Abstract
Auditory cortex (AC) is necessary for the detection of brief gaps in ongoing sounds, but not for the detection of longer gaps or other stimuli such as tones or noise. It remains unclear why this is so, and what is special about brief gaps in particular. Here, we used both optogenetic suppression and conventional lesions to show that the cortical dependence of brief gap detection hinges specifically on gap termination. We then identified a cortico-collicular gap detection circuit that amplifies cortical gap termination responses before projecting to inferior colliculus (IC) to impact behavior. We found that gaps evoked off-responses and on-responses in cortical neurons, which temporally overlapped for brief gaps, but not long gaps. This overlap specifically enhanced cortical responses to brief gaps, whereas IC neurons preferred longer gaps. Optogenetic suppression of AC reduced collicular responses specifically to brief gaps, indicating that under normal conditions, the enhanced cortical representation of brief gaps amplifies collicular gap responses. Together these mechanisms explain how and why AC contributes to the behavioral detection of brief gaps, which are critical cues for speech perception, perceptual grouping, and auditory scene analysis.
Keywords: auditory cortex, inferior colliculus, neural computation, speech perception, temporal processing
Introduction
A key function of the auditory system is to detect events in the environment, which are signaled by fluctuations in acoustic input. Events can be signaled by either increases or decreases in sound intensity, to which the auditory system is exquisitely sensitive. In speech perception, for example, brief gaps or noise bursts serve to segment and even identify phonemes in an otherwise continuous stream of speech (Lisker and Abramson 1964). The brain areas that mediate perception of sound increments and decrements show a striking asymmetry. It has been known for at least 25 years that lesions of auditory cortex (AC) have no effect on the detection of sound increments (such as noise bursts) but strongly impair the detection of brief sound decrements (such as gaps in noise) (Ison et al. 1991; Kelly et al. 1996; Bowen et al. 2003; Threlkeld et al. 2008; Masini et al. 2012). Curiously, cortical lesions have no effect on the detection of long gaps. This demonstrates that AC is required for the normal detection of brief gaps but that earlier auditory regions such as inferior colliculus (IC) are sufficient for the detection of long gaps and noise bursts. However, what constitutes “brief” or “long” has varied widely across studies, and the boundary between these remains unclear.
Gap detection deficits have been implicated in a wide range of neurodevelopmental and neurodegenerative conditions, including autism, auditory processing disorder, specific language impairment, specific reading disability, aging, and Alzheimer’s disease (Gordon-Salant and Fitzgibbons 1993; Snell 1997; Strouse et al. 1998; Snell and Frisina 2000; McArthur and Bishop 2001; Phillips et al. 2010; Bhatara et al. 2013; Iliadou et al. 2017), although the roles of gap detection in these conditions are still very much under debate (e.g., McArthur and Bishop 2001). Indeed, gap detection has emerged as a practical measure of temporal acuity and a model for temporal processing more generally. This underscores the importance of understanding the neural circuitry underlying gap detection, and how disruptions to that circuitry contribute to gap detection deficits.
It has remained a puzzle why AC should be involved in the detection of decrements but not increments. Moreover, it is unclear what is special about cortical processing of “brief” gaps, where the cutoff lies between brief and long gaps, and why AC is necessary for detection of one but not the other. A key question is whether gap encoding is simply relayed up the auditory hierarchy from IC to cortex, or is instead transformed in some way that might enhance the detection of brief gaps. Here, we sought to understand the underlying circuit mechanisms by which sound increments and decrements are encoded and transformed from IC to AC, and how these two structures are differentially involved in the behavioral detection of gaps and noise bursts.
To measure the behavioral detection of sounds, we used prepulse inhibition (PPI), in which a noise burst or a gap in continuous background noise acts as a cue that reduces the acoustic startle response. The detection of gaps in tones has been studied either with a single tone frequency (“within-channel”) or with a gap formed between tones of two frequencies (“between-channel”), which appear to recruit distinct perceptual mechanisms (Phillips et al. 1997; Formby et al. 1998; Phillips and Smith 2004). We used gaps in white noise, which engages both within-channel and between-channel mechanisms because white noise contains power at all frequencies. IC is known to be a critical region involved in PPI; sound-evoked activity flows from the auditory brainstem to IC, then to superior colliculus, and then to the pedunculo-pontine tegmental nucleus, which sends an inhibitory projection to startle-related premotor neurons in the pontine reticular nucleus (Koch 1999). A major corticofugal projection from AC to IC is known to modify the tuning of collicular neurons for numerous features, including frequency, intensity, and sound localization cues (Nakamoto et al. 2008; Bajo and King 2012; Suga 2012). This raises the possibility that this cortico-collicular projection might also mediate the cortical contribution to the behavioral detection of brief gaps.
Both noise bursts and gaps consist of sound onsets and offsets. Neurons in both IC and AC typically respond transiently to sound onsets and offsets. Which of these responses contribute to perceptual gap detection—on-responses, off-responses, or both? Previous work has shown that gap detection is impaired by the optogenetic suppression of AC just during the on-response to the resumption of noise at the end of the gap (Weible et al. 2014a, 2014b). This on-response (termed gap termination response—GTR) therefore appears to be critical for gap detection, but it remains unknown whether the off-response also contributes to gap detection. It also remains unclear whether the effects of cortical lesions result specifically from eliminating the GTR. The temporal precision of optogenetic suppression is well-suited to distinguishing the relative importance of on- and off-responses for the detection of gaps and noise bursts, but leaves open the question of whether the effects of cortical lesions are also mediated by affecting the same specific responses. To address these questions, we compared the effects of optogenetic suppression and conventional lesions of auditory cortex on gap detection and noise burst detection. We isolated the effects of on-responses and off-responses by using either the timing of optogenetic suppression, or (for lesioned animals) by designing noise bursts or gaps that did or did not terminate before detection. Both lesions and optogenetic suppression revealed that the cortical role in gap detection depended specifically on gap termination, but not on sound offset. To understand why this was the case, and what is special about brief gaps in particular, we compared gap encoding in dorsal IC and AC. We found that cortical GTRs were specifically enhanced for brief gaps relative to those in dorsal IC and that the mechanism underlying this amplification arises from the temporal summation of off- and on-responses. This mechanism represents a novel neural computation and explains the behavioral data by demonstrating that the cutoff between brief and long gaps is dictated by the limits of temporal summation. Optogenetic suppression of AC reduced brief gap responses in dorsal IC, indicating that the cortical amplification of gap responses in turn has downstream corticofugal effects on gap processing in dorsal IC. IC is known to play a critical role in PPI, our behavioral measure of gap detection (Koch 1999). This cortico-collicular amplification of brief gap responses therefore provides a potential mechanism for how AC contributes to brief gap detection.
Materials and Methods
Mice
All mice were 8–12 weeks of age at the time of surgery and were bred to the C57Bl6/J background strain. C57BL/6J mice at this age can start to develop age-related high-frequency hearing loss (Ison et al. 2007). Optogenetic suppression of cortex was performed in offspring (n = 20) of a cross between homozygotic Pvalb-IRES-Cre (“PV,” 008069; The Jackson Laboratory) and homozygotic Rosa26-CAG-LSL-ChR2H134R-eYFP (“ChR2,” Ai32, 012569; The Jackson Laboratory) lines. In these mice (PV × ChR2), ChR2 was expressed in parvalbumin (PV)-expressing interneurons. Aspiration lesion (n = 16) or control (n = 14) mice were either from the same cross or were homozygous for Pvalb-IRES-Cre. We also recorded single neuron activity from either PV × ChR2 mice (n = 11) or offspring of a cross between SOM-IRES-Cre (013044; The Jackson Laboratory) and ChR2 lines (n = 10).
Surgery
We administered dexamethasone (0.1 mg/kg) and atropine (0.03 mg/kg) pre-operatively to reduce inflammation and respiratory irregularities. Surgical anesthesia was maintained with isoflurane (1.25–2.0%). For optogenetic suppression of cortical excitatory activity, we implanted 200-μm optic fibers in each hemisphere at AP 2.3 mm (relative to bregma), ML 4.4 mm, and depth 0.5 mm below the dura (immediately dorsal to AC). For aspiration lesions, a bilateral craniotomy (2 × 3 mm) was performed overlying AC (using stereotactic coordinates) and the dura retracted. Cortical aspiration was performed by applying suction through a blunt-tipped 20-ga hypodermic needle. Sterile cold saline was applied for irrigation and to reduce bleeding. The region was then packed with sterile gelatin sponge. A thin coating of antibacterial ointment was applied over the sponge and the whole area covered with Grip Cement (Dentsply). Control mice underwent the craniotomy, but the segment of skull was left in place and covered over with Grip Cement. For single neuron recordings in AC, an array of eight tetrodes was inserted vertically through a smaller craniotomy (2 x 1 mm) dorsal to AC. The tetrode array was mounted on a microdrive which was cemented into place. For single neuron recordings in IC, the position of both lobes of the IC was first confirmed visually through a craniotomy (2.5 x 1.0 mm) made along the midline immediately posterior to the transverse sinus. Two pairs of microdrive-mounted tetrodes were then implanted bilaterally 0.5–0.75 mm from the midline, immediately ventral to the dura. This tetrode placement was selected to target dorsal IC, which receives the largest cortifugal projection from AC. Finally, a pair of optic fibers was implanted bilaterally overlying AC. A light-weight aluminum post (~1.5 g) was also cemented in place anterior to the recording array to enable head-fixed recordings in awake mice freely running on an exercise wheel. Ketoprofen (4.0 mg/kg) was administered postoperatively to minimize discomfort. Mice were housed individually after the surgery and allowed 7 days of postoperative recovery.
Behavioral Data Acquisition and Stimulus Delivery
All behavioral data were collected in a sound-attenuating chamber. Sounds were delivered from a free-field speaker directly facing the animal. The speaker was calibrated to within ±1 dB using a Brüel and Kjær 4939 1/4-inch microphone positioned where the ear would be but without the animal present. Mice were loosely restrained in a plastic tube (35-mm inner diameter, 1.5-mm wall thickness) affixed to a flat base. The head was fixed in position. The tube was perforated (~3 mm diameter) to allow effective transmission of sound, with no more than 5-dB attenuation. An open slot along the top enabled access to the implanted fibers. To measure the startle response, the tube rested on a piezo transducer. Movement signals from the piezo transducer were amplified and digitized at 10 kHz. We did not record electrophysiology during startle response behavior.
