Abstract
Purpose
The primary aim was to examine the utility of the Western Aphasia Battery–Revised (WAB-R; Kertesz, 2007) for classifying variants of primary progressive aphasia (PPA). Traditional WAB-R metrics of Aphasia Quotient (AQ), subtest scores, WAB-R classification, and several novel metrics were examined. A secondary aim was to examine these same WAB-R metrics in individuals with primary progressive apraxia of speech (PPAOS).
Method
A retrospective analysis of WAB-R records from 169 participants enrolled in a study of neurodegenerative speech and language disorders was conducted. PPA/PPAOS classification was determined by consensus review of speech, language, and cognitive profiles. Scores on each of the WAB-R subtests were obtained to derive AQ, WAB-R aphasia profile, and 3 ratios reflecting relative performance on subtests.
Results
Mean AQ was significantly higher in the PPAOS group compared to all PPA variants except primary fluent aphasia. AQ above the normal cutoff was observed for 20% of participants with PPA. Significant main effects of group were noted for each of the subtests. Follow-up comparisons most frequently discriminated PPAOS, primary agrammatic aphasia (PAA), and logopenic progressive aphasia. Primary fluent aphasia and semantic dementia (SD) subtest scores were less distinctive, with the exception of Naming for SD, which was significantly lower than for PAA and PPAOS. When the WAB-R AQ detected aphasia, a classification of anomic aphasia was most frequently observed; this pattern held true for each of the PPA variants. The mean Information Content:Naming ratio was highest for SD, and the mean Comprehension:Fluency ratio was highest for PAA.
Conclusions
In the current study, AQ underestimated the presence of PPA and WAB-R classification did not distinguish among PPA classification determined by consensus. Performance on individual subtests and relative performance across subtests demonstrated inconsistent alignment with PPA classification. We conclude the WAB-R in isolation is inadequate to detect or characterize PPA. We instead suggest utilizing the WAB-R as 1 component of a comprehensive language and motor speech assessment when PPA is suspected.
Primary progressive aphasia (PPA; Mesulam, 2001) is a class of neurodegenerative disorder with language disruption as its presenting and most prominent feature. Progressive apraxia of speech (AOS) may accompany or occur independently of progressive language impairments (Josephs et al., 2005, 2006). The language impairments observed in PPA (e.g., anomia, disrupted grammar, impaired repetition) overlap with those observed in aphasia following stroke, although the patterns of language impairment in PPA (e.g., Botha et al., 2015; Gorno-Tempini et al., 2011) are often described differently from the profiles arising from connectionist models traditionally cited in stroke (see Catani & Mesulam, 2008, for a review). The most widely applied classification system for PPA (Gorno-Tempini et al., 2011) includes the nonfluent/agrammatic variant, labeled primary agrammatic aphasia (PAA) in this study, characterized by agrammatism, halting, and effortful speech; the semantic variant, labeled semantic dementia (SD) in this study, characterized by prominent anomia and impaired single-word comprehension; and the logopenic variant, labeled logopenic progressive aphasia (LPA) in this study, characterized by anomia and impaired repetition. Participants whose performance does not align well with any of those variants are recognized either as alternative variants or as unclassifiable. Examples include a primary fluent aphasia (PFA) variant, characterized by anomia with relative sparing of grammar, repetition, and comprehension (Botha et al., 2015), and a lexical variant described as mildly impaired comprehension and repetition in the setting of fluent expression (González Victoriano, Hornauer-Hughes, Leyton Moscoso, Neumann Serra, & Vera González, 2015).
Two important goals of classification systems for PPA are to predict pathophysiology and disease course (Botha et al., 2015; Rogalski et al., 2011; Vandenberghe, 2016). As PPA has become more widely recognized outside of research and tertiary medical centers, the task of assessing speech and language impairments expressly for classification (beyond the profiling necessary for identification of relative strengths and weaknesses that inform treatment planning and assessment of treatment outcomes) is falling to community-based speech-language pathologists (SLPs). It is unlikely that SLPs practicing in traditional settings will have either access to or familiarity with the tools comprising the full batteries described in PPA research literature or the time allotted to administer such a battery under typical clinical constraints. Instead, it is more likely that SLPs will turn to assessment methods familiar to them for the assessment of language function in the setting of neurologic impairment, namely, aphasia batteries such as the Western Aphasia Battery–Revised (WAB-R; Kertesz, 2007) or the Psycholinguistic Assessments of Language Processing in Aphasia (Kay, Lesser, & Coltheart, 1996). A meaningful translation of the PPA literature, therefore, would be an exploration of how well these familiar tools function in isolation for the purpose of classifying PPA.
The speech and language battery adopted by our laboratory includes the WAB-R (Kertesz, 2007). This tool profiles relative impairments of fluency, auditory comprehension, repetition, and naming to derive a classification based on connectionist models (see Figure 1). The WAB-R further yields an overall severity rating, Aphasia Quotient (AQ), calculated from weighted scoring on each subtest (see Table 1). Studies of PPA that employ the WAB-R typically do so for participant description. For example, the AQ has been used in many studies, from our research lab and others, to document the presence and severity of neurodegenerative aphasia (e.g., Josephs et al., 2010, 2014; Karbe, Kertesz, & Polk, 1993; Mesulam et al., 2009; Thompson, Lukic, King, Mesulam, & Weintraub, 2012). A small number of studies have reported performance on specific subtests of the WAB to characterize syntactic impairments (George & Mathuranath, 2005; Tetzloff, Utianski, et al., 2018; Tetzloff, Whitwell, et al., 2018; Wicklund et al., 2014) and anomia (Gorno-Tempini et al., 2008). Only one study by González Victoriano et al. (2015, abstract only) explicitly examined the utility of WAB-R classifications for determining PPA variants. In that report, the WAB-R derived that transcortical sensory aphasia was concordant SD, Broca's aphasia with PAA, and conduction aphasia with LPA. The anomic profile aligned with a fourth variant they labeled the lexical variant.
Figure 1.
Western Aphasia Battery–Revised (WAB-R) classification criteria. AV = auditory verbal; TCM = transcortical motor; TCS = transcortical sensory.
Table 1.
Description of tasks and scoring for the Western Aphasia Battery–Revised (WAB-R).
| WAB-R subtest | Task description | Scoring |
|---|---|---|
| Information Content | Conversational questions Picture description |
Accuracy of responses to six questions Completeness of picture description |
| Fluency | Conversational questions Picture description |
0, 1 = Stereotypes or lack of verbal response 2, 4, 5 = Varied severity of telegraphic speech 3, 7, 8 = Varied severity of jargon speech 6, 9 = Varied severity of anomia 10 = Normal |
| AV Comprehension | Yes/no questions Word recognition Sequential commands |
Accuracy |
| Repetition | Word, phrase, and sentence repetition | Accuracy |
| Naming | Object naming Word fluency Sentence completion Responsive speech |
Accuracy (cued or uncued) Presence of paraphasias |
Note. AV = Auditory Verbal.
