Abstract
Metabolic syndrome is reported to play a role in the genesis and development not only of angina, arteriosclerosis, diabetes, and osteoporosis but also of prostate cancer. Hypercholesterolemia is a strong risk factor in prostate cancer development. The current study was conducted to analyze whether pretreatment serum levels of cholesterol correlate with prostate cancer metastasis. Three hundred fifty-one subjects who received a histopathological diagnosis of prostate cancer were evaluated by clinical factors such as age, body mass index (BMI), disease stage, Gleason score, prostate-specific antigen (PSA), total cholesterol, Luteinizing hormone (LH), testosterone, and free testosterone. A multivariate analysis was performed on these factors, and a statistically significant difference was identified in total cholesterol level (p =.01) and PSA (p < .001). The total cholesterol level was higher in cases of metastatic prostate cancer compared to nonmetastatic prostate cancer in this study and therefore may be a predictive factor for poor prognosis.
Keywords: hypercholesterolemia, metastasis, prostate cancer
Neither race nor geographical region greatly impacts the rate of latent prostate cancer (Haas et al., 2008). These factors do greatly impact the rate of clinical prostate cancer, which is low in Asia. Diet is one factor thought to explain why latent cancer does not progress to clinical cancer in Asia. The incidence rate and deaths associated with prostate cancer are on the rise, with an aging society and Westernization of diet regarded as likely factors in Japan (Horie, 2012). Studies have reported an increase in prostate cancer incidence in Asian men after emigration to the United States (Namiki et al., 2010). Not all prostate cancer patients require proactive treatment; actually, many die from other causes. Accurate risk assessment is thus important, along with prevention of prostate cancer deemed likely to progress and malignant prostate cancer considered likely to result in clinical death.
Lifestyle and prostate cancer are considered to be closely related, with obesity reported to be a strong risk factor in prostate cancer development (Chan et al., 2014). Metabolic syndrome, which results from obesity, is reported to be a risk factor in the development of prostate cancer as well as type 2 diabetes and cardiovascular disease, and an increase in total cholesterol level is known to be related to histopathological malignancy in the prostate as well as to invasive cancer (Schnoeller et al., 2017). A study using a genetic model of prostate cancer has reported that a high-fat diet promotes metastatic prostate cancer (Chen et al., 2018). Furthermore, highly malignant prostate cancer cells have been reported to absorb, or even produce, cholesterol independently (Stopsack et al., 2017). Higher cellular cholesterol levels were evident in bone metastasis sites compared to primary lesions in prostate cancer (Thysell et al., 2010). In particular, an increase of de novo testosterone synthesis was indicated by an upregulation of enzymes synthesizing testosterone from identified cholesterol in castration-resistant prostate cancers which were tolerant of hormone therapy (Armandari et al., 2014). These results, combined with evidence that use of cholesterol-lowering statins is associated with reduced risk of high-grade prostate cancer, suggest that serum lipid levels should be explored as modifiable risk factors for aggressive prostate cancer. This retrospective study is aimed at evaluating the correlation between pretreatment serum cholesterol levels and prostate cancer metastasis in the Japanese population.
Methods
This study examined 351 subjects who underwent needle biopsy of the prostate gland and subsequently received a histopathological diagnosis of prostate cancer between January 2008 and December 2013. All participants were Japanese. Individuals taking statin medications for hyperlipidemia were excluded from the study due to an influence on total cholesterol level. Information concerning age, body mass index (BMI), disease stage, metastasis, Gleason score, prostate-specific antigen (PSA), total cholesterol, luteinizing hormone (LH), testosterone, and free testosterone was collected from medical histories and laboratory data. Study participants ranged in age from 39 to 92 years; the median age of all enrolled patients was 70 years. The median ages of participants in the metastatic and non-metastatic groups were 73 and 70 years, respectively. The average BMI of all enrolled patients was 23.38. The average BMI values for individuals in the metastatic and non-metastatic groups were 23.4 and 23.38, respectively. There was no statistical difference between the two groups. A Lumipulse kit (Fujirebio, Tokyo, Japan) was utilized for PSA levels, which were measured using a chemiluminescence enzyme immunoassay. Testosterone levels were measured with an Architect testosterone kit (Abbott Japan, Tokyo, Japan). An ECLusys kit (Roche Diagnostics, Basel, Switzerland) was used for LH levels, which were measured by electrochemiluminescence immunoassay. Patients were separated into two study groups: one group with metastasis (including bone and lymph node) and the other group without. One-way ANOVA (SPSS21, IBM, Armonk, NY) multivariate analysis was performed to determine any differences in variables linked to prostate cancer in the groups with or without metastasis. The following variables were analyzed: age, BMI, PSA, total cholesterol, luteinizing hormone, free testosterone and total testosterone levels. A p-value of less than .05 was considered to be statistically significant.