We measured the effectiveness of three stimulus types to attenuate startle responses elicited by a 100-dB white noise burst. Schematics of each stimulus type are included as insets in the corresponding figure panels. During PPI, a brief white noise burst (60 dB) preceded the startle stimulus by a fixed interstimulus interval (either 0 or 50 ms, measured from prepulse offset to startle onset). The duration of the white noise burst varied from 1 to 256 ms. During gap detection with a white noise carrier, silent gaps were inserted into continuous background white noise (80-dB SPL). These gaps varied from 1 to 256 ms duration, matching the prepulse stimulus durations, and the gap termination preceded the startle pulse by 0, 10, 20, 30, or 50 ms, a period we refer to as the postgap interval. We defined stimulus onset asynchrony as the time between gap onset and startle pulse onset (i.e., gap duration + postgap interval). Gaps in white noise did not have a ramp at gap onset or termination. For gap detection with a pure tone carrier, silent gaps were inserted into a continuous background 8-kHz pure tone (80-dB SPL). Gap durations were 0, 32, or 256 ms in duration, with 3 ms ramps at gap onset and termination. We also presented isolated startle stimuli not preceded by a gap or prepulse. Each stimulus was presented 20 times per session, separated by a random intertrial interval of 15 ± 5 s. All stimuli were presented in a randomized sequence.
We suppressed cortical activity in PV-ChR2 mice using a 445-nm wavelength diode laser set to an output power of 6.3 mW. The resulting intensity of 200 mW/mm2, as measured at the tip of the 200-μm diameter fiber, results in the suppression of excitatory activity limited to AC (Weible et al. 2014a). Laser illumination was delivered either during the 50-ms postgap interval or during the gap; for noise bursts, illumination was delivered either during the 50-ms postburst interval or during the burst. Thus, postgap illumination was always 50 ms in duration, whereas illumination during the gap had variable durations of 1–256 ms (and the same was true for noise bursts). Rise/fall times of laser pulses were 5 μs. We measured laser power and rise/fall times with a Thorlabs PM100D power meter. We have previously characterized this optogenetic suppression method and found that tone-evoked and spontaneous activities were suppressed by 86 and 87%, respectively, with no postsuppression rebound. GTRs are completely abolished by illumination during the postgap interval (Weible et al. 2014a). Suppression of spontaneous activity (recorded in silence) begins within 3.3 ms following laser onset (initial 10% reduction) and reaches 90% of maximal suppression within 11.4 ms; activity begins to recover within 12.3 ms after laser offset and returns to 90% of baseline firing rate within 26.8 ms (see Supplementary Fig. 1). Thus, the onset of optogenetic suppression is faster than the fastest cortical sound-evoked response latencies, and the offset of suppression is faster than typical off-response latencies.
Behavioral Analysis
We quantified startle amplitudes by calculating the area of the rectified signal from the piezo transducer within a 100-ms window after startle stimulus onset. We quantified baseline movement during the 100-ms interval preceding the gap in the same way. For all behavioral experiments (lesions and optogenetic suppression), all sessions were included for analyses (i.e., no exclusion criteria were established for individual sessions). Data were collected from the same mouse for no more than four sessions, to minimize the likelihood of introducing any experience-related shifts in startle behavior at brief gap durations (Swetter et al. 2010; Weible et al. 2014b). For the aspiration lesion experiments, both lesion and control curves were normalized (within session) to the median “pure” startle response (i.e., to isolated startle stimuli not preceded by a gap or prepulse stimulus). A separate comparison was performed using non-normalized data to determine whether the lesion had any effect on the pure startle response relative to controls (Wilcoxon rank-sum test). For optogenetic experiments, because comparisons were within-animals rather than between-animals, laser-on and laser-off data within each session were normalized to that session’s median laser-off pure response. Psychometric curves were calculated using ROC analysis of startle response amplitudes, and then fit with a logistic function. For each gap duration, the ROC analysis measured how well (in percent correct) an ideal observer (binary classifier) could distinguish startle responses preceded by a gap from those that were not preceded by a gap (Green and Swets 1966). Minimum gap detection thresholds were determined from the midpoint of the logistic fit (halfway between chance and maximal performance). Because not all of the behavioral data were normally distributed, we used nonparametric statistics for all behavioral analyses. To test for main effects of optogenetic suppression or lesions on gap detection behavior, we measured median normalized startle response values for each session and used the Kruskal–Wallis test (nonparametric alternative to the Anova) across sessions. We then used the rank-sum test post-hoc to identify effects at specific gap durations. In principle, paired tests could be used for optogenetic suppression (comparing laser/no laser within mice), but the lesion data required unpaired tests (comparing lesion/no lesion across mice). To directly compare lesion results to optogenetic results requires using tests with the same power, so we used the unpaired test (rank-sum) in both cases.
Single Neuron Recording and Analysis
We implanted an array of eight tetrodes passed through a 1 × 4 array of 28-gauge stainless steel hypodermic tubing, with two tetrodes per tube. Tetrodes were made of 18-μm (25-μm coated) tungsten wire (California Fine Wire). The entire array was mounted on a custom microdrive. Tetrode data were acquired with 32-channel RHD2000 hardware (Intan Technologies) and Open Ephys software (http://open-ephys.org). A minimum threshold of 50 μV was set for the collection of spiking activity. Spiking activity of individual neurons was isolated offline using the open source spike sorting software packages Simpleclust (https://github.com/open-ephys/simpleclust) and MClust (http://redishlab.neuroscience.umn.edu/MClust/MClust.html). Measures of peak and trough waveform voltage, energy, and principal components analysis were used as waveform separation parameters in 2D cluster space. Cells were accepted for analysis only if they had a cluster boundary completely separate from adjacent cluster boundaries, and completely above threshold, on at least one 2D view. Additionally, cells with events during a 2-ms refractory window in the interspike interval histogram ≥1% of the total spike count were excluded from analysis (the median of the final population accepted for analysis was 0.14%). Single neuron recordings were performed in a sound attenuation chamber while mice were head-fixed and free to run on an exercise wheel. Sounds were delivered from a free-field speaker placed 10 cm from (and facing) the contralateral ear, which was calibrated to within ±1 dB as described above. We did not measure startle responses during electrophysiology.
We characterized neuronal responses to gap-in-noise, white noise, and paired-tone stimuli. The presentations of gaps-in-noise differed from the behavioral stimulus protocol in only two respects. First, no startle pulses were presented. This ensured that we could accurately measure the duration of GTRs and any other response profiles associated with gap presentations. Second, a shorter ITI was used (1 s vs. the 15 ± 5 s used during behavioral experiments). All gap durations were presented 25 times per recording session. From some of these cells, we also recorded responses to white noise stimuli (60 dB) with durations matching those used in our behavioral assessment of PPI (1–256 ms). All white noise durations were presented 25 times per recording session. We used gaps in tone pairs to examine the temporal summation of off- and on-responses. First, we screened cells for off- and on-responses to an array of 400-ms tones, six frequencies per octave from 4 to 64 kHz, 70 dB, 3-ms cosine-squared ramps, 500-ms interstimulus interval, and 50 repetitions. We identified cells that had only an off-response to one frequency and only an on-response to another. We then presented paired-tone stimuli at those frequencies, 70 dB, 500-ms duration, 1000-ms interstimulus interval, and separated in time by gaps of 2, 4, 8, 16, 32, 64, 128, and 256 ms (20 repetitions for each gap duration). The gaps separating tones were measured between the half-maxima of the 3-ms cosine-squared ramps on the tones. Afterwards, the recording array was moved down 40–80 μm and allowed to settle for a minimum of 2 h before screening for new cells.
We quantified GTRs as the average firing rate during the 50-ms postgap interval, and tested for significance by comparing to activity in the same time window on trials without a gap (paired t-test). To quantify off-responses, we measured the average firing rate in the 50-ms window following sound offset, for gap durations of 64–256 ms. For 32-ms gaps, we quantified the off-response as the average firing rate in a 32-ms window following sound offset, and compared with a 32-ms window on trials without a gap. We did not measure off-responses for gaps shorter than 32 ms, as they would overlap with the postgap interval. A cell was only considered to have a significant response if the response was significant for two consecutive gap durations. For example, a cell with a minimum gap threshold (MGT) of 4 ms must have exhibited a significant response following both the 4- and 8-ms gap duration (following the criterion established by Walton et al. 2008). The preferred gap duration was defined as the higher of the two consecutive greatest GTRs. We used the two-sample Kolmogorov–Smirnov test (K–S test) to compare the distribution of cells across gap durations for measures of MGT or preferred duration. Off-response durations were measured as the duration that the trial-averaged, Gaussian-smoothed (3-ms SD) firing rate was continuously above baseline firing rate.
Note that MGTs for behavioral gap detection and for neural responses were measured differently (as the midpoint of the psychometric curve in the first case, and by testing for significant spiking responses in the other). Because these tests do not have comparable sensitivity, the MGTs we report for behavior and for neural responses should not be directly compared to each other.
We computed a gap tuning index (GTI) in order to quantify how selective neuronal GTRs were for gap duration. This measure compares the responses to best gap duration to the worst gap duration, as given as follows:
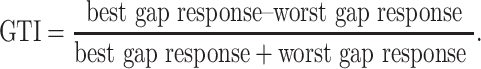 |
GTI varies from 0 to 1 and reflects the depth of modulation in the gap duration tuning curve. For robustness, we averaged the two best gap responses together, as well as the two worst gap responses. Cells without a significant GTR were excluded from this analysis. We also computed a relative amplification factor to quantify the amplification of the GTR for the preferred gap duration compared with the GTR for a long gap (for which on-responses and off-responses were well-separated in time). For each cell, we computed this amplification factor by normalizing gap responses to the GTR evoked by a 128-ms gap. Cells without a GTR for 128-ms gaps were excluded from this analysis.
To identify on-responses and off-responses to white noise stimuli, we used paired t-tests. On-responses were assessed during the 50-ms interval immediately following white noise onset. Off-responses were also assessed during a 50-ms interval, but only for white noise bursts ≥64 ms, as shorter intervals would overlap with the onset window. The same criteria were used to identify minimum white noise thresholds and preferred white noise durations as those used for the detection of gap responses. We used the two-sample K–S test to compare the distribution of cells across gap durations for the measures of minimum duration threshold or preferred duration.
