Abstract
Background:
Uruguay is the south American country which has the highest cancer incidence and mortality rates. The National Cancer Registry collects data on cancer cases nationwide since 1989 and has reached high quality standards in the last decades. This is the first report on incidence trends.
Methods:
Data from the National Cancer Registry of all new cases of invasive cancer from twelve sites diagnosed in 2002-2015 was analyzed. Age-standardized rates were calculated. Trends of incidence rates were analyzed using joinpoint regression models.
Results:
For both, men and women, incidence rates trends for all cancer sites, colo-rectal and bladder cancer remained stable. Esophageal and gastric cancers descended while thyroid and kidney cancer incidence increased. In men lung cancer decreased; testicular cancer increased, and prostate cancer increased at the beginning of the period and decreased in the final years. In women, lung cancer increased, breast cancer remained stable and cervical cancer presented a significant decline from 2005 to 2010 and reached a plateau since then.
Conclusion:
Cancer incidence dynamics are complex and affected not only by Public Health policies such as tobacco control, vaccination and screening programs, but also by environmental and life style changes and the attitude of the medical community towards the application of diagnostic and therapeutic tools. The aim of this paper is to analyze cancer incidence time trends in the country and provide possible explanations to them.
Keywords: Neoplasms; incidence; trends; Uruguay; urinary bladder neoplasms; stomach neoplasms; tobacco; Latin America; testicular germ cell tumor; testicular neoplasms; breast neoplasms; uterine cervical neoplasms; noncommunicable diseases; thyroid gland; carcinoma, transitional cell
Resumen
Introducción:
Uruguay es el país de Sudamerica que tiene las mayores tasas de incidencia y mortalidad por cáncer. El Registro Nacional de Cáncer recoge los datos de cáncer de todo el país desde 1989 y en las últimas décadas ha alcanzado los más altos estándares de calidad. Este es el primer reporte de tendencias de incidencia de cáncer de Uruguay.
Métodos:
Se analizaron los datos de todos los casos de cáncer invasivo diagnosticados entre 2002 y 2015 incluidos en el Registro Nacional de Cáncer y los de once topografías en particular. Se calcularon las tasas de incidencia estandarizada y se analizaron las tendencias utilizando los modelos de regresión de Joinpoint.
Resultados:
Las tasas de incidencia de cáncer colorrectal, vejiga y todos los sitios reunidos se mantuvieron estables tanto en hombres como en mujeres. La tasa de incidencia de cáncer de estómago y esófago disminuyeron mientras que las de tiroides y riñón aumentaron. En los hombres, el cáncer de pulmón disminuyó, el cáncer de testículo aumentó y el de próstata aumentó en un lapso inicial y decreció en los últimos años. En las mujeres el cáncer de pulmón aumentó y el de mama se mantuvo estable mientras que el cáncer de cérvix presentó un descenso significativo entre 2005 y 2010 alcanzando una meseta desde entonces.
Conclusión:
La dinámica de la incidencia de cáncer es compleja y está afectada no sólo por las políticas de Salud Pública como las campañas de control de tabaco, vacunación y programas de tamizaje sino por los cambios ambientales y de los estilos de vida y la actitud de los médicos respecto a la aplicación de técnicas diagnósticas y terapéuticas. En este trabajo se analizan las tendencias de incidencia en el país y se plantean posibles explicaciones para los cambios.
Palabras clave: Neoplasmas, incidencia, tendencia, Uruguay, neoplasias de la vejiga urinaria, neoplasias estomacales, tabaco, America Latina, tumor de células germinales testiculares, neoplasias testiculares, neoplasias de la mama, neoplasias cervicales uterinas, enfermedades no transmisibles, glándula tiroides, carcinoma de células transicionales
Remark
| 1) Why was this study conducted? |
| This study was conducted to analyze cancer incidence trends in Uruguay |
| 2) What were the most relevant results of the study? |
| Standardized incidence rates reflected significant changes: in men, decrease in lung cancer, initial rise followed by decrease in prostate cancer, and increase in testicular cancer. In women: rates increased markedly in lung cancer and remained stable in breast cancer; in cervical cancer they declined between 2005 and 2010, stabilizing since then. In both sexes an increase in kidney and thyroid cancers was observed, while stomach and esophageal cancer decreased. |
| 3) What do these results contribute? |
| The comparative analysis with the dynamics observed in other Latin American countries is a relevant input for cancer control programs. |
Introduction
Uruguay is one of the smallest countries in South America, not only because of its surface (175,000 km2) but also its population. Nevertheless, it is the country in the continent which has the highest cancer incidence and mortality rates 1 .
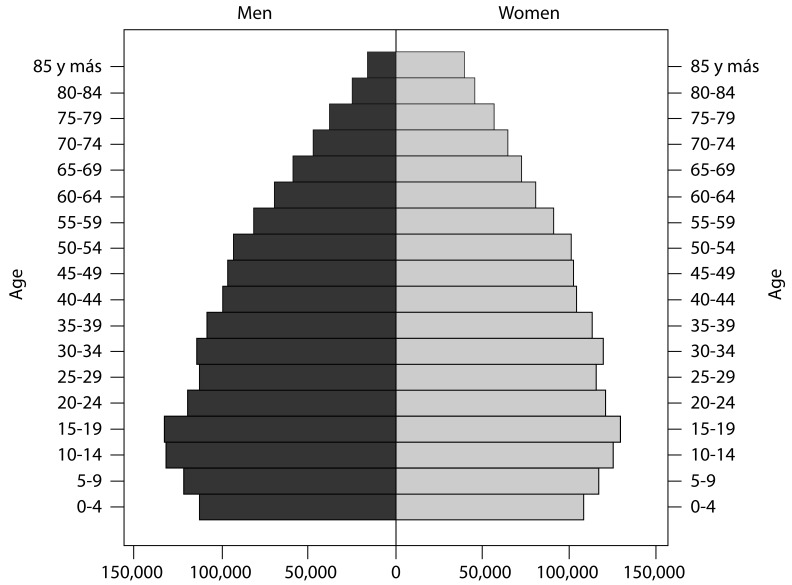
Uruguay had a population of 3,241,003 in 2004 2 and 3,286,314 in 2011 3 according to national census. The population is ethnically homogeneous (95% Western European descent) and was stable in terms of migratory behavior for the period 2002-2015. The country underwent the demo-epidemiological transition early in the 20th century and currently has a fairly aged population (Fig. 1) with 14% of the population aged 65 or more in 2011.