In a study of progressive language impairment (Kertesz, Davidson, McCabe, Takagi, & Munoz, 2003), WAB-R developers explored a novel use of WAB-R subtest scores to discriminate a group of participants with PPA from a group with Alzheimer's dementia. Specifically, these authors calculated ratios of subtest scores to illustrate that impairment on the Auditory Verbal (AV) Comprehension subtest relative to performance on other subtests reliably discriminated the two groups. The calculation of ratios based on subtest performance is appealing as it holds the potential to minimize the influence of overall aphasia severity when comparing one performance profile to another. For example, each of the variants of PPA may exhibit deficits in naming, but anomia disproportionate to deficits in fluency and repetition would be more likely in SD than other variants. The aim of the current study was to examine the utility of the WAB-R for classifying variants of PPA through a series of research questions listed below. A secondary aim was to examine how individuals with progressive AOS without aphasia (primary progressive AOS [PPAOS]) perform on the WAB-R. Two lines of reasoning motivated the secondary aim. First, in most clinical scenarios, the communication diagnosis is not known a priori—the role of the SLP is to determine the presence and severity of both language and motor speech impairments. Although there is little question that detection and characterization of AOS requires specific methods for assessing motor speech, it is of interest how the presence of AOS impacts performance on a language measure such as the WAB-R. Previous studies utilizing the WAB-R to study stroke-related communication impairment have reported discrepancy between WAB or WAB-R classification and clinical impression when AOS was present (Horner, Dawson, Heyman, & Fish, 1992; John et al., 2017; Swindell, Holland, & Fromm, 1984), but similar studies have not yet been reported in the context of PPA. Second, although our lab classifies PPAOS separately from PPA because these participants do not meet the root criteria for PPA, that of primary language impairment (Gorno-Tempini et al., 2011), other groups categorize participants with progressive AOS, with or without evidence of language impairment, as a nonfluent variant of PPA (Leyton et al., 2011; Vandenberghe, 2016). Including PPAOS in the current investigation allows for application of the findings to a broad range of PPA classification schemes.
This project addressed four research questions:
Does WAB-R AQ detect the presence of PPA and discriminate PPA from PPAOS?
Does performance on individual subtests of the WAB-R discriminate among variants of PPA and PPAOS?
Does the WAB-R classification, based on connectionist models of aphasia following stroke, discriminate variants of PPA and PPAOS?
Do ratios of relative impairment across WAB-R subtests discriminate among variants of PPA and PPAOS?
Method
All procedures performed were in accordance with the ethical standards of the institutional review board.
Participants
Participants were 169 individuals enrolled in a larger National Institutes of Health–funded study of speech and language impairments in neurodegenerative disease (see Table 2). The communication diagnosis (see Table 3) was determined by consensus agreement of two experienced SLPs (authors E. A. S., H. M. C., J. R. D., or R. L. U.) based on performance on a comprehensive communication battery (see Table 4). Details of the consensus process are provided in Botha et al. (2015).
Table 2.
Participant characteristics.
| PPA group | n (%) | Age (SD) |
M (range) |
||
|---|---|---|---|---|---|
| Years of education (range) | Disease duration (years; range) | MMSE (max, 30) | |||
| PAA (±AOS) | 50 (30) |
68.3 (9.56) |
14.4(8–20) | 3.2 (< 1–8) |
26.9 (13–30) |
| LPA | 61 (36) |
65.7 (8.32) |
15.2 (12–20) |
3.1 (< 1–6) |
21.7 (8–30) |
| PFA | 9 (5) |
69.7 (8.3) |
16.3 (140–18) |
2.9 (1–4) |
28.1 (26–30) |
| SD | 14 (8) |
62.9 (6.3) |
15.9 (12–20) |
3.7 (< 1–14) |
26.6 (16–30) |
| PPAOS | 35 (21) |
69.54 (9.4) |
15.7 (12–20) |
3.4 (< 1–9) |
29.3 (27–30) |
Note. PPA = primary progressive aphasia; MMSE = Mini-Mental State Examination (Folstein et al., 1975); max = maximum; PAA = primary agrammatic aphasia variant; AOS = apraxia of speech; LPA = logopenic progressive aphasia variant; PFA = primary fluent aphasia variant; SD = semantic dementia variant; PPAOS = primary progressive apraxia of speech.
Table 3.
Primary progressive aphasia (PPA) classification (adapted from Botha et al., 2015).
| Classification | Defining characteristics |
|---|---|
| Primary progressive apraxia of speech | Apraxia of speech (AOS) is the only speech or language disturbance at the time of testing (Josephs et al., 2012). Criteria for the diagnosis of AOS are detailed in previous work (Duffy et al., 2017; Utianski et al., 2018). No more than equivocal aphasia is present. Dysarthria may be present, but it is less prominent than AOS. |
| Agrammatic variant PPA | Aphasia is present, and core criteria for PPA are met (Gorno-Tempini et al., 2011). Verbal and/or written output are agrammatic or telegraphic. Difficulties with verbal comprehension, reading comprehension, writing, and naming may be present. In patients with anomia, the target words should be recognized when provided on the majority of items. AOS may be present but should be equal or less severe than the aphasia. Dysarthria may be present. |
| Logopenic progressive aphasia | Aphasia is present, and core criteria for PPA are met (Gorno-Tempini et al., 2011). Verbal output characteristics are not agrammatic or telegraphic. Speech may be hesitant and slow from pauses for apparent word retrieval or verbal formulation efforts. There is poor retention of spoken stimuli, resulting in poor repetition that typically increases with stimulus length and complexity. Phonemic paraphasic errors are often present. Performance on tasks involving single-word comprehension should be better than on those that involve complex sentence comprehension. Anomia is usually present, but target words should be recognized on the majority of items; there are no reports from subjects or informants that the subject does not understand the meaning of common words. |
| Semantic dementia | Aphasia is present, and core criteria for PPA are met (Gorno-Tempini et al., 2011). The dominant features are anomia and poor single-word comprehension. Verbal output is grossly normal with regard to grammar, syntax, average phrase length, and prosody, excluding pauses for word retrieval. Content may be lacking in terms of substantive nouns and verbs, replacing common words with “thing,” for example. Anomia is most striking on confrontational naming. Loss of word meaning can be supported by failure to recognize target words when provided or by statements from the patient or informant that he or she does not seem to understand or recognize the meaning of certain common words; loss of single-word meaning should be disproportionate to overall aphasia severity. Performance on single-word comprehension tasks should be poorer than comprehension of complex sentences containing individual words that are comprehended. Repetition, especially for nonlengthy stimuli, is relatively preserved, and phonological errors are rare. Other supporting features include disproportionately poor performance on word fluency tasks, especially category fluency; more difficulty reading irregular words than regularly spelled nonsense words; poor performance on semantic association or receptive vocabulary tasks (e.g., Pyramids and Palm Trees Test; Howard & Patterson, 1992); and difficulty with facial recognition. |
| Primary fluent aphasia | Aphasia is present, and core criteria for PPA are met (Gorno-Tempini et al., 2011), with anomia being the predominant feature. Speech is fluent but does not meet criteria for logopenic progressive aphasia or semantic dementia. As such, grammar, syntax, average phrase length, and prosody are normal. There is little if any loss of word meaning, and repetition of complex sentences is nearly normal. Phonemic paraphasias are absent or rare. |
Table 4.
