Abstract
Timely accrual continues to be a challenge in clinical trials. The evolution of Electronic Health Record systems and cohort selection tools like i2b2 have improved identification of potential candidate participants. However, delays in receiving relevant patient information and lack of real time patient identification cause difficulty in meeting recruitment targets. The authors have designed and developed a proof of concept platform that informs authorized study team members about potential participant matches while the patient is at a healthcare setting. This Just-In-Time Alert (JITA) application leverages Health Level 7 (HL7) messages and parses them against study eligibility criteria using Amazon Web Services (AWS) cloud technologies. When required conditions are satisfied, the rules engine triggers an alert to the study team. Our pilot tests using difficult to recruit trials currently underway at the UMass Medical School have shown significant potential by generating more than 90 patient alerts in a 90-day testing timeframe.
Introduction
Clinical trials enable the translation of biomedical research findings into safe and effective pharmaceutical products and devices. A key determinant of a successful trial is the timely recruitment, enrollment, and retention of human participants (1). Recruitment of eligible study participants falls behind schedule in more than 80% of clinical trials in progress, with 13% of studies lagging by more than 6 months (2). Further, in a study of 787 phase II/III adult National Clinical Trial Network sponsored trials launched between 2000 and 2011, Bennette et al. reported that 18% of the trials closed with low accrual or had accrued less than 50% of their target three years or more after initiation (3). Failure to achieve recruitment goals results in significant cost and increased time to market (4). Barriers to successful recruitment include FDA regulations mandating higher minimum patient enrollment per study, the stringent eligibility criteria put forth by some studies, and increasing patient privacy regulations (5). The timely recruitment of an unbiased study cohort while keeping costs low remains a pertinent challenge.
The federal mandate for electronic health records (EHR), in conjunction with the use of data warehouses and query tools, has revolutionized patient recruitment to clinical trials (6, 7). However, the full potential of EHR in clinical research has not yet been applied to the optimization of clinical trials recruitment. The lack of effective natural language processing (NLP) tools to extract pertinent information from unstructured text in EHR hinders the use of patient health information to the fullest potential (8). Non-interoperability between EHR vendors used at the various healthcare institutions, failure to update patient contact information and patient’s unwillingness to return to the clinical trial site further complicate recruitment efforts. Many study teams are looking for opportunities to approach patients and consent them while the patient is in the hospital Emergency Rooms (ED) or admitted into the hospital in an inpatient department. Significant increases in physician participation and patient enrollment at the point-of-care were reported when real-time alerts were incorporated into recruitment approaches that use computerized physician order entry (CPOE) and EHR (9-11). However, physicians are left overwhelmed with EHR alerts (12) leading to potential lack of response on patient recruitment alerts for clinical trials. Hence, a paradigm-shifting approach that alerts study teams of potential participants in real time may benefit patient recruitment. Potential benefits of such a system are twofold: 1) they engage study teams directly, and 2) alerts happen in real time while the patient is in the clinic/ED/hospital allowing study teams to approach the patient while the information is more relevant to the patient. Our main goal was to develop a proof of concept (POC) application that facilitates timely recruitment and enrollment of clinical trials participants with the potential to expand to other institutions.
Here, we present Just-In-Time Alert (JITA) - an HL7 and cloud-based secure, user friendly, highly scalable and adaptable platform that alerts study-specific researchers when a patient meets recruitment criteria at the time of clinical care, and while they are still physically present at the hospital. We showcase the technology used to develop JITA and its use in the emergency and inpatient settings.
Materials and Methods
Health Level Seven International (HL7) includes a set of standards, formats and definitions for exchanging EHR data. In our POC implementation, the JITA application processed and analyzed three types of incoming HL7 messages from healthcare organizations: patient administration messages (ADT) with information on patient admission/discharge/transfer status within a healthcare facility; observation results (ORU LABS) messages in response to laboratory test orders; and scheduling information unsolicited (SIU) messages that exchange the patient’s appointment status.
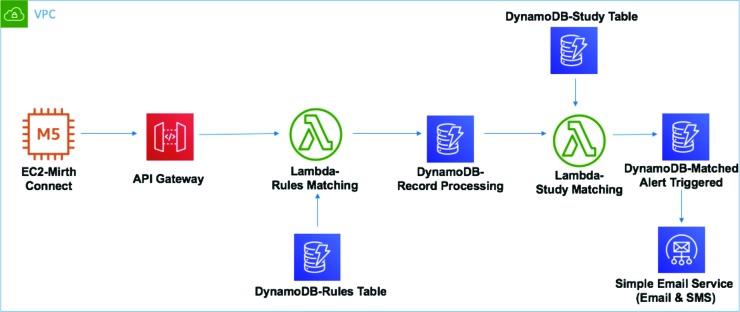
The JITA application is built upon a combination of open-source software (i.e. Mirth Connect) and AWS technologies including VPC, EC2, S3, API Gateway, Lambda, DynamoDB and Cognito (Figure 1). AWS VPC environment that we leveraged has ISO 27001, SOC1, SOC2, and FedRAMP (Medium) certifications.
