Abstract
Evidence suggests that some patients experience a higher symptom burden. However, little is known about whether this high risk phenotype persists over time. Latent transition analysis (LTA) was used to examine the probability that patients remained in the same symptom class when assessed prior to the administration of and following their next dose of chemotherapy (CTX). For the patients whose class membership remained consistent, differences in demographic and clinical characteristics, and quality of life (QOL) were evaluated. The Memorial Symptom Assessment Scale (MSAS) was used to evaluate symptom burden. LTA was used to identify subgroups of patients with distinct symptom experiences based on the occurrence of the MSAS symptoms. Of the 906 patients evaluated, 83.9% were classified in the same symptom occurrence class at both assessments. Of these 760 patients 25.0% were classified as Low-Low, 44.1% as Moderate-Moderate, and 30.9% as High-High. Compared to the Low-Low class, the other two classes were younger, more likely to be female and to report child care responsibilities, and had a lower functional status and a higher comorbidity scores. The two higher classes reported lower QOL scores. The use of LTA could assist clinicians to identify higher risk patients and initiate more aggressive interventions.
Keywords: cancer, chemotherapy, latent transition analysis, symptoms, predictive risk modeling, quality of life
INTRODUCTION
Patients receiving chemotherapy (CTX) report an average of ten co-occurring symptoms (Esther Kim et al., 2009). However, in several studies (Pud et al., 2008, Dodd et al., 2011, Dodd et al., 2010, Illi et al., 2012, Miaskowski et al., 2006), a significant amount of inter-individual variability was found in patients’ experiences with various multiple co-occurring symptoms. All five of these studies evaluated for subgroups of patients based on self-reports of the four most common symptoms associated with cancer and its treatment (i.e., pain, fatigue, sleep disturbance, depression). A consistent finding across all five studies was the identification of a subgroup of patients with low levels of all four symptoms and a subgroup of patients with high levels of all four symptoms. Equally important, patients in the “all high” subgroup reported worse functional status and poorer quality of life (QOL) outcomes.
While the aforementioned studies were limited to only four co-occurring symptoms, newer work has used the Memorial Symptom Assessment Scale (MSAS) (Portenoy et al., 1994) to identify high risk patients based on the occurrence of 32 symptoms (Ferreira et al., 2008, Gwede et al., 2008, Miaskowski et al., In press). In two of these studies (Ferreira et al., 2008, Gwede et al., 2008), two distinct symptom subgroups were identified. In the third study (Miaskowski et al., In press), four distinct symptom subgroups were found. Across these three studies, patients in the subgroup with the highest symptom occurrence rates reported decrements in functional status and QOL. Of note, across all eight studies cited above, clinical characteristics were not associated with subgroup membership. Variations in the total number of patient subgroups may be related to the heterogeneous nature of the samples in terms of cancer diagnoses and types of treatments, the number of symptoms evaluated, the dimension of the symptom experience (e.g., occurrence, severity) used to create the subgroups, and the statistical procedures used to identify the subgroups.
Recently, our research team reported on the use of latent class analysis (LCA) to identify three distinct groups of patients (i.e., latent classes) based on the relative occurrence rates for 25 symptoms on the MSAS prior to receiving their next cycle of CTX (Miaskowski et al., 2014a). Of the 582 patients evaluated, 36.1% were categorized in the low class (i.e., mean of 5.7 symptoms); 50.0% were in the moderate class (i.e., mean of 12.9 symptoms); and 13.9% were in the all high class (i.e., mean of 20.3 symptoms). Of note, patients in the all high class were significantly younger and more likely to be female and nonwhite, and had lower levels of social support, lower socioeconomic status, poorer functional status, and a higher level of comorbidity. As noted previously, no other clinical characteristics were associated with latent class membership. At the conclusion of this paper, we suggested that the use of LCA may provide an effective way to identify patients with a higher symptom burden or a high risk symptom phenotype.
A question that remains unanswered is whether or not this high risk symptom phenotype persists following the administration of CTX. In other words, do patients who were classified, using LCA, into the low, moderate, and high classes remain in those symptom classes following the administration of their next dose of CTX? To answer this research question, we used latent transition analysis (LTA) (Collins and Lanza, 2010, Lanza et al., 2003), to examine the probability that patients remained in the same class at their initial (i.e., prior to the administration of CTX) and subsequent (i.e., following the administration of their next dose of CTX) assessment. For the patients whose class membership remained consistent over the two time points, differences in demographic and clinical characteristics, as well as QOL outcomes were evaluated.
PATIENTS AND METHODS
Patients and Settings
This study is part of an ongoing, longitudinal study of the symptom experience of oncology outpatients receiving CTX (Miaskowski et al., 2014a). Eligible patients were ≥18 years of age; had a diagnosis of breast, gastrointestinal (GI), gynecological (GYN), or lung cancer; had received CTX within the preceding four weeks; were scheduled to receive at least two additional cycles of CTX; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs. A total of 1505 patients were approached and 906 consented to participate (60.2% response rate). The major reason for refusal was being overwhelmed with their cancer treatment.
Instruments
A demographic questionnaire obtained information on age, gender, ethnicity, marital status, living arrangements, education, employment status, and income. The Karnofsky Performance Status (KPS) scale (Karnofsky, 1977) was used to evaluate patients’ functional status. The Self-administered Comorbidity Questionnaire (SCQ) (Sangha et al., 2003) evaluated the occurrence, treatment, and functional impact of common comorbid conditions (e.g., diabetes, arthritis).
The MSAS was used to evaluate the occurrence, severity, frequency, and distress of 32 symptoms commonly associated with cancer and its treatment. The MSAS is a self-report questionnaire designed to measure the multidimensional experience of symptoms. Patients were asked to indicate whether or not they had experienced each symptom in the past week (i.e., symptom occurrence). If they had experienced the symptom, they were asked to rate its frequency of occurrence, severity, and distress. The reliability and validity of the MSAS is well established in studies of oncology inpatients and outpatients (Portenoy et al., 1994).
Quality of life (QOL) was evaluated using generic (i.e., Medical Outcomes Study-Short Form-12 (SF-12)) (Ware et al., 1996) and disease-specific (i.e., Quality of Life Scale-Patient Version (QOL-PV)) (Dow et al., 1996, Ferrell et al., 1995) measures. Both measures have well-established validity and reliability. Higher scores on both measures indicate a better QOL.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Board at each of the study sites. Eligible patients were approached by the research staff in the infusion unit to discuss participation in the study. Written informed consent was obtained from all patients. Depending on the length of their CTX cycles, patients completed questionnaires in their homes, a total of six times over two cycles of CTX (i.e., prior to CTX administration (Time 1 and 4), approximately 1 week after CTX administration (Time 2 and 5), approximately 2 weeks after CTX administration (Time 3 and 6)). For this analysis, symptom occurrence data from the Time 1 (i.e., recovery from previous cycle) and Time 2 (i.e., acute symptoms) assessments were analyzed. Patients were asked to report on their symptom experience for the previous week. Medical records were reviewed for disease and treatment information.
