Highlights
-
•
Understory composition was the best correlated with structural forest canopy measures.
-
•
Tree canopy LAI and the fractional cover of understory were strongly related.
Keywords: Leaf area index, LAI, Canopy cover, Canopy openness, Site fertility, Forest canopy, Hyytiälä
Abstract
Information on understory composition and its relationships with the overstory tree canopy, especially leaf area index (LAI), is crucially needed in, e.g., modeling land-atmosphere interactions and productivity of forests. There are also several global LAI products produced from satellite data which need to be validated with ground reference data. However, to date, only scarce field data on simultaneous structural properties of under- and overstory vegetation, and tree canopy LAI, have been available in boreal forests. This paper shows how understory composition and fractional cover of different species types varies in a boreal forest site, and how it is linked to structural properties of the tree layer. The study is based on 301 understory plots collected in an area of ∼16 km2 around Hyytiälä forestry field station, Finland (61°50′N, 24°17′E) in a southern boreal forest site. Forest understory plot data was accompanied with measurements of both standard forest inventory variables and optically-based canopy light transmittance data. Clear differences in average species composition between different site fertility types were observed, but also large variation within each site fertility type was noted. Forest understory composition was better correlated with structural forest canopy measures (e.g., tree canopy LAI, canopy cover, canopy openness) than with traditional forest inventory variables such as tree height or diameter. Forest canopy LAI and the fractional cover of understory were strongly related, especially in more fertile sites. Our results highlight the role of tree canopy structural metrics as modifiers of the understory light climate and growing conditions, also, in boreal forests.
1. Introduction
A boreal forest is characterized by an overstory tree layer with a short statured understory vegetation layer containing woody shrubs, mosses and lichens. Information regarding understory composition of different species in forests is required in many research fields: both in ecological research, dealing with growth, survival and regeneration (e.g., Tonteri et al., 2016, Nilsson and Wardle, 2005) and modeling of ecosystem functioning (e.g., photosynthetic production and carbon balance [e.g., Messier et al., 1998, Kolari et al., 2006]), and even in vegetation remote sensing (e.g., Miller et al., 1997, Pisek et al., 2012). The influence of understory vegetation on forest reflectance can also be observed in global satellite-based vegetation products (Majasalmi et al., 2015), and thus better quantification of both layers (i.e., the tree layer and understory layer) is necessary for improved mapping and monitoring of global land surfaces from optical satellite data. Although there are plenty of studies published from boreal forests that comprise data from all the vegetation layers (i.e., both understory and overstory), they do not usually report at stand-level canopy leaf area index (LAI, m2/m2) values for large numbers of stands, nor do they explicitly link the canopy LAI to understory cover fractions, even though such data are urgently needed in, e.g., physically-based reflectance modeling or remote sensing of forests. The optical LAI is a key variable in the radiative transfer equation for vegetation (see e.g., Ross, 1981), and thus cannot be replaced with any other ecological variable in, e.g., physically-based models in remote sensing of forests or in land surface modeling (as part of climate models).
In a boreal forest, availability of soil nutrients and soil physical properties, such as moisture, temperature and ventilation (as described by site fertility type), and the local climate control the abundance and composition of understory vegetation (Cajander, 1949). The lower understory layer, also called ‘forest floor’ or ‘ground floor’, is composed of mosses, litter or lichens, and is often covered by an ‘upper understory’ composed of, e.g., graminoids, herbs, or dwarf shrubs. As boreal forests have a low number of different tree species compared to other forested biomes, a large portion of the species richness (i.e., biodiversity) in a boreal forest is linked to variation in understory species composition (Barbier et al., 2008, Kuuluvainen, 2002, Roberts, 2004), some of which also have economical value (e.g., berries).
Forest canopy is one of the major determinants of the microhabitat within a forest, and thus also has an impact on understory composition. The tree layer influences understory vegetation by modifying the light conditions it creates for the understory (Tonteri et al., 2016) and by, e.g., its litterfall and nutrient recycling (Ukonmaanaho et al., 2008, Nilsson and Wardle, 2005). For example, canopy shading during daytime results in lower temperatures for the understory than for the overstory canopy, whereas the opposite is true at night (Niinemets & Valladares, 2004). Thus, there are differences in understory air humidity and vapor pressure deficit. As temperature affects both photosynthesis and respiration, and humidity on stomatal conductance (Sellin et al., 2010), forest canopy shading can also have direct implications on carbon gain. Increasing the proportion of conifers typically decreases soil pH and soil nutrient availability, which results in decreases in soil fertility (Roberts, 2004, Barbier et al., 2008).
Understory vegetation has a short-term impact on tree seedling regeneration and a long-term impact on decomposition, nutrient cycling and buildup of soil nutrients (Nilsson & Wardle, 2005). For example, increasing densities of black crowberry (Empetrum nigrum) have been frequently associated with reduced forest tree stand productivity (e.g., Wardle et al., 2003), and the exclusion of bilberry (Vaccinium myrtillus) has been shown to exert a negative effect on Norway spruce (Picea abies) seedlings (Jäderlund et al., 1997). Seedlings planted onto ground dominated by lichens have shown superior growth to those planted within other ground-layer vegetation, whereas seedlings planted on feather moss (Ptilium crista-castrensis) grow poorly, despite the ability of moss to retain moisture (Steijlen et al., 1995). Understory has been shown to significantly contribute to nutrient cycling in forests (Gilliam, 2007), and especially in the spring when potential for nutrient leakage is the greatest, the spring ephemerals may capture the nutrients, and re-release them later during the growing season via decomposition of understory plant material (e.g., Gerken Golay et al., 2016).