Figure 1. Population structure by age and sex. Uruguay. 2011.
Source: Instituto Nacional de Estadística
In average, more than 16,000 new cancer cases are registered every year and more than 8,000 deaths are caused by this disease 4 . Cancer has been the second cause of death in Uruguay for decades, accounting for almost a quarter (24.6%) of the total deaths in 2017 5 .
The age-standardized incidence rate for the 2011-2015 period for all cancer sites excluding non-melanoma skin cancer for both sexes is 256.8 per 100,000, close to the International Agency for Research in Cancer (IARC) estimates for all countries with very high human development index 1 .
Summarized mortality data are available since 1952 and true mortality databases exist since 1990; this enabled several cancer mortality trends analysis 4 , 6 . The present work is the first analysis of incidence trends for the main cancer sites in the country. It is important to emphasize that both incidence and mortality trends provide relevant information to understand the epidemiological dynamics of most cancers. For sites that are known to have very high lethality, such as lung or pancreatic cancer, difference between mortality and incidence trends is scarce. In other sites, either because they operate well-known improvements in the survival of patients or because of the development of more sensitive diagnostic techniques, the incidence trends may have very different courses from those observed in the trends of mortality. The report of cancer incidence trends is still not frequent in Latin America most likely because the majority of cancer registries in the region are very young, with the exception of the Cali Registry that has been able to describe the incidence in this urban area since 1962 and the 30-year trend analysis of Quito 7 - 9 .
The aim of this article is to analyze recent cancer incidence trends in Uruguay of the most relevant sites, namely breast cancer, prostate, lung, colorectum, kidney, urothelial, stomach, esophagus, cervix, thyroid, testis and all cancer sites excluding non-melanoma skin cancer.
Materials and Methods
The National Cancer Registry of Uruguay (NCRU) records cancer incidence at the national level since 1991. It actively collects data along the country through 30 registrars. NCRU accesses all the death certificates of the whole country and records cancer deaths, linked to their corresponding incidence, when it is already registered. Not registered cases identified through a death certificate are traced back to medical records. New cases are checked for duplication, coded according to International Classification of Diseases for Oncology 3ed (ICD-O-3) 10 (ICD-O-1 was used until 2005). IARC rules for multiple primaries are followed. Quality control procedures are performed both manually and automatically through IARC/IACR Check and conversion programs 11 and NCRU software.
National Cancer Registry of Uruguay data have been included in Cancer Incidence in Five Continents X and XI meeting the highest level of quality standards at IARC scale 12 - 14 . Regarding quality indicators, the percentage of cases morphologically verified was 78% in males and 82% in females, and the percentage of records abstracted from death certificate only was 10.1% in males and 8.8% in females. There were no cases with unknown age. The percentage of ill defined cancers was 5% (unknown and non-specific primary site, C76 and C80) 13 .
All incident cases of invasive neoplasias collected by NCRU for years 2002 to 2015 were analyzed. Cases from the sites with higher incidence were selected. Incidence trends of the 12 most relevant sites in Uruguay are described in this article, corresponding to codes C50 (female breast cancer),C61(prostate), C33-C34 (lung), C18-C21 (colon, rectum and annus), C64-C65 (kidney and renal pelvis), C66-C68 (bladder, urethra and ureter), C16 (stomach), C15 (esophagus), C53 (cervix uteri), C73 (thyroid), C62 (testis) and all cancer sites excluding non-melanoma skin cancer.
Person-years at risk were estimated using the country’s population by linear interpolation of census data from 1996, 2004 and 2011 2 , 3 , 15 . Age-standardized incidence rates were calculated by the direct method, using the world standard population 16 . Rates are expressed per 100,000 person-years. Join-point regression models were used to identify change points in incidence trends using publicly accessible software Join point version 4.7 from the Surveillance Research Program of the US National Cancer Institute 17 , 18 . The Join point analysis uses years as an independent variable and identifies time segments, in the log linear slope of the trend, that are defined by points of significant change in the trend, so called join points. Thus, the annual percent change was obtained representing the average percent increase or decrease in cancer rates per year over each specified period of time. In describing the change, the terms ‘‘increase’’ or ‘‘decrease’’ were used only when the annual percent change was significantly different from zero (two-sided p values <0.05); otherwise the term ‘‘stable’’ was used.
In order to graphically represent trends, Locally Weighted Scatterplot Smoothing (LOWESS) curves were fitted to provide smoothed lines through the scatterplot of the incidence rates by year. A bandwidth of 0.4 was used, e.g. 40% of the data were used in smoothing each point. Statistic analysis were carried out using STATA (version 15) and R (version 3.3.1).
Results
Age-standardized incidence rate at the beginning and the end of the period for each cancer site are presented in Table 1. Summarized information on the annual percent change for the twelve cancer sites selected for male and female during the studied period are presented in Table 2. Incidence trends plots are shown in Figures 2 and 3.