Data Analysis
Latent class analysis
LCA identifies latent classes based on an observed response pattern using categorical variables (Collins and Lanza, 2010, Lanza et al., 2003, Nylund et al., 2007a, Vermunt and Magdison, 2002). Prior to identifying the LTA model that described patients’ transitions from classes at Time 1 to classes at Time 2, separate LCAs were done to identify subgroups of patients with similar symptom experiences at each of the two assessments. These analyses provided an estimate of the number of classes that might be expected at each assessment, so as to inform the LTA modeling of the class transitions.
The LCAs and LTA were done using the symptom occurrence data from the MSAS. In order to have a sufficient number of patients with each symptom to perform the LCA and LTA, we identified the MSAS symptoms that occurred in at least 40% of the patients. This criterion was selected to provide assurance that sufficient information was available to identify classes that were not sample-specific, due to infrequent reports of symptoms. A total of 25 out of 32 symptoms from the MSAS occurred in >40% of the patients. Following the identification of the number of latent classes at each assessment with LCA, the estimation of latent transition classes was performed.
The final number of latent classes for each LCA was selected based on the Bayesian Information Criterion (BIC), the Vuong, Lo, Mendel, and Rubin likelihood ratio test (VLMR), and entropy. Typically, the best fitting LCA model has the lowest BIC. This BIC criterion can be supplemented by an evaluation of the VLMR (Nylund et al., 2007b) which tests whether a model with K classes fits the data better than a model with one fewer class (the K-1 class model). If the VLMR is significant, it supports the K-class model as fitting the data better. If it is not significant, it indicates that too many classes were extracted and that the K-1 class model fits the data better than the K-class model. In addition, well-fitting models produce entropy values of ≥.80 (Celeux and Soromenho, 1996). Finally, well-fitting models “make sense” conceptually and the estimated classes differ as might be expected on variables not used in the generation of the model. Because the VLMR is not available for LTA, the best fitting model was determined based on its BIC and entropy values.
Latent Transition Analysis
LTA allows for the identification of individuals who transition from latent classes at one point in time, to the same latent classes at a subsequent point in time, as well as individuals who move to different classes (Collins and Lanza, 2010, Lanza et al., 2003, Nylund et al., 2007a). Symptom occurrence data from the MSAS was used to identify the latent classes of patients with similar symptom experiences at two time points during their CTX cycle (i.e., Time 1 – week prior to CTX administration and Time 2 – week following CTX administration).
The LCA and LTA models were estimated using Mplus™ Version 7 (Muthen and Muthen, 1998-2014). Estimation was carried out with robust Maximum-Likelihood (MLR) and the Expectation-Maximization (EM) algorithm (Muthen and Shedden, 1999). Given that the observed variables were dichotomies, estimation was carried out with a logit link. To protect against solutions that were identified based on a local maximum, from 800 to as many as 6,000 random starts were used in the estimation of the model. This approach ensured that the best fitting log-likelihood was replicated with multiple models (Muthen and Muthen, 1998-2014).
Differences in demographic and clinical characteristics and QOL outcomes among the LTA classes, were evaluated using analyses of variance, Kruskal-Wallis, or Chi-Square tests with Bonferroni corrected post hoc contrasts, using SPSS version 22 (IBM, Armonk, NY). A p-value of <.05 was considered statistically significant. All comparisons among the classes used actual values. Adjustments were not made for missing data. Therefore, the cohort for each of these analyses was dependent on the largest set of complete data among groups.
RESULTS
Latent Class Analyses
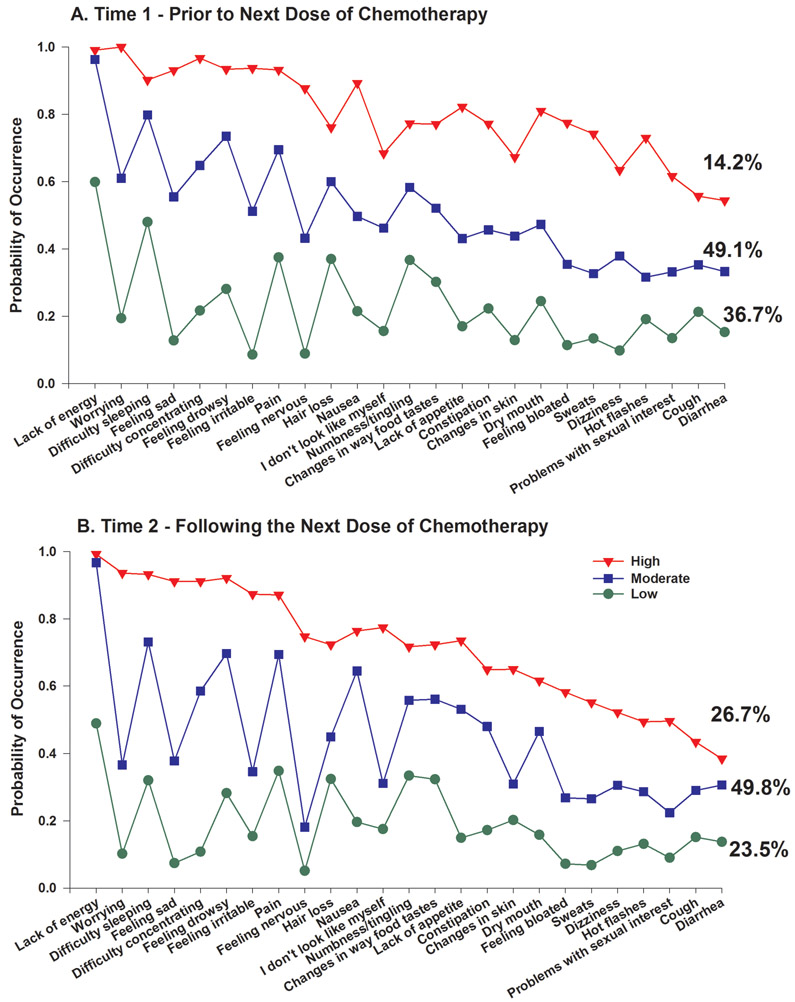
A total of 906 patients completed the MSAS at Time 1 and Time 2. A separate LCA was done for each time point. As shown in Table 1, a three-class solution fit the data best at each time point. For both time points, the BIC was lower for the 3-class compared to the 2-class solution and the VLMR was significant. While the BIC for the 4-class solution was smaller, the VLMR was not significant. These consistent results for the two time points suggested an upper limit to the number of classes that might be found over the two assessments with the LTA. The probability of occurrence of the 25 MSAS symptoms for the three class solutions for the Time 1 and Time 2 assessments are illustrated in Figures 1A and 1B, respectively.