Although the biomass of understory species is small compared to that of trees, their biomass turnover is fast – the share of standing shrub biomass replaced yearly is around 62% for bilberry and 39% for lingonberry (Vaccinium vitis-idaea) (Wardle & Zackrisson, 2005), meaning that the net primary productivity of these shrubs is half that of the trees (Nilsson & Wardle, 2005). It has also been observed that photosynthesis of the moss understory in black spruce (Picea mariana) forests may account for 10 to 50% of whole-forest gross photosynthesis (Goulden & Crill, 1997).
Vegetation studies conducted in the boreal region have tended to focus on trees, and while many studies have also looked at forest understory composition, simultaneous explicit quantification of forest canopy structural characteristics, such as LAI, influencing the understory light climate has been lacking (e.g., Messier et al., 1998, Kolari et al., 2006). In particular, little empirical data is available for simultaneous measurements of both the understory and tree canopy structural characteristics, such as LAI and canopy openness (CO), due to the laborious and expensive nature of field campaigns required for collecting such data. Previously, Tonteri et al. (2016) reported how the cover of different plant species has changed during a 20-year period as a function of forest management activities in boreal forests. Their results showed that after a clear-cut in an old forest, the cover of shade and semi-shade tolerant understory species decreased strongly (>70%), and their recovery was slow. Understory species which were adapted to semi-light conditions decreased clearly (20–60%) after clear-cut, but their recovery was fairly fast – reaching the initial cover level at stand age of ca. 30 years. Light-demanding species increased after clear-cutting, and decreased after intermediate cuttings, thus demonstrating the key role of forest management activities as modifiers of boreal understory species compositions (Tonteri et al., 2016). However, Tonteri et al. (2016) did not analyze how measurements of overstory canopy structure (e.g., LAI) and understory composition are linked. As both the understory and tree canopy layer develop synergistically, simultaneous measurements of both layers are necessary. Thus, an increased understanding of understory properties may also have important implications for production-oriented forest ecosystem management.
While today’s dynamic vegetation models (DVM) contain a large number of traits to characterize different plant functional types, the model predictions may be regarded as tentative due to large uncertainties. They may be one of the largest sources of uncertainty in current Earth system models (ESMs) (e.g., Fischer et al., 2016, Fisher et al., 2017). The key for improving these models lays in developing better plant trait databases, and enhancing the mechanistic understanding regarding light and competition (e.g., Laanisto & Niinemets, 2015) in forests. As forest understory species composition cannot easily be mapped using remote sensing data, it must be predicted from overstory canopy properties such as LAI.
In this paper, we show how boreal forest understory composition and fractional cover vary in different site fertility types, and analyze how understory properties depend on tree-layer canopy structure (e.g., LAI). Although fires are the strongest ecosystem modifier for most of the boreal region (e.g., Hart & Chen, 2006), they are not common in managed Finnish forests, and thus discussion regarding the role of fire as a stand-replacing disturbance driving the change in understory composition is omitted here.
2. Methods
2.1. Study site
Field data was collected in an area of ∼16 km2 around Hyytiälä forestry field station, Finland (61°50′N, 24°17′E) in a southern boreal forest site during peak growing season (June 24 – July 17, 2013). The mean annual temperature and precipitation for the area are 3 °C and 700 mm, respectively. The study area is dominated by Norway spruce, Scots pine (Pinus sylvestris) and birches (Betula pubescens and Betula pendula). Monocultural birch stands are rare, i.e., deciduous stands are often mixed with other tree species. The area is under common forest management practices with periodical thinnings (e.g., the rotation period varies from 60 to 120 years).
The study area was divided into 16 subareas (each 1 km × 1 km). Within each 1 km2 subarea, a cluster of 20 plots was located using a systematic sampling scheme. The distance between the plots within each cluster was 100 m in the south-north direction, and 150 m in the east–west direction. If the individual plot location did not fall in a forest (e.g., it was on a road), it was moved in steps of 10 m (but not exceeding a total of 30 m) in either of the cardinal directions. The total number of plots was 320, of which 307 were located in a forest. In this study, we report values of 301 plots (instead of 307) because six plots were located on bare rock (i.e., they had no understory vegetation cover). In other words, bare rock covered, in our data, 2% of the forest area. For each plot, information on soil type (i.e., mineral soil or peatland), and thus also the presence/absence of ditches, was recorded. Examples of species compositions representing the different site fertility types are shown in Table 1. More details are provided in Majasalmi et al. (2015). Based on summary statistics, the two most fertile site types (i.e., herb-rich and mesic) had the highest shares of deciduous tree species (Table 2). Sub-xeric sites had some deciduous trees, but were mainly dominated by conifers, whereas xeric sites had only pine trees, which also had the smallest variation in forest canopy LAI, and the highest canopy openness values.
Table 1.
Example species compositions in different site fertility types.
| Tree species | Dwarf shrubs | Pteridophytes + herbaceous | Graminoids | Mosses | Lichens | |
|---|---|---|---|---|---|---|
| Herb-rich | Picea abies | Vaccinium myrtillus | Oxalis acetosella | Deschampsia flexuosa | Pleurozium schreberi | |
| Betula spp. | Vaccinium vitis-idaea | Athyrium filix-femina | Calamagrostis spp. | Dicranum spp. | ||
| Gymnocarpium dryopteris | Ptilium crista-castrensis | |||||
| Rhytidiadelphus triquetrus | ||||||
| Mesic | Picea abies | Vaccinium myrtillus | Equisetum sylvaticum | Deschampsia flexuosa | Pleurozium schreberi | |
| Pinus sylvestris | Vaccinium vitis-idaea | Dryopteris carthusiana | carex spp. | Hylocomium splendens | ||
| Betula spp. | Linnaea borealis | Melampyrum sylvaticum | Dicranum spp. | |||
| Sub-xeric | Pinus sylvestris | Vaccinium myrtillus | Luzula pilosa | Deschampsia flexuosa | Pleurozium schreberi | |
| Vaccinium vitis-idaea | Maianthemum bifolium | Dicranum spp. | ||||
| Calluna vulgaris | Trientalis europaea | |||||
| Xeric | Pinus sylvestris | Vaccinium vitis-idaea | Cladina arbuscula | |||
| Calluna vulgaris | Cladina rangiferina | |||||
| Empetrum nigrum | Cladonia stellaris |
Table 2.
