Abstract
Relative to males, female rats can show enhanced contextual fear generalization (demonstrating a fear response in a safe or neutral context) dependent on estrogen receptor activation. The current experiment aimed to extend this finding to cued fear conditioning. Females in low-estrogen phases of the estrous cycle showed good discrimination, similar to males, between a conditional stimulus that predicted shock (CS+) and an equally familiar one that did not (CS−), while females in the proestrus (high estrogen) phase demonstrated similar levels of fear between the CS+ and CS−. These results demonstrate that cued fear generalization is similarly influenced by endogenous estrogens.
Forming precise fear-based memories is impaired in several neuropsychiatric diseases (e.g., Giustino et al. 2016). These fear- and anxiety-related disorders, such as generalized anxiety disorder (GAD) and posttraumatic stress disorder (PTSD), constitute the most prevalent class of psychiatric illnesses, with a lifetime occurrence of over 30% in the United States alone (Kessler et al. 2005). Interestingly, these disorders occur more frequently in women than men, even when controlling for external variables (cause/severity of trauma, age, etc.; Kessler et al. 1995, 2012).
Pavlovian conditioning (Pavlov 1927) in rodents provides an ideal method to understand the fundamental differences between males and females in the development of these disorders. In a typical preparation, a neutral conditional stimulus (CS; e.g., a tone) is paired with an aversive unconditional stimulus (UCS; e.g., a shock). Rats will demonstrate a conditional fear response (such as freezing) to this CS during subsequent testing (e.g., Goode et al. 2017). Freezing to a CS that has consistently been paired with a shock (CS+) can be compared to a neutral CS that has never been paired with a shock (CS−). This learning typically results in higher freezing to the CS+ than the CS−, indicating that the animal is successfully discriminating between fearful and safe stimuli (e.g., Ferrara et al. 2017).
Impairments in this type of stimulus discrimination can lead to generalization of fear responding to safe (or novel) stimuli. Stimulus generalization is a hallmark symptom of both PTSD and GAD, which both occur disproportionately in women. Recent work has begun to investigate the potential role for estradiol in context fear generalization (Lynch et al. 2013). For example, Lynch et al. (2014) conditioned animals using a passive avoidance procedure in which the dark side of a two-sided black and white chamber is paired with footshock. They found that ovarectomized female rats injected with estradiol benzoate 1 h before training showed increased contextual generalization of this learning (demonstrated by increased latency to cross from the light to the dark side of the chamber in a neutral context) relative to vehicle-injected controls. Similar work found that naturally cycling female mice display increased contextual fear generalization when compared to males (Keiser et al. 2017). Interestingly, when comparing female subjects across all phases of estrous to males using a cued fear conditioning paradigm, Foilb et al. (2018) found that female rodents showed better discrimination between a CS that predicts shock (CS+) and a CS predictive of nothing (CS−). It is worth noting that they did not track the estrous phase, so the influence of fluctuating hormones is still unclear.
Based on the findings from Lynch et al. (2014, 2016) and others (e.g., Keiser et al. 2017), we hypothesized that high levels of endogenous estrogens (measured using estrous phase as a proxy measure) would correspond with impaired fear discrimination and enhanced generalization using a discrete cue fear discrimination paradigm previously used in our laboratory (Ferrara et al. 2017). In rodents, the estrous cycle is 4–5 d in length and consists of four distinct phases: proestrus, estrous, metestrus, and diestrus (Nelson 2011). Notably, changes in the estrous phase are correlated with fluctuating levels of several steroid hormones, including progesterone and estrogen. A high level of estrogen is primarily characteristic of the proestrus phase (Milad et al. 2009). We, therefore, expected that males and low-estrogen females (in the estrus, metestrus, or diestrus phases) would show good discrimination between the CS+ and CS−, but that high-estrogen females (in proestrus phase) would show similar levels of freezing in the CS+ and the CS− (indicative of fear generalization).
