Abstract
Introduction
Laparoscopic gynecologic surgery is one of the most well-known procedures. Pneumoperitoneum with carbon dioxide insufflation can cause unfavorable hemodynamic effects due to catecholamine and vasopressin release.
Aim
To examine the effects of stellate ganglion block on hemodynamic response and postoperative pain.
Material and methods
In a prospective double blinded randomized parallel study we included 40 patients with ASA physical status I and II, aged between 18 and 50 years with a gynecologic problem candidate for laparoscopic surgery under general anesthesia. The patients were randomly divided into two groups. Fifteen minutes before anesthesia induction, the patients underwent ultrasound guided stellate ganglion block with 10 ml of lidocaine 1% and the control group underwent stellate ganglion block using 10 ml of distilled water as placebo. After induction of general anesthesia, systolic and diastolic blood pressure and heart rate were recorded, especially after blowing of CO2 gas, the position change, depletion of CO2, and tracheal extubation in recovery. The postoperative pain was calculated using the visual analogue scale (VAS) at three times (0, 30, and 24 h after surgery).
Results
Our results showed that mean systolic and diastolic blood pressure and heart rate did not show any significant difference at the measurement times (p > 0.05), and mean VAS of patients in the two groups was significantly different for the three measurement times except 24 h after surgery (p < 0.05).
Conclusions
Stellate ganglion block before laparoscopic gynecologic surgery has no significant effect on intraoperative and postoperative hemodynamic responses; however, it can decrease VAS in the early postoperative period.
Keywords: stellate ganglion block, hemodynamic responses, laparoscopy, gynecology
Introduction
Laparoscopic procedures have become more frequent in the last decades. In this regard, laparoscopic gynecologic surgery is one of the most well-known procedures [1–3]. Carbon dioxide (CO2) insufflation induces pneumoperitoneum, which along with hypercapnia can cause unfavorable hemodynamic effects due to catecholamine and vasopressin release. Any imbalance of the sympathetic and parasympathetic branches of the autonomic nervous system occurring usually during laparoscopic surgery not only can cause fluctuating hemodynamic conditions but also can cause and maintain pain and inflammation. These changes could lead to an unexpected increase of mean arterial blood pressure (MAP), heart rate (HR), and systemic vascular resistance and diminished cardiac output leading to hypertension (HTN), arrhythmia, tachycardia and bradycardia [4]. In fact, hemodynamic changes occur following the release of catecholamine such as epinephrine (E), norepinephrine (NE) and dopamine (DA) via the adrenal medulla. During stress, E and NE are secreted by the adrenal medulla into the blood circulation [5]. Regarding pain induction during surgery, peripheral and central sensitization processes take place, affecting the sympathetic ganglia, afferent and efferent, spinal and supraspinal levels, and release of inflammatory neuropeptides [6]. Various pharmacologic agents have been used to diminish these vasopressor reactions such as opioids, magnesium, β-blockers, α2 agonists and local anesthetics [7–11]. A study showed that stellate ganglion block in patients with upper limb surgery following trauma reduces postoperative pain scores and morphine consumption [12, 13]. There are some studies that showed the antihypertensive effects of stellate ganglion block via effects on endothelin-1 (ET-1) and endothelial nitric oxide synthase (endothelial NOS) in blood vessels [14, 15].
Aim
In this study, we examined the effects of stellate ganglion block (SGB) on the hemodynamic response and postoperative pain.
Material and methods
After obtaining approval from the ethics committee of Iran University of Medical Sciences (IR.IUMS.REC1395.95-03-218-29652) and registration in the Iranian Registry of Clinical Trials (IRCT2017012510599N14), we performed a prospective double blinded randomized parallel study. All procedures were designed, conducted, and reported in compliance with the Declaration of Helsinki.
Sample size in this study was calculated using the measured visual analogue scale (VAS) in both case and control groups in the previously done study. Considering a confidence coefficient of 0.05 and statistical power of 80%, and d = 2.6, we found a sample size of 20 in each group and in total 40.