Language batteries used to determine primary progressive aphasia/primary progressive apraxia of speech classification.
| WAB-R Part 1 (Kertesz, 2007) WAB-R Part 2: Supplemental reading and writing tasks (Kertesz, 2007) 22-item version of the Token Test (De Renzi & Vignolo, 1962) 15-item Boston Naming Test (Lansing et al., 1999) Letter Fluency (FAS; Loonstra et al., 2001) Action (Verb) Fluency (Woods et al., 2005) The Pyramids and Palm Trees Test (Howard & Patterson, 1992) Examination of oral structure and function Eight-item assessment of nonverbal oral apraxia Alternate motion rates Sequential motion rates Repetition of words and sentences of increasing length and complexity |
Note. WAB-R = Western Aphasia Battery–Revised.
WAB-R Measures
Scores on each of the subtests contributing to the AQ calculation were extracted, along with the WAB-R aphasia classification (see Figure 1). Ratios were calculated to describe relative impairments among Comprehension, Fluency, Information Content, and Naming subscores (Kertesz, 2007). The Comprehension:Naming ratio reflects the WAB-R's composite score for the three auditory comprehension tasks relative to the composite score for the four naming and word-finding tasks. The Comprehension:Fluency ratio reflects the WAB-R's composite score for the auditory comprehension tasks relative to the composite rating of fluency, grammatical competence, and paraphasias derived from the spontaneous speech tasks. Finally, the Information Content:Naming ratio reflects the rating of information communicated during the spontaneous speech tasks relative to the composite score for the naming and word-finding tasks.
Data Analysis
Descriptive statistics for each dependent variable were calculated for each PPA/PPAOS variant. Specifically, simple counts were obtained for each of the eight WAB-R aphasia profiles. Mean, range, and standard error were calculated for WAB-R AQ, each of the WAB-R subtest scores, and each of the three performance ratios derived from subtest scores. Between-groups analyses of variance were performed to determine differences among variants. Welch's analysis of variance tests were performed where assumptions for equal variance were violated. Follow-up comparisons were performed using Tukey–Kramer honestly significant difference (HSD) at an α level of .05, correcting for multiple tests; the Dunn method for joint ranking was employed when assumptions for equal variance were violated. Statistical analyses were performed using JMP computer software (Version 13.0.0; SAS Institute, Inc.).
Results
AQ
Mean AQ for each of the PPA and PPAOS groups is summarized in Table 5. A significant main effect of group was observed, F(4, 36.5) = 40.25, p < .0001. Follow-up comparisons were performed using the Dunn method for joint ranking at an α level of .05, correcting for multiple tests. The mean AQ for the PPAOS group fell in the normal range and was significantly higher than all other groups except PFA. The mean AQ for the PFA group was also significantly higher than LPA. AQs for the PAA, LPA, and SD groups did not differ significantly from each other. One participant with PPAOS scored below the normal cutoff (93.8), and 27 participants with PPA had AQs above the normal cutoff. Based on the current sample, therefore, sensitivity of the WAB-R cutoff score for detecting aphasia was 79.9% and specificity was 97%.
Table 5.
Western Aphasia Battery–Revised (WAB-R) Aphasia Quotient (AQ).
| Variable | PAA (± AOS) | LPA | PFA | SD | PPAOS | Groups combined |
|---|---|---|---|---|---|---|
| Mean WAB-R AQ (range) |
84.28B,C
(49–98.8) |
78.6C
(33.5–98.8) |
92.7A,B
(86–97) |
86B,C
(64–98.4) |
97.5A
(93–100) |
85.5
(33.5–100) |
| Number (%) of participants with AQ in the normal range (above 93.8) | 14 (28) |
5 (8) |
4 (44) |
4 (29) |
34 (97) |
61
(36) |
Note. Values not designated by the same superscripted letters (A, B, or C) are significantly different (p < .05). The values bolded in the final column reflect combined data from all groups and were not included in statistical analysis. PAA = primary agrammatic aphasia variant; AOS = apraxia of speech; LPA = logopenic progressive aphasia variant; PFA = primary fluent aphasia variant; SD = semantic dementia variant; PPAOS = primary progressive apraxia of speech.
WAB-R Subtests
Table 6 details mean performance on each of the WAB-R subtests (visualized in Figure 2). A significant main effect of group was observed for each of the WAB-R subtests. Follow-up comparisons were performed using Tukey–Kramer HSD where the assumption for equal variance was upheld (AV Comprehension) and using the Dunn method for joint ranking where assumption of equal variance was violated (Information Content, Fluency, Repetition, and Naming). Alpha level was set at .05, correcting for multiple tests.
Table 6.
Western Aphasia Battery–Revised subtest scores.
| Subtest |
M (range) |
||||
|---|---|---|---|---|---|
| PAA (± AOS) | LPA | PFA | SD | PPAOS | |
| Information Content | 9.08B
(3–10) |
7.96C
(3–10) |
9.77A,B
(9–10) |
9.28A,B
(7–10) |
9.97A
(9–10) |
| Fluency | 6.78B
(2–10) |
7.65B
(3–10) |
8.88A,B
(8–9) |
8.5B
(6–10) |
9.6A
(9–10) |
| AV Comprehension | 9.54B
(7–10) |
8.92C
(5.5–10) |
10A,B
(10) |
9.42A,B,C
(8–10) |
9.97A
(9–10) |
| Repetition | 8.2B
(0–10) |
7.5 C
(2.1–9.8) |
9.44A,B
(8.6–10) |
9.0A,B
(5.6–10) |
9.66A
(8.8–10) |
| Naming | 8.61B
(4.3–10) |
7.32C
(0.6–10) |
8.37B,C
(6.4–9.9) |
6.64C
(2.4–9.2) |
9.58A
(8.8–10) |
Note. Values not designated by the same superscripted letters (A, B, or C) are significantly different (p < .05). PAA = primary agrammatic aphasia variant; AOS = apraxia of speech; LPA = logopenic progressive aphasia variant; PFA = primary fluent aphasia variant; SD = semantic dementia variant; PPAOS = primary progressive apraxia of speech; AV = Auditory Verbal.
Figure 2.
Mean performance on each of the Western Aphasia Battery–Revised subtests. Error bars reflect standard error. PAA = primary agrammatic aphasia variant; AOS = apraxia of speech; LPA = logopenic progressive aphasia variant; PFA = primary fluent aphasia variant; SD = semantic dementia variant; PPAOS = primary progressive apraxia of speech.
For Information Content, F(4, 35.08) = 22.2, p < .0001, the LPA group exhibited a lower mean score compared to all other groups; the PAA mean score was also lower than the PPAOS mean score. The PAA, PFA, and SD groups did not differ from each other.
The PPAOS group demonstrated a higher mean Fluency score than all other groups except PFA, F(4, 46.6) = 35.29, p < .0001. The remaining comparisons were not significantly different.
For AV Comprehension, F(4, 164) = 8.97, p < .0001, mean score for PPAOS was significantly higher than that for PAA, which was significantly higher than that for LPA. Mean score for PFA was also higher than that for LPA. Mean score for SD did not differ significantly from any other group.