Subjects were male and female Long Evans rats from Envigo (n = 42; Madison, WI). Rats were individually housed with free access to water and rat chow. The animal colony was maintained at a 14:10-h light–dark cycle with all experiments occurring under the light portion of the cycle. All experiments were approved by the UW-Milwaukee Institutional Animal Care and Use Committee.
Female rats were subject to at least 3 d of handling in preparation of vaginal swab collection. Cotton swabs with tips no wider than 2 mm and no longer than 5 mm were autoclaved prior to use. To collect vaginal cytological samples, autoclaved cotton swabs were first soaked in sterile dH2O. Soaked swabs were then gently inserted into the vagina, the vaginal wall swabbed, and the swab gently removed. The cotton tip was then lightly rolled on to a prelabeled slide. Once dry, the estrous phase was identified via light microscopy. To determine if females were naturally cycling, the estrous phase was tracked through at least three complete cycles (12–15 d). The collection of vaginal epithelial samples occurred at the same time each day.
Prior to training, all rats were transported to the room where training would occur via a transportation cart and handled for 2-min each for three consecutive days. Rats were placed in Context A for differential fear auditory conditioning. Context A was comprised of four identical Plexiglas and stainless-steel chambers each housed inside a separate sound-attenuating box. Each outer box was illuminated with a 7.5-W house light and was ventilated with a small fan. The background noise level in each of these outer boxes ranged from 46 to –50 dB. The floors of the Plexiglas chambers in Context A were made of evenly spaced stainless-steel rods through which the footshock (UCS) was delivered. Each chamber was cleaned and the inside wiped down with 5% ammonium hydroxide between animals. During the training session, rats received 10 counterbalanced CS+ and CS− presentations. CS+ cues were always paired with a shock (1 sec, 0.5 mA), and CS− cues were never paired with shock. The average intertrial interval (ITI) between each tone presentation was 70 sec. The tone duration was 10 sec with a frequency of either 1 kHz (65 dB) or 7 kHz (55 dB) and frequencies were counterbalanced between groups.
Auditory CS testing took place in Context B 24 h after conditioning. The chamber floors in Context B were composed of an opaque, white piece of plastic. The chambers of Context B were wiped with 5% acetic acid before each test session. Rats received five discrete tone presentations of the CS− then five of the CS+ (30 sec; 60 sec ITI) following a 60 sec stimulus-free period. Freezing was defined as the cessation of all movement excluding respiration and was automatically scored in real-time with FreezeScan 1.0 detection software (Clever Sys, Inc.) calibrated to a trained human observer.
As our primary interest was in examining the contributions of high-estrogen levels, female subjects were separated based on the estrus phase at the time of retrieval testing. We compared animals in their proestrus phase (High E) to animals in all other phases (Low E). Crucially, there were no differences between freezing levels in animals in estrus, metestrus, or diestrus phases. Both groups were compared to males of the same age (previous work has demonstrated good discrimination in males using this conditioning preparation; Ferrara et al. 2017). Two animals were excluded for showing baselines freezing rates over 20% in the neutral context (Zs = 3.69, 3.27; Field 2005), leaving final groups sizes at n = 10 (Males), n = 5 (High E), and n = 25 (Low E). One excluded animal was from the estrus group and the second was from the metestrus group.
Results from the testing day are displayed in Figure 1. A 3 (Group: Male, Female High E, and Female Low E) × 5 (Trial Type: Pre-CS, CS−, CS+, ITI, and Post-CS) ANOVA found a main effect of Group, F(2,37) = 10.87, MSE = 1076.57, P < 0.001, , trial, F(4,148) = 62.54, MSE = 390.48, P < 0.001, , and an interaction between the two, F(8,148) = 2.45, MSE = 390.48, P = 0.016, . While there were no group differences during the baseline period, planned comparisons found that males differed from both groups of females during the CS− (High E: P = 0.001, Low E: P = 0.002), the CS+ (High E: P = 0.01, Low E: P = 0.006), the ITI (High E: P = 0.001, Low E: P = 0.002), and the post-CS period (High E: P = 0.03, Low E: P = 0.007), reflecting overall lower rates of freezing in males when compared to females. High-estrogen and low-estrogen females did not differ from each other during any trial. While males (P < 0.001) and low-estrogen females (P < 0.001) froze less during the CS− than the CS+, this was not true of high-estrogen females (P = 0.42), highlighted in Figure 3A.