Table 1 –
Latent Class Solutions and Fit Indices for the Time 1 and Time 2 Assessments Using Symptom Occurrence Ratingsa
| Time 1 Assessment – Prior to Next Dose of Chemotherapy | |||||
|---|---|---|---|---|---|
| Model | LL | AIC | BIC | VLMR | Entropy |
| 2 Class | −13465.34 | 27032.68 | 27277.55 | 2375.10**** | .84 |
| 3 Classb | −13217.76 | 26589.51 | 26959.21 | 495.17**** | .83 |
| 4 Class | −13084.89 | 26375.78 | 26870.31 | 265.73ns | .80 |
| Time 2 Assessment – Following the Next Dose of Chemotherapy | |||||
| 2 Class | −13286.83 | 26675.67 | 26920.53 | 2320.59**** | .83 |
| 3 Classb | −13021.47 | 26196.94 | 26566.64 | 530.73**** | .81 |
| 4 Class | −12892.35 | 25990.71 | 26485.24 | 258.23ns | .79 |
Not significant
p < .05
p < .01
p < .001
p < .0001
In order to have a sufficient number of patients with each symptom to perform the latent class analyses, the MSAS symptoms that occurred in at least 40% of the patients were identified. This criterion was selected to provide assurance that sufficient information was available to identify classes that were not sample-specific, due to infrequent reports of symptoms. A total of 25 out of 32 symptoms from the MSAS occurred in >40% of the patients.
The 3-class solution was selected because the VLMR was significant for the 3-class solution, indicating that three classes fit the data better than two classes, and the VLMR was not significant for the 4-class solution, indicating that too many classes had been extracted.
Note. LL = log-likelihood; AIC = Akaike Information Criterion, BIC = Bayesian Information Criterion; VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test for the K vs. K-1 model.
Figure 1.
A – Probability of symptom occurrence for each of the latent classes for the 25 symptoms on the Memorial Symptom Assessment Scale that occurred in ≥40% of the total sample (n=760) at Time 1 (i.e., Prior to next dose of chemotherapy).
B - Probability of symptom occurrence for each of the latent classes for the 25 symptoms on the Memorial Symptom Assessment Scale that occurred in ≥40% of the total sample (n=760) at Time 2 (i.e., Following next dose of chemotherapy).
Latent Transition Analysis
First, the LTA models were fit with two classes at each of the two assessments. Then the models were fit with three classes at each of the two time points. To ensure that the LTA classes at the two times were comparable, the LTA models were fit assuming measurement invariance for latent class indicators at each time.
As shown in Table 2, the BIC was smaller for the 3-to-3-class LTA and entropy remained above .80. As shown in Table 3, an LTA solution with 3 classes at each assessment produces nine classes in the joint distribution. Clearly, an inspection of Table 3 shows that the proportion of cases in the off-diagonal classes is much smaller than for the classes on the diagonal and that five of the off-diagonal classes consist of less than 3% of the sample. Therefore, we did not estimate models beyond three classes at each assessment.
Table 2 -.
Latent Transition Solutions and Fit Indices for Two-to-Two and Three-to-Three Classes Using Symptom Occurrence Ratingsa for Time 1 to Time 2
| Model | LL | AIC | BIC | Entropy |
|---|---|---|---|---|
| 2 classes | −26645.54 | 53397.07 | 53651.95 | .86 |
| 3 classesb | −26073.41 | 52312.82 | 52711.97 | .85 |
In order to have a sufficient number of patients with each symptom to perform the latent class analyses, the MSAS symptoms that occurred in at least 40% of the patients were identified. This criterion was selected to provide assurance that sufficient information was available to identify classes that were not sample-specific, due to infrequent reports of symptoms. A total of 25 out of 32 symptoms from the MSAS occurred in >40% of the patients.
The 3-to-3-class solution was selected because the BIC was smaller than the 2-to-2-class solution.
Note. LL = log-likelihood; AIC = Akaike Information Criterion, BIC = Bayesian Information Criterion.
Table 3 -.
Latent Transition Class Counts and Proportions for Three-to-Three Classes Using Symptom Occurrence Ratings for Time 1 to Time 2
| A. Three-Class to Three-Class Solution | |||
|---|---|---|---|
| Time | Class | Count | Proportion |
| 1 | 1 | 264.73 | 0.292 |
| 2 | 374.81 | 0.414 | |
| 3 | 266.45 | 0.294 | |
| 2 | 1 | 251.18 | 0.277 |
| 2 | 434.76 | 0.480 | |
| 3 | 220.06 | 0.243 | |
| B. Three-Class to Three-Class Pattern | |||
|---|---|---|---|
| T1 Class | T2 Class | Count | Proportion |
| 1 | 1 | 224.39 | 0.248 |
| 1 | 2 | 35.33 | 0.039 |
| 1 | 3 | 5.01 | 0.006 |
| 2 | 1 | 22.77 | 0.025 |
| 2 | 2 | 318.08 | 0.351 |
| 2 | 3 | 33.97 | 0.037 |
| 3 | 1 | 4.02 | 0.004 |
| 3 | 2 | 81.34 | 0.090 |
| 3 | 3 | 181.08 | 0.200 |
| C. Actual Classification of Patients Based on Their Most Likely Latent Class Pattern from Time 1 to Time 2 | ||||
|---|---|---|---|---|
| Class | Latent Class Pattern |
Number of Patients |
Percentage of Patients |
Class Names |
| 1 | 1 - 1 | 235 | 25.94 | High-High |
| 2 | 1 – 2 | 25 | 2.76 | High-Moderate |
| 3 | 1 - 3 | 3 | 0.33 | High-Low |
| 4 | 2 – 1 | 13 | 1.43 | Moderate-High |
| 5 | 2 – 2 | 335 | 36.98 | Moderate-Moderate |
| 6 | 2 – 3 | 27 | 2.98 | Moderate-Low |
| 7 | 3 – 1 | 3 | 0.33 | Low-High |
| 8 | 3 – 2 | 75 | 8.28 | Low-Moderate |
| 9 | 3 - 3 | 190 | 20.97 | Low-Low |
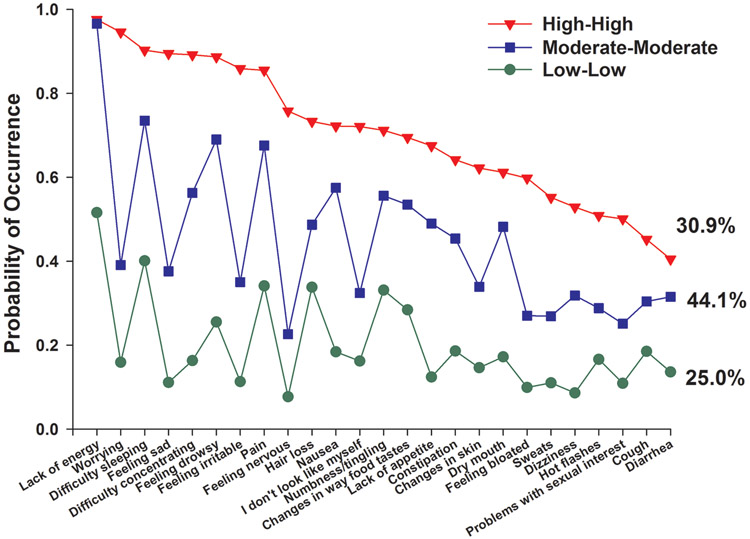
As shown in Table 3C, of the 906 patients who had evaluable data at Time 1 and Time 2, 760 (83.9%) were classified in the same symptom occurrence classes at both assessments. Of these 760 patients, based on the relative occurrence rates for the 25 MSAS symptoms across the latent classes, 25.0% were classified as Low-Low, 44.1% as Moderate-Moderate, and 30.9% as High-High ( Figure 2).