Mean Repetition score was lower in the LPA group compared to all other groups, F(4, 37.8) = 26.35, p < .0001. Mean Repetition score for the PAA group was significantly lower than that for the PPAOS group. No other comparisons achieved statistical significance.
Finally, for Naming, F(4, 35.15) = 25.99, p < .0001, the PPAOS group demonstrated a higher mean score compared to all other groups; the PAA group demonstrated a higher mean score compared to the LPA and SD groups. Mean Naming score for the LPA, PFA, and SD groups did not differ significantly.
WAB-R Aphasia Profiles
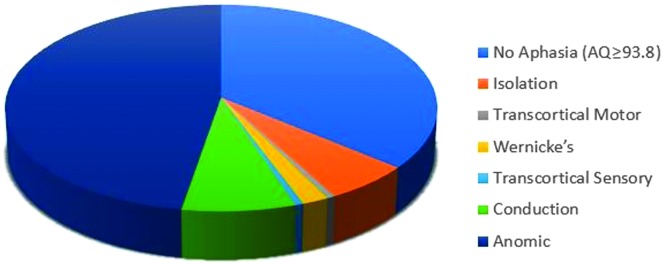
When WAB-R AQ indicated the presence of aphasia, the most common classification was anomic aphasia, accounting for 60% of the sample (see Figure 3). It included participants from each PPA variant group and PPAOS (see Table 7). Two groups (PFA and PPAOS) displayed only anomic profiles; all participants in the SD group except one displayed anomic profiles.
Figure 3.
Western Aphasia Battery–Revised classifications across all participants.
Table 7.
Western Aphasia Battery–Revised (WAB-R) profiles.
| PPA group | Number of WAB-R profile classifications (%) in PPA variant or PPAOS |
No aphasia |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonfluent profiles |
Fluent profiles |
||||||||
| Global | Broca's | Isolation | Transcortical Motor | Wernicke's | Transcortical Sensory | Conduction | Anomic | (AQ > 93.8) | |
| PAA (± AOS) | 6 (12) | 1 (2) | 3 (6) | 26 (52) | 14 (28) | ||||
| LPA | 4 (7) | 3 (4) | 1 (2) | 9 (15) | 39 (64) | 5 (8) | |||
| PFA | 5 (56) | 4 (44) | |||||||
| SD | 1 (7) | 9 (64) | 4 (29) | ||||||
| PPAOS | 1 (3) | 34 (97) | |||||||
Note. PPA = primary progressive aphasia; PPAOS = primary progressive apraxia of speech; AQ = Aphasia Quotient; PAA = primary agrammatic aphasia variant; AOS = apraxia of speech; LPA = logopenic progressive aphasia variant; PFA = primary fluent aphasia variant; SD = semantic dementia variant.
Conduction aphasia was the next most common profile, comprising 9.7% of the sample. Isolation aphasia comprised all of the nonfluent profiles, except for one transcortical motor profile. The majority of the nonfluent profiles were from the PAA group, whereas the remaining were from the LPA group. The PAA and LPA groups included WAB-R profiles more varied than the other groups, but non-anomic profiles were much less common than anomic profiles even for these two groups.
Performance Ratios
Comprehension:Naming
Although a significant main effect of group was detected, F(4, 164) = 3.05, p = .018, follow-up comparisons did not survive correction for multiple tests using Tukey–Kramer HSD at an α level of .05. Visual inspection reveals a trend for the SD group to exhibit the highest mean ratio, with mean ratios in the PAA and PPAOS groups closer to 1.0 (see Table 8).
Table 8.
Western Aphasia Battery–Revised performance ratios.
| Ratio |
M (range) |
||||
|---|---|---|---|---|---|
| PAA (± AOS) |
LPA | PFA | SD | PPAOS | |
| Comprehension:Naming | 1.12 (0.88–2.1) |
1.44 (0.84–10.1) |
1.21 (1.01–1.56) |
1.68 (1.1–3.3) |
1.01 (0.98–1.1) |
| Comprehension:Fluency | 1.6A
(0.99–4.5) |
1.21B
(0.78–2.67) |
1.12B
(1.11–1.25) |
1.12B
(0.89–1.5) |
1.03B
(0.9–1.1) |
| Information Content:Naming | 1.06B
(0.47–1.8) |
1.2B
(0.54–5) |
1.18A,B
(0.98–1.56) |
1.61A
(1.1–2.9) |
1.04B
(0.93–1.1) |
Note. Values not designated by the same superscripted letters (A, B, or C) are significantly different (p < .05). PAA = primary agrammatic aphasia variant; AOS = apraxia of speech; LPA = logopenic progressive aphasia variant; PFA = primary fluent aphasia variant; SD = semantic dementia variant; PPAOS = primary progressive apraxia of speech.
Comprehension:Fluency
A significant group effect was detected, F(4, 164) = 11.64, p < .0001. Follow-up comparisons performed using Tukey–Kramer HSD at an α level of .05, correcting for multiple tests, revealed the PAA group exhibited the highest mean ratio. The other groups had ratios close to 1.0 and were not significantly different from each other.
Information Content:Naming
A significant group effect was detected, F(4, 164) = 5.84, p = .0002. Follow-up comparisons were performed using Tukey–Kramer HSD at an α level of .05, correcting for multiple tests. Compared to all other groups except the PFA group, participants in the SD group had the highest mean ratio. No other comparisons were significantly different.
Combined Ratios
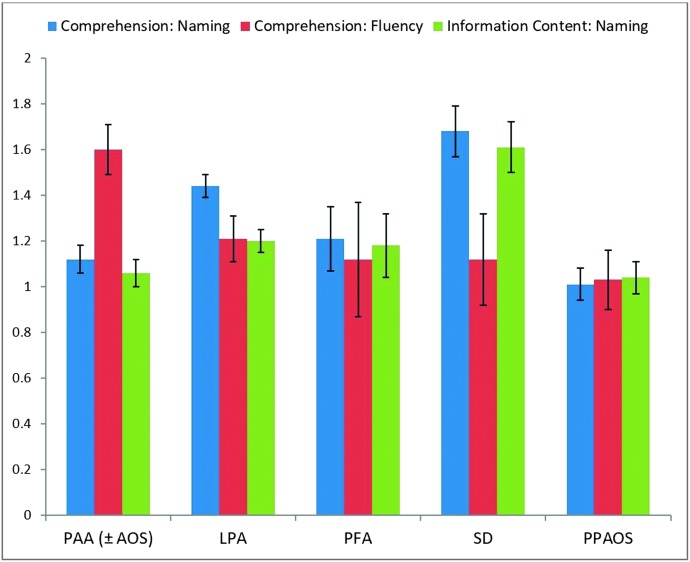
The relative magnitude of the three ratios for each of the PPA variants and PPAOS was further considered. As can be appreciated in Figure 4, the PAA ratio profile was characterized by relatively high Comprehension:Fluency ratio, with the other ratios closer to 1.0. Examination of individual performance data revealed that 56% of all participants in the PAA group demonstrated this profile. When considering only those participants with AQ lower than 93.8, 78% of the participants with PAA aligned with the profile described for the group as a whole.
Figure 4.