Figure 1.
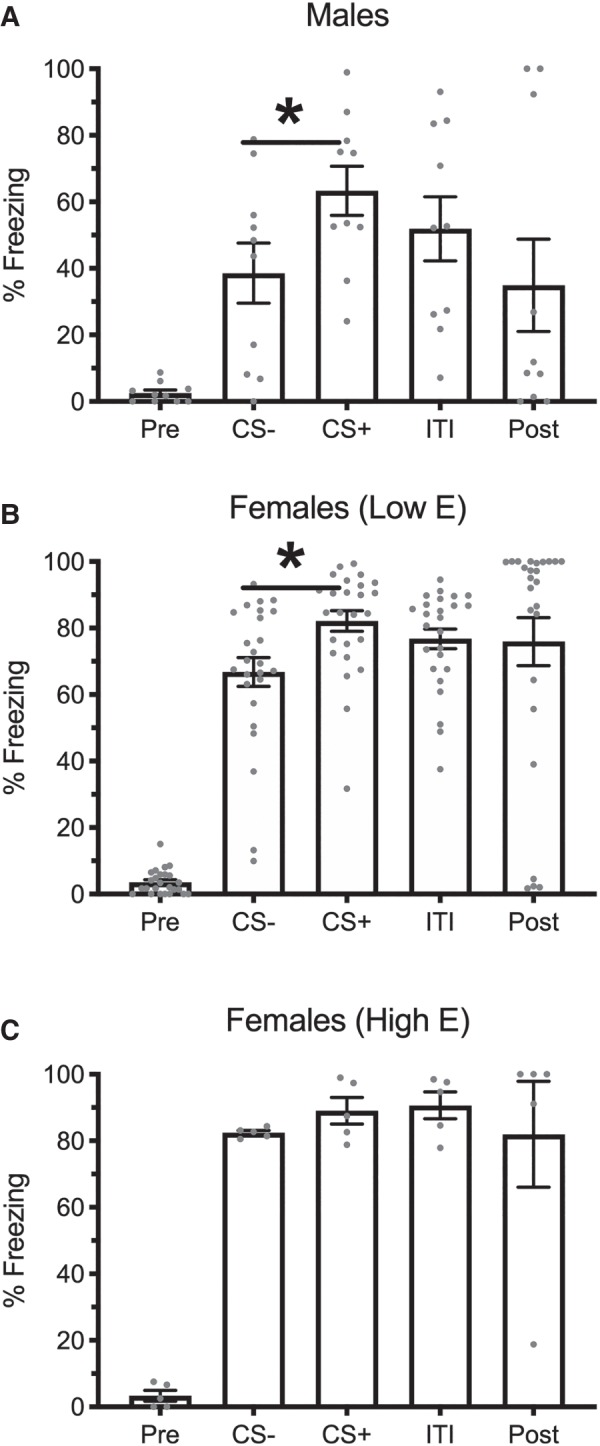
Means and SEM during the pre-CS, CS−, CS+, ITI, and post-CS periods during the retrieval session for males (A), low-estrogen females (B), and high-estrogen females (C). Asterisks indicate P < 0.05 between CS− and CS+. Groups were constructed based on the estrous phase at the time of retrieval.
Figure 3.