We computed duration tuning curves based on the responses across gap or noise burst durations. We then classified neurons with significant GTRs or white noise responses as all-pass, band-pass, short-pass, or long-pass, as has been done previously (Casseday et al. 1994; Fuzessery and Hall 1999). All-pass neurons responded above 50% of the peak response amplitude across all stimulus durations. Band-pass neurons exhibited ≤50% firing rate at durations both shorter and longer than the peak duration. Short-pass neurons preferred brief durations and exhibited a decrease to ≤50% of the peak firing rate at longer durations. Long-pass neurons typically preferred longer durations and exhibited a decrease to ≤50% of the peak firing rate at shorter durations. In a few instances, the peak firing rate was achieved to two nonconsecutive stimulus durations, separated by a minimum 50% decrease. These neurons were classified as dual-pass and were excluded from group comparisons for pass-types, as they were: 1) only very infrequently detected and 2) have generally not been described in the duration tuning literature. The width of tuning was defined as the number of consecutive gap durations around the peak with a firing rate above the 50% of peak threshold. For example, the short-pass cell in Supplementary Figure 5a would have a width of four, while the band-pass cell in Supplementary Figure 5c would have a width of two.
Histology
All brains were sectioned coronally. We verified that optic fibers and recording tetrodes accurately targeted AC or dorsal IC using the structure of the hippocampus and the rhinal fissure as rostrocaudal and dorsoventral landmarks (section thickness: 100 μm [fibers] or 50 μm [tetrodes]). To reconstruct the position and size of aspiration lesions, a photomicrograph was taken of every other section encompassing the entirety of the lesion (section thickness: 100 μm). Photomicrographs were then matched to atlas sections (Paxinos and Franklin 2001) based on the rostrocaudal morphology of the hippocampus, and the boundaries of dorsal, primary, and ventral auditory cortical regions from those atlas sections applied to the photomicrographs. We calculated the total volume of damage, section-by-section, as a percentage of auditory cortical volume.
Results
Optogenetic Suppression
First, we compared the effects of optogenetic suppression and conventional lesions of AC on gap detection and noise burst detection. We measured gap detection and noise burst detection using a PPI behavioral paradigm, in which either a noise burst or a gap in continuous background noise acted as a prepulse to reduce the acoustic startle response. To optogenetically suppress AC, we implanted optical fibers bilaterally over AC in PV-ChR2 mice (see Methods) and used a laser power (200 mW/mm2) that we have previously shown to suppress activity throughout but not beyond AC (Weible et al. 2014a). We first tested the importance of on-responses and off-responses for gap detection. To isolate the effects of on-responses, we suppressed AC during the 50-ms postgap interval (Fig. 1a, inset), when the GTR occurs. Suppression of GTRs during the postgap interval significantly impaired gap detection. We quantified gap detection performance by using ROC analysis to ask whether an ideal observer could detect the gap based on the attenuation of the startle response, producing a psychometric curve for gap detection (Fig. 1a). Optogenetic suppression of the GTR shifted the psychometric curve to the right, such that a longer gap was required to reach the same level of performance. Figure 1b shows the same data plotted as startle response amplitudes. Because gap detection corresponds to the attenuation of the startle response, optogenetic suppression shifted the curve upwards (less attenuation), indicating significantly impaired gap detection (Kruskal–Wallis df = 1, X2 = 22.65, P < 0.0001). Gap detection was impaired for brief gap durations (2–64 ms, rank-sum P < 0.05) but not for longer gaps. Optogenetic suppression had no effect on 1-ms gaps, which were below detection threshold. This is consistent with previous work and demonstrates that the detection of brief gaps depends critically on the GTR (Weible et al. 2014a, 2014b). In contrast, cortical suppression during the gap itself had no effect on gap detection (Fig. 1c,d). This suggests that unlike the GTR, off-responses that occur during the gap do not contribute to gap detection.
Figure 1.

Optogenetic suppression of cortical activity during the postgap interval, but not the gap itself, impairs gap detection. We suppressed cortical activity by optogenetically activating PV-expressing inhibitory interneurons during either the 50-ms postgap interval or during gaps ranging from 1 to 256 ms in duration. (A) Suppression of cortical activity during the postgap interval robustly impaired gap detection. Gap detection was impaired for brief gaps (≤64 ms) but not for longer gaps. Inset shows a diagram of the stimulus, with laser illumination shown in blue, and an example of a neuronal response with both off-responses and on-responses. Laser illumination during the postgap interval suppresses on-responses (GTRs). We quantified gap detection as the percent correct with which an ideal observer could detect gaps using ROC analysis of startle response amplitudes. MGTs were measured halfway between chance (50%) and maximal performance, and are indicated by vertical gray lines. Under control conditions, the MGT was 2.9 ms, and during postgap suppression, the MGT increased to 9.3 ms. Error bars show standard error across sessions (n = 9 mice, 25 sessions). (B) Startle attenuation values underlying the ROC analysis in (A). Post-hoc analyses identified differences for gaps 2–64 ms in duration. Values are normalized to the median startle response without a gap. (C,D) In contrast to the effects of suppression during the postgap interval, cortical suppression during the gap itself (see inset) had no effect on gap detection, measured as percent correct (C), startle attenuation (D), or MGT (n = 4 mice, 10 sessions). (E,F) Cortical suppression had no effect on conventional PPI using a 60-dB noise burst ranging from 1 to 256 ms in duration as the prepulse, regardless of whether we delivered laser illumination during the 50-ms postburst interval (E; n = 3 mice, 6 sessions), or during the noise burst (F; n = 5 mice, 8 sessions). The interval between prepulse termination and startle pulse onset was fixed at 50 ms. (*: P < 0.05, rank-sum post-hoc).
We then tested the importance of on-responses and off-responses for conventional PPI, using brief bursts of noise as the prepulse. Optogenetic suppression of AC had no effect on conventional PPI, regardless of whether the off-response was suppressed (Fig. 1e) or the on-response was suppressed (Fig. 1f). Thus, we found a striking asymmetry in the cortical dependence of increment and decrement detection. Suppression of auditory cortex impaired gap detection, but not noise burst detection. The impairment of gap detection only occurred with the suppression of GTRs, but not with the suppression of off-responses during the gap. Furthermore, gap detection was only impaired for brief gaps, i.e., durations of 64 ms and shorter.
Auditory Cortical Lesions
Auditory cortical lesions impair the detection of brief gaps. Detection of longer gaps is unaffected, although the boundary between brief and long has not been well characterized and estimates vary (15–75 ms; Ison et al. 1991; Kelly et al. 1996; Bowen et al. 2003; Threlkeld et al. 2008; Masini et al. 2012). The fact that only GTR suppression impairs gap detection suggests that the effects of cortical lesions might also be mediated specifically through their elimination of the GTR (although, of course, they also eliminate all other aspects of cortical gap responses). To isolate the effects of off-responses and on-responses, we used noise bursts or gaps that did or did not terminate before the startle stimulus. First, we confirmed that aspiration lesions of AC impaired the detection of brief gaps. Damage to AC was extensive, averaging >85% removal bilaterally across animals (see Supplemental Fig. 2). Lesions significantly impaired gap detection compared with controls (Fig. 2a,b; Kruskal–Wallis df = 1, X2 = 18.5, P < 0.0001). Detection was impaired for gaps 2–32 ms in duration (rank-sum P < 0.05). Cortical lesions and optogenetic suppression produced similar impairments of gap detection (Fig. 2d; Kruskal–Wallis df = 1, X2 = 1.5, P = 0.2). This confirms previous work and clarifies that “brief” gaps (those for which detection is impaired by cortical lesions or suppression) are those less than 32–64 ms (Ison et al. 1991; Kelly et al. 1996; Bowen et al. 2003; Threlkeld et al. 2008).
Figure 2.

Lesions of AC impair brief gap detection. (A) Cortical lesions significantly impaired gap detection and increased the MGT by 23 ms (from 6.4 to 29.5 ms). Gap detection was impaired for brief gaps (≤32 ms) but not for longer gaps. Inset shows a diagram of the stimulus (the same as in Fig. 1). (B) Lesioned mice showed significantly less startle attenuation across gap durations compared with controls (lesions: n = 10 mice, 30 sessions; controls: n = 8 mice, 21 sessions). Post-hoc analysis revealed significant differences between lesion and control mice for individual gaps 2–32 ms in duration (rank-sum). (C) Importantly, these effects cannot be explained by differences in sensitivity to the startle stimulus, since raw startle amplitudes (measured in arbitrary units) were comparable between groups for trials without a gap. (D) Impairment of gap detection was similar for lesions and optogenetic suppression of AC. (*: P < 0.05, rank-sum post-hoc). Blue dots show the difference between laser-on and laser-off trials, red dots show the difference between lesion and control groups, and the dashed line corresponds to no effect.
To study the specific role of gap terminations in gap detection with lesioned mice, we used the stimulus shown in Figure 3a (inset), in which a gap precedes the startle stimulus but does not terminate before the startle stimulus. Thus, only the noise offset (and the off-response to it) contributes to startle attenuation. Mice performed well at detecting gaps without a termination, but cortical lesions had no effect on this performance (Fig. 3a), consistent with previous work (Bowen et al. 2003; Yu et al. 2016). We varied the postgap interval from 50 (as in Figs 1 and 2) to 0 ms (i.e., gaps that did not terminate before the startle pulse) and found that the effect of lesions on gap detection decreased smoothly with decreasing postgap intervals (Fig. 3b–e). Cortical lesion effects were significant for postgap intervals of 30 and 50 ms, trended toward significance with a 20-ms postgap interval, but were absent with postgap intervals of 10 and 0 ms. This is consistent with the idea that the effects of lesions are mediated by elimination of cortical GTRs. Indeed, this predicts that GTRs have a response latency between 10 and 20 ms, which would explain why GTRs would not have a chance to affect gap detection if the postgap interval is shorter than this. Thus, cortical lesions have no effect on pure offset detection in a gap detection paradigm and instead only have an effect when the gap is terminated by a resumption of noise that lasts long enough for the evoked GTR to precede the startle response.