Summary statistics (i.e., mean ± standard deviation values) for tree canopy layer in different site fertility types. Abbreviations: n is the number of plots, LAI is tree canopy leaf area index, CO is canopy openness, CC is canopy cover, fDecid., fSpruce and fPine are the fractions of tree species, BA is stand basal area (m2/ha), H is the basal area weighted mean tree height (m), CL is crown length (m), DBH is the median tree diameter-at-breast-height (cm).
| Fertility | n | LAI | CO | CC | fDecid. | fSpruce | fPine | BA | H | CL | DBH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Herb-rich | 28 | 4.6 ± 1.3 | 0.09 ± 0.06 | 0.8 ± 0.12 | 0.33 ± 0.38 | 0.57 ± 0.39 | 0.09 ± 0.25 | 23.7 ± 11 | 20 ± 5.0 | 11.1 ± 3.5 | 22.6 ± 7.4 |
| Mesic | 231 | 3.4 ± 1.5 | 0.23 ± 0.18 | 0.63 ± 0.21 | 0.19 ± 0.3 | 0.47 ± 0.38 | 0.34 ± 0.38 | 16.6 ± 8.6 | 15.8 ± 6.8 | 8.3 ± 3.8 | 18.1 ± 8.7 |
| Sub-xeric | 38 | 2.7 ± 0.9 | 0.32 ± 0.14 | 0.52 ± 0.17 | 0 ± 0.01 | 0.11 ± 0.28 | 0.88 ± 0.28 | 15.2 ± 5.8 | 15.4 ± 5.5 | 7.2 ± 2.7 | 18.7 ± 7.5 |
| Xeric | 4 | 2.2 ± 0.3 | 0.39 ± 0.07 | 0.41 ± 0.06 | 0 ± 0 | 0 ± 0 | 1 ± 0 | 11 ± 6.7 | 10.7 ± 6.8 | 6.3 ± 2.7 | 11.9 ± 9.4 |
2.2. Measurements of understory
First, the understory layer in each plot was classified into four site fertility types: herb-rich, mesic, sub-xeric, and xeric. Next, for each plot, the cover fractions of understory were obtained from two 1 m × 1 m understory sub-plots, located 4 m west and east from the plot center. A traditional sampling quadrat was used to visually estimate the vertical cover fractions. Fractional cover of the upper understory layer (in the vertical direction) was estimated for: (1) dwarf shrubs, (2) pteridophytes and herbaceous species (later called ‘herbs’), and (3) graminoids. Similarly, the ground layer fractional cover was estimated separately for: (1) mosses, (2) lichens, and (3) litter (including all non-photosynthetic material). To obtain vertical cover fractions, the upper understory layer was assumed to overlay equally all forest floor components. All cover fractions were estimated by the same person.
2.3. Measurements of tree canopy
Tree canopy structure was quantified by measuring both standard forest inventory variables and canopy light transmittance. Forest inventory variables included basal area (BA, m2/ha), which quantifies the cross-section of tree stems at breast height (1.3 m above the ground) by tree species, and the basal area weighted mean tree height (H, m), crown length (CL, m), and diameter at breast height (DBH, cm). Canopy light transmittance data was used to obtain three different forest canopy structural measures: effective canopy leaf area index (LAIeff), canopy openness (CO) and canopy cover (CC).
The optical data was measured using the LAI-2000 device (LI-COR, 1992), which measures blue light (320–490 nm) transmittance through plant canopies with a hemispherical lens. Canopy transmittance data was obtained by combining data from two instruments recording simultaneously below and above the tree canopy (e.g., in a radiation tower or in the middle of an open, nearby field). Measurements were performed when the sun was lower than 16° from the horizon, or in fully overcast conditions, to avoid direct radiation reaching the sensors. Optical data was always collected after all other field measurements had been completed in order to avoid walking on the understory plots. The sampling scheme used to collect the canopy transmittance data comprised eight measurement points in each plot: two points in each cardinal direction from the plot center, at 4 m and 8 m distance from the center.
The sensor’s field-of-view extends over almost 150° and is divided into five concentric rings (ranges: 0°–13°, 16°–28°, 32°–43°, 47°–58° and 61°–74°) centered at zenith angles θ that are weighted according to the part of the hemisphere that they cover. The LAI estimation method is based on the inversion of canopy transmittance according to Beer’s law (Monsi & Saeki, 1953), and LAIeff was computed as (LI-COR, 1992):
| (1) |
where T(θ) is the measured angular canopy gap fraction at point j.
As the conifer forest canopy LAIeff underestimates the ‘true’ forest LAI due to needles clustering into shoots, a standard correction procedure (i.e., a shoot-level clumping correction) was applied by dividing the LAIeff with a basal-area-weighted mean shoot-level clumping factor (see Majasalmi et al., 2013 for description of method). The shoot-level clumping factors were 0.59 for pine (Smolander et al., 1994) and 0.64 for spruce (Stenberg et al., 1995). For deciduous species, no clumping correction was applied.