Performance ratio profiles. Error bars reflect standard error. PAA = primary agrammatic aphasia variant; AOS = apraxia of speech; LPA = logopenic progressive aphasia variant; PFA = primary fluent aphasia variant; SD = semantic dementia variant; PPAOS = primary progressive apraxia of speech.
The SD ratio profile was characterized by relatively high Comprehension:Naming and Information Content:Naming ratios, with Comprehension:Fluency closer to 1.0. Examination of individual performance data revealed that 70% of participants in the SD group with an abnormal AQ demonstrated this profile.
The LPA group profile was characterized by a comparatively higher Comprehension:Naming ratio relative to Comprehension:Fluency and Information Content:Naming ratios. However, only 20% of individual participants' profiles aligned with this group profile. Also observed in the LPA group were profiles typical of the SD group and relatively flat profiles seen in the PFA and PPAOS groups discussed below.
The PFA and PPAOS groups demonstrated ratio profiles that were relatively flat (i.e., ratios were equivalent). Examination of individual performance revealed 67% of the participants with PFA and 100% of the participants with PPAOS displayed flat ratio profiles.
Discussion
The current study examined how performance on a commonly used aphasia battery, the WAB-R, might be used to classify variants of PPA and PPAOS. Each research question will be considered in turn.
Does WAB-R AQ Detect the Presence of PPA and Discriminate PPA From PPAOS?
Mean AQ for each of the PPA variants fell below the cutoff for normal performance (93.8). In contrast, the mean AQ for the PPAOS group was above the cutoff for normal and was significantly higher than each of the PPA variants except PFA. At the group comparison level, therefore, AQ was a satisfactory metric for detecting PPA (as determined by consensus diagnosis) and discriminating the most common variants of PPA from PPAOS. Unfortunately, when considered at the individual participant level, sensitivity of the AQ cutoff for detecting aphasia when it was present was not ideal, as 20% of participants with PPA had AQs in the normal range. Prior reports acknowledged a subset of participants with PPA with AQs above the cutoff for abnormal performance (Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Thompson, Cho, et al., 2012; Thompson, Lukic, et al., 2012). It is therefore not surprising that several authors have recommended broader language batteries for detecting and describing PPA (Gorno-Tempini et al., 2011; Harris et al., 2013; Mesulam et al., 2009, 2012; Thompson, Lukic, et al., 2012; Wicklund et al., 2014).
Although AQ sensitivity for detecting aphasia was modest for the current PPA sample, specificity for discriminating participants without language impairment was quite high. Only one participant with PPAOS, clinically judged to have normal language functioning, had an AQ below the cutoff. In that particular case, Fluency, Repetition, and performance on the animal fluency task, which contributes to the Naming subtest score, were disproportionately impaired relative to comprehension, picture naming, and integrity of language assessed in the written modality, a pattern that has been described in previous work (Josephs et al., 2014; Scheffel, Duffy, Strand, & Josephs, 2015). Other researchers have similarly noted that AQ, heavily weighted for items requiring spoken expression (Crary & Gonzalez-Rothi, 1989), may be overly and artifactually sensitive to motor speech disorders (Hula, Donovan, Kendall, & Gonzalez-Rothi, 2010). Researchers and clinicians must therefore consider the potential spurious impact of motor speech impairment on ratings of fluency and rapid word naming when using such tasks to detect aphasia.
Does Performance on Individual Subtests of the WAB-R Discriminate Variants of PPA and PPAOS?
The group differences observed across several subtests inconsistently aligned with expectations given the classification criteria adopted. Specifically, although mean Fluency score in the PAA group was numerically lower than those in all other groups, the statistical difference was not significant. Nonetheless, the pattern of performance across groups tended in the predicted direction. This is reassuring, recognizing that the Fluency score is rated subjectively and that the scale is not interval but ordinal (Hula et al., 2010), with scores for “fluent” and “nonfluent” subtypes dispersed throughout the scale (see Table 1). Moreover, it is recognized that individual speakers may exhibit features of several different fluency scores within the same sample (Trupe, 1984).
The pattern of mean Naming score across groups tended in the predicted direction, specifically with the SD group demonstrating the numerically lowest mean Naming score. However, the difference in mean Naming score in the SD group was statistically significant only in comparison with the PAA and PPAOS groups.
Finally, the LPA group demonstrated a significantly lower mean Repetition score compared to all other groups. The LPA group also exhibited the lowest mean score on the Information Content (statistically lower than all other groups) and AV Comprehension (statistically lower than all other groups except the SD group) subtests. This is somewhat unexpected, in that loss of word meaning in SD might be expected to result in a disproportionately low AV Comprehension score. The finding is likely attributable to the specific tasks that comprise the AV Comprehension score. Leyton et al. (2011) found that single-word comprehension better discriminated the semantic variant from the other groups than did sentence comprehension, yet single-word comprehension accounts for only 4.8 of the 10 points of the WAB-R AV Comprehension score. An additional 1.2 points reflect comprehension of short phrases, and the remaining 4 points reflect comprehension of sentences of increasing length. Impoverished working memory likely accounts for the LPA group performing poorly on comprehension tasks involving longer sentences (Owens et al., 2018).
Does the WAB-R Classification, Based on Connectionist Models of Aphasia Following Stroke, Discriminate Variants of PPA and PPAOS?
The profile of performance across the various subtests on the WAB-R yields a classification of aphasia based on traditional connectionist models of language function (Kertesz, 2007). The anomic profile accounted for 60% of the current sample when AQ was below the normal cutoff and included four variants of PPA. Indeed, the anomic profile was the most common in every group. This finding has been reported previously for patients with PPA (Karbe et al., 1993) and for patients with aphasia after stroke (Swindell et al., 1984).
The transcortical motor, transcortical sensory, and Wernicke's profiles were each displayed by participants in only one PPA variant group (PAA, LPA, and LPA, respectively) and, in these cases, aligned with traditional descriptions of the variants. Unfortunately, these accounted for only 3% of the entire sample. The remaining WAB-R profiles were not specific to any one PPA variant: The isolation profile was exhibited by both participants with PAA and LPA, and the conduction profile was exhibited, by participants with PAA, LPA, and SD.
Overall, the WAB-R classification system performed poorly for identifying PPA variant. The current findings contrast with those of González Victoriano et al. (2015), which reported good alignment between WAB-R profiles and aphasia variants in their study of 58 participants with PPA. The full details of the clinical examinations and definition of clinical groups were not reported so it is difficult to reconcile the difference in findings. One possibility is that the González Victoriano et al. participants demonstrated more severe aphasia overall and were, therefore, more likely to exhibit non-anomic profiles (Kang et al., 2010; Karbe et al., 1993).
Do Ratios of Relative Impairment Across WAB-R Subtests Discriminate Variants of PPA and PPAOS?
Kertesz (2007) was the first to describe performance ratios, reporting that such ratios discriminated participants with PPA from those with Alzheimer's dementia. The current study considered how individual ratios and the profile of ratios varied across PPA variants and PPAOS. The absolute magnitude of ratios was consistent with the characteristic features of variants. Specifically, the Comprehension:Naming and Information Content:Naming ratios were numerically highest for the SD group, the variant with predominant anomia. Similarly, the Comprehension:Fluency ratio was statistically highest for the PAA group, the variant distinguished by effortful, hesitant spoken expression.