Figure 3.

Lesion effects require gap terminations. (A) For gaps without a termination, cortical lesions had no effect. We used gaps that did not terminate before the startle pulse (see inset) such that the postgap interval was 0 ms. Mice were able to detect these gaps, with MGTs that were no different in control and lesioned mice (control: 3.0 ms, n = 7 mice, 10 sessions, lesion: 3.1 ms, n = 6 mice, 12 sessions, n.s.). (B) We varied the postgap interval from 0 to 50 ms. The effect of cortical lesions was prominent with a postgap interval of 50 ms and progressively decreased as we decreased the postgap interval towards 0 ms. Sample sizes for 50 ms: control, 8 mice 21 sessions, lesion, 10 mice 30 sessions (same data as Fig. 2a); 30 ms: control, 7 mice 10 sessions, lesion, 6 mice 12 sessions; 20 ms: control, 7 mice 10 sessions, lesion, 6 mice 12 sessions; 10 ms: control, 7 mice 10 sessions, lesion, 7 mice 10 sessions; and 0 ms: control, 9 mice 12 sessions, lesion, 7 mice 10 sessions. Kruskal–Wallis 30-ms postgap interval: df = 1, X2 = 14.9, P = 0.0001; 0–20 ms postgap interval: n.s. (C) MGT increased for longer postgap intervals, much more for lesioned mice than for control mice. (D,E) Gap detection performance depended significantly on the postgap interval. In both lesion (D) and control (E) mice, longer postgap intervals shifted psychometric curves towards longer gaps, increasing the MGT.
Interestingly, the shorter the postgap interval, the better mice were at detecting gaps (Fig. 3d,e). We wondered whether this was a specific effect of this period of resumed noise, or whether it was simply an effect of stimulus onset asynchrony. As the postgap interval becomes shorter, the separation between the gap onset and the startle stimulus also becomes shorter. To disambiguate these two features, we examined gap detection as a function of stimulus onset asynchrony (see Supplementary Fig. 3) and found that shorter postgap intervals produced better gap detection even after controlling for the change in stimulus onset asynchrony. Taken together, these results show that off-responses alone can mediate gap detection (see Fig. 3a). But since optogenetically suppressing cortical off-responses during the gap had no effect, and cortical lesions had no effect on detection of gaps containing only offsets, subcortical off-responses must be sufficient to mediate the detection of gaps containing only offsets.
As with optogenetic suppression (Fig. 1e,f), we found that cortical lesions had no impact on conventional PPI (see Supplementary Fig. 4a). This is consistent with previous work (Davis and Gendelman 1977; Bowen et al. 2003) and indicates that AC is not required for the detection of noise bursts. To specifically test the cortical role for onset detection, we used a variant of PPI in which the prepulse did not terminate before the startle stimulus, such that only the noise onset contributes to PPI. We found that cortical lesions produced no deficit in this onset detection task (see Supplementary Fig. 4b), from which we conclude that AC is not required for noise onset or burst detection. We also noted that noise onsets alone produced weak and unreliable PPI (see Supplementary Fig. 4b), consistent with previous work (Bowen et al. 2003).
These lesion experiments indicate cortical involvement in brief gap detection, but not noise burst detection. Taken together, the optogenetic suppression and lesion experiments show that the cortical dependence of brief gap detection hinges specifically on gap termination. This suggests that cortical responses to the gap termination make a critical contribution to gap detection by influencing downstream midbrain components of the startle response pathway. This raises several questions. What is special about brief gaps compared with longer gaps? How do auditory cortical neurons respond to brief gaps? Do cortical neurons encode brief gaps differently than inferior collicular neurons? Do cortical gap responses have any influence on those in the IC? To address these questions, we recorded gap responses from individual neurons in AC and IC in awake mice, and tested the effect of cortical suppression on collicular gap responses.
Gap Encoding in AC
We used chronically implanted tetrodes to record from auditory cortical neurons in awake mice during presentations of gap stimuli that matched those used in our behavioral experiments, except that we omitted the startle pulse and shortened the intertrial interval to 1 s. We histologically verified the placement of the tetrodes in AC (Fig. 4a). Neurons showed several distinct types of gap responses. Figure 4b shows an example of a typical auditory cortical neuron showing robust GTRs. This cell was tuned to gap duration such that the largest GTR was evoked by a gap of 16 ms (Fig. 4c). We recorded gap responses from a total of 534 neurons (complete dataset at http://www.uoneuro.uoregon.edu/wehr/data). Of these, 39% (210/534) showed significant GTRs (we determined significance by comparing spiking during the 50-ms postgap interval to that during background noise without a gap, see Methods). GTRs had a latency of 15.5 ± 13.8 ms (median ± IQR), consistent with the prediction based on the behavioral lesion results above. These cells showed a broad range of preferred gap durations (Fig. 4e; median preferred gap duration was 16 ms, tuning based on GTRs). Cells also ranged from tightly to broadly tuned for gap duration. We quantified the selectivity of GTRs for gap duration using a Gap Tuning Index (GTI), which ranges from 0 (untuned) to 1 (tightly tuned, see Methods). Figure 4f shows the range of GTI for all cells with significant GTRs; median GTI was 0.63 ± 0.45 IQR. A few cells, like the example in Figure 4g, responded with only an off-response locked to noise offset (23/534, or 4%). Much more common were cells like the example in Figure 4h, which showed both off-responses and GTRs (108/534, 20% of all cells, or 51% of the 210 cells that showed GTRs). For these cells, distinct off-responses and GTRs can be seen for long gap durations. But for gaps shorter than about 32–64 ms, the two responses are no longer distinct. Although it is impossible to assign any given spike in the postgap interval as having been evoked by noise offset or noise onset, it is tempting to infer that the two responses summate and produce a stronger net GTR for brief gaps. Indeed, the GTRs of this cell were tuned to 16 ms, where the two types of responses overlapped completely (Fig. 4h,i). This observation led us to the following working hypothesis: cortical GTRs for brief gaps can be amplified by the temporal summation of off-responses and on-responses. This idea is supported by our previous finding that off-responses and on-responses in AC are driven by largely non-overlapping sets of synapses (Scholl et al. 2010), which would allow these responses to summate for the first time in AC without interference from synaptic depression or forward suppression. This specific cortical amplification of brief-gap GTRs could in turn explain why and how cortical lesions or optogenetic suppression of the cortical GTR specifically affect the detection of brief gaps.
Figure 4.

Gap encoding in AC. (A) Neurons were located in AC. About 39% of cortical neurons (210/534) exhibited a significant burst in spiking activity during the postgap interval, referred to as a GTR. (B,C) Example of a neuron showing robust GTRs to brief gaps. Stimuli are indicated by horizontal gray bars, aligned to gap termination. (C) Preferred gap duration for this cell was 16 ms. Error bars show SEM. (D) Distribution of MGTs. Neurons in AC had a median MGT (i.e., the smallest detectable GTR) of 2 ms. (E) The distribution of preferred gap durations was approximately uniform for durations at or above the behavioral detection threshold. (F) Cells varied widely in their selectivity for gap duration, as measured with the GTI. (G) Example of a neuron showing robust off-responses. (H,I) Example of a neuron showing both off-responses and on-responses. (I) Preferred gap duration for this cell was 16 ms. (J) Relative amplification factor of preferred gap responses. For each cell, we computed the amplification factor by normalizing GTRs to that evoked by a 128-ms gap. Responses are aligned to the preferred gap duration for each cell. Relative gap duration (the x-axis) is measured in units of octave distance from best gap duration (e.g., 8–16 ms). Dashed horizontal line at 1 corresponds to the GTR evoked by a 128-ms gap. Average of 432 cells with a nonzero 128-ms gap response. (K) Off-responses for n = 111 cells (gray traces) that showed significant off-responses. Black trace shows the population-averaged off-response. Red dashed line shows the median off-response duration (75 ms). (L) Distribution of off-response durations matches behavioral effect size. Blue and red curves show behavioral gap detection deficits caused by optogenetic suppression and cortical lesions, respectively (the same data as Fig. 2D). Black curve shows the distribution of off-response durations, plotted as survival fraction. Each point indicates the percentage of cells with off-responses longer than a given gap duration. The y-axis for blue and red curves is at left; y-axis for black curve is at right.
Several observations about gap duration tuning in our recorded cells support this hypothesis. First, we found that GTRs evoked by preferred brief gaps were amplified relative to isolated on-responses, such as the GTR evoked by a long gap. To quantify this effect, we computed an amplification factor (Fig. 4j), which showed a sharp peak that reflected a 2.6-fold amplification of the preferred GTR across the population. This suggests that gap responses are boosted by the overlapping off-response within a narrow range around the preferred gap duration. Second, “off–on” cells, which had both significant off-responses and GTRs at long (≥64 ms) gap durations, had shorter MGTs (df = 208, t = 3.0, P = 0.003) and preferred briefer gap durations (df = 208, t = 2.6, P = 0.009) compared with “on-only” cells that showed only GTRs (n = 108 “off–on” cells, n = 102 “on-only” cells). In addition, the nature of gap duration tuning appeared to differ across these populations. To investigate this further, and to compare with previous findings, we turned to a commonly used method of characterizing gap-duration-tuning, by categorizing cells as either short-pass, band-pass, long-pass, or all-pass (examples and group data shown in Supplementary Fig. 5) (Casseday et al. 1994; Fuzessery and Hall 1999). The proportion of short-pass, band-pass, long-pass, and all-pass response types differed between groups (χ2 = 8.9, P = 0.03), with more band-pass and fewer long-pass responses seen from off–on cells (off–on: short: n = 5, band: n = 56, long: n = 38, all: n = 5; on-only: short: n = 9, band: n = 43, long: n = 48, all: n = 0). This is consistent with the idea that off- and on-responses merge for brief gaps in off–on cells, amplifying those gap responses and thereby producing short-pass or band-pass tuning for brief gaps. Nearly all short- and band-pass responses were tuned to gaps ≤32 ms in duration (101/113 or 89%), which matches the cutoff for which cortical lesions affected perceptual gap detection (see Fig. 2a). For long-pass responses, the tuning width (i.e., the range of gap durations that evoked at least half-maximal responses) was significantly wider for off–on cells than for on-only cells (5.8 ± 2.4 SD durations vs. 4.0 ± 2.3 SD durations; df = 84, t = 3.5, P = 0.0007), indicating that long-pass off–on cells had greater responses to brief gaps than long-pass on-only cells. In addition, mean GTR firing rates for the two groups differed significantly across gap durations, with higher firing rates evoked by brief gaps for off–on cells (df = 1, F = 27.8, P = 1.4 × 10−7, Anova). Finally, this hypothesis predicts that “brief” gaps (i.e., those amplified by this cortical mechanism and for which cortical manipulations impair gap detection) are those for which off-responses and on-responses overlap in time. This overlap is determined by the duration of cortical off-responses. To test whether off-response durations matched the behavioral definition of “brief” gap durations, we measured off-response durations in our sample of cortical neurons. Median off-response duration was 75 ± 62 ms (Fig. 4k, n = 111 cells with significant off-responses, ±IQR), which matches well with the cutoff for brief gaps as determined by optogenetic suppression (≤64 ms) and lesions (≤32 ms). Indeed, the dependence of lesion and suppression effects on gap duration matched the distribution of off-response durations (Fig. 4l). As gap durations increase beyond the population of off-response durations, cortical manipulations cease to have any effect. For the shortest gaps, cortical manipulations have little effect because mice perform poorly near their detection threshold.