Figure 2.
Probability of symptom occurrence for each of the latent transition classes for the 25 symptoms on the Memorial Symptom Assessment Scale that occurred in ≥40% of the total sample (n=760).
Differences in Patient Characteristics Among the Three Consistent LTA Classes
Table 4 summarizes the differences in demographic and clinical characteristics among the three consistent LTA classes. Compared to the Low-Low class, patients in the High-High and Moderate-Moderate classes were significantly younger, more likely to be female, more likely to report having child care responsibilities, and had a lower KPS score and a higher comorbidity score. In terms of specific comorbid conditions, compared to the Low-Low class, a higher percentage of patients in the High-High class reported the occurrence of anemia, depression, and back pain. With the exception of the KPS and comorbidity scores, as well as cancer diagnosis, none of the other clinical characteristics (i.e., time since diagnosis, types and number of prior treatments, presence or number of metastatic sites) differed among the LTA classes. For cancer diagnosis, pairwise contrasts found that compared to the High-High class, a higher percentage of patients in the Low-Low class had a GI cancer. Patients in the High-High class reported the occurrence of a significantly higher number of MSAS symptoms (19.3 ± 4.2) than patients in the Moderate class (12.4 ± 3.0). Patients in the Moderate-Moderate class reported a significantly higher number of symptoms than patients in the Low-Low class (5.5 ± 2.7).
Table 4 –
Differences in Demographic and Clinical Characteristics Among the Three Latent Transition Analysis Classes (n=760)
| Characteristic | Low-Low (1) n=190 25.0% |
Moderate-Moderate (2) n=335 44.1% |
High-High (3) n=235 30.9% |
Statistics |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 61.4 (10.6) | 57.0 (11.9) | 54.3 (12.1) | F=19.9, p<.0001 1 > 2 > 3 |
| Education (years) | 15.7 (3.2) | 16.5 (2.8) | 16.1 (3.0) | F=4.34, p=.013 1 < 2 |
| Body mass index (kg/m2) | 26.1 (5.6) | 26.3 (5.8) | 25.9 (5.6) | F=0.30, p=.741 |
| Karnofsky Performance Status score | 87.4 (9.7) | 79.7 (11.8) | 74.8 (12.0) | F=58.25, p<.0001 1 > 2 > 3 |
| Self-administered Comorbidity Questionnaire score | 4.5 (2.5) | 5.5 (3.1) | 6.4 (3.3) | F=19.85, p<.0001 1 < 2 < 3 |
| Time since diagnosis (mean in years) | 1.9 (3.2) | 2.1 (3.7) | 2.1 (4.1) | KW, p=.871 |
| Time since diagnosis (median in years) | 0.44 | 0.44 | 0.44 | |
| Number of prior cancer treatments | 1.6 (1.6) | 1.7 (1.5) | 1.8 (1.5) | F=1.34, p=.263 |
| Number of metastatic sites including lymph node involvementa | 1.3 (1.2) | 1.2 (1.2) | 1.2 (1.2) | F=0.42, p=.656 |
| Number of metastatic sites excluding lymph node involvement | 0.9 (1.1) | 0.8 (1.1) | 0.8 (1.0) | F=0.74, p=.477 |
| Mean number of MSAS symptoms (out of 32) | 5.5 (2.7) | 12.4 (3.0) | 19.3 (4.2) | F=880.63, p<.0001 1 < 2 < 3 |
| % (n) | % (n) | % (n) | ||
| Gender (% female) | 68.9 (131) | 78.2 (262) | 89.8 (211) | X2=31.96, p<.0001 1 < 2 < 3 |
| Self-reported ethnicity | ||||
| White | 71.0 (130) | 72.9 (240) | 68.4 (158) | X2=1.37, p=.505 |
| Non-white | 29.0 (53) | 27.1 (89) | 31.6 (73) | |
| Married or partnered (% yes) | 70.1 (131) | 67.1 (222) | 62.7 (146) | X2=2.64, p=.266 |
| Lives alone (% yes) | 17.0 (32) | 19.9 (66) | 23.9 (56) | X2=3.16, p=.206 |
| Currently employed (% yes) | 37.2 (70) | 37.4 (125) | 28.6 (67) | X2=5.43, p=.066 |
| Annual household income | KW=9.47, p=.230 | |||
| Less than $30,000 | 16.7 (27) | 15.9 (49) | 22.0 (46) | |
| $30,000 to $70,000 | 24.1 (39) | 22.7 (70) | 18.7 (39) | |
| $70,000 to $100,000 | 17.9 (29) | 13.6 (42) | 19.6 (41) | |
| Greater than $100,000 | 41.4 (67) | 47.9 (148) | 39.7 (83) | |
| Child care responsibilities (% yes) | 15.7 (29) | 24.0 (79) | 29.1 (67) | X2=10.40, p=.006 1 < 2 and 3 |
| Elder care responsibilities (% yes) | 6.9 (12) | 9.9 (30) | 7.0 (15) | X2=1.95, p=.378 |
| Common comorbidities (% yes) | ||||
| Heart disease | 4.7 (9) | 5.7 (19) | 4.3 (10) | X2=0.62, p=.733 |
| High blood pressure | 34.7 (66) | 31.9 (107) | 27.2 (64) | X2=2.92, p=.233 |
| Lung disease | 12.6 (24) | 11.0 (37) | 11.9 (28) | X2=0.31, p=.857 |
| Diabetes | 8.4 (16) | 9.3 (31) | 7.7 (18) | X2=0.45, p=.797 |
| Ulcer or stomach disease | 2.6 (5) | 4.2 (14) | 6.4 (15) | X2=3.58, p=.167 |
| Kidney disease | 0.0 (0) | 0.9 (3) | 1.7 (4) | X2=3.34, p=.188 |
| Liver disease | 7.4 (14) | 3.3 (11) | 7.7 (18) | X2=6.34, p=.042 No significant pw contrasts |
| Anemia | 6.3 (12) | 13.1 (44) | 18.3 (43) | X2=13.32, p=.001 1<3 |
| Depression | 5.8 (11) | 14.9 (50) | 39.1 (92) | X2=82.81, p<.0001 1<2<3 |
| Osteoarthritis | 10.5 (20) | 9.6 (32) | 16.2 (38) | X2=6.214, p=.045 No significant pw contrasts |
| Back pain | 15.8 (30) | 24.8 (83) | 37.4 (88) | X2=26.19, p<.0001 1 and 2 <3 |
| Rheumatoid arthritis | 3.2 (6) | 3.9 (13) | 3.4 (8) | X2=0.21, p=.902 |
| Cancer diagnosis | X2=23.36, p=.001 | |||
| Breast cancer | 34.7 (66) | 41.5 (139 | 46.0 (108 | 1>3 |
| Gastrointestinal cancer | 35.8 (68) | 27.5 (92) | 21.3 (50) | |
| Gynecological cancer | 14.7 (28) | 16.7 (56) | 24.7 (58) | |
| Lung cancer | 14.7 (28) | 14.3 (48) | 8.1 (19) | |
| Prior cancer treatment | X2=11.25, p=.081 | |||
| No prior treatment | 27.6 (51) | 23.4 (77) | 15.9 (37) | |
| Only surgery, CTX, or RT | 35.1 (65) | 41.6 (137) | 45.9 (107) | |
| Surgery and CTX, or surgery and RT, or CTX and RT | 23.8 (44) | 20.1 (66) | 21.5 (50) | |
| Surgery and CTX and RT | 13.5 (25) | 14.9 (49) | 26.7 (39) | |
| Metastatic sites | X2=6.33, p=.387 | |||
| No metastasis | 29.1 (55) | 33.3 (111) | 36.1 (84) | |
| Only lymph node metastasis | 20.1 (38) | 23.1 (77) | 21.0 (49) | |
| Only metastatic disease in other sites | 28.6 (54) | 23.7 (79) | 19.7 (46) | |
| Metastatic disease in lymph nodes and other sites | 22.2 (42) | 19.8 (66) | 23.2 (54) | |
Total number of metastatic sites evaluated was 9.