We included patients with ASA physical status I and II, aged between 18 and 50 years with a gynecologic problem candidate for laparoscopic surgery under general anesthesia. The patients with the following criteria were not included in the study: a body mass index more than 30, history of substance abuse, inability to understand the VAS, inability to use patient-controlled analgesia (PCA) and allergy to local anesthetic agents. The patients were evaluated a day before surgery to assess their fitness for the proposed surgical procedure under general anesthesia. After obtaining written, informed consent, the patients were randomly divided into two groups. Our blinding technique was as follows: At first we prepared a randomized list of patients in two groups. Then whenever any patient entered the study we performed randomized group selection on them. At this stage none of the research group if where in contact with the studied patient where not aware of the type of group specification. The randomized allocation was formerly prepared in closed envelopes by a person who was not present while opening the envelopes. The patients were trained to use the PCA pump and the VAS a day before the surgery and were reminded before transfer to the operating room and theater and in the post-anesthesia care unit. All patients fasted for 8 h before surgery and premedicated with oral lorazepam 1 mg and ranitidine 150 mg in the night before surgery and 2 h before surgery. In both groups, a peripheral vein was taken in the operating room for the patient’s routine monitoring including non-invasive blood pressure (NIBP), ECG, invasive blood pressure (IBP), end-tidal carbon dioxide (ETCO2), bispectral index (BIS) and peripheral oxygen saturation (SPO2). Heart rate and blood pressure were recorded. Intermittent pneumatic compression (IPC) was used for all patients. Fifteen minutes before anesthesia induction, the patients underwent ultrasound guided stellate ganglion block with 10 ml of lidocaine 1% and the control group underwent stellate ganglion block using 10 ml of distilled water as placebo. Due to the fact that most evaluations were done during surgery and we wanted to see the Horner’s symptoms shortly after, and in terms of pain assessment the evaluation was up to 24 h, we used lidocaine in this study. After 5 min, the symptoms of the block were evaluated with Horner’s symptoms which include thinning of the occipital gaps, enophthalmos, meiosis, facial anesthesia and flushing. Subsequently, midazolam 25 µg/kg and fentanyl 3 µg/kg were administered as premedication. Anesthesia was induced using propofol 2 mg/kg and atracurium 0.5 mg/kg. Tracheal intubation was performed in both groups with direct laryngoscopy. Maintenance of anesthesia was done using oxygen 5 l/min, propofol 100 µg/kg/min and atracurium 10 mg/kg every 15 min. All hemodynamic parameters of patients such as systolic and diastolic blood pressure, heart rate and arterial oxygen saturation were recorded by a collaborator physician who was not aware of group specification of the patient. These data were recorded especially after blowing of CO2 gas, after the position change (Trendelenburg), after depletion of the CO2 and after tracheal extubation in recovery.
After the surgery, the postoperative pain was calculated using the VAS at three times (0, 30, and 24 h after surgery).
Results
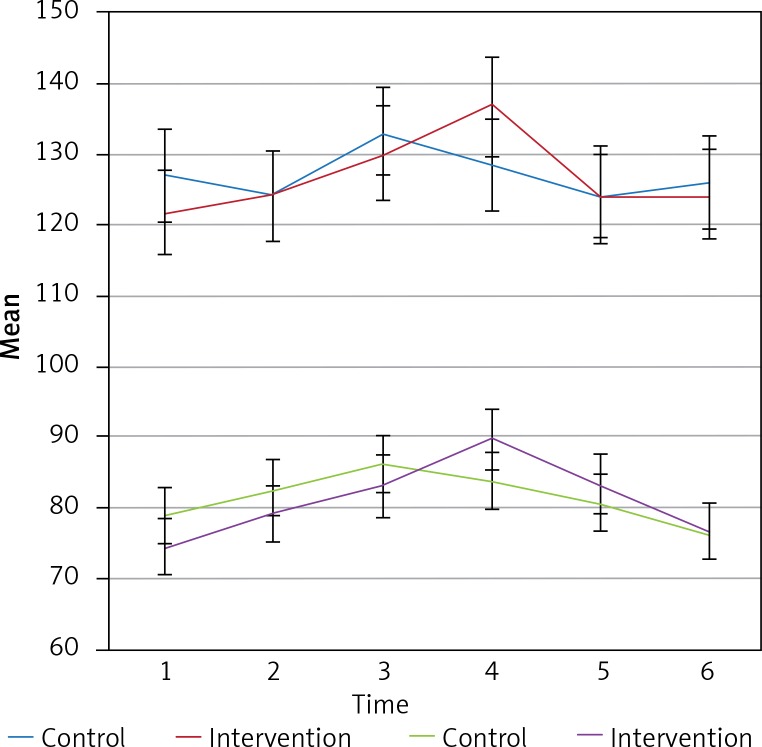
In the present study, 40 patients were divided into two stellate ganglion block (n = 20) and control (n = 20) groups. Mean age, weight and height of patients in the two groups are shown in Table I. The mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the patients for the two groups at different time points including before induction (time 1), after intubation (time 2), after administration of inhalational anesthetic agents (time 3), after position change (time 4) and two other points (time 5 and 6) were recorded. The results showed that the mean SBP and DBP did not show any significant difference at the measurement times (p > 0.05). Table II shows the comparison of the mean SBP and DBP for the two groups at the six recorded times.