In order to test the hypothesis that cortical GTRs for brief gaps are amplified by temporal summation of off- and on-responses, it would be ideal to “tag” spikes as being evoked by either sound offset or sound onset. If we could independently manipulate off-responses or on-responses, we could test whether and how they summate to amplify brief gap responses. To address this, we took advantage of the fact that off-responses and on-responses in cortical neurons typically show different frequency tuning (Recanzone 2000; Qin et al. 2007; Fishman and Steinschneider 2009; Scholl et al. 2010). We composed gap stimuli from pure tones that differed in frequency, and chose frequency pairs for which one tone (FON) preferentially evoked an on-response and the other tone (FOFF) evoked an off-response. Figure 5a,b shows an example of such a cell. In this cell, FOFF tones evoked off-responses (open blue arrows), and FOFF–FON pairs evoked robust GTRs that were tuned to gaps of 16 ms (black arrows). For long gaps (256 ms), FOFF–FON off-responses and on-responses were separated in time. But for shorter gaps, they overlapped in time, amplifying the GTR and producing a maximal response for gaps of 8–16 ms. Replacing either the trailing tone (e.g., FOFF–FOFF, blue arrows) or the leading tone (e.g., FON–FON, red arrows) or both (e.g., FON–FOFF, purple arrows) removed the on-response, the off-response, or both. Any of these manipulations dramatically reduced the GTR and also the tuning of this cell for brief gaps (Fig. 5b). This indicates that the GTR for brief gaps was amplified in this cell by temporal summation of off- and on-responses. We recorded from 214 cells in AC, for which 86 showed selective on-responses for one tone and off-responses for another, allowing us to test for summation. Across the population, the average tuning of gap responses was very similar to this example cell (Fig. 5c). Gaps formed by tone pairs that evoked an off–on sequence (black curve) showed marked tuning for short gaps, for which the off- and on-responses overlapped in time (black arrows). Eliminating either the on-response or the off-response by changing the component tones strongly reduced GTR amplitudes and tuning for brief gaps (red, blue, and purple arrows). Similarly, peak firing rate evoked by a cell’s preferred gap duration was strongly reduced by replacing any of the component tones (Fig. 5d). These data provide strong support for the hypothesis that brief gap responses in AC are amplified by temporal summation of off- and on-responses. Although tone-pair gaps are different stimuli than gaps in noise (which allowed us to independently manipulate off- and on-responses), it seems likely that the same mechanism amplifies GTRs for gaps in noise (as in Fig. 4j). Because white noise contains power at all frequencies, it is likely to activate both off-responsive synapses and on-responsive synapses onto cortical neurons (in response to noise offsets and onsets, respectively). Because these sets of synapses are largely non-overlapping, the net synaptic drive could sum with little interference from synaptic depression, thereby producing amplification. To confirm that mice can behaviorally detect gaps in pure tones, we measured gap detection in a separate cohort of eight mice for 32- and 256-ms gaps in a continuous 8-kHz tone; mice showed significant gap detection for both gap durations (32 ms: P = 0.046, 256 ms: P = 0.002, n = 8 mice, rank-sum).
Figure 5.

Amplification by temporal summation of off- and on-responses. (A) Response of an example cortical neuron to gaps formed between two pure tones. Blue bars (FOFF): 11.3-kHz tones that selectively evoked off-responses (open blue arrows); red bars (FON): 36-kHz tones that selectively evoked on-responses (red arrow). Each column is a different gap duration. For an FOFF–FON sequence (second row), the longest gap (256 ms, far right column) evoked off-responses and on-responses that were well-separated in time. With progressively shorter FOFF–FON gaps, these off-responses and on-responses merged and produced maximal GTRs for 16-ms gaps (black arrow). Selectively removing either the on-response (blue arrow, top row) or off-response (red arrow, fourth row) or both (purple arrow) by changing the component tones abolished this amplification, indicating that it is due to temporal summation of off- and on-responses. (B) Gap duration tuning curves based on GTRs of the cell in (A). Black arrow shows the amplification of GTRs for 16-ms gaps. Red, blue and purple arrows show the loss of amplification when on- or off-responses are eliminated. (C) Population average gap duration tuning curves for 86 cells for which we were able to identify on- and off-selective tone frequencies. Effects and arrows are as described in (A) and (B). (D) Population average responses to preferred gap duration for each tone-pair combination. Effects and arrows are as described in (A) and (B). Note that the difference between (C) and (D) is that responses in (D) are specifically evoked by the preferred gap duration for each cell, whereas responses in (C) are just averaged across cells.
If GTRs are amplified by a temporal overlap of off- and on-responses, why is this amplification not maximal for the shortest gaps? In other words, why does the black curve in Figure 5c peak at an intermediate gap duration, instead of at the shortest gap duration where the off- and on-responses coincide completely? One explanation is that the latencies for off-responses are typically longer than for on-responses (in our sample, the median difference in latency was 7 ms (n = 64 cells)). This suggests that off- and on-responses will coincide completely at an intermediate gap duration that compensates for this latency difference.
These results suggest that even though cortical GTRs are temporally locked to noise onset at the termination of a brief gap, they are synaptically distinct from the on-responses evoked by bursts of noise. In other words, cortical neurons encode increments differently depending on whether they occur as gap terminations or as noise burst onsets. To test this idea directly, we recorded the responses to noise bursts (60 dB, 1–256 ms in duration) in 171 of the 534 cells for which we also recorded gap-in-noise responses. Most cells (70%, or 119/171) showed significant on-responses, which were tuned for duration. Preferred noise burst duration was approximately uniformly distributed across all durations tested (1–256 ms). Less than half of these cells (55/119) also responded to gaps with a GTR, whereas 8/171 cells (5%) responded to gaps with a GTR but had no on-response to noise bursts. The duration tuning for one type of stimulus had no predictive value about their tuning to the other. Duration tuning for noise bursts and gaps was uncorrelated (r = 0.01, P = 0.93), as were the minimum duration thresholds for bursts and gaps (r = 0.07, P = 0.64). The distributions of preferred and minimum durations across the population also differed significantly (P < 0.0001 and P = 0.0005, respectively, two sample K–S test). These data suggest that on-responses to noise bursts and GTRs for gaps are distinct phenomena, with different tuning properties and thresholds. This suggests that the cellular and/or synaptic mechanisms that shape duration tuning are likely to differ between noise burst and gap stimuli.
Cortical Modulation of Gap Encoding in IC
IC is known to be a critical region involved in the inhibition of the startle response (Koch 1999). It remains unclear which subdivision of IC is critical for PPI (Leitner and Cohen 1985; Li et al. 1998). Because AC is critically involved when startle attenuation is produced by gaps, we hypothesized that gap-evoked activity in cortex flows back down to dorsal IC (dIC) through cortico-collicular descending projections to activate the PPI pathway. Furthermore, because AC amplifies GTRs for brief gaps, we hypothesized that this descending pathway should in turn amplify dIC GTRs for brief gaps. These hypotheses make two key predictions. First, cortical neurons should be more likely to prefer short gaps than dIC neurons. Second, optogenetic suppression of AC should suppress brief gap responses in dIC neurons, thereby shifting their gap tuning towards longer gaps.
To test these predictions, we recorded from dorsal IC (a secondary subdivision of IC that receives a major descending projection from A1 and is an integral component of the PPI pathway (Winer and Schreiner 2005)) in awake PV-ChR2 mice that were implanted with optical fibers bilaterally over AC (Fig. 6a). Overall, dIC neurons were more likely to respond to gaps than cortical neurons, with 57% (67/117) of collicular neurons exhibiting a GTR compared with 39% in cortex (χ2 = 12.6, P = 0.0004). Compared with AC, however, far fewer dIC neurons preferred brief gaps, and more dIC neurons preferred long gaps (Fig. 6b). Thus, the distribution of preferred gap durations in dIC and AC was significantly different (P = 0.0008, two sample K–S test). The median preferred gap duration in dIC was 32 ms, compared to 16 ms in AC. Similarly, population-averaged gap tuning curves were significantly different, with dIC neurons firing more spikes for long gaps and cortical neurons firing more spikes for short gaps (Fig. 6c, interaction df = 9, F = 11.3, P = 2 × 10−17, Anova). The amplification of GTRs evoked by brief gaps was significantly smaller for dIC neurons than cortical neurons (Fig. 6d, 1.5-fold compared with 2.6-fold, P = 1.8 × 10−5, rank-sum). Most dIC neurons showed long-pass tuning for gap duration (67% or 45/67, Fig. 6e). Compared with AC, dIC neurons were less likely to show short- or band-pass tuning, and more likely to show long-pass tuning (χ2 = 14.2, P = 0.002). Similarly, there were proportionately more “on-only” and fewer “off–on” cells in dIC than in AC (P = 0.0016, Fisher’s exact test). In contrast to cortical gap responses, these results demonstrate that dIC gap responses generally scale in amplitude with gap duration, consistent with previous descriptions of IC gap responses (Finlayson 1999, 2002; Walton et al. 2008).