CC was approximated as 1- T(θ) of the smallest zenith angle ring (of 0-13°), whereas CO (which is also sometimes called canopy diffuse non-interceptance) was obtained as (LI-COR, 1992):
| (2) |
where Γ is the intensity distribution of the sky radiation above the canopy.
3. Results and discussion
3.1. Understory composition and fractional cover
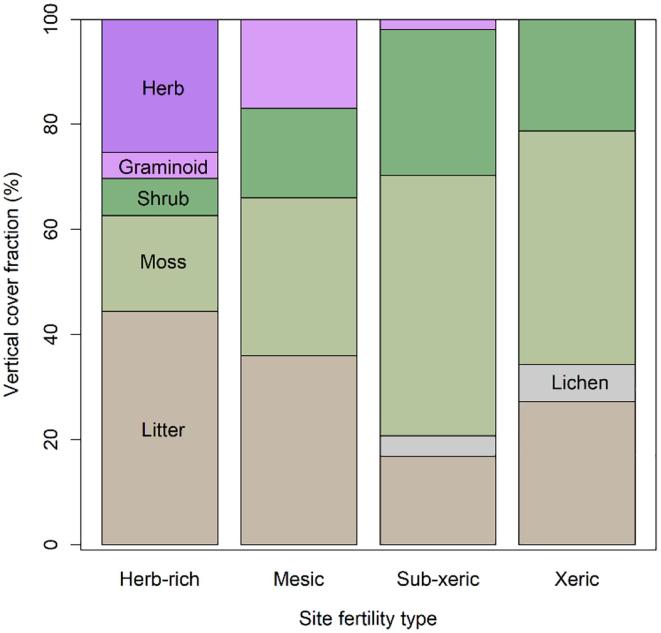
Litter and mosses constituted on average over 60% of the cover fraction in all site fertility types (Fig. 1). The fractional cover of moss on average increased when moving from fertile towards more infertile sites. In general, the fractional covers of litter, herbs and graminoids, on the other hand, decreased when moving from fertile to infertile site types. Among the four site fertility types, the two most fertile site types (i.e., herb-rich and mesic) had the highest litter fractions (Fig. 1).
Fig. 1.
Mean vertical cover fractions (%) of forest floor and understory species for the four site fertility types.
The most variation in vertical fractional cover within a site fertility type was observed for the mesic type plots (Fig. 2). Compared with herb-rich and mesic site types, the sub-xeric and xeric site types had less variation in vertical cover fractions of forest floor and understory species composition. In the mesic sites, the forest floor was sometimes almost completely covered by litter or moss, and the cover fraction of the upper understory species (i.e., shrubs, graminoids and herbs) was up to 60–80%. In herb-rich sites, on the other hand, the coverage of shrubs and graminoid species was at maximum around 45%. In sub-xeric and xeric site fertility types, the largest variation in vertical cover fractions was also observed for litter (up to 79% and 74% for sub-xeric and xeric) and moss (up to 88% and 74%, respectively). Some lichens were present in sub-xeric and xeric types, and none had any herbaceous plants. Both site fertility types also had fairly high coverage of shrubs (on average 28% and 21%, and reaching up to 70% and 32%, respectively). While the average species composition of different site fertility types shows clear differences (Fig. 1), it must be noted that within site fertility type, variation in species composition is large (Fig. 2).
Fig. 2.
Variation in vertical cover fraction of forest floor and understory species composition in: (a) Herb-rich, (b) Mesic, (c) Sub-xeric, and (d) Xeric site fertility types. Mean, maximum and minimum are denoted with symbols, and standard deviation as a dark line with whiskers.
3.2. Relationship between understory and canopy structure
Vertical fractions of understory cover showed moderate correlation with optically-based forest canopy structural variables (i.e., LAI, CO and CC). The observed trend was that LAI and CC were negatively correlated with the cover fraction of upper understory (i.e., the sum of fractional covers of shrubs, herbs and graminoids) (r = −0.48 and −0.34, respectively), while positive correlation was observed between cover fraction of upper understory and CO (r = 0.38) (Table 3). Increasing LAI and CC denote denser forest canopies, which are more closed and cast more shadows. In contrast to LAI and CC, increasing CO indicates sparser forest canopies and thus also that more light is able to reach the forest understory layer.
Table 3.
Correlation matrix of different forest ground floor and understory compositions and forest canopy properties. Understory components: vertical cover fraction of litter, lichen, moss, shrub, herbs and graminoids, ‘Upper story’ is the sum of vertical cover fraction of shrubs, herbs and graminoids. Canopy structural metrics: LAI is the canopy leaf area index, and fDecid., fSpruce and fPine are the fractions of tree species in overstory.