Table I.
Mean age, weight and height of patients in the two groups
| Variable | Stellate ganglion block Mean ± SD | Control Mean ± SD |
|---|---|---|
| Age [years] | 36.56 ±12.23 | 32.25 ±9.90 |
| Weight [kg] | 74.9 ±33.47 | 70.20 ±15.92 |
| Height [cm] | 151.6 ±39.2 | 164.55 ±3.43 |
Table II.
Comparison of mean systolic and diastolic blood pressure at the six recorded times
| Variable | Mean | SD | P-value | |
|---|---|---|---|---|
| SBP (time 1) | Control | 127.077 | 22.240 | 0.442 |
| Stellate ganglion block | 121.68 | 12.75 | ||
| DBP (time 1) | Control | 78.77 | 17.64 | 0.446 |
| Stellate ganglion block | 74.58 | 13.08 | ||
| SBP (time 2) | Control | 123.92 | 37.92 | 0.983 |
| Stellate ganglion block | 124.17 | 23.74 | ||
| DBP (time 2) | Control | 82.62 | 32.92 | 0.746 |
| Stellate ganglion block | 79.22 | 25.02 | ||
| SBP (time 3) | Control | 132.46 | 22.78 | 0.770 |
| Stellate ganglion block | 130.11 | 21.77 | ||
| DBP (time 3) | Control | 86.31 | 19.06 | 0.606 |
| Stellate ganglion block | 83.26 | 14.04 | ||
| SBP (time 4) | Control | 128.31 | 18.53 | 0.216 |
| Stellate ganglion block | 136.72 | 18.12 | ||
| DBP (time 4) | Control | 83.62 | 15.25 | 0.200 |
| Stellate ganglion block | 89.79 | 11.42 | ||
| SBP (time 5) | Control | 124.62 | 14.07 | 0.869 |
| Stellate ganglion block | 123.68 | 16.46 | ||
| DBP (time 5) | Control | 80.85 | 14.02 | 0.712 |
| Stellate ganglion block | 83.16 | 19.09 | ||
| SBP (time 6) | Control | 125.91 | 13.48 | 0.755 |
| Stellate ganglion block | 124.21 | 14.48 | ||
| DBP (time 6) | Control | 76.45 | 18.15 | 0.997 |
| Stellate ganglion block | 76.47 | 14.34 | ||
Intergroup effects for the mean SBP and DBP for both groups at the measurement times showed no significant changes in the systolic and diastolic blood pressure over the 6 measurement times (p > 0.05). Also, the results of intergroup analysis indicated that differences between the two groups were not statistically significant (p > 0.05). Figure 1 shows the trend and error bar, the mean and standard deviation SBP and DBP for the two groups at the six measurement times.
Figure 1.