Results
Clinical and laboratory data were compared and analyzed in patients with and without metastasis of prostate cancer (Table 1). The PSA levels for the two groups at the time of diagnosis were 281.4 ng/ml and 18.6 ng/ml, respectively. In terms of the Gleason score, which provides a histopathological context, 13/50 cases (26%) had a Gleason score of eight or above in the metastatic group and 95/301 cases (31.6%) in the nonmetastatic group. The difference between the mean values of the two groups of patients with or without metastasis was compared by the independent samples t-test. A simple logistic regression analysis evaluating the risk of metastasis identified a statistically significant difference in bone and lymph node metastasis for total cholesterol value (p = .009), PSA (p < .001), and LH (p = .006), but not for age (p = .083), BMI (p = .975), free testosterone (p = .305), or total testosterone (p = .201). When a multivariate analysis was performed on these factors, a statistically significant difference was identified in total cholesterol level (p = .01) and PSA (p < .001) (Table 2). The linear association between total cholesterol and PSA levels is shown in Figure 1. There was a statistically significant but weak positive correlation between serum PSA level and total cholesterol (r = 0.119, p = .037).
Table 1.
Comparison Between the Group With and Without Metastasis.
| Variable | Total patients | Patients with metastasis | Patients without metastasis |
|---|---|---|---|
| No. | 351 | 50 | 301 |
| Age (yr) | 69.8 | 71.8 | 69.5 |
| Age (median, range) | 70 (39–92) | 73 (54–89) | 70 (39–92) |
| Body mass index | 23.38 | 23.40 | 23.38 |
| Total cholesterol | 191.18 | 204.86** | 189.16 |
| Prostate specific antigen | 68.61 | 281.35** | 18.60 |
| Luteinizing hormone | 9.75 | 12.69** | 9.20 |
| Free testosterone | 6.47 | 6.84 | 6.41 |
| Total testosterone | 496.06 | 525.42 | 491.19 |
p <.01.
Table 2.
Logistic Regression Analysis Evaluating Risk of Metastasis (Multiple Regression).
| Variable | OR (95% CI) | p |
|---|---|---|
| T-cho | 1.017 (1.004–1.030) | .01 |
| PSA | 1.026 (1.016–1.036) | <.001 |
| LH | 1.044 (0.994–1.097) | .085 |
Figure 1.
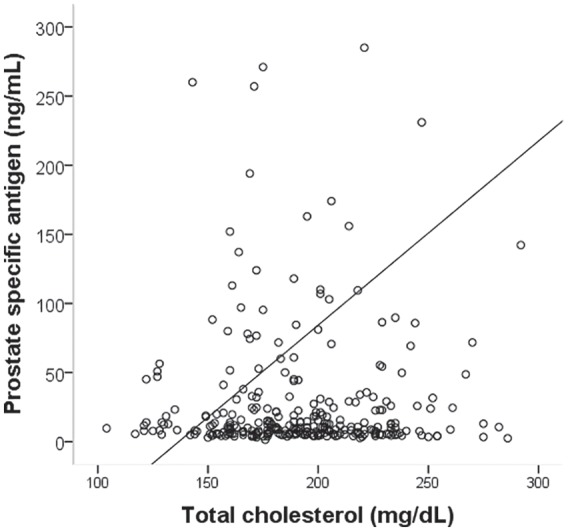
Correlation Between Serum Total Cholesterol and PSA in Men With Prostate Cancer
Discussion
Many studies reported that a diet rich in fats as well as an excess of cholesterol is an accepted risk factor for malignant prostate cancer (Masko et al., 2013). Analysis of 4974 participants in a REDUCE (Reduction by Dutasteride of Prostate Cancer Events) study identified that high total serum cholesterol was associated with an increased risk of high-grade prostate cancer (Jamnagerwalla et al., 2018). Those reports, together with those in the current study, suggest that high serum cholesterol associates with prostate cancer metastasis in a setting where biopsies ensure complete cancer ascertainment. These data also support a role for cholesterol, a modifiable risk factor, in aggressive prostate cancer. In fact, cholesterol is the precursor to androgens, essential for prostate cancer development and growth (Pelton et al., 2012). Furthermore, androgens modulate the expression of multiple lipid transporters in prostate cancer cells, and the expression of lipid transporters is enhanced in bone metastasis when compared to localized prostate cancer diseases (Tousignant et al., 2019). Prostate cancer cells that have malignant potential may already have the ability to incorporate cholesterol and produce androgen.
Interestingly, among white men without prostate cancer, total cholesterol was positively correlated with PSA levels (Zapata et al., 2015). In contrast, among black men with abnormal lipid levels, total cholesterol was not significantly related to PSA levels. In this study, there was a weak association between total cholesterol and PSA levels in Japanese patients with prostate cancer. Although patient background significantly differs among these studies, PSA levels may be affected by nutritional status, race, other lipids status and clinical stages of cancer.
A prostate lifestyle trial in which patients adopted a low-fat, plant-based diet, exercised, and practiced stress management, reported the possibility of allowing patients choosing active surveillance to delay conventional treatment for prostate cancer (Frattaroli et al., 2008). These observational and clinical studies identified that dietary changes and pharmacologic intervention may impact prostate cancer development and progression.
Furthermore, data increasingly indicate that statin use might selectively lower the risk of advanced prostate cancer (Alfaqih et al., 2017). Together with findings that the antineoplastic effect of statins might arise from a number of cholesterol-mediated mechanisms that affect pathways essential for cancer progression, this study supports an association between serum cholesterol and an increased risk of metastatic prostate cancer.