Figure 6.

Brief gap responses in dorsal IC are amplified by descending auditory cortical input. (A) Only neurons from tetrode tracks histologically verified as being within dorsal IC were included. N = 5 mice. (B) Gap duration tuning in dorsal IC was significantly different from that in AC (P = 0.0008, two sample K–S test). Cumulative histogram of best gap durations for each population. (C) Averaged across the population, brief gaps evoked more spikes in cortical neurons. Note that the notch at 64 ms (green curve) matches the limit of cortical off–on temporal integration. Green: cortical GTRs, Black: IC GTRs. To allow direct comparison of the shapes of the IC and cortical distributions, we normalized GTRs to the maximal GTR for each cell, then averaged across cells, and then normalized to the population maximum. (D) Relative amplification factor of preferred gap responses for IC neurons (black) and AC neurons (green). Format as in Figure 4J. (E) We classified dorsal IC GTRs as: 1) short-pass, 2) band-pass, or 3) long-pass. The distribution of preferred gap durations across tuning classes in IC (black points) differed from those in cortex (green points), with more long-pass and fewer band-pass and short-pass responses in dorsal IC. (F) Gap responses in an example IC neuron. On control trials (black lines), this neuron showed GTRs for all gap durations ≥4 ms. When we optogenetically suppressed auditory cortex on interleaved trials (blue lines, suppression during the 50-ms postgap interval), GTRs for brief gaps (4–32 ms) were suppressed, but longer gaps were unaffected. From this, we infer that auditory cortex amplified GTRs in this neuron for 4–32 ms gaps. (G) Averaged across the population (n = 67 cells), cortical suppression significantly reduced IC GTRs for brief gaps (1–64 ms, except for 2 ms) but not for longer gaps. Responses are normalized to the peak laser-off response for each cell. (H) Cortical suppression significantly shifted the distribution of preferred IC gap durations (based on GTRs) towards longer gaps. The lack of optogenetic impact for gaps 2–8 ms could be a floor effect, due to the small number of IC cells with preferred gaps in this range. (I) Effects of cortical suppression on IC gap responses matched behavioral effects of lesions and optogenetic suppression. Blue and red curves show behavioral gap detection deficits caused by optogenetic suppression and cortical lesions, respectively (the same data as Fig. 2D). Black curve shows percentage change in IC GTRs caused by cortical suppression (i.e., the difference between black and blue curves in panel G). (*: P < 0.05, post-hoc).
When we optogenetically suppressed AC during the post-gap interval, GTRs for brief gaps were suppressed in dIC neurons. For example, the dIC neuron in Figure 6f showed GTRs for gaps of 4–128 ms on control trials (black traces with no illumination). On laser trials (blue traces), the GTRs for gaps of 4–32 ms were markedly suppressed, but responses to longer gaps were unaffected. From this, we infer that AC must be amplifying the dIC responses to brief gaps under control conditions. Across the population, cortical suppression modestly but significantly decreased the spiking responses evoked by short gaps, but not long gaps (Fig. 6g, df = 9, F = 12.1, P < 0.0005, Anova). As a result, dIC gap responses shifted even more towards longer gaps. Dorsal IC neurons became more likely to prefer long gaps and less likely to prefer short gaps (Fig. 6h). The median preferred gap duration increased from 32 to 64 ms (P = 0.0002, rank-sum). These effects were specific to gap-responsive dIC neurons, because dIC cells with significant GTRs were much more likely to show an effect of cortical suppression than cells without gap responses (20/67 responsive vs. 4/50 nonresponsive, χ2 = 8.4, P = 0.004). Moreover, laser delivered in the absence of a gap (i.e., just during background noise) had no effect on dIC spiking (P = 0.97, rank-sum, n = 117 dIC neurons). The range of brief gap durations over which cortical suppression affected dIC gap responses was remarkably similar to the range over which cortical lesions or suppression affected behavioral gap detection (Fig. 6i). These results demonstrate that the suppression of cortical GTRs in turn suppresses collicular GTRs for brief gaps. From this, we conclude that AC amplifies the responses to brief gaps in dorsal inferior collicular neurons.
Discussion
Here we have identified a cortico-collicular gap detection circuit in which auditory cortex amplifies neural responses to brief gaps before sending this information back to inferior colliculus, a key component of the PPI pathway. We found that neurons in dorsal IC (dIC), relatively early in the auditory pathway, preferred much longer gaps than cortical neurons. In contrast, cortical neurons were equally likely to prefer brief or long gaps. Consequently, brief gaps evoked markedly greater spiking activity in cortex than in dIC. Thus, the representation of brief gaps is enhanced rather than faithfully relayed as it flows up the auditory pathway. Information about sound offsets and onsets is known to ascend through separate synaptic channels to AC (He 2001; Scholl et al. 2010). Thus, in cortical neurons, off-responses and on-responses that temporally overlap for brief gaps can sum independently. This mechanism amplified cortical GTRs for brief gaps, which explains why and how more cortical neurons preferred brief gaps. Optogenetic cortical suppression revealed that this enhanced cortical representation in turn influences dIC, and modestly but significantly amplifies collicular responses to brief gaps. Because IC is involved in mediating PPI (Parham and Willott 1990; Koch 1999), it seems likely that cortical GTRs impact gap detection behavior via this descending pathway. This mechanism provides an explanation for our finding that lesions or optogenetic suppression of cortical GTRs impaired the detection of brief gaps, but not of long gaps. Together, these results resolve several related questions. They explain why and how cortex is necessary for normal detection of brief gaps, and clarify that the delineation between brief and long gaps (~64 ms) stems from the limits of temporal integration of off- and on-responses. In turn, this temporal integration is limited by the duration of off-responses (~75 ms). These mechanisms are likely to play a key role not just in the perceptual detection of gaps, as we have shown here, but also in other temporal processing challenges faced by the auditory system, such as the detection and identification of speech sounds (especially voice onset time), species-specific vocalizations, or other important events characterized by acoustic fluctuations in the environment.
Layers 5 and 6 neurons in AC are known to project directly to dIC, and this large corticofugal projection shapes sound processing in IC neurons (Bajo and King 2012; Suga 2012). It seems likely that this direct pathway is responsible for the cortical amplification of brief gap responses that we observed in dIC neurons. However, it is important to note that we did not test whether a direct monosynaptic projection from AC to dIC accounts for this result; it is also possible that the amplification we observed could involve an indirect pathway from AC to dIC through some other brain structure (such as the medial geniculate nucleus). One way to resolve this ambiguity would be to optogenetically suppress cortico-collicular layer 5/6 neurons to determine if they specifically amplify brief gap responses in dIC. The relative contributions of the dorsal, central, and external subdivisions of IC to PPI or gap detection also remain unclear. Lesions of the entire IC abolish PPI (Leitner and Cohen 1985; Li et al. 1998), but it will be interesting to test the effects of more specific manipulations on PPI and gap detection. Recent work has also shown projections from AC to both superior colliculus and the pedunculo-pontine tegmental nucleus (Schofield and Motts 2009; Schofield 2010; Bajo et al. 2010a, 2010b), raising the possibility that AC influences gap detection through those pathways in addition to its effects on IC.
Our behavioral paradigm for measuring gap detection is based on inhibition of the acoustic startle response. As such, it provides unambiguous evidence that the animal has behaviorally detected the gap, but leaves open the question to what extent the animal is perceptually aware of the gap. Because the inhibition of the startle reflex depends on brainstem and midbrain circuitry, some have argued that it may be automatic and is not necessarily a measure of auditory perception (Lauer et al. 2017). Unlike PPI, however, brief gap detection requires cortex, suggesting that it is plausible that gaps may reach perceptual awareness. Furthermore, gap detection thresholds measured using operant Go/No-Go procedures are identical to those measured using startle-based measures (1–2 ms in mice in both cases; (Walton et al. 1997; Allen et al. 2008; Radziwon et al. 2009)), suggesting that these two approaches may be measuring the same fundamental perceptual events. It will be of great interest to test whether the mechanisms we have identified here also underlie gap detection as measured with operant psychophysical procedures.
This off–on temporal summation mechanism for the amplification of brief gap responses represents a novel neural computation, although mechanisms of a similar nature have been proposed to support temporal integration before. For example, neurons in the dorsal zone of AC typically show selectivity for the duration of tones or noise bursts (He et al. 1997), which has been proposed to involve the temporal integration of on- and off-responses (He 2001). Similarly, the dynamics of click train encoding in AC appear to be shaped by the interplay of click responses, post-response suppression, and subsequent rebound activity (Christianson et al. 2011). In bat IC, many neurons are tuned to tone duration, which has been shown to be constructed from the temporal interaction of sustained inhibition and delayed excitation (Casseday et al. 1994; Ehrlich et al. 1997; Fuzessery and Hall 1999). In principle, an excitatory-inhibitory interaction of this sort could also contribute to gap duration tuning in AC. Our results do not rule out this possibility but do support an alternative account for the construction of gap duration tuning based on the summation of excitatory off- and on-responses. Off-responses also appear to contribute to the encoding of brief gaps in auditory thalamus, because both thalamic off-responses and brief gap responses are selectively impaired in a mouse model of developmental disorders (Anderson and Linden 2016). In previous work, we found that manipulating cortical activity before and after a gap had opposing effects on behavioral gap detection, suggesting the existence of a temporal comparison process that compares pregap and postgap activity (Weible et al. 2014b). The mechanism we have described here involves off- and on-responses evoked by the gap; these events occur during and following the gap. This off–on summation mechanism thus appears to act independently of any temporal comparison with pregap activity.
If off-responses critically contribute to the amplification of gap responses, why then did we see no behavioral effect of optogenetic suppression of off-responses during the gap (Fig. 1c)? It is important to note here that the suppression of neural activity during the gap only affects off-responses for long gaps—long enough that the off-response occurs within the gap, and therefore does not overlap in time with the GTR. For gaps brief enough that the off-response extends beyond the end of the gap and overlaps with the GTR, illumination just during the gap would fail to suppress the critical portion of the off-response contributing to brief gap detection. We conclude that off-responses do not contribute to gap detection for long gaps but do contribute to gap detection for short gaps, and that the boundary between short and long is dictated by the limits of temporal integration of on- and off-responses.