| Understory components |
Canopy structural metrics |
Tree species fractions |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lichen | Moss | Shrub | Herb | Graminoid | Upper story | LAI | Canopy cover | Canopy openness | fDecid. | fSpruce | fPine | |
| Litter | −0.18 | −0.75 | −0.50 | 0.14 | 0.08 | −0.26 | 0.24 | 0.28 | −0.19 | 0.30 | 0.06 | −0.27 |
| Lichen | 0.17 | 0.07 | −0.18 | −0.06 | −0.17 | −0.08 | −0.11 | 0.06 | −0.14 | −0.20 | 0.28 | |
| Moss | 0.26 | −0.49 | −0.23 | −0.43 | 0.12 | −0.01 | −0.09 | −0.44 | 0.03 | 0.29 | ||
| Shrub | −0.44 | −0.24 | 0.30 | −0.11 | −0.12 | 0.06 | −0.32 | −0.15 | 0.36 | |||
| Herb | −0.18 | 0.54 | −0.38 | −0.30 | 0.37 | 0.33 | 0.05 | −0.28 | ||||
| Graminoid | 0.23 | 0.04 | 0.14 | −0.10 | 0.29 | 0.00 | −0.21 | |||||
| Upper story | −0.48 | −0.34 | 0.38 | 0.25 | −0.08 | −0.10 | ||||||
| LAI | 0.79 | −0.87 | −0.27 | 0.37 | −0.16 | |||||||
| canopy cover | −0.92 | 0.10 | 0.13 | −0.20 | ||||||||
| canopy openness | 0.04 | −0.22 | 0.18 | |||||||||
| fDecid. | −0.29 | −0.44 | ||||||||||
| fSpruce | −0.73 | |||||||||||
Vertical cover fractions of ground floor species groups (i.e., lichen and moss) did not show any strong relationships with the canopy structural metrics. Herbaceous species was the only species group which had a cover fraction that strongly correlated with canopy structure (r = -0.38 for LAI, and r = 0.37 for CO). For the other species groups, the correlations were very weak or non-existent (Table 3). As expected, the litter fraction not only correlated with canopy LAI and CC (r = 0.24 and 0.28, respectively), but also with the fraction of deciduous species (r = 0.30). In other words, larger trees with more shadow casting and annual leaf fall increase the amount of litter on the forest floor. Lichen cover was the most correlated with the fraction of pine trees in a stand (r = 0.28), as both pines and lichens are adapted to grow in dry, open and arid conditions.
The fractional cover of shrubs was the most correlated with the fraction of pine trees in a stand (r = 0.36), because many shrub species, such as lingonberry, are abundant in dry sites. The fractional cover of mosses was negatively correlated with the fraction of deciduous tree species (r = −0.44), and moderately correlated with the fraction of pines (r = 0.29). Fractional cover of graminoids and herbs was the best correlated with the fraction of deciduous tree species (r = 0.29 and 0.33), as expected.
From the traditional forest inventory variables, BA was the most correlated with fractional cover of the upper understory layer (r = -0.35), but otherwise the correlations were clearly lower than for optically-based canopy structural variables (i.e., −0.07, −0.12 and −0.14 for CL, DBH and H, respectively. Data not shown).
LAI was the most correlated with the upper understory fraction in both herb-rich and mesic sites (r = −0.57 and −0.58, respectively) (Table 4). In general, CO was more correlated with upper understory fraction than CC in all site fertility types. In sub-xeric and xeric sites, CO was the best correlated with upper understory fraction (r = 0.39). Regrouping the data according to dominating tree species showed that in pine-dominated sites, none of the tree canopy characteristics had strong relationships with upper understory fraction, whereas in spruce-dominated sites, especially LAI had a clear correlation with the upper understory fraction (r = −0.60). In deciduous stands, on the other hand, all canopy structural characteristics were correlated with upper understory fraction (r varied from −0.35 to −0.38). These results demonstrate the role of dominant tree species in modifying the light conditions inside a forest, and thus also their role in influencing understory fractional composition and cover.
Table 4.
Linear correlation (r) between upper understory fraction and canopy structural characteristics by site fertility type and dominating tree species. LAI is the canopy leaf area index.
| Herb-rich | Mesic | Sub-xeric + Xeric | Pine | Spruce | Decid. | ||
|---|---|---|---|---|---|---|---|
| LAI | −0.57 | −0.58 | −0.37 | −0.23 | −0.60 | −0.38 | |
| Canopy cover | −0.09 | −0.46 | −0.25 | −0.18 | −0.48 | −0.35 | |
| Canopy openness | 0.37 | 0.48 | 0.39 | 0.22 | 0.49 | 0.37 |
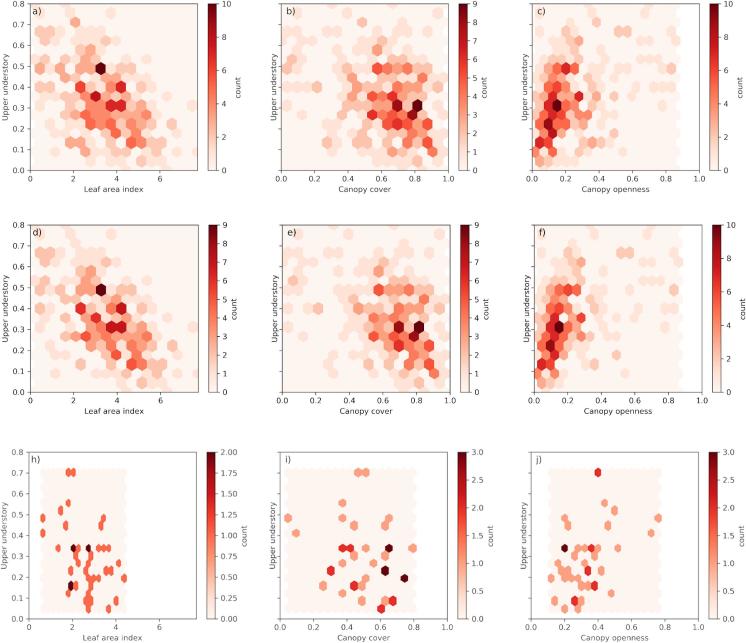
Linear models were fitted to illustrate the relationships of vertical cover fraction of the upper understory and LAI, CC and CO (Table 5, Fig. 3). First, data from all plots were pooled together, and then the plots were divided into two groups: fertile sites (herb-rich + mesic) and less fertile sites (sub-xeric + xeric) to fit the linear models separately. Using data from all plots, the model intercept term between forest upper understory and LAI was 0.53, between forest upper understory and CC was 0.51, and between forest upper understory and CO was 0.25 (Table 5, Fig. 3 abc). A simple interpretation of the intercept terms is that after a clear-cut harvest, the vertical cover fraction of upper understory would be either 50% following the LAI and CC models, or 25% in the case of the CO model. When the models were fitted to data only from the fertile sites (Table 5, Fig. 3 def), the intercepts were higher (for LAI: 0.56, CC: 0.55, and CO: 0.26) than when using data from all plots. On the other hand, when the models were fit using only data from the less fertile (i.e., sub-xeric and xeric) sites, the values of the intercepts were lower (i.e., for LAI 0.47, CC: 0.41, and CO: 0.14, respectively) than using all data (Table 5, Fig. 3 hij). As our data did not contain any information regarding the age of the trees or timings of the past harvests, we cannot draw strong conclusions based on the intercept values.