There are limitations to the design of this study, a retrospective, should be considered. As the design did not limit cholesterol levels, the possibility of artificial effects due to the other confounding factors cannot be excluded. A further limitation of this study is its relatively small sample size. This is regarded as a study that will hopefully lead to further investigations including larger numbers of participants and prognostic analysis.
Conclusions
Higher total cholesterol levels in cases of metastatic prostate cancer were identified compared to nonmetastatic prostate cancer and may be a predictive factor for poor prognosis. Further research on exercise, functional foods and nutrients involved in reducing total cholesterol level in prostate cancer prevention will yield a potential approach to measuring chemopreventive effects.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent for Publication: The report approval was obtained from the Hospital Research Ethics Board (Approval number: 14-079)
ORCID iD: Hisamitsu Ide  https://orcid.org/0000-0001-6350-9814
https://orcid.org/0000-0001-6350-9814
References
- Alfaqih M. A., Allott E. H., Hamilton R. J., Freeman M. R., Freedland S. J. (2017). The current evidence on statin use and prostate cancer prevention: are we there yet? Nature Reviews Urology, 14(2), 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armandari I., Hamid A. R., Verhaegh G., Schalken J. (2014). Intratumoral steroidogenesis in castration-resistant prostate cancer: a target for therapy. Prostate International, 2(3), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. M., Van Blarigan E. L., Kenfield S. A. (2014). What should we tell prostate cancer patients about ( -secondary) prevention? Current Opinion in Urology, 24(3), 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Zhang J., Sampieri K., Clohessy J. G., Mendez L., Gonzalez-Billalabeitia E., Liu X. S., Lee Y. R., Fung J., Katon J. M., Menon A. V., Webster K. A., Ng C., Palumbieri M. D., Diolombi M. S., Breitkopf S. B., Teruya-Feldstein J., Signoretti S., Bronson R. T., . . . Pandolfi P. P. (2018). An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nature Genetics, 50(2), 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattaroli J., Weidner G., Dnistrian A. M., Kemp C., Daubenmier J. J., Marlin R. O., Crutchfield L., Yglecias L., Carroll P. R., Ornish D. (2008). Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology, 72(6), 1319–1323. [DOI] [PubMed] [Google Scholar]
- Haas G. P., Delongchamps N., Brawley O. W., Wang C. Y., de la Roza G. (2008). The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Canadian Journal of Urology, 15(1), 3866–3871. [PMC free article] [PubMed] [Google Scholar]
- Horie S. (2012). Chemoprevention of prostate cancer: soy isoflavones and curcumin. Korean Journal of Urology, 53(10), 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnagerwalla J., Howard L. E., Allott E. H., Vidal A. C., Moreira D. M., Castro-Santamaria R., Andriole G. L., Freeman M. R., Freedland S. J. (2018). Serum cholesterol and risk of high-grade prostate cancer: results from the REDUCE study. Prostate Cancer and Prostatic Diseases, 21(2), 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masko E. M., Allott E. H., Freedland S. J. (2013). The relationship between nutrition and prostate cancer: is more always better? European Urology, 63(5), 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki M., Akaza H., Lee S. E., Song J. M., Umbas R., Zhou L., Lee B. C., Cheng C., Chung M. K., Fukagai T., Hinotsu S., Horie S. (2010). Prostate Cancer Working Group report. Japanese Journal of Clinical Oncology, 40(Suppl 1), i70–75. [DOI] [PubMed] [Google Scholar]
- Pelton K., Freeman M. R., Solomon K. R. (2012). Cholesterol and prostate cancer. Current Opinion in Pharmacology, 12(6), 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoeller T. J., Jentzmik F., Schrader A. J., Steinestel J. (2017). Influence of serum cholesterol level and statin treatment on prostate cancer aggressiveness. Oncotarget, 8(29), 47110–47120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopsack K. H., Gerke T. A., Andren O., Andersson S. O., Giovannucci E. L., Mucci L. A., Rider J. R. (2017). Cholesterol uptake and regulation in high-grade and lethal prostate cancers. Carcinogenesis, 38(8), 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thysell E., Surowiec I., Hornberg E., Crnalic S., Widmark A., Johansson A. I., Stattin P., Bergh A., Moritz T., Antti H., Wikstrom P. (2010). Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PlOS One, 5(12), e14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant K. D., Rockstroh A., Taherian Fard A., Lehman M. L., Wang C., McPherson S. J., Philp L. K., Bartonicek N., Dinger M. E., Sadowski M. C. (2019). Lipid uptake is an androgen-enhanced lipid supply pathway associated with prostate cancer disease progression and bone metastasis. Molecular Cancer Research, 17(5), 1166–1179. [DOI] [PubMed] [Google Scholar]
- Zapata D., Howard L. E., Allott E. H., Hamilton R. J., Goldberg K., Freedland S. J. (2015). Is PSA related to serum cholesterol and does the relationship differ between black and white men? Prostate, 75(16), 1877–1885. [DOI] [PubMed] [Google Scholar]