Abbreviations: CTX = chemotherapy, kg = kilograms, KW = Kruskal Wallis, m2 = meters squared, pw= pair-wise, RT = radiation therapy, SD = standard deviation
Differences in Quality of Life Scores Among the Latent Classes
As shown in Table 5, except for the spiritual well-being subscale, post hoc contrasts revealed that patients in the High-High class reported significantly lower scores on the QOL-PV subscale and total scores than patients in the Moderate-Moderate class. Patients in the Moderate-Moderate class reported significantly lower QOL-PV scores than patients in the Low-Low class.
Table 5 –
Differences in Disease Specific and Generic Quality of Life Scores Among the Three Latent Transition Analysis Classes (n=760)
| Characteristic | Low-Low (1) n=190 25.0% |
Moderate- Moderate (2) n=335 44.1% |
High-High (3) n=235 30.9% |
Statistics |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| MULTIDIMENSIONAL QUALITY OF LIFE SCALE | ||||
| Physical well-being | 8.1 (1.3) | 6.6 (1.6) | 5.5 (1.7) | F=153.26, p < .001 1 > 2 > 3 |
| Psychological well-being | 6.9 (1.5) | 5.7 (1.7) | 4.1 (1.5) | F=170.35 p < .001 1 > 2 > 3 |
| Social well-being | 7.1 (1.7) | 5.8 (1.8) | 4.4 (1.7) | F=119.97, p<.0001 1 > 2 > 3 |
| Spiritual well-being | 5.4 (2.2) | 5.3 (2.1) | 5.2 (2.1) | F=0.58, p = .562 |
| Total QOL score | 6.9 (1.2) | 5.8 (1.3) | 4.6 (1.2) | F=183.88 p < .001 1 > 2 > 3 |
| SF12 SCORES | ||||
| Physical functioning | 65.6 (34.0) | 48.0 (32.8) | 42.3 (32.5) | F=25.89, p < .001 1 > 2 and 3 |
| Role physical | 70.6 (26.2) | 51.1 (27.6) | 40.5 (26.8) | F=63.54, p < .001 1 > 2 > 3 |
| Bodily pain | 91.3 (17.9) | 76.4 (25.9) | 64.1 (29.2) | F=58.64, p<.0001 1 > 2 > 3 |
| General health | 73.1 (22.6) | 64.1 (27.3) | 51.9 (28.8) | F=32.58, p < .001 1 > 2 > 3 |
| Vitality | 62.9 (22.6) | 42.0 (24.5) | 35.0 (24.8) | F=72.61, p < .001 1 > 2 > 3 |
| Social functioning | 85.9 (21.8) | 67.1 (29.5) | 52.1 (30.3) | F=73.36, p<.0001 1 > 2 > 3 |
| Role emotional | 87.7 (22.0) | 78.2 (25.5) | 62.8 (27.8) | F=50.89, p < .001 1 > 2 > 3 |
| Mental health | 83.9 (16.9) | 74.6 (18.2) | 57.7 (20.2) | F=108.83, p < .001 1 > 2 > 3 |
| Physical Component Summary score | 46.2 (9.2) | 40.0 (10.3) | 38.3 (10.0) | F=33.07, p<.0001 1 > 2 and 3 |
| Mental Component Summary score | 55.3 (8.6) | 50.2 (9.4) | 42.5 (9.8) | F=92.45, p<.0001 1 > 2 > 3 |
Abbreviations: SD = standard deviation
In terms of the SF-12 subscale and physical component summary (PCS) and mental component summary (MCS) scores, except for the physical functioning and PCS scores, post hoc contrasts revealed the same pattern of between group differences in QOL scores (i.e., High-High < Moderate-Moderate < Low-Low). For the physical functioning and PCS scores, the pattern of between class differences was Moderate-Moderate class and High-High class < Low-Low class.
DISCUSSION
To our knowledge, this study is the first to use LTA to evaluate for a stable symptom phenotype based on patients’ experiences with 25 common symptoms before and following a dose of CTX. Consistent with our previous report that used LCA to evaluate 582 patients in the current sample prior to their next dose of CTX (Miaskowski et al., 2014a), three distinct subgroups of patients with consistent symptom experiences were identified by LTA. In addition, the mean number of symptoms reported by each latent class is relatively consistent across both studies. While in the previous study, as well as in the Time 1 LCA done with this larger sample (Figure 1A), approximately 14% of the patients were categorized in the All High class, in the current LTA study, using both the pretreatment and post-treatment assessments, 30.9% of the patients were categorized in the High-High class (Figure 2). This finding suggests that two or more assessments may be warranted to categorize those patients who are in a “stable” lower or higher risk symptom phenotype. In addition, it should be noted that 75% of the patients in this study experienced moderate to high occurrence rates for 25 common symptoms from prior to through the first week following their dose of CTX.
As shown in Table 2, for 83.9% of the patients (n=760), their most likely latent class pattern remained the same from Time 1 to Time 2. In terms of patients who transitioned from a lower to a higher symptom class, 9.7% went up one class and 0.3% went up two classes. In terms of patients whose transition pattern was the opposite, 5.7% went down one class and 0.3% went down two classes. Given the small sample sizes for each of these groups, one cannot readily evaluate the exact reasons for these different patterns of transition.