Table 1. Age standardized incidence rates and Joinpoint analyses for 2002 to 2015 in Uruguay. Women.
| Standardized incidence rate (per 100.000 person-year) | Period | APC | APC 95% CI | |||
|---|---|---|---|---|---|---|
| 2002 | 2015 | |||||
| Esophagus (C15) | 3.0 | 1.7 | 2002 | 2015 | -3.7* | -5.4;-2.0 |
| Stomach (C16) | 6.7 | 5.5 | 2002 | 2015 | -1.7* | -2.8;-0.5 |
| Colorectum(C18-C21) | 24.9 | 27.5 | 2002 | 2015 | 0.3 | -0.3;1.0 |
| Lung (C33-C34) | 8.7 | 14.0 | 2002 | 2015 | 3.3* | 2.3;4.3 |
| Kidney (C64-C65) | 6.0 | 7.8 | 2002 | 2015 | 2.8* | 1.4;4.2 |
| Urothelial (C66-C68) | 3.0 | 2.5 | 2002 | 2015 | -0.4 | -2.5;1.8 |
| Thyroid (C73) | 4.7 | 14.6 | 2002 | 2015 | 8.7* | 6.7;10.8 |
| Breast (C50) | 77.3 | 71.1 | 2002 | 2015 | -0.3 | -0.9;0.2 |
| Cervix (C53) | 17.6 | 14.8 | 2002 | 2005 | 2.4 | -4.4;9.7 |
| 2005 | 2010 | -5.1* | -9.3;-0.7 | |||
| 2010 | 2015 | 0.0 | -3.3;3.3 | |||
| ACS† | 228.6 | 232.3 | 2002 | 2015 | 0.0 | -0.4;0.4 |
†All cancer sites (excluding non melanoma of skin)* The estimated annual percent change is significantly different from 0 (two-side p <0.05); APC: annual percentage change
Table 2. Age standardized incidence rates and Joinpoint analyses for 2002 to 2015 in Uruguay. Men.
| Standardized incidence rate (per 100.000 person-year) | Period | APC | APC 95% CI | |||
|---|---|---|---|---|---|---|
| 2002 | 2015 | |||||
| Esophagus (C15) | 12.2 | 7.1 | 2002 | 2015 | -4.8* | -6.4;-3.2 |
| Stomach (C16) | 16.9 | 12.1 | 2002 | 2015 | -1.8* | -2.6;-0.9 |
| Colorectum(C18-C21) | 33.9 | 36.1 | 2002 | 2015 | 0.3 | -0.7;1.3 |
| Lung (C33-C34) | 55.9 | 43.9 | 2002 | 2015 | -1.7* | -2.3;-1.1 |
| Kidney (C64-C65) | 12.2 | 18.0 | 2002 | 2015 | 2.8* | 1.8;3.8 |
| Urothelial (C66-C68) | 17.9 | 13.3 | 2002 | 2015 | -0.2 | -1.4;1.0 |
| Thyroid (C73) | 1.5 | 2.6 | 2002 | 2015 | 7.3* | 4.6;10.0 |
| Prostate (C61) | 47.1 | 51.2 | 2002 | 2004 | 12.8* | 4.7;21.5 |
| 2004 | 2011 | -0.1 | -1.3;1.1 | |||
| 2011 | 2015 | -4.0* | -6.1;-1.8 | |||
| Testis (C62) | 5.1 | 7.6 | 2002 | 2015 | 3.3 | 2.3;4.4 |
| ACS† | 300.0 | 279.2 | 2002 | 2015 | -0.5 | -1.1;0 |
†All cancer sites (excluding non melanoma of skin)* The estimated annual percent change is significantly different from 0 (two-side p < 0.05); APC: annual percent change.
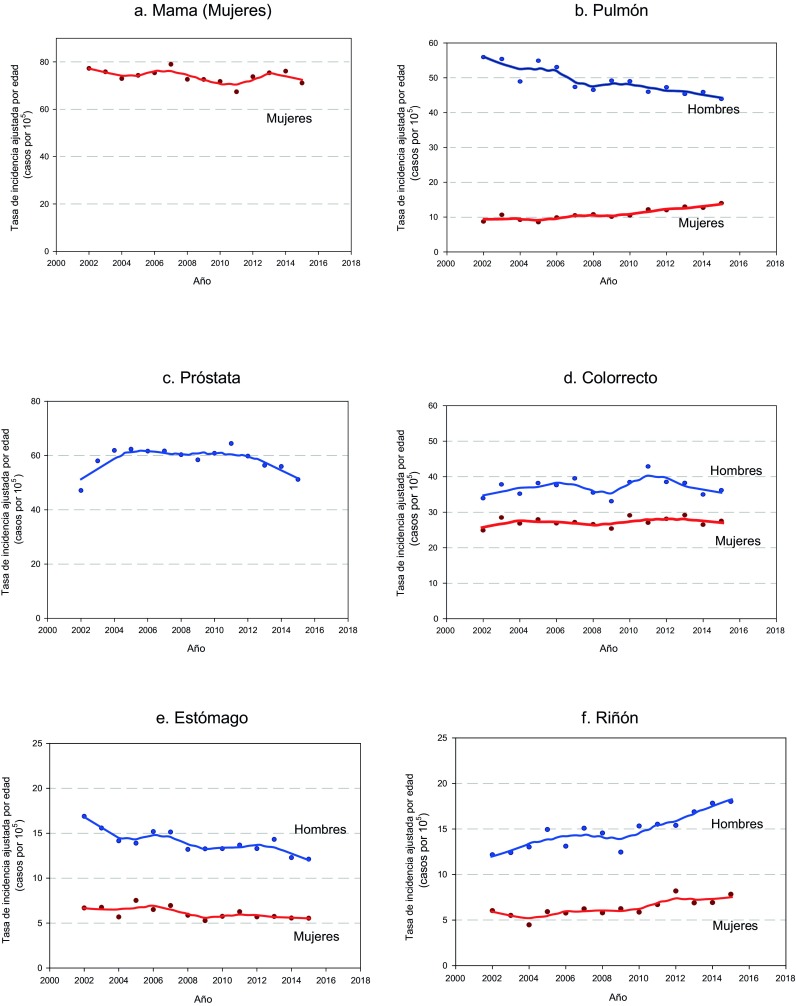
Figure 2. Cancer incidence trends in Uruguay. 2002-2015. a. Breast b. Lung c. Prostate. d. Colorectal cancer. e. Stomach f. Kidney.