Beyond each of the independent ratios is the relative size of each ratio for each variant. Notably, the PAA group exhibited a ratio profile in which Comprehension:Fluency was quite high and the other ratios were closer to 1.0. In contrast, the SD group's profile was characterized by relatively high Comprehension:Naming and Information Content:Naming ratios, with the Comprehension:Fluency ratio closer to 1.0. These ratio profiles are visually quite distinctive (see Figure 4). It is particularly meaningful that the performance of the majority of individual participants aligned with the ratio profiles described for PAA and SD at the group level. Less distinctive but roughly as consistent were the relatively flat profiles displayed by the PFA and PPAOS groups, whose performance was near ceiling for each of the WAB-R subtests.
Only the LPA group failed to demonstrate a consistent ratio profile. This group was further distinguished by exhibiting the most severe aphasia overall and displaying the widest range of performance on each of the WAB-R subtests except Fluency. The variability in WAB-R performance in this group likely reflects heterogeneity in language ability but could be further influenced by nonaphasic cognitive deficits common in LPA (Butts et al., 2015; Owens et al., 2018). Although the lower scores on Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) exhibited by the participants with LPA in the current study offer some support for this hypothesis, evidence from cognitive measures relying less heavily on language function than does the Mini-Mental State Examination is required to address this question adequately.
General Discussion
Accumulating evidence demonstrates relationships among clinical classification, rate and nature of progression, site of lesion, and pathophysiology in PPA (see Botha et al., 2015; Gorno-Tempini et al., 2011; Vandenberghe, 2016, for reviews). Fully characterizing language deficits, including developing a profile of relative strengths and weaknesses, may contribute meaningfully to differential diagnosis, education of patients and their families about likely progression, and provision of appropriate interventions to maximize language function and address effective compensatory strategies (Beeson et al., 2011; Farrajota et al., 2012; Henry, Beeson, & Rapcsak, 2008a, 2008b; Henry et al., 2018; Taylor-Rubin et al., 2017).
A single abstract (González Victoriano et al., 2015) aside, there is little disagreement that performance on a single standard aphasia test is insufficient for establishing the presence and nature of PPA. To that end, most labs studying PPA have established a diverse language battery, typically supplemented by neurologic, cognitive, and motor speech evaluations that more reliably detect the presence of PPA and classify PPA variants. Unfortunately, these batteries are quite extensive and, therefore, time consuming to administer and, perhaps more importantly, may include measures that are unfamiliar or unavailable to SLPs in traditional practice. It is therefore likely that clinicians will turn to tools they are more familiar with, including aphasia batteries such as the WAB-R.
In this study, four potential applications of WAB-R scores were examined for usefulness in detecting and classifying variants of PPA and PPAOS. Of these, the AQ performed well at the group level but showed poor sensitivity to the presence of aphasia when examined at the individual participant level. Performance on individual subtests showed significant group effects but considerable overlap in ranges of scores across variants. Performance ratios based on relative impairment across subtests and profiles of these ratios were, particularly when combined with AQ, discriminated 70% or more of PAA and SD variants.
Limitations
The current study examined the feasibility of using the WAB-R to detect and classify PPA. It is important to note, however, that the experimental sample was limited to participants with PPA or PPAOS. Clinical scenarios are rarely so narrowly defined. The current data should be considered in conjunction with other work describing WAB or WAB-R performance in other neurodegenerative conditions, such as Alzheimer's dementia (Horner et al., 1992; Kertesz & Clydesdale, 1994; Kertesz et al., 2003), Lewy body dementia (Misch et al., 2014), or frontotemporal dementia (Hutchinson & Mathias, 2007), which may also present with progressive language impairment.
A second limitation is that the consensus diagnosis was determined with consideration of WAB-R scores as one component of a much broader language and motor speech battery. Thus, there is some risk that agreement between WAB-R classification and consensus diagnosis was overestimated given the lack of independence of variables. Given this potential bias, agreement on the presence of aphasia was surprisingly poor for the current sample. However, the comparatively good agreement between subtest scores and consensus diagnosis might also have been overestimated by the availability of said scores at the time that consensus diagnosis was established. These confounds necessarily limit the strength of the interpretations that can be drawn from the current data set.
This project examined agreement of WAB-R indices with PPA diagnoses established by consensus using the Botha et al. (2015) criteria. The findings do not speak to the use of WAB-R scores in other PPA classification schemes. For example, established algorithms for classifying PPA (Leyton et al., 2011; Vandenberghe, 2016) discriminate among variants of PPA by considering the presence or absence of motor speech disorder or agrammatism, impaired comprehension, and impaired repetition. Although these algorithms were developed to be used with language tasks other than the WAB-R, future research could populate the algorithms with pertinent WAB-R measures. It is unlikely that WAB-R subtest scores will be most effective for this purpose, as these scores are composite scores reflecting performance on either multiple tasks or tasks varying in levels of complexity. For example, the WAB-R Repetition subtest involves word, short phrase, and sentence repetition, whereas sentence repetition alone is the preferred metric for populating PPA classification algorithms (Vandenberghe, 2016). Similarly, the WAB-R AV Comprehension subtest includes single-word comprehension and comprehension of longer elements, yet single-word recognition is noted to be a more sensitive metric for PPA (Vandenberghe, 2016).
Conclusions
Based on the current findings, the following recommendations are offered:
Because the WAB-R alone is insufficient to detect or fully characterize PPA and the motor speech deficits that may accompany PPA, it should be considered for use only as one component of a larger communication assessment battery.
A thorough motor speech evaluation should be conducted to assess the presence, nature, and severity of dysarthria and/or AOS.
Consider using other measures of aphasia described in the literature (Botha et al., 2015; Leyton et al., 2011; Mesulam et al., 2009; Vandenberghe et al., 2005) that may be more sensitive to the key impairments discriminating among PPA variants. Such measures include the Northwestern Anagram Test (Weintraub et al., 2009), the Sydney Language Battery (Savage et al., 2013), and the Syntax subtests of the Boston Diagnostic Aphasia Examination (Goodglass, Kaplan, & Barresi, 2000).
AQs above the normal cutoff should be interpreted with caution. Confirm that performance on other language measures is normal before dismissing the presence of aphasia.
The connectionist classification provided by the WAB-R is insufficient to establish PPA classification. Performance ratio profiles with relatively high or low Comprehension:Fluency ratios can support the clinical impression of PAA and SD, respectively, with moderate confidence. Based on the findings of Kertesz et al. (2003), performance ratio profiles with relatively low Comprehension:Naming and Comprehension:Fluency ratios should raise suspicion for Alzheimer's dementia.
As systematic study of PPA continues, recommendations for clinical detection and characterization of the PPA will be refined. Ideally, such methods will not only address diagnostic needs but also identify treatment targets and assess benefits of intervention.
Acknowledgments
The study was funded by National Institutes of Health Grants R01 DC010367 (PI: K. A. Josephs) and R01 DC12519 (PI: J. L. Whitwell). The authors acknowledge the patients who participated in this study and their family members who made their participation possible. The authors express gratitude to Sarah Boland for her contributions as study coordinator.