Figure 1.
JITA AWS technology stack.
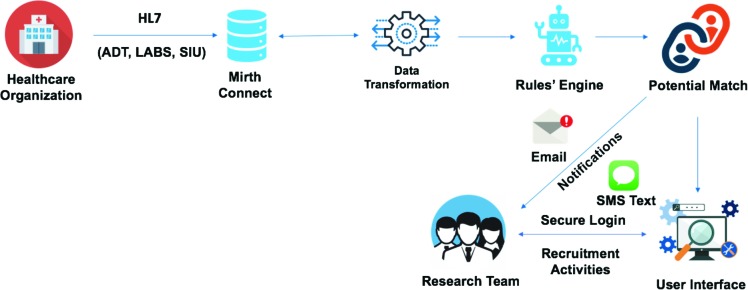
A Mirth Connect instance in AWS EC2 receives all incoming HL7 messages streamed in real time over secure and encrypted channels from healthcare organizations. The Mirth Connect converts every such incoming HL7 message from the native pipe-delimited format into a JavaScript Object Notation (JSON), a light weight format for storing and transporting data. This enables the processing, querying and parsing of messages more efficiently. A robust rules engine is built as the core of the application to filter all incoming messages, and individual rules are created at the message type level to define the inclusion or exclusion criteria. After each HL7 message is converted to JSON, the application performs an http call to the AWS API Gateway, invoking the AWS Lambda-based parsing functionality. Each message is evaluated against all existing rules. All rules based on applicable message types for a study have to be satisfied in order for a potential candidate to be selected. An alert is then generated as an SMS or email (based on preference) using the AWS Simple Email Service. The overall workflow is presented in Figure 2.
Figure 2.
The JITA Workflow.
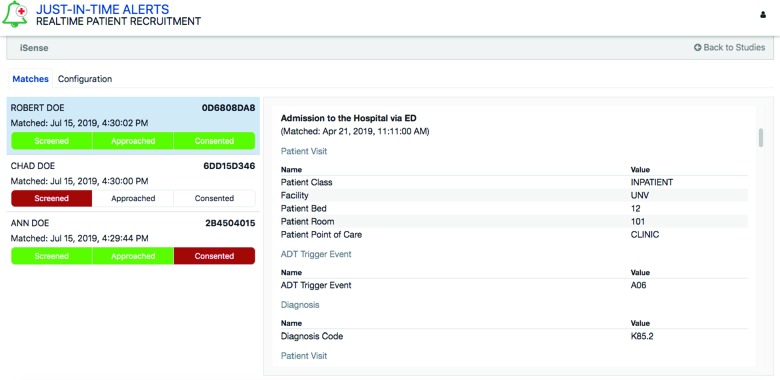
The JITA application includes a browser-based user interface hosted in AWS as the front end for all authorized study team members to review detailed alerts-related information securely. DynamoDB is used as the back end for storing the participant and study information. All users must log into the application via a single sign on with Cognito authentication technology in AWS. Access is based on membership or association with studies, ensuring that only authorized study team members will see specific study-related alerts upon login. A color-coded workflow is also provided in the user interface that allows study team members to flag each patient as ‘Eligible’, ‘Approached’, or ‘Consented’ as they work through the recruitment process (Figure 3).
Figure 3.
The JITA user interface.
Results
We piloted the functionality and usability of the JITA application using an ongoing trial that is facing challenges recruiting participants. The Emergency Medicine Department (ED) at our institution is conducting a clinical trial on wearable biosensors to evaluate the evolution of opioid tolerance on acute pancreatitis patients who present in the ED with elevated lipase values. Recruitment efforts using our central research data warehouse and i2b2 identified potential candidates after they were discharged from the hospital, thus missing the opportunity and minimizing effectiveness of recruitment efforts. The study team then had to constantly watch the physical ED dashboard and seek potential patients that meet the study eligibility by continuously combing through the EHR of ED patients, both of which were proving to be unwieldy. JITA leveraged the ADT and LAB messages to identify potential candidate patients. When patients are admitted to the hospital from the ED for clinical care, the authorized study team member received the JITA alert. The study team member could then login and obtain pertinent information on the ‘potential participant’ such as patient demographics, medical record number, matching date/time, patient room and bed number so that they can formulate a plan of action to review patient eligibility, to approach the patient and obtain consent if applicable. Over the course of three months in the piloting phase, the study has received over 90 patient alerts.
Encouraged by the preliminary results, another clinical research team from the Gastroenterology department at UMass is currently participating in further testing the JITA application. The team has received 11 patient alerts in less than 45 days and was able to approach 10 patients and successfully consent and recruit 2 patients so far.
Discussion
Studies that rely solely on the patients to respond to researchers in order to join a study have shown to have low enrollment rates (13). We leveraged the HL7 messages and AWS to develop JITA to alert researchers of an IRB approved research study, allowing them to approach a patient while they are still in the hospital. In this way JITA addresses both physician and patient-related barriers to recruitment and participation in clinical trials.