Table 5.
Example linear fits between upper understory fraction and canopy structural characteristics using all plots or subsets containing the more fertile (i.e., herb-rich and mesic), and less fertile (i.e., sub-xeric and xeric) plots. LAI is the canopy leaf area index, RMSE is the root mean squared error and r is the correlation coefficient.
| Data | Characteristics | Visualization | Intercept | Slope | RMSE | r |
|---|---|---|---|---|---|---|
| All plots | LAI | Fig. 3a | 0.53 | −0.06 | 0.15 | −0.48 |
| All plots | Canopy cover | Fig. 3b | 0.51 | −0.27 | 0.16 | −0.34 |
| All plots | Canopy openness | Fig. 3c | 0.25 | 0.36 | 0.15 | 0.38 |
| Herb-rich + Mesic | LAI | Fig. 3d | 0.56 | −0.06 | 0.14 | −0.54 |
| Herb-rich + Mesic | Canopy cover | Fig. 3e | 0.55 | −0.32 | 0.15 | −0.4 |
| Herb-rich + Mesic | Canopy openness | Fig. 3f | 0.26 | 0.41 | 0.15 | 0.43 |
| Sub-xeric + Xeric | LAI | Fig. 3h | 0.47 | −0.07 | 0.15 | −0.37 |
| Sub-xeric + Xeric | Canopy cover | Fig. 3i | 0.41 | −0.24 | 0.16 | −0.25 |
| Sub-xeric + Xeric | Canopy openness | Fig. 3j | 0.14 | 0.46 | 0.15 | 0.39 |
Fig. 3.
Frequency plots showing the number of observations between upper understory and (a) Leaf area index (LAI) using data from all plots, (b) Canopy cover (CC) using data from all plots, and (c) Canopy openness (CO) using data from all plots, (def) LAI, CC and CO using only data from fertile (herb-rich and mesic) plots, (hij) LAI, CC and CO using only data from less fertile (sub-xeric and xeric) plots. The mosaic of hexagons with a color scale shows the number of observations falling into each hexagon bin.
Although the correlations between LAI, CC and CO and forest understory properties remained moderate, we were able to demonstrate quantitatively the direction and magnitude of how boreal forest canopy structure (e.g., LAI) links with forest understory composition. Thus, our data can be used to describe the dynamics between, e.g., remote sensing data based estimates of LAI, CC and CO and understory composition, for which data has not been previously available. Uncertainty in our data is the largest for the xeric site type, for which only four plots were available in the study area.
Due to the important role of boreal understories in ecosystem functioning, information on climate sensitivity of the understory composition (e.g., Weigel et al., 2019) is needed to estimate climate change impacts on forest ecosystems. Typically, remote sensing data is used to set the initial state of forest in land components of the ESMs, which employ integrated land surface model constituents, such as DVMs. Many of the current DVMs contain a description of shrubs, as in the Community Land Model (Oleson et al., 2013), mosses or both mosses and shrubs, as in JULES (Chadburn et al., 2015) and BIOME4 (Kaplan et al., 2003), and lichens and bryophytes in JSBACH (Porada et al., 2013) models. In addition, the newest version of the ORCHIDEE (version: ORC‐HL‐VEGv1.0 by Druel et al., 2017) allows simulation of cover changes of mosses and shrubs alongside grasses and trees (Druel et al., 2019). Although the newest ORCHIDEE version does not directly simulate multiple vegetation layers (i.e., understory vegetation with mosses/grass/shrubs under trees), it employs calibrated key parameters which allow modeling of the dynamic competition of mosses and shrubs with grasses and trees in the boreal and Arctic zones. These recent developments demonstrate that the role of understory vegetation as a driver of boreal forest dynamics has been acknowledged. While various remote sensing systems are available for quantifying forest canopy structures and species, very little information is obtained from the understory due to tree canopy masking, especially in forests with dense canopies and large LAI. Due to problems in initial mapping of forest understory fractional cover and composition, these can be modeled based on tree canopy properties, such as LAI, as was demonstrated in this paper.
The focus in this paper was to provide means to characterize the relationships between tree canopy structure and understory compositions in the context of, e.g., physically-based remote sensing of forests or land surface modeling. We are aware that data needs vary between different disciplines (e.g., ecophysiology, plant community sciences, etc.) and thus we provide the raw and processed data (doi: 10.17632/dyt4nkp583.1), so that all users can analyze the data to suit their needs.
4. Conclusions
The results showed that, in a boreal forest area, the more fertile sites have more variation in species compositions than infertile sites, but also that the variation in species compositions within site fertility types is large. As forest canopy structure influences forest understory composition through light climate, our data (doi: https://doi.org/10.17632/dyt4nkp583.1) can be used to provide a link between remotely sensed estimates of forest canopy LAI, CC and CO and understory composition. As forest canopies mask the understory vegetation, little information can be obtained from remote sensing data on forest understory composition in forests with high LAI and closed canopies. Thus, for better characterization of different boreal forest understory compositions in, e.g., different DVMs, data linking forest understory properties with tree canopy properties are crucially needed.