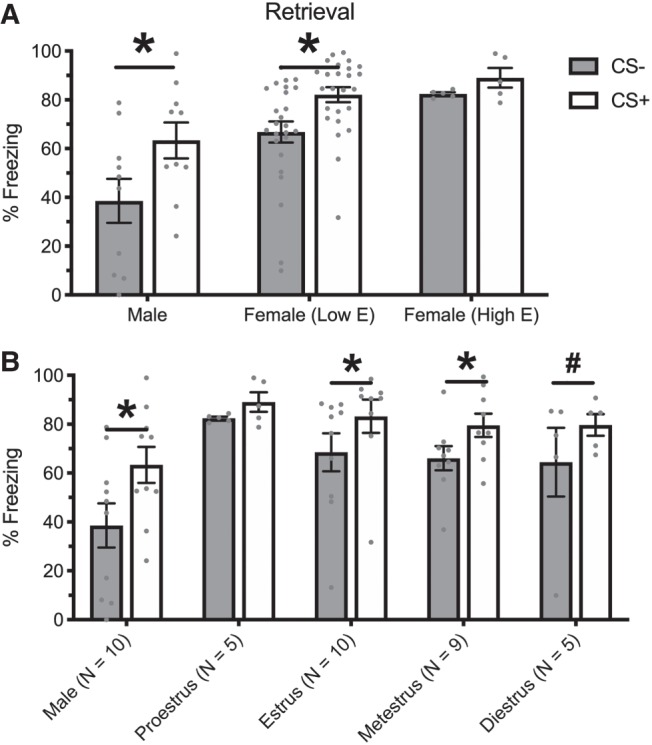
Means and SEM during the CS− and CS+ periods during the retrieval session separated into males, and High E and Low E females (A) as well as examining each individual phase of the estrous cycle (B). Asterisks indicate P < 0.05 between CS− and CS+.
To isolate the effect of phase on retrieval, a similar ANOVA was run to assess responding during the retrieval session as a function of phase at training (depicted in Fig. 2) and found a main effect of Group, F(2,37) = 9.92, MSE = 199.24, P < 0.001, , trial, F(4,148) = 69.71, MSE = 372.63, P < 0.001, , and an interaction between the two, F(8,148) = 3.45, MSE = 372.63, P = 0.001, . Importantly, all three groups showed less responding during the CS− than the CS+ (Male: P < 0.001; High E Female: P = 0.03, Low E Female: P = 0.001). These results suggest that the reduced discrimination was a function of estrogen level at the time of retrieval rather than at the time of training.
Figure 2.
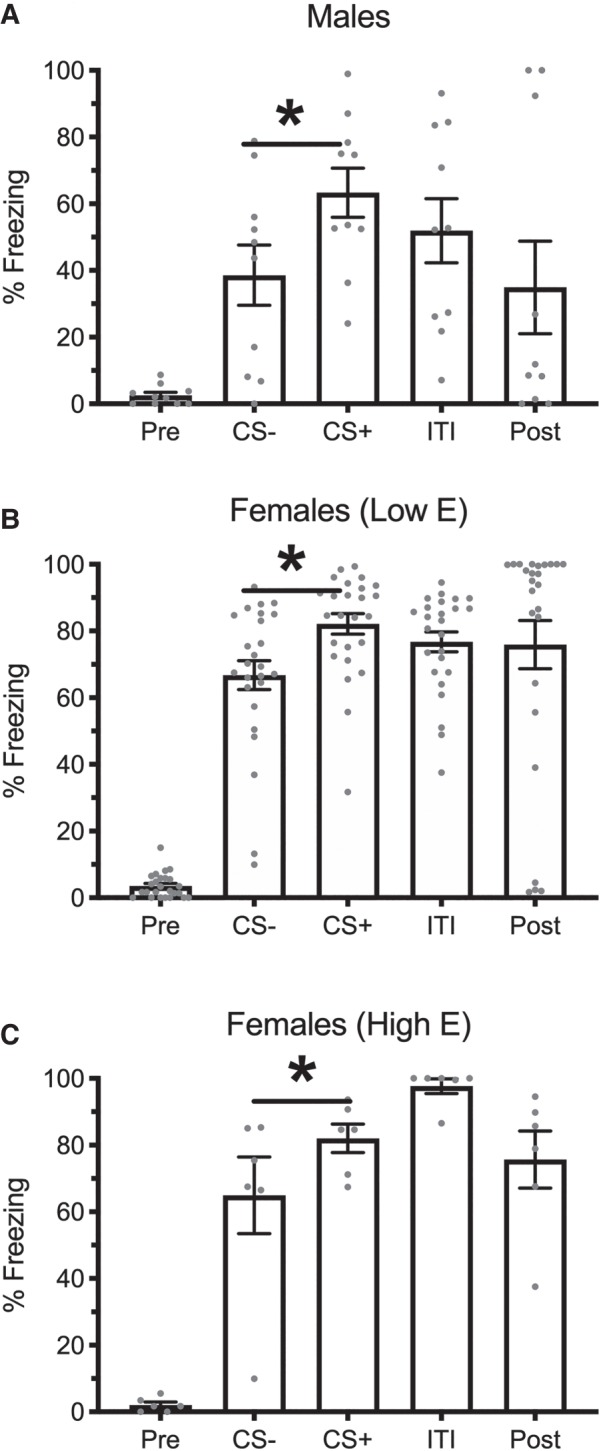
Means and SEM during the pre-CS, CS−, CS+, ITI, and post-CS periods during the retrieval session for males (A), low-estrogen females (B), and high-estrogen females (C). Asterisks indicate P < 0.05 between CS− and CS+. Groups were constructed based on the estrous phase at the time of acquisition.