As shown in Table 6, the six symptoms that were among the top eleven in occurrence rates across the three LTA classes were lack of energy (0.512 to 0.976), difficulty sleeping (0.401 to 0.946), pain (0.341 to 0.855), hair loss (0.338 to 0.733), feeling drowsy (0.255 to 0.877), and nausea (0.184 to 0.727). Of note, lack of energy, difficulty sleeping, anxiety, and pain are symptoms commonly associated with cytokine-induced sickness behavior (Illi et al., 2012, Wang et al., 2010, Myers, 2008, Dantzer et al., 2008). However, while depression is often reported to be a symptom associated with sickness behavior (Walker et al., 2014, Harrison et al., 2009, Dantzer, 2006), it (i.e., feeling sad on the MSAS) was only found in the top eleven occurring symptoms in patients in the High-High class. In fact, in the High-High class, worrying (0.946), feeling irritable (0.895), and feeling nervous (0.758) had very high occurrence rates. None of the four psychological symptoms were found in the top 11 occurring symptoms in the other two LTA classes. This finding suggests that in addition to interventions to treat physical symptoms, patients in the High-High class require more in depth mental health evaluation and more proactive and aggressive management of their psychological symptoms. This approach is warranted given the substantial body of evidence that has documented the negative long-term sequelae of ongoing and high levels of psychological distress in cancer patients (Stanton et al., 2015).
Table 6 –
Probability of Occurrence for the 25 MSAS Symptoms For Each of the Three Latent Transition Class in Descending Order of Occurrence
| Rank Order |
High-High | P | Moderate-Moderate | P | Low-Low | P |
|---|---|---|---|---|---|---|
| 1 | Lack of energy | .976 | Lack of energy | .966 | Lack of energy | .512 |
| 2 | Worrying | .946 | Difficulty sleeping | .735 | Difficulty sleeping | .401 |
| 3 | Difficulty sleeping | .903 | Feeling drowsy | .690 | Pain | .341 |
| 4 | Feeling sad | .895 | Pain | .676 | Hair loss | .338 |
| 5 | Difficulty concentrating | .892 | Nausea | .575 | Numbness and tingling in hands/feet | .331 |
| 6 | Feeling drowsy | .887 | Difficulty concentrating | .563 | Changes in the way food tastes | .284 |
| 7 | Feeling irritable | .859 | Numbness and tingling in hands/feet | .556 | Feeling drowsy | .255 |
| 8 | Pain | .855 | Changes in the way food tastes | .535 | Constipation | .186 |
| 9 | Feeling nervous | .758 | Lack of appetite | .490 | Cough | .185 |
| 10 | Hair loss | .733 | Hair loss | .487 | Nausea | .184 |
| 11 | Nausea | .727 | Dry mouth | .482 | Dry mouth | .172 |
| 12 | I don’t look like myself | .721 | Constipation | .454 | Hot flashes | .166 |
| 13 | Numbness and tingling in hands/feet | .712 | Worrying | .391 | Difficulty concentrating | .163 |
| 14 | Changes in the way food tastes | .695 | Feeling sad | .376 | I don’t look like myself | .162 |
| 15 | Lack of appetite | .675 | Dizziness | .368 | Worrying | .159 |
| 16 | Constipation | .642 | Feeling irritable | .350 | Changes in skin | .146 |
| 17 | Changes in skin | .622 | Changes in skin | .339 | Diarrhea | .136 |
| 18 | Dry mouth | .612 | I don’t look like myself | .324 | Lack of appetite | .124 |
| 19 | Feeling bloated | .598 | Diarrhea | .315 | Feeling irritable | .113 |
| 20 | Sweats | .552 | Cough | .304 | Feeling sad | .111 |
| 21 | Dizziness | .529 | Hot flashes | .288 | Sweats | .110 |
| 22 | Hot flashes | .509 | Feeling bloated | .270 | Problems with sexual interest | .109 |
| 23 | Problems with sexual interest | .501 | Sweats | .269 | Feeling bloated | .099 |
| 24 | Cough | .452 | Problems with sexual interest | .251 | Dizziness | .086 |
| 25 | Diarrhea | .405 | Feeling nervous | .226 | Feeling nervous | .077 |
Consistent with our previous report in the same sample (Miaskowski et al., 2014a), as well as reports by others (Gwede et al., 2008, Ferreira et al., 2008), KPS and SCQ scores were associated with LTA class membership. While associations between a higher symptom burden and a higher level of comorbidity, as well as poorer functional status, are reported consistently in oncology patients (Pud et al., 2008, Dodd et al., 2011, Dodd et al., 2010, Miaskowski et al., 2006, Ferreira et al., 2008, Gwede et al., 2008), additional research is warranted to further explicate these relationships. For example, the most common comorbid conditions in this sample were high blood pressure (31.2%), back pain (26.4%), and depression (20.1%). Many of the chronic conditions listed in Table 4 are associated with both acute and chronic symptoms. Therefore, future studies need to assess the impact of the symptoms associated with cancer and its treatment, as well as the symptoms associated with other chronic conditions, on latent class membership. In addition, future longitudinal studies need to evaluate, using statistical procedures like parallel process growth modeling (Cheong et al., 2003, Rose et al., 2009), whether increases in symptom burden are associated with decreases in functional status or vice versa. Similar approaches could be used to evaluate for changes in patients’ symptom burden in relationship to changes in their comorbidity profiles.
While the majority of the characteristics associated with cancer and its treatment did not predict LTA class membership, compared to the High-High class, a relatively higher percentage of patients with GI cancer were in the Low-Low class. The exact reasons for this difference are not readily apparent and warrant investigation in future studies.
Compared to the Low-Low class, patients in the Moderate-Moderate and High-High classes were almost a half or a whole decade younger, respectively. While the association between younger age and higher symptom burden is reported in previous studies (Illi et al., 2012, Miaskowski et al., 2014a, Cataldo et al., 2013, Ritchie et al., 2014), the underlying physiologic and psychological mechanisms for this association remain to be determined. However, because recent evidence suggests that an overlap exists between molecular mechanisms that govern both aging and cancer (Coppede, 2013, Kong et al., 2013, Menck and Munford, 2014, Teschendorff et al., 2013), patients with cancer may experience “premature biological aging” that is associated with a higher symptom burden. Alternatively, “chronologically” older patients may receive lower doses of CTX (Townsley et al., 2005, Kumar et al., 2007) or have a “response shift” in their perception of symptoms (Sprangers and Schwartz, 1999, Schwartz and Sprangers, 1999).
While female gender, years of education, and child care responsibilities were associated with a higher symptom burden in the current study, findings regarding these characteristics are inconsistent across studies (Dodd et al., 2011, Illi et al., 2012, Miaskowski et al., 2014b, Baldwin et al., 2010, Cheung et al., 2011, Miaskowski, 2004). In addition, while other studies found that being a member of an ethnic minority and reporting a lower socioeconomic status were associated with more severe symptoms (Miaskowski et al., 2014a), these associations were not found in the current study. Additional research is warranted to confirm or refute these inconstant findings.