CRediT authorship contribution statement
Titta Majasalmi: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. Miina Rautiainen: Resources, Writing - review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Anni-Sofia Hoppi and Johanna Karjalainen for field work.
Funding
This study has received funding from Aalto ENG postdoctoral funds and from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 771049). The text reflects only the author’s view and the Agency is not responsible for any use that may be made of the information it contains.
Data availability
The raw and processed data are stored in Mendeley Data repository with doi 10.17632/dyt4nkp583.1.
References
- Barbier S., Gosselin F., Balandier P. Influence of tree species on understory vegetation diversity and mechanisms involved—a critical review for temperate and boreal forests. For. Ecol. Manage. 2008;254:1–15. doi: 10.1016/j.foreco.2007.09.038. [DOI] [Google Scholar]
- Cajander Aimo. Forest types and their significance. Acta For. Fenn. 1949;56(5) [Google Scholar]
- Chadburn S., Burke E., Essery R., Boike J., Langer M., Heikenfeld M., Cox P., Friedlingstein P. An improved representation of physical permafrost dynamics in the JULES land-surface model. Geosci. Model Dev. 2015;8:1493–1508. [Google Scholar]
- Druel A., Ciais P., Krinner G., Peylin P. Modeling the vegetation dynamics of northern shrubs and mosses in the ORCHIDEE land surface model. J. Adv. Model. Earth Syst. 2019 doi: 10.1029/2018MS001531. [DOI] [Google Scholar]
- Druel A., Peylin P., Krinner G., Ciais P., Viovy N., Peregon A., Bastrikov V., Kosykh N.P., Mironycheva-Tokareva N.P. Towards a more detailed representation of high-latitude vegetation in the global land surface model ORCHIDEE (ORC-HL-VEGv1.0) Geosci. Model Dev. 2017;10:4693–4722. doi: 10.5194/gmd-10-4693-2017. [DOI] [Google Scholar]
- Fisher R.A., Koven C.D., Anderegg W.R., Christoffersen B.O., Dietze M.C., Farrior C.E., Lichstein J.W. Vegetation demographics in Earth System Models: A review of progress and priorities. Glob. Change Biol. 2017;24:35–54. doi: 10.1111/gcb.13910. [DOI] [PubMed] [Google Scholar]
- Fischer R., Bohn F., de Paula M.D., Dislich C., Groeneveld J., Gutiérrez A.G., Pütz S. Lessons learned from applying a forest gap model to understand ecosystem and carbon dynamics of complex tropical forests. Ecol. Model. 2016;326:124–133. doi: 10.1016/j.ecolmodel.2015.11.018. [DOI] [Google Scholar]
- Gilliam F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience. 2007;57:845–858. doi: 10.1641/B571007. [DOI] [Google Scholar]
- Gerken Golay M., Thompson J., Kolka R. Carbon, nitrogen and phosphorus storage across a growing season by the herbaceous layer in urban and preserved temperate hardwood forests. Appl. Veg. Sci. 2016;19:689–699. doi: 10.1111/avsc.12253. [DOI] [Google Scholar]
- Goulden M.L., Crill P.M. Automated measurements of CO2 exchange at the moss surface of a black spruce forest. Tree Physiol. 1997;17:537–542. doi: 10.1093/treephys/17.8-9.537. [DOI] [PubMed] [Google Scholar]
- Hart S.A., Chen H.Y. Understory vegetation dynamics of North American boreal forests. Crit. Rev. Plant Sci. 2006;25:381–397. doi: 10.1080/07352680600819286. [DOI] [Google Scholar]
- Jäderlund A., Zackrisson O., Dahlberg A., Nilsson M.C. Interference of Vaccinium myrtillus on establishment, growth, and nutrition of Picea abies seedlings in a northern boreal site. Can. J. For. Res. 1997;27:2017–2025. doi: 10.1139/x97-185. [DOI] [Google Scholar]
- Kaplan J.O., Bigelow N.H., Prentice I.C., Harrison S.P., Bartlein P.J., Christensen T.R., Cramer W., Matveyeva N.V., McGuire A.D., Murray D.F., Razzhivin V.Y., Smith B., Walker D.A., Anderson P.M., Andreev A.A., Brubaker L.B., Edwards M.E., Lozhkin A.V. Climate change and Arctic ecosystems: 2. Modeling, paleodata-model comparisons, and future projections. J. Geophys. Res. 2003;108(D19):8171. doi: 10.1029/2002JD002559. [DOI] [Google Scholar]
- Kolari P., Pumpanen J., Kulmala L., Ilvesniemi H., Nikinmaa E., Grönholm T., Hari P. Forest floor vegetation plays an important role in photosynthetic production of boreal forests. For. Ecol. Manage. 2006;221:241–248. doi: 10.1016/j.foreco.2005.10.021. [DOI] [Google Scholar]
- Kuuluvainen T. Natural variability of forests as a reference for restoring and managing biological diversity in boreal Fennoscandia. Silva Fennica. 2002;36:97–125. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.467.3524&rep=rep1&type=pdf [Google Scholar]
- Laanisto L., Niinemets Ü. Polytolerance to abiotic stresses: how universal is the shade–drought tolerance trade-off in woody species? Glob. Ecol. Biogeogr. 2015;24:571–580. doi: 10.1111/geb.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI-COR . LI-COR Inc.; Lincoln, Nebraska: 1992. LAI-2000 Plant Canopy Analyzer: Instruction Manual. https://licor.app.boxenterprise.net/s/q6hrj6s79psn7o8z2b2s. [Google Scholar]