By using an AWS-based pipeline and encryption, the JITA platform ensures that patient medical information and researcher notification occurs in a secure manner. Using AWS also enables cost optimization as the charges are based on services used. In addition, conversion of HL7 messages to more structured JSON format and use of serverless lambda functions eliminate the need for expensive architecture, while allowing efficient scalability. This simple but operative infrastructure is generalizable and can be expanded to include more HL7 message types and can be easily adapted to other patient treatment centers.
Performance of the JITA platform is highly dependent on the quality of patient information entered and exchanged through HL7 at the point-of-care. Incorrect information can result in reduced accuracy. An initial screening step performed by the researcher before rolling the study into production can catch such errors. During our pilot testing of JITA at our institution, we did not encounter many such issues. In the piloting phase, JITA exhibited specificity and sensitivity rates of 86% and 78%, respectively and a false positive rate of 14%. We are currently taking measures to further optimize workflows and quality control steps for parsing of HL7 messages by the rules engine aiming to improve specificity. One approach is to finetune the time in the admission process when an alert is sent out. This will reduce the number of false alerts in cases where patient ICD-10 codes may have been updated, but increases the risk that a patient may no longer be physically in the ED/in-patient units for the research team to approach for consenting.
A potential limiting factor in the expansion of JITA to other institutions is the inadequate interoperability between EHR software. However, prevalence of HL7 standard adoption and government regulations on the performance standards for interoperability between EHR software offers promise that JITA can significantly improve clinical trial recruitment if widely adopted.
Conclusion
We leveraged HL7 and AWS to develop a secure platform to identify eligible clinical trials subjects in real time. This tool will be particularly helpful for research involving acute and urgent conditions such as stroke, as well as providing the face-to-face recruitment of patients to clinical trials. We piloted JITA in 2 ongoing clinical trials and succeeded in increasing the number and efficiency of alerts. Further work is in progress to evaluate the effect on recruitment to clinical trials at the point-of-care.
Acknowledgements
Research reported in this manuscript was supported by NCATS of the National Institutes of Health under award number: UL1TR000161 (KL, JM). The content of this publication does not necessarily represent the official views of the National Institutes of Health.
Figures & Table
References
- 1.Huang GD, Bull J, Johnston McKee K, Mahon E, Harper B, Roberts JN. Clinical trials recruitment planning: A proposed framework from the Clinical Trials Transformation Initiative. Contemp Clin Trials. 2018;66:74–9. doi: 10.1016/j.cct.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan J. Subject Recruitment and Retention: Barriers to Success. Applied Clinical Trials. 2004 [Google Scholar]
- 3.Bennette CS, Ramsey SD, McDermott CL, Carlson JJ, Basu A, Veenstra DL. Predicting Low Accrual in the National Cancer Institute’s Cooperative Group Clinical Trials. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks L, Power E. Using technology to address recruitment issues in the clinical trial process. Trends Biotechnol. 2002;20(3):105–9. doi: 10.1016/s0167-7799(02)01881-4. [DOI] [PubMed] [Google Scholar]
- 5.May M. Twenty-five ways clinical trials have changed in the last 25 years. Nat Med. 2019;25(1):2–5. doi: 10.1038/s41591-018-0314-1. [DOI] [PubMed] [Google Scholar]
- 6.Effoe VS, Katula JA, Kirk JK, Pedley CF, Bollhalter LY, Brown WM. The use of electronic medical records for recruitment in clinical trials: findings from the Lifestyle Intervention for Treatment of Diabetes trial. Trials. 2016;17(1):496. doi: 10.1186/s13063-016-1631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Embi PJ, Jain A, Clark J, Bizjack S, Hornung R, Harris CM. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005;165(19):2272–7. doi: 10.1001/archinte.165.19.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dernoncourt RFSaF. Secondary Analysis of Electronic Health Records. Improving Patient Cohort Identification. Using Natural Language Processing: Springer. 2016 [PubMed] [Google Scholar]
- 9.Weiner DL, Butte AJ, Hibberd PL, Fleisher GR. Computerized recruiting for clinical trials in real time. Ann Emerg Med. 2003;41(2):242–6. doi: 10.1067/mem.2003.52. [DOI] [PubMed] [Google Scholar]
- 10.Weng C, Batres C, Borda T, Weiskopf NG, Wilcox AB, Bigger JT. A real-time screening alert improves patient recruitment efficiency. AMIA Annu Symp Proc. 2011;2011:1489–98. [PMC free article] [PubMed] [Google Scholar]
- 11.Simon LE, Rauchwerger AS, Chettipally UK, Babakhanian L, Vinson DR, Warton EM. Text message alerts to emergency physicians identifying potential study candidates increase clinical trial enrollment. J Am Med Inform Assoc. 2019 doi: 10.1093/jamia/ocz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parry N. Physicians Spend 1 Hour on EHR Alerts per Day Medscape 2016. Accessed 08 08 2019. [Google Scholar]
- 13.Wienroth M, Caffrey L, Wolfe C, McKevitt C. Patient-initiated recruitment for clinical research: Evaluation of an outpatient letter research statement. Health Expect. 2018;21(2):494–500. doi: 10.1111/hex.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]