Funding Statement
The study was funded by National Institutes of Health Grants R01 DC010367 (PI: K. A. Josephs) and R01 DC12519 (PI: J. L. Whitwell).
References
- Beeson P. M., King R. M., Bonakdarpour B., Henry M. L., Cho H., & Rapcsak S. Z. (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience, 45(3), 724–736. https://doi.org/10.1007/s12031-011-9579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H., Duffy J. R., Whitwell J. L., Strand E. A., Machulda M. M., Schwarz C. G., … Josephs K. A. (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. https://doi.org/10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts A. M., Machulda M. M., Duffy J. R., Strand E. A., Whitwell J. L., & Josephs K. A. (2015). Neuropsychological profiles differ among the three variants of primary progressive aphasia. Journal of the International Neuropsychological Society, 21(6), 429–435. https://doi.org/10.1017/S1355617715000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., & Mesulam M. (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex, 44(8), 953–961. https://doi.org/10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary M. A., & Gonzalez-Rothi L. J. (1989). Predicting the Western Aphasia Battery Aphasia Quotient. Journal of Speech and Hearing Disorders, 54(2), 163–166. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2468826 [DOI] [PubMed] [Google Scholar]
- De Renzi E., & Vignolo L. A. (1962). The Token Test: A sensitive test to detect receptive disturbances in aphasics. Brain, 85, 665–678. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14026018 [DOI] [PubMed] [Google Scholar]
- Duffy J. R., Hanley H., Utianski R., Clark H., Strand E., Josephs K. A., & Whitwell J. L. (2017). Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain Language, 168, 84–94. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28187331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrajota L., Maruta C., Maroco J., Martins I. P., Guerreiro M., & de Mendonça A. (2012). Speech therapy in primary progressive aphasia: A pilot study. Dementia and Geriatric Cognitive Disorders Extra, 2(1), 321–331. https://doi.org/10.1159/000341602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R. (1975). “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1202204 [DOI] [PubMed] [Google Scholar]
- George A., & Mathuranath P. S. (2005). Primary progressive aphasia: A comparative study of progressive nonfluent aphasia and semantic dementia. Neurology India, 53(2), 162–165. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16010052 [DOI] [PubMed] [Google Scholar]
- González Victoriano R., Hornauer-Hughes A., Leyton Moscoso C., Neumann Serra S., & Vera González R. (2015). Clinical characterisation of primary progressive aphasia cases using Western Aphasia Battery (WAB-R). Journal of the Neurological Sciences, 357, e449 https://doi.org/10.1016/j.jns.2015.09.101 [Google Scholar]
- Goodglass H., Kaplan E., & Barresi B. (2000). Boston Diagnostic Aphasia Examination–Third Edition Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Gorno-Tempini M. L., Brambati S. M., Ginex V., Ogar J., Dronkers N. F., Marcone A., … Miller B. L. (2008). The logopenic/phonological variant of primary progressive aphasia. Neurology, 71(16), 1227–1234. https://doi.org/10.1212/01.wnl.0000320506.79811.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., … Grossman M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. M., Gall C., Thompson J. C., Richardson A. M., Neary D., du Plessis D., … Jones M. (2013). Classification and pathology of primary progressive aphasia. Neurology, 81(21), 1832–1839. https://doi.org/10.1212/01.wnl.0000436070.28137.7b [DOI] [PubMed] [Google Scholar]
- Henry M. L., Beeson P. M., & Rapcsak S. Z. (2008a). Treatment for anomia in semantic dementia. Seminar in Speech and Language, 29(1), 60–70. https://doi.org/10.1055/s-2008-1061625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., Beeson P. M., & Rapcsak S. Z. (2008b). Treatment for lexical retrieval in progressive aphasia. Aphasiology, 22(7–8), 826–838. https://doi.org/10.1080/02687030701820055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., Hubbard H. I., Grasso S. M., Mandelli M. L., Wilson S. M., Sathishkumar M. T., … Gorno-Tempini M. L. (2018). Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain, 141(6), 1799–1814. https://doi.org/10.1093/brain/awy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner J., Dawson D. V., Heyman A., & Fish A. M. (1992). The usefulness of the Western Aphasia Battery for differential diagnosis of Alzheimer dementia and focal stroke syndromes: Preliminary evidence. Brain and Language, 42(1), 77–88. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1547470 [DOI] [PubMed] [Google Scholar]
- Howard D., & Patterson K. (1992). The Pyramids and Palm Trees Test. New York, NY: Pearson. [Google Scholar]
- Hula W., Donovan N., Kendall D., & Gonzalez-Rothi L. J. (2010). Item response theory analysis of the Western Aphasia Battery. Aphasiology, 24, 1326–1341. [Google Scholar]
- Hutchinson A. D., & Mathias J. L. (2007). Neuropsychological deficits in frontotemporal dementia and Alzheimer's disease: A meta-analytic review. Journal of Neurology, Neurosurgery, & Psychiatry, 78(9), 917–928. https://doi.org/10.1136/jnnp.2006.100669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John A. A., Javali M., Mahale R., Mehta A., Acharya P. T., & Srinivasa R. (2017). Clinical impression and Western Aphasia Battery classification of aphasia in acute ischemic stroke: Is there a discrepancy? Journal of Neurosciences Rural Practice, 8(1), 74–78. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28149086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Boeve B. F., Duffy J. R., Smith G. E., Knopman D. S., Parisi J. E., … Dickson D. W. (2005). Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase, 11(4), 283–296. https://doi.org/10.1080/13554790590963004 [DOI] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Fossett T. R., Strand E. A., Claassen D. O., Whitwell J. L., & Peller P. J. (2010). Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Archives of Neurology, 67(5), 596–605. https://doi.org/10.1001/archneurol.2010.78 [DOI] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M., Senjem M. L., Gunter J. L., … Whitwell J. L. (2014). The evolution of primary progressive apraxia of speech. Brain, 137(10), 2783–2795. https://doi.org/10.1093/brain/awu223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M, Senjem M. L., Master A. V., … Whitwell J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain: A Journal of Neurology, 135(5), 1522–1536. https://doi.org/10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Whitwell J. L., Layton K. F., Parisi J. E., … Petersen R. C. (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129(6), 1385–1398. https://doi.org/10.1093/brain/awl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E. K., Sohn H. M., Han M. K., Kim W., Han T. R., & Paik N. J. (2010). Severity of post-stroke aphasia according to aphasia type and lesion location in Koreans. Journal of Korean Medical Science, 25(1), 123–127. https://doi.org/10.3346/jkms.2010.25.1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbe H., Kertesz A., & Polk M. (1993). Profiles of language impairment in primary progressive aphasia. Archives of Neurology, 50(2), 193–201. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8431139 [DOI] [PubMed] [Google Scholar]
- Kay J., Lesser R., & Coltheart M. (1996). Psycholinguistic Assessments of Language Processing in Aphasia (PALPA): An introduction. Aphasiology, 10(2), 159–180. https://doi.org/10.1080/02687039608248403 [Google Scholar]
