent patients and ICD carriers with recent history of appropriate shocks. Conversely, the majority of ILR and percutaneous left atrial appendage (LAA) closure interventions were interrupted. In compelling cases, as for inpatients with cryptogenic stroke and no evidence of AF on telemetry, ILR was directly implanted at bedside. To further minimize contact, alternative forms of long-term external monitoring were considered whenever applicable [17, 18]. LAA closure device placement in patients who can be on oral anticoagulation was deferred.
Device/lead extraction
As for both local patients and those referred by spoke hospitals, CIED extraction procedures were restricted to urgent indications only, as in patients with sepsis, endocarditis, or pocket infection. Lead extraction procedures were limited to patients with evidence of right ventricular lead fracture or dislocation, with priority for PM-dependent cases or patients with secondary prevention ICD implants.
Endomyocardial biopsies
As a referral center for arrhythmic myocarditis [19–21], the service of EMB was restricted to patients with clinically suspected fulminant myocarditis, following evidence of normal coronary arteries at coronary angiography. Whenever possible, EMB was performed directly at the bedside, by echocardiographic guidance, instead of standard fluoroscopy-guided EP lab procedure. As for semi-urgent EMB, indications [22, 23] were restricted to symptomatic patients with evidence of troponin release; patients with arrhythmic myocarditis expected to resolve following the acute inflammatory phase; and symptomatic patients with inflammatory cardiomyopathy with a primary prevention ICD-sparing strategy.
ECV
For patients with unstable arrhythmias, ECV procedures were performed directly at the bedside, to both maintain a clean EP lab environment and avoid the risks of in-hospital contagion depending on patient mobilization. Close coordination between anesthesia and the EP lab teams was necessary to spare time and optimize resources. PPE was always used by healthcare personnel during ECV and periprocedural assistance, including sedation and transesophageal echocardiogram (TEE) whenever indicated. In patients COVID-19-positive patients with ECV-refractory AF or undergoing early recurrences, rate control strategy is of choice at our institution. TEE for ECV that can be done after appropriate period of anticoagulation, as well as for routine assessment of valves or LAA closure devices, was all postponed. Also, since TEE is an aerosol-generating procedure and subjects healthcare providers to high risk, especially given PPE shortage, ECV was generally reserved as a latter choice after symptoms could not be controlled on optimum medical therapy. CT scans were also considered as an alternative way to rule out LAA thrombi.
On-call service
Independent of the cardiology department, our EP unit offers a 24-h 7/7 on-call service for all inpatients at both ER and any hospital department. During the COVID-19 infection, the service was maintained to ensure assistance to all patients with cardiac arrhythmias. Furthermore, under needed precautions, the service was extended to the novel “COVID units.” However, the service was redesigned in order to minimize the mobilization of both consultants and patients. In detail, all clinical consultations, including ECG reporting, arrhythmia interpretation, and antiarrhythmic treatments, were performed via web or telephone. In patients with PM/ICD undergoing surgery, magnet placement for deactivation was considered a first-line approach as it does not interfere with the sterile operating field. For CIED carriers undergoing magnetic resonance imaging (MRI), the necessity of EP assistance was exceptional, since most exams had non-urgent indication and were postponed; whenever applicable, MRI was replaced by CT scan, as a time-saving technique allowing limited personnel exposure. For patients undergoing daily radiotherapy, CIED interrogation was performed by EP at a dedicated clean room.
Outpatient clinic
During the last 2 months, we significantly restricted also our outpatient clinic activity. In particular, the service maintained was limited to ensure only CIED interrogations and urgent arrhythmologic clinical evaluations. Importantly, device interrogation programmers and cables undergo disinfection before and after each visit. However, all CIED carriers were encouraged to continue or activate remote home-monitoring service [24], whenever applicable: this is recommended also by the recent guidelines [3]. Direct inspection of CIED incision sites was restricted to patients with abnormal wound healing or hematomas; as an alternative, telehealth and picture analysis via secure emails were considered in uncomplicated cases.
All our specialized outpatient activities were withheld, including routine follow-up of patients after ventricular arrhythmias ablation, left atrial appendage closure, genetic diseases, cardiomyopathies, and clinical arrhythmology. For both outpatients and inpatients, tilt-table tests were also postponed. In selected cases, as for the multidisciplinary ambulatory for arrhythmic myocarditis, telemedicine was applied to maintain follow-up quarantined patients unable to get access to the hospital.
For patients on antiarrhythmic drugs or borderline QTc values, ECG monitoring was performed via secure emails in the majority of cases. Mobile-associated tools were occasionally adopted for both QTc and cardiac rhythm monitoring [25].
Conclusions
At the time of the COVID-19 pandemic outbreak, targeted reorganization of resources is the cornerstone to both assist the infected patients and offer the most cost-effective EP support. In compliance with the current recommendations [2], all non-urgent EP procedures should be postponed, while particular attention should be paid to life-threatening conditions requiring urgent interventions. We presented how a referral EP center rapidly reorganized its activities to adapt to the ongoing healthcare emergency. In particular, in the context of a pre-existing hub-and-spoke model for complex EP procedures like VT ablation or CIED extractions, our center is still capable of offering a fast and safe way to manage urgent cases needing assistance. Maximal precautions are currently adopted to both screen for COVID-19 positivity and contain the disease transmission.