While 100% of males showed clear discrimination (in which CS+ freezing was higher than CS− freezing) between cues, 76% of low-estrogen females showed this effect and 60% of high-estrogen females showed this effect. Of the animals in each group that demonstrated this effect, the average discrimination score (calculated by subtracting freezing in the CS− from freezing during the CS+) was 24.75 for males, 22.99 for low-estrogen females, and 12.05 for high-estrogen females, suggesting that even in animals that did discriminate, this effect was smallest for high-estrogen females. When a discrimination index was calculated by examining CS− freezing as a proportion of CS+ freezing the means followed a similar numerical trend (Males: M = 0.53; Low E Females: M = 0.81; High E Females: M = 0.93). Discrimination did not differ substantially across the nonproestrus phases of the estrous cycle, with animals showing a significant difference (or trend toward significance) between the CS+ and CS− in the estrus (P = 0.01), metestrus (P = 0.036), and diestrus (P = 0.074) phases (Fig. 3B).
This result demonstrates that while both males and low-estrogen females can discriminate between a CS that signals shock and a neutral CS, this discrimination was not evident in female rodents in the proestrus phase. This experiment replicates and expands on the results from Lynch et al. (2013, 2014) in at least two important ways. First, it demonstrates a role for estrogens in cued fear discrimination, similar to previous work using contextual fear conditioning. Second, it shows that endogenous estrogens likely influence generalization of fear behavior in a similar manner to systemic estradiol injections. It should also be noted that female rats froze more than male rats, regardless of cycle. This result seems to be in accord with some research that demonstrates differential involvement of brain structures (such as the prefrontal cortex) recruited during fear conditioning as well as alterations in excitatory and inhibitory sensitivity within the amygdala across the estrous cycle (Blume et al. 2017; Kirry et al. 2019).
Previous research has demonstrated systemic estrogens and estradiol are associated with general improvements in learning and memory (for review, see Frick et al. 2015), with typical results demonstrating an effect of estrogen on the ability to more readily acquire both fear learning (Jasnow et al. 2006) and extinction learning (in which conditional responding is decreased following repeated presentations of the CS without the UCS; Milad et al. 2009; Zeidan et al. 2011). If the ability to discriminate between a fear-provoking CS+ and a neutral or safe CS− is representative of good or successful learning, then the body of work demonstrating increased generalization (and thus, less discrimination) lies in stark contrast to those results. One potential way to reconcile this is that generalized fear responding might be seen as an enhanced or potentiated fear learning. In line with this interpretation, some work has demonstrated that factors that result in enhanced memory performance following cued fear conditioning (e.g., increased CREB levels in the auditory thalamus), also result in increased freezing to neutral tones (Han et al. 2008). Similarly, Keiser et al. (2017) demonstrated that, while females showed increased contextual generalization, this was accompanied by an overall increase in fear to the acquisition context. The current results mirror this latter finding using a cued fear conditioning paradigm.