- Majasalmi T., Rautiainen M., Stenberg P., Manninen T. Validation of MODIS and GEOV1 fPAR products in a boreal forest site in Finland. Remote Sensing. 2015;7:1359–1379. https://www.mdpi.com/2072-4292/7/2/1359 [Google Scholar]
- Majasalmi T., Rautiainen M., Stenberg P., Lukeš P. An assessment of ground reference methods for estimating LAI of boreal forests. For. Ecol. Manage. 2013;292:10–18. doi: 10.1016/j.foreco.2012.12.017. [DOI] [Google Scholar]
- Messier C., Parent S., Bergeron Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998;9:511–520. doi: 10.2307/3237266. [DOI] [Google Scholar]
- Miller J.R., White H.P., Chen J.M., Peddle D.R., McDermid G., Fournier R.A., LeDrew E. Seasonal change in understory reflectance of boreal forests and influence on canopy vegetation indices. J. Geophys. Res.: Atmos. 1997;102(D24):29475–29482. doi: 10.1029/97JD02558. [DOI] [Google Scholar]
- Monsi M., Saeki T. The light factor in plant communities and its significance for dry matter production. Japan. J. Bot. 1953;14:22–52. [Google Scholar]
- Niinemets Ü., Valladares F. Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: optimality and constraints. Plant Biol. 2004;6:254–268. doi: 10.1055/s-2004-817881. [DOI] [PubMed] [Google Scholar]
- Nilsson M.C., Wardle D.A. Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005;3:421–428. doi: 10.1890/1540-9295(2005)003[0421:UVAAFE]2.0.CO;2. [DOI] [Google Scholar]
- Oleson, K., Lawrence, D., Bonan, G., Drewniak, B., Huang, M., Koven, C., Levis, S., Li, F., Riley, W., Subin, Z., Swenson, S., Thornton, P., Bozbiyik, A., Fisher, R., Heald, C., Kluzek, E., Lamarque, J.‐F., Lawrence, P., Leung, L., Lipscomb, W., Muszala, S., Ricciuto, D., Sacks, W., Sun, Y., Tang, J. & Yang, Z.‐L. (2013). Technical description of version 4.5 of the Community Land Model (CLM), https://doi.org/10.5065/D6RR1W7M.
- Pisek J., Rautiainen M., Heiskanen J., Mõttus M. Retrieval of seasonal dynamics of forest understory reflectance in a Northern European boreal forest from MODIS BRDF data. Remote Sens. Environ. 2012;117:464–468. doi: 10.1016/j.rse.2011.09.012. [DOI] [Google Scholar]
- Porada P., Weber B., Elbert W., Pöschl U., Kleidon A. Estimating global carbon uptake by lichens and bryophytes with a process-based model. Biogeosciences. 2013;10:6989–7033. [Google Scholar]
- Roberts M.R. Response of the herbaceous layer to natural disturbance in North American forests. Can. J. Bot. 2004;82:1273–1283. doi: 10.1139/b04-091. [DOI] [Google Scholar]
- Ross J. Springer; The Hague: 1981. The radiation regime and architecture of plant stands; p. 391. [Google Scholar]
- Sellin A., Õunapuu E., Karusion A. Experimental evidence supporting the concept of light-mediated modulation of stem hydraulic conductance. Tree Physiol. 2010;30:1528–1535. doi: 10.1093/treephys/tpq091. [DOI] [PubMed] [Google Scholar]
- Smolander H., Stenberg P., Linder S. Dependence of light interception efficiency of Scots pine shoots on structural parameters. Tree Physiol. 1994;14:971–980. doi: 10.1093/treephys/14.7-8-9.971. [DOI] [PubMed] [Google Scholar]
- Steijlen I., Nilsson M.C., Zackrisson O. Seed regeneration of Scots pine in boreal forest stands dominated by lichen and feather moss. Can. J. For. Res. 1995;25:713–723. doi: 10.1139/x95-079. [DOI] [Google Scholar]
- Stenberg P., Linder S., Smolander H. Variation in the ratio of shoot silhouette area to needle area in fertilized and unfertilized Norway spruce trees. Tree Physiol. 1995;15:705–712. doi: 10.1093/treephys/15.11.705. [DOI] [PubMed] [Google Scholar]
- Tonteri T., Salemaa M., Rautio P., Hallikainen V., Korpela L., Merilä P. Forest management regulates temporal change in the cover of boreal plant species. For. Ecol. Manage. 2016;381:115–124. doi: 10.1016/j.foreco.2016.09.015. [DOI] [Google Scholar]
- Ukonmaanaho L., Merilä P., Nöjd P., Nieminen T.M. Litterfall production and nutrient return to the forest floor in Scots pine and Norway spruce stands in Finland. Boreal Environ. Res. 2008;13:67–91. http://urn.fi/URN:NBN:fi-fe2016091323703 [Google Scholar]
- Wardle D.A., Zackrisson O. Effects of species and functional group loss on island ecosystem properties. Nature. 2005;435:806. doi: 10.1038/nature03611. [DOI] [PubMed] [Google Scholar]
- Wardle D.A., Hörnberg G., Zackrisson O., Kalela-Brundin M., Coomes D.A. Long-term effects of wildfire on ecosystem properties across an island area gradient. Science. 2003;300:972–975. doi: 10.1126/science.1082709. [DOI] [PubMed] [Google Scholar]
- Weigel R., Gilles J., Klisz M., Manthey M., Kreyling J. Forest understory vegetation is more related to soil than to climate towards the cold distribution margin of European beech. J. Veg. Sci. 2019;30:746–755. doi: 10.1111/jvs.12759. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw and processed data are stored in Mendeley Data repository with doi 10.17632/dyt4nkp583.1.