Figure 3. Cancer incidence trends in Uruguay. 2002-2015. a. Urothelial cancers b. Testis c. Esophageal d. Cervix uteri e. Thyroid f. All cancers.
The age-standardized incidence rate for all cancer sites excluding non-melanoma skin cancer remained stable along the period for male and female.
In males, esophageal, gastric and lung cancer age-standardized incidence rate decreased while kidney, thyroid and testicular cancer age-standardized incidence rate increased. Colorectal and urothelial cancer age-standardized incidence rate remained stable. Regarding prostate cancer, age-standardized incidence rate increased until 2004, remained stable between 2004 and 2011 and finally decreased from 2011 on.
In females esophageal and gastric cancer age-standardized incidence rate decreased but they present smother slopes than men. Kidney and thyroid cancer age-standardized incidence rate increased. Opposite to their masculine counterparts, lung cancer age-standardized incidence rate in women sharply increased. Breast, colorectal and urothelial cancer age-standardized incidence rate remained stable. Concerning to cervix cancer, age-standardized incidence rate remained stable until 2005, present a significant decline from 2005 to 2010 and reached a plateau since then.
Discussion
Female breast cancer
For at least 50 years, breast cancer has been by far the main cancer death cause in women in Uruguay, and the most frequent malignancy diagnosed, with the exception of non-melanoma cancer of the skin 1 . In most countries around the world breast cancer incidence is increasing, while time trends in those with high human development index have reached a peak and start to decrease 19 .Time trends in Uruguay remain stable, in contrast to mortality trends that decrease 1% per year since 1990 4 . In recent years the scientific community witnessed a noteworthy improvement in breast cancer treatments and in Uruguay universal access to most of those new therapies (i.e. Her2 blockers) is granted. This probably explains, partially at least, the decreasing trends in mortality. On the other hand, strong policies addressed to improve mammographic screening coverage were implemented since 2006, such as the access at no cost to the technique for women between 50 to 69 and the obligation by law for the employers to provide their employees one payed day off to attend their screening test 20 . If those strategies were successful and a broadened coverage was achieved, an increase in the detection of in situ carcinomas could lead to a decline in incidence trends in the future.
Lung cancer
In males in Uruguay, lung cancer is the second most frequent malignancy diagnosed, but it is still the first cause of cancer death. In women, it holds the fifth place in cancer incidence but it reached in the last years the third place in mortality 21 .Lung cancer incidence decreases steadily since the early 2000’s in men while they increase sharply in women. Lung cancer trends are strongly associated to tobacco epidemics, with a latency period of about three decades 22 . Previous analysis using age-period-cohort models have explained lung cancer trends in terms of a cohort effect 23 , 24 . That means that the birth cohorts of men and women increasingly exposed to tobacco, contributed progressively to the increase of lung cancer age-standardized incidence rate, with some decades of delay, due to the latency of the clinical expression. We have recently analyzed this dynamic in Uruguay using age-period-cohort models, showing similar results. A descend in specific rates in the younger cohorts are translates in global trends descend in men. Successive cohorts of women behave just the opposite, with increasing specific rates for the younger compared to the older. The youngest cohorts of women, however, seem to display a specific rates decreasing pattern. Although the small number of cases for this cohorts makes us cautious, if confirmed in the next years could allow to forecast a soften in the burden of the disease in the future 25 , 26 . This prognosis is also supported by the decrease in the prevalence of smokers among young people as was observed in several rounds of the Global Adults Tobacco Survey (GATS) 27 , 28 . Those results enables us to be reasonably optimistic regarding a future drop in the age-standardized incidence rate in women as it’s seen in men.
Prostate cancer
For at least 15 years, prostate cancer has been the most frequent malignancy diagnosed in Uruguayan men. Time trends showed three different stages. At the begging of the period (2002-2004) age-standardized incidence rate increased. We believe that these are indeed the final years of a longer phase of rate’s growth that began with the massive application of prostate-specific antigen testing. The second phase showed stabilization of the rates that finally decreased since 2011. The latter phases correspond probably to more conservative attitudes of the medical community towards the routine application of prostate-specific antigen testing in healthy men. This trend pattern presenting a peak, a stable period and decrease was also observed in developed countries some decades ago. The SEER for instance has reported in USA a sharp peak in the mid-nineties, followed by a plateau and finally a 6.5% decrease in the rates per year since 2007 29 .
Colorectal cancer
Colorectal cancer incidence and mortality rates have great variability worldwide, up to ten fold when comparing countries with highest versus lowest human development index, although these rates are increasing in the latter group consistently with life style changes 30 . Uruguayan rates are in the highest quintile in the world. Colorectal cancer is among the top three cancer sites for both men and women. Time trends analysis shows stabilization in incidence rates for both genders all along the period. Uruguayan population has high prevalence of many risk factors associated with colon cancer risk such as obesity, sedentary lifestyle and high red meat intake 27 , 28 .On the other hand, early detection guidelines and screening programs were implemented in order to detect premalignant lesions, but the adherence of the population to those programs is low. It seems essential to keep working on primary and secondary prevention to achieve some decrease in the rates.
Stomach cancer
Gastric cancer age-standardized incidence rate in Uruguay decreased for both, men and women. Mortality rates have decreased since 1950 6 . Given the high lethality of this malignancy, those decreasing mortality rates surely corresponded to decreasing incidence rates as a result of the massive introduction of domestic refrigerators and changes in food preservation techniques, as well as hygiene improvements. The global decrease of incidence rates in more recent years is probably related to a decrease in the rate of Helicobacter pylori infection which is the strongest risk factor for stomach cancer identified to date 31 . Infection decrease is a consequence of medical awareness and antibiotic treatment rates are expected to continue decreasing as a consequence of preventive measures and healthy lifestyle promotion targeting smoking cessation, reducing salted and preserved food and increasing fresh fruits and vegetables consumption.