The trend of the SBP and DBP for the two groups at the six measurement times
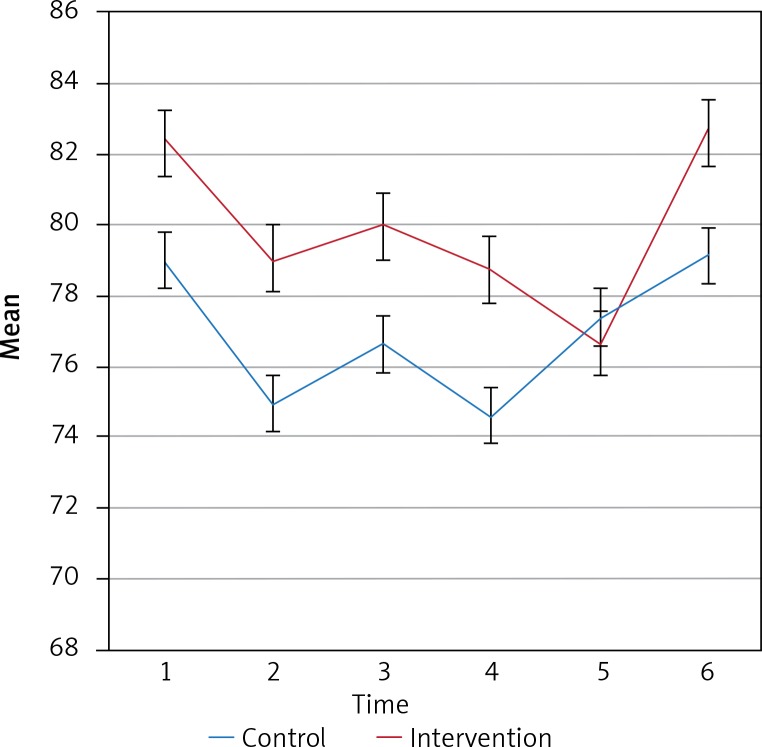
Mean heart rate (HR) for both groups were evaluated at the six times. The results showed that mean HR of patients in the two groups was not significantly different over the six measurement times (p > 0.005). Table III shows the mean HR for the two groups at the six measurement times.
Table III.
Comparison of mean HR for the two groups over the six times
| Variable | Mean | SD | P-value | |
|---|---|---|---|---|
| HR (time 1) | Control | 78.92 | 13.62 | 0.593 |
| Stellate ganglion block | 82.28 | 18.37 | ||
| HR (time 2) | Control | 74.92 | 13.39 | 0.385 |
| Stellate ganglion block | 79.00 | 14.80 | ||
| HR (time 3) | Control | 76.58 | 10.73 | 0.527 |
| Stellate ganglion block | 79.94 | 15.87 | ||
| HR (time 4) | Control | 74.58 | 9.53 | 0.397 |
| Stellate ganglion block | 78.67 | 14.43 | ||
| HR (time 5) | Control | 77.33 | 8.44 | 0.861 |
| Stellate ganglion block | 76.61 | 12.35 | ||
| HR (time 6) | Control | 79.09 | 11.14 | 0.415 |
| Stellate ganglion block | 82.56 | 10.83 | ||
Intergroup effects for the mean HR of the two groups at the six measurement times showed no significant changes in the mean HR over the six measurement times (p = 0.0408). Also, the results of intergroup analysis indicated that differences between the two groups were not statistically significant (p = 0.388). Figure 2 shows the trend and error bar, and the mean HR for the two groups over the six measurement times.
Figure 2.
The trend of the HR for the two groups over the six measurement times
Mean VAS for both groups was evaluated at three times including before the beginning of anesthesia, and 30 min and 24 h after surgery. The results showed that the mean VAS of patients in the two groups was significantly different for the three measurement times except 24 h after surgery (p < 0.05). Table IV shows the mean VAS for both groups at the three measurement times.
Table IV.