For both the generic (SF-12) and disease-specific (MQOLS-PV) measures of QOL, as symptom burden increased, QOL decreased. The decrements in QOL among the three latent classes represent not only statistically significant, but clinically meaningful decreases in QOL with effect sizes ranging from 0.44 to 1.54 (Sloan et al., 2003, Osoba, 1999). Taken together and consistent with previous reports (Illi et al., 2012, Miaskowski et al., 2014a, Ferreira et al., 2008, Gwede et al., 2008), these findings provide evidence of the significant negative impact that multiple co-occurring symptoms have on patients’ ability to function and other QOL outcomes.
Several study limitations need to be acknowledged. Because patients were recruited at different points in their CTX treatment, symptom occurrence rates prior to the initiation of CTX are not available. In addition, the CTX drugs used varied based on the patients’ diagnoses and stages of disease. While we cannot rule out the potential contributions of these clinical characteristics to the patients’ symptom experiences, the relatively similar percentages of cancer diagnoses, evidence of metastatic disease, time since cancer diagnosis, and types of previous treatments suggest that the three LTA classes were relatively similar in terms of disease and treatment characteristics. While it is possible that patients in the Low-Low class were receiving more aggressive symptom management interventions, the occurrence rates for fatigue, sleep disturbance, and pain were high across the three LTA classes.
In the era of precision medicine (National Research Council, 2011) and big data (Yoo et al., 2014), coupled with the use of electronic medical records and smart phone technology (e.g. the ASyMS/eSMART© system that is being evaluated as part of a grant from the European Commission (Kearney et al., 2009, Maguire et al., 2015)), it is conceivable that symptom data will be collected in “real time” from oncology patients receiving CTX. The use of analytic approaches like LTA, or the development of more sophisticated algorithms using techniques like machine learning (Bastanlar and Ozuysal, 2014, Yoo et al., 2014), will allow clinicians to analyze patients’ phenotypic and molecular data on an ongoing basis. The integration of these types of information across multiple patients will assist clinicians to identify patients at highest risk for the most severe symptom profiles and to pre-emptively or more aggressively treat their most common and severe symptoms. This type of risk profiling and aggressive symptom management should reduce oncology patients’ symptom burden and improve their QOL.
Acknowledgements:
This study was funded by the National Cancer Institute (CA134900). In addition, this project received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement number 602289.
Contributor Information
Christine Miaskowski, School of Nursing, University of California, San Francisco, CA 94143.
Bruce A. Cooper, School of Nursing, University of California, San Francisco, CA 94143.
Bradley Aouizerat, School of Nursing, University of California, San Francisco, CA 94143.
Michelle Melisko, School of Medicine, University of California, San Francisco, CA 94143.
Lee-May Chen, School of Medicine, University of California, San Francisco, CA 94143.
Laura Dunn, School of Medicine, University of California, San Francisco, CA 94143.
Xiao Hu, School of Nursing, University of California, San Francisco, CA 94143.
Judy Mastick, School of Nursing, University of California, San Francisco, CA 94143.
Jon D. Levine, School of Medicine, University of California, San Francisco, CA 94143.
Marilyn Hammer, New York University College of Nursing, New York, NY 10010.
Fay Wright, New York University College of Nursing, New York, NY 10010.
Jenny Harris, Florence Nightingale Faculty of Nursing and Midwifery, King’s College London, London, England.
Jo Armes, Florence Nightingale Faculty of Nursing and Midwifery, King’s College London, London, England.
Eileen Furlong, School of Nursing, Midwifery, and Health Systems, University College Dublin, Dublin, Ireland.
Patricia Fox, School of Nursing, Midwifery, and Health Systems, University College Dublin, Dublin, Ireland.
Emma Ream, School of Health Sciences, University of Surrey, Guilford, England.
Roma Maguire, School of Health Sciences, University of Surrey, Guilford, England.
Nora Kearney, School of Health Sciences, University of Surrey, Guilford, England.
REFERENCES
- Baldwin CM, Ervin AM, Mays MZ, Robbins J, Shafazand S, Walsleben J & Weaver T (2010) Sleep disturbances, quality of life, and ethnicity: the sleep heart health study. Journal of Clinical Sleep Medicine 6, 176–183. [PMC free article] [PubMed] [Google Scholar]
- Bastanlar Y & Ozuysal M (2014) Introduction to machine learning. Methods in Molecular Biology 1107, 105–128. [DOI] [PubMed] [Google Scholar]
- Cataldo JK, Paul S, Cooper B, Skerman H, Alexander K, Aouizerat B, Blackman V, Merriman J, Dunn L, Ritchie C, Yates P & Miaskowski C (2013) Differences in the symptom experience of older versus younger oncology outpatients: a cross-sectional study. BMC Cancer 13, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeux G & Soromenho G (1996) An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification 13, 195–212. [Google Scholar]
- Cheong J, Mackinnon DP & Khoo ST (2003) Investigation of mediational processes using parallel process latent growth curve modeling. Structural Equation Modeling-A Multidisciplinary Journal 10, 238–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WY, Le LW, Gagliese L & Zimmermann C (2011) Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Supportive Care in Cancer 19, 417–423. [DOI] [PubMed] [Google Scholar]
- Collins LM & Lanza ST (2010) Latent class and latent transition analysis: with applications in the Social, Behavioral, and Health Science, John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- Coppede F (2013) The epidemiology of premature aging and associated comorbidities. Clinical Interventions in Aging 8, 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R (2006) Cytokine, sickness behavior, and depression. Neurology Clinics 24, 441–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'connor JC, Freund GG, Johnson RW & Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd MJ, Cho MH, Cooper BA & Miaskowski C (2010) The effect of symptom clusters on functional status and quality of life in women with breast cancer. European Journal of Oncology Nursing 14, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd MJ, Cho MH, Cooper BA, Petersen J, Bank KA, Lee KA & Miaskowski C (2011) Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncology Nursing Forum 38, 33–42. [DOI] [PubMed] [Google Scholar]
- Dow KH, Ferrell BR, Leigh S, Ly J & Gulasekaram P (1996) An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Research and Treatment 39, 261–273. [DOI] [PubMed] [Google Scholar]
- Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T & Miaskowski C (2009) A review of the prevalence and impact of multiple symptoms in oncology patients. Journal of Pain and Symptom Management 37, 715–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira KA, Kimura M, Teixeira MJ, Mendoza TR, Da Nobrega JC, Graziani SR & Takagaki TY (2008) Impact of cancer-related symptom synergisms on health-related quality of life and performance status. Journal of Pain and Symptom Management 35, 604–616. [DOI] [PubMed] [Google Scholar]
- Ferrell BR, Dow KH & Grant M (1995) Measurement of the quality of life in cancer survivors. Quality of Life Research 4, 523–531. [DOI] [PubMed] [Google Scholar]