Kidney cancer
Worldwide, renal cancer is two to three times more frequent in men than in women; age-standardized incidence rate in more developed countries is three times higher than in developing countries. For both, Uruguayan men and women, rates are placed in the higher quintile 1 . A striking increase in the rates was observed globally, in particular in north America, Europe and some Asian countries 33 , 35 . In several populations this was partially attributed to over diagnosis 36 . In USA, a worrisome increase of the diagnosis of renal cell carcinoma among the age 22 and 39 was detected 37 .
In Uruguay kidney is among the sites that present the greatest increase in incidence rates. An increase in mortality rates was also described in the country 2 . According to European studies; a risk fraction of up to 25% is attributable to obesity and overweight 38 ,which prevalence increased alarmingly in many countries, including Uruguay 27 , 28 , 39 .
Urothelial cancers
Incidence rates for Uruguayan men are close to those of USA and Canada, but they double those of the neighboring countries. That difference related to the region was not identified for women 1 . age-standardized incidence rate time trends remained stable along the studied period for both men and women. Mortality rates in men decreased slowly but steadily since 1990 (annual percent change: -0.6; CI 95% -1.1,-0.3) 4 . Similar trends behavior (incidence increase and mortality decrease) is observed in southern European countries 40 , 41 . A possible explanation could be an increased proportion of non-muscle invasive tumors among incident cases.
Testicular cancer
Although the number of cases is relatively low compared to other sites, the relevance of this malignancy lies in the fact that it affects mainly boys, adolescents and men below the age 50. In Uruguay age-standardized incidence rate steadily increased placing this malignancy among the top ten cancer sites in males in the most recent years. Globally our country is in the highest quintile of incidence, together with many European countries, USA, Canada, Australia and Argentina 1 . For many of those countries incidence increased during the second half of the twentieth century 42 . This trends persist at the beginning of the XXIst century, although a deceleration was observed particularly in the countries affected by the highest rates such as Denmark and Iceland 43 . In several of the most affected countries a decrease in male fertility was observed during the last decades 44 , 45 . Some studies suggest therefore a relation between testicular cancer risk and male infertility 46 , 47 . We know that the global fertility rate in our country is low (1.88 children per woman in 2018) 48 but there are no specific data on males fertility. Expanded access to fertility treatments and the creation in recent years of a database with nationwide data about infertile couples may provide a chance in the future to link this information with cancer incidence data. Other possible line of investigation could be the use of cannabis as risk factor for testicular cancer. Recent evidence suggests that regular and prolonged use of TCH could be a risk factor to develop germ cell tumors, especially the non-seminoma variants 49 , 50 . An increase in Marijuana use was observed in our country in the last two decades; eventually boosted by the Cannabis market regulation since 2015 51 .
Esophageal cancer
In Uruguay esophageal cancer age-standardized incidence rate have been historically among the highest of the region, especially for men. A marked descend in age-standardized incidence rate was observed along the study period for men and females.
The most frequent pathological types are squamous cell carcinomas and adenocarcinomas. Both have different etiologies and clinicopathological characteristics 52 - 54 . squamous cell carcinomas generally occurs in younger individuals and in countries with lower levels of development (poorer socio-economic contexts), while adenocarcinomas occurs predominantly in high income populations 52 . Globally over 98% of malignant tumors of the esophagus are either squamous cell carcinomas or adenocarcinomas 55 , although the proportion of both types is very heterogeneous when different geographical areas are compared. In those areas displaying the highest rates, the most common type is squamous cell carcinomas from the most proximal segments of the organ, while adenocarcinomas of the lower third and gastro-esophageal union is more frequent in areas where age-standardized incidence rate are low. Those patterns reflect the distribution of differential risk factors for each histological.
The most important risk factors for squamous cell carcinomas are the consumption of tobacco and alcohol, as well as the thermal damage caused by the consumption of hot drinks (mainly infusions). Tobacco is also a risk factor for adenocarcinomas as well as overweight and obesity and the presence of gastro-esophageal reflux 52 - 54 , 56 .
Thermal damage caused by hot drinks has been proposed as an important factor. For example, in Iran, the high incidence and mortality due to esophagus cancer has been attributed to tea consumption at very high temperatures 56 . Mate, a traditional infusion based on Ilex paraguayensis, whose consumption is widespread in Argentina, southern Brazil, Uruguay and Paraguay has also been identified as a risk factor for cancer of the esophagus, as well as for other malignant tumors, especially of the upper aero-digestive tract but also for bladder and kidney cancers 57 - 60 . With the exception of Paraguay where Ilex paraguayensis infusion is consumed cold (Tereré), in the rest of the countries mentioned above, it is usually consumed at high temperatures. Several studies, mostly of the case-control type, have shown significantly high risks in heavy consumers 58 , 60 . Two factors have been proposed in the possible mechanism of carcinogenesis by this agent: thermal damage from exposure of mucous membranes at elevated temperatures, and the presence in the infusion of aromatic polycyclic hydrocarbons, in particular Benzopyrene. This problem has been and is currently intensively studied, particularly in our environment both experimentally and epidemiologically 60 - 62 .
In 1991 the IARC, in its monograph on the possible carcinogenic effect of xanthines (coffee, tea, mate, methylxanthines) had ruled out the carcinogenic effect of mate itself (placing it in Group 3: not classifiable in relation to its carcinogenicity in humans) and classified the hot drink mate in group 2A: “probably carcinogenic in humans” 58 . A committee of experts from the IARC 63 has recently reviewed the new available evidence and consequently the qualification of mate as a carcinogenic agent, this report emphasizes the role of thermal damage and deepens the pathological and molecular mechanisms involved. However, IARC did not change its classification as a carcinogen.