Given the high levels of freezing in both the current results and Keiser et al. (2017) it is difficult to conclusively state that any lack of discrimination was due to a failure to discriminate instead of an overall ceiling effect. One potential explanation is that estrogen might correlate with higher levels of anxiety leading to a potentiated fear response. However, previous work has demonstrated that female rodents in the proestrus phase show decreased anxiety-like responding as measured by an elevated-plus maze task (Marcondes et al. 2001), suggesting that the effect reported here is not due to increased anxiety. In a similar vein, overall differences between males and females in the present manuscript may have been due to the swabbing procedure.
The current results provide a crucial step in furthering our understanding of how estrogens mediate fear learning by demonstrating that female rats in the proestrus phase of their cycle show a generalized fear response to a neutral cue relative to both females in other phases as well as to male controls.
Acknowledgments
This work was supported by the National Institutes of Health R01MH069558 (F.J.H.) and F32MH120938 (S.T.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.051185.119.
References
- Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, DeJoseph MR, Urban JH, Rosenkranz AJ. 2017. Sex- and estrus-dependent differences in rat basolateral amygdala. J Neurosci 37: 10567–10586. 10.1523/JNEUROSCI.0758-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara NC, Cullen PK, Pullins SP, Rotondo EK, Helmstetter FJ. 2017. Input from the medial geniculate nucleus modulates amygdala encoding of fear memory discrimination. Learn Mem 24: 414–421. 10.1101/lm.044131.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. 2005. Discovering statistics using SPSS. Sage, Thousand Oaks, CA. [Google Scholar]
- Foilb AR, Bals J, Sarlitto MC, Christianson JP. 2018. Sex differences in fear discrimination do not manifest as differences in conditioned inhibition. Learn Mem 25: 49–53. 10.1101/lm.045500.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM. 2015. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem 22: 472–493. 10.1101/lm.037267.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Fitzgerald PJ, Maren S. 2016. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem 130: 26–33. 10.1016/j.nlm.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Holloway-Erickson CM, Maren S. 2017. Extinction after fear memory reactivation fails to eliminate renewal in rats. Neurobiol Learn Mem 142: 41–47. 10.1016/j.nlm.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J-H, Yiu AP, Cole CJ, Hsiang H-L, Neve RL, Josselyn SA. 2008. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn Mem 15: 443–453. 10.1101/lm.993608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. 2006. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav 49: 197–205. 10.1016/j.yhbeh.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC. 2017. Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology 42: 397–407. 10.1038/npp.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060. 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62: 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. 2012. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21: 169–184. 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirry AJ, Durigan DJ, Twining RC, Gilmartin MR. 2019. Estrous cycle stage gates sex differences in prefrontal muscarinic control of fear memory formation. Neurobiol Learn Mem 161: 26–36. 10.1016/j.nlm.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Lynch J III, Cullen PK, Jasnow AM, Riccio DC. 2013. Sex differences in the generalization of fear as a function of retention intervals. Learn Mem 20: 628–632. 10.1101/lm.032011.113 [DOI] [PubMed] [Google Scholar]
- Lynch JF III, Dejanovic D, Winiecki P, Mulvany J, Ortiz S, Riccio DC, Jasnow AM. 2014. Activation of ERβ modulates fear generalization through an effect on memory retrieval. Horm Behav 66: 421–429. 10.1016/j.yhbeh.2014.06.017 [DOI] [PubMed] [Google Scholar]
- Lynch JF, Winiecki P, Vanderhoof T, Riccio DC, Jasnow AM. 2016. Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol Learn Mem 130: 83–92. 10.1016/j.nlm.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. 2001. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 74: 435–440. 10.1016/S0031-9384(01)00593-5 [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. 2009. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164: 887–895. 10.1016/j.neuroscience.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ. 2011. An introduction to behavioral neuroendocrinology. Sinauer, Sunderland, MA. [Google Scholar]
- Pavlov IP. 2010. Conditional reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford University Press, Oxford, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70: 920–927. 10.1016/j.biopsych.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