- Gwede CK, Small BJ, Munster PN, Andrykowski MA & Jacobsen PB (2008) Exploring the differential experience of breast cancer treatment-related symptoms: a cluster analytic approach. Supportive Care in Cancer 16, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A & Critchley HD (2009) Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry 66, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, Dodd M, Dhruva A, Paul SM, Baggott C, Cataldo J, Langford D, Schmidt B & Aouizerat BE (2012) Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine 58, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky D (1977) Performance scale, Plenum Press, New York. [Google Scholar]
- Kearney N, Mccann L, Norrie J, Taylor L, Gray P, Mcgee-Lennon M, Sage M, Miller M & Maguire R (2009) Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Supportive Care in Cancer 17, 437–444. [DOI] [PubMed] [Google Scholar]
- Kong CM, Lee XW & Wang X (2013) Telomere shortening in human diseases. FEBS Journal 280, 3180–3193. [DOI] [PubMed] [Google Scholar]
- Kumar A, Soares HP, Balducci L, Djulbegovic B & National Cancer I (2007) Treatment tolerance and efficacy in geriatric oncology: a systematic review of phase III randomized trials conducted by five National Cancer Institute-sponsored cooperative groups. Journal of Clinical Oncology 25, 1272–1276. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Flaherty BP & Collins LM (2003) Latent class and latent transition analysis In: Handbook of Psychology: Research Methods in Psychology, (eds SCHINKA JA & VELICER WF), pp. 663–685. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- Maguire R, Ream E, Richardson A, Connaghan J, Johnston B, Kotronoulas G, Pedersen V, Mcphelim J, Pattison N, Smith A, Webster L, Taylor A & Kearney N (2015) Development of a novel remote patient monitoring system: the advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nursing 38, E37–47. [DOI] [PubMed] [Google Scholar]
- Menck CF & Munford V (2014) DNA repair diseases: What do they tell us about cancer and aging? Genetic and Molecular Biology 37, 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C (2004) Gender differences in pain, fatigue, and depression in patients with cancer. Journal of the National Cancer Institute 32, 139–143. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Melisko M, Chen LM, Mastick J, West C, Paul SM, Dunn LB, Schmidt BL, Hammer M, Cartwright F, Wright F, Langford DJ, Lee K & Aouizerat BE (2014a) Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer 120, 2371–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, West C, Cho M & Bank A (2006) Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncology Nursing Forum 33, E79–89. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Dunn L, Ritchie C, Paul SM, Cooper B, Aouizerat BE, Alexander K, Skerman H & Yates P (In press) Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. Journal of Pain and Symptom Management. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Paul SM, Cooper B, West C, Levine JD, Elboim C, Hamolsky D, Abrams G, Luce J, Dhruva A, Langford DJ, Merriman JD, Kober K, Baggott C, Leutwyler H & Aouizerat BE (2014b) Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. European Journal of Oncology Nursing 18, 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B & Shedden K (1999) Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics 55, 463–469. [DOI] [PubMed] [Google Scholar]
- Muthen LK & Muthen BO (1998-2014) Mplus User's Guide (7th ed.), 7th edn, Muthen & Muthen, Los Angeles, CA. [Google Scholar]
- Myers JS (2008) Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncology Nursing Forum 35, 802–807. [DOI] [PubMed] [Google Scholar]
- National Research Council. (2011) Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease, The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Nylund K, Bellmore A, Nishina A & Graham S (2007a) Subtypes, severity, and structural stability of peer victimization: what does latent class analysis say? Child Development 78, 1706–1722. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T & Muthen BO (2007b) Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling 14, 535–569. [Google Scholar]
- Osoba D (1999) Interpreting the meaningfulness of changes in health-related quality of life scores: lessons from studies in adults. International Journal of Cancer 12, 132–137. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L & et al. (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer 30A, 1326–1336. [DOI] [PubMed] [Google Scholar]
- Pud D, Ben Ami S, Cooper BA, Aouizerat BE, Cohen D, Radiano R, Naveh P, Nikkhou-Abeles R, Hagbi V, Kachta O, Yaffe A & Miaskowski C (2008) The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. Journal of Pain and Symptom Management 35, 162–170. [DOI] [PubMed] [Google Scholar]
- Ritchie C, Dunn LB, Paul SM, Cooper BA, Skerman H, Merriman JD, Aouizerat B, Alexander K, Yates P, Cataldo J & Miaskowski C (2014) Differences in the symptom experience of older oncology outpatients. Journal of Pain and Symptom Management 47, 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Kypriotakis G, Bowman KF, Einstadter D, O'toole EE, Mechekano R & Dawson NV (2009) Patterns of adaptation in patients living long term with advanced cancer. Cancer 115, 4298–4310. [DOI] [PubMed] [Google Scholar]
- Sangha O, Stucki G, Liang MH, Fossel AH & Katz JN (2003) The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism 49, 156–163. [DOI] [PubMed] [Google Scholar]
- Schwartz CE & Sprangers MA (1999) Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Social Science and Medicine 48, 1531–1548. [DOI] [PubMed] [Google Scholar]
- Sloan JA, Cella D, Frost MH, Guyatt G & Osoba D (2003) Quality of life III: translating the science of quality-of-life assessment into clinical practice-an example-driven approach for practicing clinicians and clinical researchers. Clinical Therapeutics 25 Suppl D, D1–5. [DOI] [PubMed] [Google Scholar]
- Sprangers MA & Schwartz CE (1999) The challenge of response shift for quality-of-life-based clinical oncology research. Annal of Oncology 10, 747–749. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Rowland JH & Ganz PA (2015) Life after diagnosis and treatment of cancer in adulthood: Contributions from psychosocial oncology research. American Psychologist 70, 159–174. [DOI] [PubMed] [Google Scholar]
- Teschendorff AE, West J & Beck S (2013) Age-associated epigenetic drift: implications, and a case of epigenetic thrift? Human Molecular Genetics 22, R7–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley C, Pond GR, Peloza B, Kok J, Naidoo K, Dale D, Herbert C, Holowaty E, Straus S & Siu LL (2005) Analysis of treatment practices for elderly cancer patients in Ontario, Canada. Journal of Clinical Oncology 23, 3802–3810. [DOI] [PubMed] [Google Scholar]
- Vermunt JK & Magdison J (2002) Latent class cluster analyses, Cambridge University Press, New York. [Google Scholar]
- Walker AK, Kavelaars A, Heijnen CJ & Dantzer R (2014) Neuroinflammation and comorbidity of pain and depression. Pharmacology Reviews 66, 80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM & Liao Z (2010) Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behavior and Immunity 24, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J Jr., Kosinski M & Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 34, 220–233. [DOI] [PubMed] [Google Scholar]
- Yoo C, Ramirez L & Liuzzi J (2014) Big data analysis using modern statistical and machine learning methods in medicine. International Neurourology Journal 18, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]