Our results reveal striking differences in how AC and dorsal IC encode gaps. We found that IC neurons tend to prefer long gaps and that their gap responses generally scale with gap duration. This agrees with the previous studies of gap encoding in IC (Walton et al. 1997, 2008; Barsz et al. 1998; Finlayson 1999, 2002). In contrast, the gap encoding that we found for auditory cortical neurons differs from that described in the previous studies of AC. We report for the first time that cortical neurons were well-tuned for gap duration and that their preferred gap durations were uniformly distributed across the behaviorally detectable range. Thus, although MGTs are similar in both IC and AC (1–4 ms, (Eggermont 1995a, 1999; Walton et al. 1997, 2008; Barsz et al. 1998; Finlayson 1999, 2002; Liu et al. 2010; Recanzone et al. 2011; Kirby and Middlebrooks 2012), preferred gap durations (the gap durations that evoke the maximal response) are significantly shorter in cortex than in IC. MGTs in auditory thalamus are also similar to those in IC and AC (Anderson and Linden 2016), but it is unclear whether the distribution of preferred gap durations in thalamus resembles those in IC, those in cortex, or lies somewhere in between. In contrast to our findings, previous studies of cortical gap encoding have reported that gap responses generally scale with gap duration, similar to those in IC (Eggermont 1995a, 1999; Liu et al. 2010; Recanzone et al. 2011; Kirby and Middlebrooks 2012). Of the many methodological differences among these studies (e.g., species, anesthesia, multi-unit vs. single-unit recording, ranges of durations tested, or continuous background noise vs. tone or noise pairs), two seem most likely to account for our different results. First, most previous studies recorded from anesthetized animals. Off-responses have been reported to be much less common under anesthesia (Qin et al. 2007), and indeed, we found significantly more off-responsive or off-on-responsive neurons than reported previously (χ2 = 9.4, P = 0.009) (Eggermont 1999). Because off-responses critically contribute to the amplification and resulting enhanced representation of brief gaps, the fact that this enhanced representation has not previously been reported could be a consequence of the reduction of off-responses by anesthesia. Second, many previous studies used gaps formed by pairs of brief tones or noise bursts, rather than inserted into continuous background noise as we used here. Off-responses scale with sound duration such that a brief sound is likely to evoke little or no off-response (Scholl et al. 2010). Thus, tuning for brief gaps, which we have shown to depend on off-responses, is unlikely to occur for gaps formed from pairs of brief sounds, even when studied in awake animals (Liu et al. 2010; Recanzone et al. 2011). For this reason, we used relatively long tones (500 ms) to create gaps from tone pairs to test for temporal summation on off- and on-responses (Fig. 5). This also suggests that the encoding of gaps in natural sounds (such as speech) is expected to depend strongly on context. Indeed, when tested with a brief leading noise (<50 ms) that mimics onset time in isolated consonant-vowel pairs, or with isolated actual consonant-vowel pairs, cortical neurons have much longer gap thresholds (~30 ms) that match the categorical voice onset time boundaries typical of consonants (Eggermont 1995a, 1995b, 1999, 2000). Fluent natural speech spans a wide dynamic range of durations, with isolated consonants and continuous background noise lying at opposite extremes of this spectrum. This suggests that gap thresholds, gap duration tuning, and the contribution of off-responses to these properties varies considerably depending on the surrounding speech in which they are embedded. How gap encoding contributes to phoneme segmentation and identification in fluent, continuous speech is therefore likely to be complex and context-dependent.
Human psychophysical studies have shown that gap detection thresholds are lower when the sounds before and after the gap are pure tones or narrow-band noise of the same frequency (“within-channel”), and thresholds are higher when the sounds are of different frequencies (“between-channel”). Between-channel gap detection thresholds also depend on the frequency separation of the two sounds (Phillips et al. 1997; Formby et al. 1998; Phillips and Smith 2004). This suggests that within-channel and between-channel gap detection use different mechanisms. Our behavioral gap detection experiments used white noise, which should engage both within- and between-channel mechanisms because it contains power at all frequencies, whereas our paired-tone experiment (Fig. 5) is strictly between-channel. Because the on- and off-responses of cortical neurons are tuned to different frequencies, only a small population of neurons is likely to show both on- and off-responses to a given pure tone frequency. This suggests that the cortical temporal summation mechanism we have described here contributes primarily to between-channel gap detection (i.e., that using white noise or tones of different frequencies).
Consistent with previous work, we found that cortical cells were tuned for the duration of noise bursts (Brugge and Merzenich 1973; He et al. 1997). However, we found that tuning for noise burst duration was independent of tuning for gap duration, suggesting that these forms of duration tuning likely arise from distinct mechanisms. Although noise bursts, like gaps, have both onsets and offsets, it is unlikely that the amplification mechanism we describe for brief gaps also operates on noise bursts. Off-responses are likely generated by inhibitory rebound in the auditory brainstem, which takes time to develop (Kuwada and Batra 1999; Kopp-Scheinpflug et al. 2011, 2018). Thus, because brief sounds evoke little or no off-response, a noise burst long enough (>100 ms) to evoke even a moderate off-response would produce non-overlapping on- and off-responses.
Several phenomenological models for gap detection have been proposed, which generally fall into two classes. The first are “energy-detector models” that detect the drop in acoustic energy created by the gap (Mathews and Pfafflin 1965; Green and Swets 1966; Buunen and van Valkenburg 1979; Buss and Florentine 1985; Forrest and Green 1987). The neuronal off-responses that we and others have observed are consistent with the output of such models. We also found that offsets alone can drive behavioral gap detection (Fig. 3a), which further agrees with this class of models. Because cortical lesions had no effect on offset-only gap detection, energy-detector models resemble the aspects of gap detection mediated by IC. In contrast, our finding that cortical GTRs critically contribute to the detection of gaps that include an onset is inconsistent with energy-detector models. Similarly, cortical suppression during the gap had no effect on behavior, whereas such a manipulation should impair gap detection by an energy-detector model. Thus, these models do not appear consistent with the aspects of gap detection mediated by AC. A second class of models instead relies on detecting the resumption of noise following the gap (Plomp 1964; Oxenham 1997; Phillips et al. 2002; Weible et al. 2014b). Cortical GTRs are consistent with the output of such onset-detection models, and the importance of these GTRs for gap detection behavior supports these models. However, we found that off-responses make a critical contribution by amplifying GTRs for brief gaps, which is inconsistent with onset-detection models. Plomp proposed a model in which onset detection is impaired by the decay of sensation after sound offset (Plomp 1964). This predicts that gap detection scales with gap duration, which indeed matches our behavioral results and those of others (Ison 1982; Ison and Pinckney 1983; Ison et al. 1991; Kelly et al. 1996; Threlkeld et al. 2008; Weible et al. 2014b). However, this model predicts a similar scaling for GTRs, which matches IC gap encoding but is inconsistent with the tuning for brief gaps that we found in AC. Plomp’s model also predicts that suppression during the gap should enhance gap detection, whereas we found it had no effect. Thus, our findings are consistent with aspects of each of these models but reveal a more complex picture in which the auditory midbrain and cortex make distinct contributions to gap detection.
Gaps not only signal environmental events and support segmentation of auditory streams, but are also critical for the identification of sounds such as phonemes that are differentiated by gap duration. The mechanisms we have described here are therefore likely to contribute to speech perception, perceptual grouping, and auditory scene analysis. The corticofugal projection from AC to IC has been shown to modify the tuning of collicular neurons for numerous features, including frequency, intensity, and sound localization cues (Bajo and King 2012; Suga 2012). We recorded from dorsal IC, which receives the largest corticofugal projection, but all three IC subdivisions receive cortical feedback, including central IC, which provides ascending lemniscal input to auditory thalamus and cortex (Winer et al. 1998). Gap detection therefore adds to an emerging picture in which auditory cortex transforms sound representations and then projects them back to IC to specifically and systematically shape its own input. This corticofugal system, in addition to directly impacting gap detection behavior, appears to serve more generally to tune input to auditory cortex in order to focus on signals of interest. Understanding how this system operates dynamically during temporal processing tasks such as speech perception and auditory scene analysis remains an important challenge.
Funding
National Institutes of Health National Institute on Deafness and Other Communication Disorders (R01 DC-015828).
Notes
We would like to thank Miya Ott for her assistance with histology. Conflict of Interest: None declared.