Squamous cell carcinomas and adenocarcinomas of the oesophagus have different incidence trends. Histological information from many cases especially in the earlier years of the study period is missing. This poses difficulties when time trend analysis discriminated by histological type is performed. However, from the evolution of squamous cell carcinomas/adenocarcinomas rates ratio we infer that the descend in age-standardized incidence rate of esophageal cancer in our country is mainly explained by the descend of age-standardized incidence rate of squamous cell carcinomas, especially in men (unpublished data), similarly to observed data globally 64 , 65
Cervix uteri cancer
Because of its close relation to human papilloma virus infection and variables associated with that infection, cervical cancer is strongly linked to social inequity. This illness affects mainly people from low and middle income countries but also the poorest population from high income countries 32 . Globally cervical cancer incidence declined during the last 30 years not only in high income countries but also in many low and middle income countries that improved their general life conditions 66 . Uruguay has intermediate age-standardized incidence rate in the global context (third quintile). It is the third more frequently diagnosed malignant tumor in women, only surpassed by breast and colon cancer and it holds the fifth place in mortality. age-standardized incidence rate in Uruguay declined steadily between 2005 and 2010. In most recent years (2010-2015) incidence reached a plateau. Meanwhile mortality rates steadily declined since 1998 (annual percent change -1.5) 4 .
In Uruguay a Cervical Cancer Prevention Program based on PAP smear testing is running since 1994; but more recently, the Program was enhanced. In 2006 it was expanded nationwide and by this time, as it was described for breast cancer, several strategies to overcome economical and social barriers and enable access to the test were implemented 67 . Unlike breast cancer or other malignancies, treatments for cervical cancer have changed little in the last two decades. We believe that the sustained decrease in mortality support the idea that the incidence stabilization is indeed reflecting earlier detection and probably a lead time bias related to the health policies described above. Since 2009, human papilloma virus vaccination is available in the country and in 2012 the vaccine became free of charge for the girls aged 11 to 12. In 2019 began the vaccination of boys in the same age group. Therefore it seems reasonable to expect a further decrease in incidence rates for the years to come.
Another factor that may be considered when analyzing cervical cancer trends is the number of births per woman since multiparity is associated with risk of cervical precancer an cancer among human papilloma virus infected women 68 . Fertility rates have steadily decreased from 3 in 1970-1975 to 2 in 2010-2015 69 .
Thyroid cancer
This illness used to be until recent years relatively infrequent. In the last decades an unexpected increase in the age-standardized incidence rate worldwide, mainly driven by papillar carcinoma in young women was observed. The underlying cause of the rise of incidence remains unclear. Some unrelated events could be at least part of the explanation. Some researchers point out that papillar thyroid cancer increase was observed in many countries that implemented iodine supplementation 70 . On the other hand, the recent increase in the rates is attributed to the extended use of diagnostic ultrasound. One example is the Republic of Korea where following the implementation of an aggressive screening program age-standardized incidence rate reached 88.6 cases per 100,000 people/year 71 , 72 . In Uruguay mortality rates are very low for both genders (0.35 males, 0.31 females for the period 2009-2013), stable in men and decreasing in women (annual percent change 1990-2016: -1.8) 4 .
Although a screening program was not implemented, the wide availability of diagnostic ultrasound and most likely a greater awareness of health professionals to this pathology are probably part of the reason that explains the sharp increase in the incidence observed in the study period, more evident in women.
All cancer sites
Age-standardized incidence rate for all cancer sites excluding non-melanoma cancer remains stable all along the study period for both, men and women, although the dynamics are far more complex. In men the decrease of lung cancer incidence was counteracted by the increase of prostate cancer at the beginning of the century. Kidney and urothelial cancer incidence rates have increased while esophageal and stomach cancer rates have decreased. In women, the decline of cervical, esophageal and gastric cancer incidence is partially offset by the increase of thyroid, lung and kidney cancer. Among those, lung cancer is the most worrisome, because of its high lethality.
Conclusions
The analysis of cancer incidence trends provides relevant information to better understand the causes of the disease, evaluate the actions undertaken to reduce its burden and guide new changes to Public health policies, as well aschanges in lifestyle and health professional practices. It is also intended to guide future research on known or suspected risk factors.
Although the fact of comprising only 14 years imposes certain limitations, this article is intended to provide an initial approach to the understanding of changes in cancer dynamics observed at the beginning of the XXIst century. Other point of interest of this article is to communicate cancer incidence trends from a Latin American country in a national scale, even considering the small population of Uruguay.
As it has been previously stressed, Uruguayan demographic characteristics are probably very different from that of most Latin American countries. Uruguay has started earlier its demographic transition at the regional context. Hence, the characterization of the dynamics of the epidemiological profile, particularly regarding noncommunicable diseases, may be useful to anticipate events and challenges that other countries of the region could experiment in the near future.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2018. [Google Scholar]
- 2.Instituto Nacional de Estadística Censo 2004 Fase I. 2005. [2016 Nov 8]. Available from: http://www.ine.gub.uy/web/guest/censo-2004-fase-i.
- 3.Instituto Nacional de Estadística Censos 2011; 2012. [2016 Oct 5]. Available from: http://www.ine.gub.uy/censos-2011.
- 4.Comisión Honoraria de Lucha contra el cáncer Registro Nacional de Cáncer. 2019. [2019 Apr 11]. Available from: https://www.comisioncancer.org.uy/categoria/Registro-Nacional-de-Cancer-14.
- 5.Ministerio de Salud Estadísticas Vitales. [2017 May 16]. Available from: http://www.msp.gub.uy/EstVitales/#services.