Acknowledgments
All people working hard in our nation in this challenging period are strongly acknowledged for their massive efforts and daily care for critically ill patients suffering from SARS-CoV-2 infection. In particular, we would like to thank nurses, anesthesiologists, infectious diseases specialists, and the whole emergency personnel working at our institution.
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Availability of data and material
Available upon request to the corresponding author.
Code availability
N/A.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patrizio Mazzone and Giovanni Peretto contributed equally to this work.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frontera A, Martin C, Vlachos K, Sgubin G. Regional air pollution persistence links to covid19 infection zoning. J Infect. 2020. 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed]
- 3.Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam M, et al. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force; electrophysiology section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020. 10.1161/CIRCULATIONAHA.120.047063. [DOI] [PMC free article] [PubMed]
- 4.Deng Y, Liu W, Liu K, Fang YY, Shang J, zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J. 2020. 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020. 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed]
- 7.Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020. 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed]
- 8.Peretto G, Sala S, De Luca G, et al. Impact of systemic immune-mediated diseases on clinical features and prognosis of patients with biopsy-proved myocarditis. Int J Cardiol. 2019;280:110–116. doi: 10.1016/j.ijcard.2018.11.104. [DOI] [PubMed] [Google Scholar]
- 9.Peretto G, Sala S, Rizzo S, de Luca G, Campochiaro C, Sartorelli S, Benedetti G, Palmisano A, Esposito A, Tresoldi M, Thiene G, Basso C, Della Bella P. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16:793–801. doi: 10.1016/j.hrthm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020. 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed]
- 11.Chen X, Zhao H, Sun L, Zhu W, Zhang F. Electrocardiogram characteristics and arrhythmic events during fever in patients with fever-induced Brugada syndrome. Cardiology. 2020;145:130–135. doi: 10.1159/000505642. [DOI] [PubMed] [Google Scholar]
- 12.Evaluating the interaction risk of experimental COVID-19 therapies. Liverpool Drug Interactions Group, University of Liverpool, www.covid19-druginteractions.org
- 13.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Della Bella P, Baratto F, Tsiachris D, et al. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation. 2013;127:1359–1368. doi: 10.1161/CIRCULATIONAHA.112.000872. [DOI] [PubMed] [Google Scholar]
- 15.Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, Baratto F, Berruezo A, Wijnmaalen AP. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol. 2011;4:653–659. doi: 10.1161/CIRCEP.111.962217. [DOI] [PubMed] [Google Scholar]
- 16.Mazzone P, Tsiachris D, Marzi A, Ciconte G, Paglino G, Sora N, Gulletta S, Vergara P, Della Bella P. Advanced techniques for chronic lead extraction: heading from the laser towards the evolution system. Europace. 2013;15:1771–1776. doi: 10.1093/europace/eut126. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, Cantillon DJ, Dilaveris P, Dubner SJ, el-Sherif N, Krol J, Kurpesa M, la Rovere MT, Lobodzinski SS, Locati ET, Mittal S, Olshansky B, Piotrowicz E, Saxon L, Stone PH, Tereshchenko L, Turitto G, Wimmer NJ, Verrier RL, Zareba W, Piotrowicz R. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 2017;14:e55–e96. doi: 10.1016/j.hrthm.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Kaura A, Sztriha L, Chan FK, Aeron-Thomas J, Gall N, Piechowski-Jozwiak B, Teo JT. Early prolonged ambulatory cardiac monitoring in stroke (EPACS): an open-label randomised controlled trial. Eur J Med Res. 2019;24:25. doi: 10.1186/s40001-019-0383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peretto G, Sala S, Rizzo S, Palmisano A, Esposito A, de Cobelli F, Campochiaro C, de Luca G, Foppoli L, Dagna L, Thiene G, Basso C, Della Bella P. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Peretto G, Sala S, Basso C, Della BP. Programmed ventricular stimulation in patients with active vs previous arrhythmic myocarditis. J Cardiovasc Electrophysiol. 2020;31:692–701. doi: 10.1111/jce.14374. [DOI] [PubMed] [Google Scholar]
- 21.Peretto G, Sala S, Della BP. Diagnostic and therapeutic approach to myocarditis patients presenting with arrhythmias. G Ital Cardiol. 2020;21:187–194. doi: 10.1714/3306.32767. [DOI] [PubMed] [Google Scholar]
- 22.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Heart Rhythm. 2018;15:e190–e252. doi: 10.1016/j.hrthm.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, van Veldhuisen D, Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace. 2015;17:1601–1687. doi: 10.1093/europace/euv319. [DOI] [PubMed] [Google Scholar]
- 24.Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, McLean RC, Mittal S, Morichelli L, Patton KK, Raitt MH, Pietro Ricci R, Rickard J, Schoenfeld MH, Serwer GA, Shea J, Varosy P, Verma A, Yu CM. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation. 2017;136:1784–1794. doi: 10.1161/CIRCULATIONAHA.117.030583. [DOI] [PubMed] [Google Scholar]