Supplementary Material
References
- Allen PD, Schmuck N, Ison JR, Walton JP. 2008. Kv1.1 channel subunits are not necessary for high temporal acuity in behavioral and electrophysiological gap detection. Hear Res. 246:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LA, Linden JF. 2016. Mind the gap: two dissociable mechanisms of temporal processing in the auditory system. J Neurosci. 36:1977–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, King AJ. 2012. Cortical modulation of auditory processing in the midbrain. Front Neural Circuit. 6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, King AJ. 2010a. The non-lemniscal auditory cortex in ferrets: convergence of corticotectal inputs in the superior colliculus. Front Neuroanat. 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. 2010b. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci. 13:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsz K, Benson PK, Walton JP. 1998. Gap encoding by inferior collicular neurons is altered by minimal changes in signal envelope. Hear Res. 115:13–26. [DOI] [PubMed] [Google Scholar]
- Bhatara A, Babikian T, Laugeson E, Tachdjian R, Sininger YS. 2013. Impaired timing and frequency discrimination in high-functioning autism spectrum disorders. J Autism Dev Disord. 43:2312–2328. [DOI] [PubMed] [Google Scholar]
- Bowen GP, Lin D, Taylor MK, Ison JR. 2003. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 13:815–822. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Merzenich MM. 1973. Responses of neurons in auditory cortex of the macaque monkey to monaural and binaural stimulation. J Neurophysiol. 36:1138–1158. [DOI] [PubMed] [Google Scholar]
- Buss S, Florentine M. 1985. Gap detection in normal and impaired listeners: the effect of level and frequency In: Michelsen A, editor. Time resolution in auditory systems. Berlin: Springer. [Google Scholar]
- Buunen TJ, van Valkenburg DA. 1979. Auditory detection of a single gap in noise. J Acoust Soc Am. 65:534–536. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Ehrlich D, Covey E. 1994. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science. 264:847–850. [DOI] [PubMed] [Google Scholar]
- Christianson GB, Sahani M, Linden JF. 2011. Depth-dependent temporal response properties in core auditory cortex. J Neurosci. 31:12837–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Gendelman PM. 1977. Plasticity of the acoustic startle response in the acutely decerebrate rat. J Comp Physiol Psychol. 91:549–563. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. 1995a. Neural correlates of gap detection and auditory fusion in cat auditory cortex. Neuroreport. 6:1645–1648. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. 1995b. Representation of a voice onset time continuum in primary auditory cortex of the cat. J Acoust Soc Am. 98:911–920. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. 1999. Neural correlates of gap detection in three auditory cortical fields in the cat. J Neurophysiol. 81:2570–2581. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. 2000. Neural responses in primary auditory cortex mimic psychophysical, across-frequency-channel, gap-detection thresholds. J Neurophysiol. 84:1453–1463. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Casseday JH, Covey E. 1997. Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol. 77:2360–2372. [DOI] [PubMed] [Google Scholar]
- Finlayson PG. 1999. Post-stimulatory suppression, facilitation and tuning for delays shape responses of inferior colliculus neurons to sequential pure tones. Hear Res. 131:177–194. [DOI] [PubMed] [Google Scholar]
- Finlayson PG. 2002. Paired-tone stimuli reveal reductions and alterations in temporal processing in inferior colliculus neurons of aged animals. J Assoc Res Otolaryngol. 3:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman YI, Steinschneider M. 2009. Temporally dynamic frequency tuning of population responses in monkey primary auditory cortex. Hear Res. 254:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formby C, Gerber MJ, Sherlock LP, Magder LS. 1998. Evidence for an across-frequency, between-channel process in asymptotic monaural temporal gap detection. J Acoust Soc Am. 103:3554–3560. [DOI] [PubMed] [Google Scholar]
- Forrest TG, Green DM. 1987. Detection of partially filled gaps in noise and the temporal modulation transfer function. J Acoust Soc Am. 82:1933–1943. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM, Hall JC. 1999. Sound duration selectivity in the pallid bat inferior colliculus. Hear Res. 137:137–154. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. 1993. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 36:1276–1285. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. 1966. Signal detection theory and psychophysics. New York: Wiley. [Google Scholar]
- He J. 2001. On and off pathways segregated at the auditory thalamus of the Guinea pig. J Neurosci. 21:8672–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Hashikawa T, Ojima H, Kinouchi Y. 1997. Temporal integration and duration tuning in the dorsal zone of cat auditory cortex. J Neurosci. 17:2615–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadou VV, Bamiou DE, Sidiras C, Moschopoulos NP, Tsolaki M, Nimatoudis I, Chermak GD. 2017. The use of the gaps-in-noise test as an index of the enhanced left temporal cortical thinning associated with the transition between mild cognitive impairment and Alzheimer's disease. J Am Acad Audiol. 28:463–471. [DOI] [PubMed] [Google Scholar]
- Ison JR. 1982. Temporal acuity in auditory function in the rat: reflex inhibition by brief gaps in noise. J Comp Physiol Psychol. 96:945–954. [PubMed] [Google Scholar]
- Ison JR, O'Connor K, Bowen GP, Bocirnea A. 1991. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci. 105:33–40. [DOI] [PubMed] [Google Scholar]
- Ison JR, Pinckney LA. 1983. Reflex inhibition in humans: sensitivity to brief silent periods in white noise. Percept Psychophys. 34:84–88. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O'Neill WE. 2007. Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J Assoc Res Otolaryngol. 8:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, Phillips DP. 1996. Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius). Behav Neurosci. 110:542–550. [DOI] [PubMed] [Google Scholar]
- Kirby AE, Middlebrooks JC. 2012. Unanesthetized auditory cortex exhibits multiple codes for gaps in cochlear implant pulse trains. J Assoc Res Otolaryngol. 13:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. 1999. The neurobiology of startle. Prog Neurobiol. 59:107–128. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Sinclair JL, Linden JF. 2018. When sound stops: offset responses in the auditory system. Trends Neurosci. 41:712–728. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tozer AJ, Robinson SW, Tempel BL, Hennig MH, Forsythe ID. 2011. The sound of silence: ionic mechanisms encoding sound termination. Neuron. 71:911–925. [DOI] [PubMed] [Google Scholar]
- Kuwada S, Batra R. 1999. Coding of sound envelopes by inhibitory rebound in neurons of the superior olivary complex in the unanesthetized rabbit. J Neurosci. 19:2273–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Behrens D, Klump G. 2017. Acoustic startle modification as a tool for evaluating auditory function of the mouse: progress, pitfalls, and potential. Neurosci Biobehav Rev. 77:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner DS, Cohen ME. 1985. Role of the inferior colliculus in the inhibition of acoustic startle in the rat. Physiol Behav. 34:65–70. [DOI] [PubMed] [Google Scholar]
- Li L, Korngut LM, Frost BJ, Beninger RJ. 1998. Prepulse inhibition following lesions of the inferior colliculus: prepulse intensity functions. Physiol Behav. 65:133–139. [DOI] [PubMed] [Google Scholar]
- Lisker L, Abramson AS. 1964. A cross-language study of voicing in initial stops: acoustical measurements. Word. 20:384–422. [Google Scholar]
- Liu Y, Qin L, Zhang X, Dong C, Sato Y. 2010. Neural correlates of auditory temporal-interval discrimination in cats. Behav Brain Res. 215:28–38. [DOI] [PubMed] [Google Scholar]
- Masini CV, Babb JA, Nyhuis TJ, Day HE, Campeau S. 2012. Auditory cortex lesions do not disrupt habituation of HPA axis responses to repeated noise stress. Brain Res. 1443:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MV, Pfafflin SM. 1965. Effect of filter type on energy-detection models for auditory signal detection. J Acoust Soc Am. 38:1055–1056. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DV. 2001. Auditory perceptual processing in people with reading and oral language impairments: current issues and recommendations. Dyslexia. 7:150–170. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ. 1997. Increment and decrement detection in sinusoids as a measure of temporal resolution. J Acoust Soc Am. 102:1779–1790. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Jones SJ, Palmer AR. 2008. Descending projections from auditory cortex modulate sensitivity in the midbrain to cues for spatial position. J Neurophysiol. 99:2347–2356. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. 2001. The mouse brain in stereotaxic coordinates, Ed 2. San Diego: Academic. [Google Scholar]
- Parham K, Willott JF. 1990. Effects of inferior colliculus lesions on the acoustic startle response. Behav Neurosci. 104:831–840. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Comeau M, Andrus JN. 2010. Auditory temporal gap detection in children with and without auditory processing disorder. J Am Acad Audiol. 21:404–408. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Hall SE, Boehnke SE. 2002. Central auditory onset responses, and temporal asymmetries in auditory perception. Hear Res. 167:192–205. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Smith JC. 2004. Correlations among within-channel and between-channel auditory gap-detection thresholds in normal listeners. Perception. 33:371–378. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Taylor TL, Hall SE, Carr MM, Mossop JE. 1997. Detection of silent intervals between noises activating different perceptual channels: some properties of “central” auditory gap detection. J Acoust Soc Am. 101:3694–3705. [DOI] [PubMed] [Google Scholar]
- Plomp R. 1964. Rate of decay of auditory sensation. J Acoust Soc Am. 36:277–282. [Google Scholar]
- Qin L, Chimoto S, Sakai M, Wang J, Sato Y. 2007. Comparison between offset and onset responses of primary auditory cortex ON-OFF neurons in awake cats. J Neurophysiol. 97:3421–3431. [DOI] [PubMed] [Google Scholar]
- Radziwon KE, June KM, Stolzberg DJ, Xu-Friedman MA, Salvi RJ, Dent ML. 2009. Behaviorally measured audiograms and gap detection thresholds in CBA/CaJ mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 195:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH. 2000. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 150:104–118. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Engle JR, Juarez-Salinas DL. 2011. Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey. Hear Res. 271:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR. 2010. Projections from auditory cortex to midbrain cholinergic neurons that project to the inferior colliculus. Neuroscience. 166:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Motts SD. 2009. Projections from auditory cortex to cholinergic cells in the midbrain tegmentum of Guinea pigs. Brain Res Bull. 80:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Gao X, Wehr M. 2010. Nonoverlapping sets of synapses drive on responses and off responses in auditory cortex. Neuron. 65:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell KB. 1997. Age-related changes in temporal gap detection. J Acoust Soc Am. 101:2214–2220. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. 2000. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 107:1615–1626. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. 1998. Temporal processing in the aging auditory system. J Acoust Soc Am. 104:2385–2399. [DOI] [PubMed] [Google Scholar]
- Suga N. 2012. Tuning shifts of the auditory system by corticocortical and corticofugal projections and conditioning. Neurosci Biobehav Rev. 36:969–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swetter BJ, Fitch RH, Markus EJ. 2010. Age-related decline in auditory plasticity: experience dependent changes in gap detection as measured by prepulse inhibition in young and aged rats. Behav Neurosci. 124:370–380. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, Penley SC, Rosen GD, Fitch RH. 2008. Detection of silent gaps in white noise following cortical deactivation in rats. Neuroreport. 19:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Barsz K, Wilson WW. 2008. Sensorineural hearing loss and neural correlates of temporal acuity in the inferior colliculus of the C57BL/6 mouse. J Assoc Res Otolaryngol. 9:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, O'Neill WE. 1997. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol A. 181:161–176. [DOI] [PubMed] [Google Scholar]
- Weible AP, Liu C, Niell CM, Wehr M. 2014a. Auditory cortex is required for fear potentiation of gap detection. J Neurosci. 34:15437–15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Moore AK, Liu C, DeBlander L, Wu H, Kentros C, Wehr M. 2014b. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr Biol. 24:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Diehl JJ, Hefti BJ. 1998. Auditory cortical projections to the cat inferior colliculus. J Comp Neurol. 400:147–174. [PubMed] [Google Scholar]
- Winer JA, Schreiner C. 2005. The inferior colliculus: with 168 illustrations. New York, NY: Springer. [Google Scholar]
- Yu H, Vikhe Patil K, Han C, Fabella B, Canlon B, Someya S, Cederroth CR. 2016. GLAST deficiency in mice exacerbates gap detection deficits in a model of salicylate-induced tinnitus. Front Behav Neurosci. 10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