- 6.De Stefani E, Fierro L, Barrios E, Ronco A. Cancer mortality trends in Uruguay 1953-1991. Int J Cancer. 1994;56(5):634–639. doi: 10.1002/ijc.2910560505. [DOI] [PubMed] [Google Scholar]
- 7.Sierra MS, Soerjomataram I, Antoni S, Laversanne M, Piñeros M, de Vries E. Cancer patterns and trends in Central and South America. Cancer Epidemiol. 2016;44:S23–S42. doi: 10.1016/j.canep.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Corral CF, Cueva AP, Yépez MJ, Tarupi MW. Trends in cancer incidence and mortality over three decades in Quito - Ecuador. Colomb Med (Cali) 2018;49(1):35–41. doi: 10.25100/cm.v49i1.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bravo LE, Collazos T, Collazos P, García LS, Correa P. Trends of cancer incidence and mortality in Cali, Colombia 50 years experience. Colomb Med (Cali) 2012;43(4):246–255. [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L. Parkin DM et al . International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 11.Ferlay J, Burkhard C, Whelan S, Parkin DM. Check and conversion programs check and conversion programs. Lyon: 2005. [2016 Jun 29]. Available from: http://www.iacr.com.fr/index.php?option=com_content&view=article&id=72:iarccrgtools&catid=68&Itemid=445. [Google Scholar]
- 12.Forman D, Bray F, Brewster DH, Gombe MC, Kohler B, Piñeros M. Cancer Incidence in Five Continents, Vol. X. Lyon, France: International Agency for research on Cancer; 2014. [Google Scholar]
- 13.Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, et al. Cancer Incidence in Five Continents. Lyon: International Agency for Research on Cancer; 2017. (Vol. XI). [Google Scholar]
- 14.IARC. WHO . Globocan. Cancer today. Data Sources and methods. 2018. [2019 Feb 6]. Available from: http://gco.iarc.fr/today/data-sources-methods. [Google Scholar]
- 15.Instituto Nacional de Estadística Censos 1963-1996. 1997. [2016 Oct 3]. Available from: http://www.ine.gub.uy/web/guest/censos-1963-1996.
- 16.Segi M. Cancer mortality for selected sites in 24 countries (1950-57) Department of Public Health, Tohoku University of Medicine; Sendai, Japan: 1960. [Google Scholar]
- 17.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute . Statistical Methodology and Applications Branch, Survilliance Research Program. Joinpoint Regression Program, Ver 4.7.0.0. Bethesda: Maryland, Estados Unidos; 2019. [Google Scholar]
- 19.Schnitt SJ, Lakhani SR. Stewart BW, Wild C. World cancer report 2014. Lyon, France: IARC-WHO; 2014. Breast cancer; pp. 362–373. [Google Scholar]
- 20.Ministerio de Salud Pública . Guía de práctica clínica de detección temprana del cáncer de mama. Uruguay: MSP; 2015. [2017 May 31]. Available from: http://www.msp.gub.uy/sites/default/files/archivos_adjuntos/Iniciativas%20sanitarias%20%28guia%20deteccion%20cancer%20mama%29.pdf. [Google Scholar]
- 21.Barrios E, Musetti C, Alonso R, Garau M. V Atlas de mortalidad por cáncer en el Uruguay 2009-2013. Montevideo: Comisión Honoraria de Lucha Contra el Cáncer; 2015. [Google Scholar]
- 22.Thun MJ, Henley S. Tobacco. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3era. Ed. Oxford University Press; 2006. pp. 217–242. [Google Scholar]
- 23.Bray F, Tyczynski JE, Parkin DM. Going up or coming down The changing phases of the lung cancer epidemic from 1967 to 1999 in the 15 European Union countries. Eur J Cancer. 2004;40(1):96–125. doi: 10.1016/j.ejca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Bray F, Weiderpass EE. Lung cancer mortality trends in 36 European countries secular trends and birth cohort patterns by sex and region 1970-2007. Int J Cancer. 2010;126(6):1454–1466. doi: 10.1002/ijc.24855. [DOI] [PubMed] [Google Scholar]
- 25.Barrios E, Garau M, Musetti C, Alonso R. A decade of tobacco control planning in Uruguay: analysis of the lung cancer epidemiological situation; 37th International Association of Cancer registries Annual Scientific Conference; 2015; Mumbai, India. [Google Scholar]
- 26.Alonso R, Piñeros M, Laversanne M, Musetti C, Garau M, Barrios E, et al. Lung cancer incidence trends in Uruguay 1990-2014: An age-period-cohort analysis. Cancer Epidemiol. 2018;55:17–22. doi: 10.1016/j.canep.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Ministerio de Salud Pública . 1a Encuesta Nacional de Factores de Riesgo de Enfermedades Crónicas No Transmisibles. Montevideo: 2006. http://www.msp.gub.uy/sites/default/files/archivos_adjuntos/1er_enfrecnt_2006_1.pdf [Google Scholar]
- 28.Ministerio de Salud Publica [2018 Sep 18];2a Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles. 2013 Available from: http://www.msp.gub.uy/publicaci%C3%B3n/2%C2%AA-encuesta-nacional-de-factores-de-riesgo-de-enfermedades-no-transmisibles.
- 29.Negoita S, Feuer EJ, Mariotto A, Cronin KA, Petkov VI, Hussey SK. Annual report to the nation on the status of cancer, part II Recent changes in prostate cancer trends and disease characteristics. Cancer. 2018;124(13):2801–2814. doi: 10.1002/cncr.31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 31.Shibata A, Parsonnet J. Stomach Cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3era. Ed. Oxford University Press; 2006. [Google Scholar]
- 32.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 33.Song W, Jeon HG. Incidence of kidney, bladder, and prostate cancers in Korea An update. Korean J Urol. 2015;56(6):422–428. doi: 10.4111/kju.2015.56.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De P, Otterstatter MC, Semenciw R, Ellison LF, Marrett LD, Dryer D. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986-2007. Cancer Causes Control. 2014;25(10):1271–1281. doi: 10.1007/s10552-014-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Znaor A, Laversanne M, Bray F. Less overdiagnosis of kidney cancer an age-period-cohort analysis of incidence trends in 16 populations worldwide. Int J Cancer. 2017;141(5):925–932. doi: 10.1002/ijc.30799. [DOI] [PubMed] [Google Scholar]
- 37.King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease United States 2001 to 2010. J Urol. 2014;191(6):1665–1670. doi: 10.1016/j.juro.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergström A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91(3):421–430. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 39.WHO Obesity and overweight. 2019. [2019 Jun 25].