- Kertesz A. (2007). Western Aphasia Battery–Revised. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Kertesz A., & Clydesdale S. (1994). Neuropsychological deficits in vascular dementia vs Alzheimer's disease. Frontal lobe deficits prominent in vascular dementia. Archives of Neurology, 51(12), 1226–1231. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7986178 [DOI] [PubMed] [Google Scholar]
- Kertesz A., Davidson W., McCabe P., Takagi K., & Munoz D. (2003). Primary progressive aphasia: Diagnosis, varieties, evolution. Journal of the International Neuropsychology Society, 9(5), 710–719. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12901777 [DOI] [PubMed] [Google Scholar]
- Lansing A. E., Ivnik R. J., Cullum C. M., & Randolph C. (1999). An empirically derived short form of the Boston naming test. Archives of Clinical Neuropsychology, 14(6), 481–487. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14590575 [PubMed] [Google Scholar]
- Leyton C. E., Villemagne V. L., Savage S., Pike K. E., Ballard K. J., Piguet O., … Hodges J. R. (2011). Subtypes of progressive aphasia: Application of the International Consensus Criteria and validation using β-amyloid imaging. Brain, 134(10), 3030–3043. https://doi.org/10.1093/brain/awr216 [DOI] [PubMed] [Google Scholar]
- Loonstra A. S., Tarlow A. R., & Sellers A. H. (2001). COWAT metanorms across age, education, and gender. Applied Neuropsychology, 8(3), 161–166. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11686651 [DOI] [PubMed] [Google Scholar]
- Mesulam M. (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11310619 [PubMed] [Google Scholar]
- Mesulam M., Wieneke C., Rogalski E., Cobia D., Thompson C., & Weintraub S. (2009). Quantitative template for subtyping primary progressive aphasia. Archives of Neurology, 66(12), 1545–1551. https://doi.org/10.1001/archneurol.2009.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M., Wieneke C., Thompson C., Rogalski E., & Weintraub S. (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135(5), 1537–1553. https://doi.org/10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misch M. R., Mitchell S., Francis P. L., Sherborn K., Meradje K., McNeely A. A., … Masellis M. (2014). Differentiating between visual hallucination-free dementia with Lewy bodies and corticobasal syndrome on the basis of neuropsychology and perfusion single-photon emission computed tomography. Alzheimer's Research & Therapy, 6(9), 71 https://doi.org/10.1186/s13195-014-0071-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. E., Machulda M. M., Duffy J. R., Strand E. A., Clark H. M., Boland S., … Josephs K. A. (2018). Patterns of neuropsychological dysfunction and cortical volume changes in logopenic aphasia. Journal of Alzheimer's Disease, 66(3), 1015–1025. https://doi.org/10.3233/JAD-171175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E., Cobia D., Harrison T. M., Wieneke C., Weintraub S., & Mesulam M. M. (2011). Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology, 76(21), 1804–1810. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21606451. https://doi.org/10.1212/WNL.0b013e31821ccd3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage S., Hsieh S., Leslie F., Foxe D., Piguet O., & Hodges J. R. (2013). Distinguishing subtypes in primary progressive aphasia: Application of the Sydney Language Battery. Dementia and Geriatric Cognitive Disorders, 35(3–4), 208–218. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23467307. https://doi.org/10.1159/000346389 [DOI] [PubMed] [Google Scholar]
- Scheffel L., Duffy J. R., Strand E. A., & Josephs K. A. (2015). Word fluency test performance in primary progressive aphasia and primary progressive apraxia of speech. Paper presented at the Clinical Aphasiology Conference, Monterey, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell C. S., Holland A., & Fromm D. (1984). Classification of aphasia: WAB type versus clinical impression. In Clinical Aphasiology: Proceedings of the Conference 1984 (pp. 48–54). Minneapolis, MN: BRK Publishers. [Google Scholar]
- Taylor-Rubin C., Croot K., Power E., Savage S. A., Hodges J. R., & Togher L. (2017). Communication behaviors associated with successful conversation in semantic variant primary progressive aphasia. International Psychogeriatrics, 29(10), 1619–1632. https://doi.org/10.1017/S1041610217000813 [DOI] [PubMed] [Google Scholar]
- Tetzloff K. A., Utianski R. L., Duffy J. R., Clark H. M., Strand E. A., Josephs K. A., & Whitwell J. L. (2018). Quantitative analysis of agrammatism in agrammatic primary progressive aphasia and dominant apraxia of speech. Journal of Speech, Language, and Hearing Research, 61(9), 2337–2346. https://doi.org/10.1044/2018_JSLHR-L-17-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff K. A., Whitwell J. L., Utianski R. L., Duffy J. R., Clark H. M., Machulda M. M., … Josephs K. A. (2018). Quantitative assessment of grammar in amyloid-negative logopenic aphasia. Brain and Language, 186, 26–31. https://doi.org/10.1016/j.bandl.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. K., Cho S., Price C., Wieneke C., Bonakdarpour B., Rogalski E., … Mesulam M. (2012). Semantic interference during object naming in agrammatic and logopenic primary progressive aphasia (PPA). Brain and Language, 120(3), 237–250. https://doi.org/10.1016/j.bandl.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. K., Lukic S., King M. C., Mesulam M., & Weintraub S. (2012). Verb and noun deficits in stroke-induced and primary progressive aphasia: The Northwestern Naming Battery. Aphasiology, 26(5), 632–655. https://doi.org/10.1080/02687038.2012.676852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupe E. (1984). Reliability of rating spontaneous speech in the Western Aphasia Battery: Implications for classification. In Clinical Aphasiology: Proceedings of the Conference 1984 (pp. 55–69). Minneapolis, MN: BRK Publishers. [Google Scholar]
- Utianski R. L., Duffy J. R., Clark H. M., Strand E. A., Boland S. M., Machulda M. M., … Josephs K. A. (2018). Clinical progression in four cases of primary progressive apraxia of speech. American Journal of Speech-Language Pathology, 27(4), 1303–1318. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30458509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R. (2016). Classification of the primary progressive aphasias: Principles and review of progress since 2011. Alzheimer's Research & Therapy, 8, 16 https://doi.org/10.1186/s13195-016-0185-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R., Vandenbulcke M., Weintraub S., Johnson N., Porke K., Thompson C. K., & Mesulam M. M. (2005). Paradoxical features of word finding difficulty in primary progressive aphasia. Annals of Neurology, 57(2), 204–209. https://doi.org/10.1002/ana.20362 [DOI] [PubMed] [Google Scholar]
- Weintraub S., Mesulam M., Wieneke C., Rademaker A., Rogalski E. J., & Thompson C. K. (2009). The Northwestern Anagram Test: Measuring sentence production in primary progressive aphasia. American Journal of Alzheimer's Disease & Other Dementias, 24(5), 408–416. https://doi.org/10.1177/1533317509343104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund M. R., Duffy J. R., Strand E. A., Machulda M. M., Whitwell J. L., & Josephs K. A. (2014). Quantitative application of the primary progressive aphasia consensus criteria. Neurology, 82(13), 1119–1126. https://doi.org/10.1212/WNL.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Scott J. C., Sires D. A., Grant I., Heaton R. K., Troster A. I., & . HIV Neurobehavioral Research Center Group. (2005). Action (verb) fluency: Test–retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society, 11(4), 408–415. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16209421 [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a