- 40.Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF. The global epidemiology of bladder cancer a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8(1):1129–1129. doi: 10.1038/s41598-018-19199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality A Global Overview and Recent Trends. Eur Urol. 2017;71(1):96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Park JS, Kim J, Elghiaty A, Ham WS. Recent global trends in testicular cancer incidence and mortality. Medicine (Baltimore) 2018;97(37):e12390. doi: 10.1097/MD.0000000000012390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ylönen O, Jyrkkiö S Pukkala E Syvänen K Boström PJ. Time trends and occupational variation in the incidence of testicular cancer in the Nordic countries. BJU Int. 2018;122(3):384–393. doi: 10.1111/bju.14148. [DOI] [PubMed] [Google Scholar]
- 44.Sengupta P, Dutta S, Krajewska-Kulak E. The disappearing sperms analysis of reports published between 1980 and 2015. Am J Mens Health. 2017;11(4):1279–1304. doi: 10.1177/1557988316643383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlsen E, Giwercman A, Keiding N, Skakkeblek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169(4):351–356. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker JA, Buck GM, Vena JE, Moysich KB. Fertility patterns prior to testicular cancer diagnosis. Cancer Causes Control. 2005;16(3):295–299. doi: 10.1007/s10552-004-4024-2. [DOI] [PubMed] [Google Scholar]
- 48.worldbank Indicators | Data. 2019. [2019 Jul 31]. Available from: https://data.worldbank.org/indicator.
- 49.Callaghan RC, Allebeck P, Akre O, McGlynn KA, Sidorchuk A. Cannabis use and incidence of testicular cancer A 42-year follow-up of swedishmen between 1970 and 2011. Cancer Epidemiol Biomarkers Prev. 2017;26(11):1644–1652. doi: 10.1158/1055-9965.EPI-17-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer a systematic review and meta-analysis. BMC Cancer. 2015;15(1):897–897. doi: 10.1186/s12885-015-1905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monitor Cannabis Evolución del consumo de cannabis en Uruguay y mercados regulados. 2019. [2019 Jun 25]. Available from: http://monitorcannabis.uy/evolucion-del-consumo-de-cannabis-en-uruguay-y-mercados-regulados/
- 52.Montgomery EA. Stewart BW, Wild C. World cancer report 2014. Lyon: IARC-WHO; 2014. Oesophageal cancer; pp. 374–382. [Google Scholar]
- 53.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Blackstock AW. Esophageal Cancer. Semin Radiat Oncol. 2007;17(1):1–1. [Google Scholar]
- 55.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO . Classification of Tumours of the Digestive System. Fourth Edition . Lyon: IARC; 2010. Tumours of the Oesophagus Cap 1. [Google Scholar]
- 56.Chen Y, Tong Y, Yang C, Gan Y, Sun H, Bi H. Consumption of hot beverages and foods and the risk of esophageal cancer a meta-analysis of observational studies. BMC Cancer. 2015;15:449–449. doi: 10.1186/s12885-015-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vassallo A, Correa P, De Stéfani E, Cendán M, Zavala D, Chen V. Esophageal cancer in Uruguay a case-control study. J Natl Cancer Inst. 1985;75(6):1005–1009. [PubMed] [Google Scholar]
- 58.International Agency for Research on Cancer . Coffee, tea, mate, methylxanthines and methylglyoxal. Lyon: 1991. pp. 513–513. (IARC monographs on the evaluation of carcinogenic risks to humans). [PMC free article] [PubMed] [Google Scholar]
- 59.Lubin JH, De Stefani E, Abnet CC, Acosta G, Boffetta P, Victora C. Maté drinking and esophageal squamous cell carcinoma in South America pooled results from two large multicenter case-control studies. Cancer Epidemiol Biomarkers Prev. 2014;23(1):107–116. doi: 10.1158/1055-9965.EPI-13-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.IARC . Report of the Advisory Group to recommend Priorities for IARC Monographs during 2015-2019. Lyon: 2014. (Monographs on the evaluation of Carcinogenic Risks to Humans). [Google Scholar]
- 61.Candreva EC, Keszenman DJ, Barrios E, Gelós U, Nunes E. Mutagenicity induced by hyperthermia, hot mate infusion, and hot caffeine in Saccharomyces cerevisiae. Cancer Res. 1993;53(23):5750–5753. [PubMed] [Google Scholar]
- 62.De Stefani E, Moore M, Aune D, Deneo-Pellegrini H, Ronco AL, Boffetta P. Maté consumption and risk of cancer a multi-site case-control study in Uruguay. Asian Pac J Cancer Prev. 2011;12(4):1089–1093. [PubMed] [Google Scholar]
- 63.Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma a meta-analysis. Am J Gastroenterol. 2014;109(6):822–827. doi: 10.1038/ajg.2014.71. [DOI] [PubMed] [Google Scholar]
- 64.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 65.Bosetti C, Levi F, Ferlay J, Garavello W, Lucchini F, Bertuccio P. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122(5):1118–1129. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 66.Prat J, Franceschi S. Lyon, France: Stewart BW and Wild C IARC-WHO; 2014. Cancers of the female reproductive organs; pp. 465–481. [Google Scholar]
- 67.Nozar DMF, Briozzo L. Cáncer de cuello uterino en Uruguay Controversias en la prevención. Rev Med Uru. 2017;33(1):64–70. [Google Scholar]
- 68.Schiffman MH, Hildesheim A. Cervical Cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3era. Ed. Oxford University Press; 2006. [Google Scholar]
- 69.The Word Bank Fertility rate, total (births per woman) - Uruguay | Data. 2019. [ 2019 Dec 11].
- 70.Schottenfeld D, Fraumeni JF. Cancer epidemiology and prevention. 3era. Ed. Oxford University Press; 2006. [Google Scholar]
- 71.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 72.Ahn HS, Welch HG. South Korea's Thyroid-Cancer "Epidemic" - Turning the Tide. N Engl J Med. 2015;373(24):2389–2390. doi: 10.1056/NEJMc1507622. [DOI] [PubMed] [Google Scholar]