Comparison of mean VAS for both groups at the three measurement times
| Variable | Mean | SD | P-value | |
|---|---|---|---|---|
| VAS (time 1) | Control | 8.77 | 1.59 | 0.005 |
| Stellate ganglion block | 6.61 | 2.25 | ||
| VAS (time 2) | Control | 7.07 | 1.89 | 0.003 |
| Stellate ganglion block | 4.4 | 2.41 | ||
| VAS (time 3) | Control | 2.75 | 1.60 | 0.061 |
| Stellate ganglion block | 1.33 | 0.82 | ||
Discussion
In this study, the effects of stellate ganglion block on the hemodynamic response and postoperative pain in laparoscopic surgery were investigated. The mean systolic blood pressure and diastolic blood pressure of the patients for the two groups at different time points including before induction, after intubation, after administration of inhalational anesthetics agents, after position change and two other points were recorded to be compared with the control group. The results showed that the mean SBP and DBP did not differ significantly at the six measurement times. Intergroup effects for the mean SBP and DBP for both groups at the measurement times showed no significant changes in the systolic and diastolic blood pressure over the six measurement times. Also, the mean heart rate for both groups was evaluated at the six times and the results showed that between the two groups it was not significantly different at the six measurement times. Intergroup effects for the mean heart rate of the two groups at the six measurement times showed no significant changes over the six measurement times. Also, the results of intergroup analysis indicated that differences between the two groups were not statistically significant. The mean VAS for both groups was evaluated at three times including after extubation (0), and 30 min and 24 h after surgery. The results showed that the mean VAS of patients in the two groups was significantly different for the three measurement times except 24 h after surgery.
In a study of Kumar et al. patients were administered a local anesthetic drug using ultrasound-guided stellate ganglion block under general anesthesia with postoperative analgesia by patient-controlled analgesia. Also cardiovascular variables and visual analogue scale pain scores were recorded. Opioid consumption was significantly reduced in the patients receiving an anesthetic agent using SGB assessed by VAS scores. This study revealed an analgesic effect of pre-operative stellate ganglion block in patients under general anesthesia [16].
We used Homer principles to find the exact place to administer the local anesthetic of interest and the results were acceptable. But some studies showed that Horner’s syndrome is not an appropriate indicator of correct placement to administer the drug of interest during stellate ganglion block [17, 18]. In addition, after induction of general anesthesia, the assessment of cardiovascular features of Horner’s syndrome such as enophthalmos, ptosis and miosis will be complex and unreliable due to the use of neuromuscular relaxant medications and opioids, which is a limitation to our study [19]. The role of stellate ganglion block is well known in patients with chronic pain because of the reduction in sympathetic tone, and prevention of central sensitization helping to have normal somatic sensation [20, 21]. De La Vega Costa et al. reported that SGB has minor effects on the hemodynamic status [22], which is similar to our study. In the current study we did not find major hemodynamic consequences in patients after performing SGB.
This study has novelty in terms of assessing the effects of a pain control procedure on hemodynamic status as well. It gives new ideas about other consequences of pain procedures.
Our study had some limitations, for example using short-acting lidocaine. We recommend performing this procedure with long-acting drugs such as ropivacaine.
Conclusions
Stellate ganglion block before laparoscopic gynecologic surgery has no significant effect on intraoperative and postoperative hemodynamic responses; however, it can decrease VAS in the early postoperative period.
Acknowledgments
The authors would like to thank the Rasoul Akram Hospital Clinical Research Development Center (RCRDC) for its technical assistance.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Farag S, Padilla PF, Smith KA, et al. Management, prevention, and sequelae of adhesions in women undergoing laparoscopic gynecologic surgery: a systematic review. J Minim Invasive Gynecol. 2018;25:1194–216. doi: 10.1016/j.jmig.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Tinelli A, Gasbarro N, Lupo P, et al. Safe introduction of ancillary trocars. JSLS. 2012;16:276–9. doi: 10.4293/108680812X13427982376464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitale SG, Gasbarro N, Lagana AS. Safe introduction of ancillary trocars in gynecological surgery: the “yellow island” anatomical landmark. Ann Ital Chir. 2016;87:608–11. [PubMed] [Google Scholar]
- 4.Hakeem A, Iqbal A, Dawood M, et al. Effects of CO2 pneumoperitoneum on arterial partial pressure of carbon dioxide Ph, end tidal carbon dioxide and bicarbonate in patients during laparoscopic cholecystectomy. J Evol Med Dent Sci. 2016;5:2299–302. [Google Scholar]
- 5.Goto KA, Ishii NA, Kizuka TO, et al. The impact of metabolic stress on hormonal responses and muscular adaptations. Med Sci Sports Exerc. 2005;37:955–63. [PubMed] [Google Scholar]
- 6.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–7. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102:248–57. doi: 10.1213/01.ANE.0000181289.09675.7D. [DOI] [PubMed] [Google Scholar]
- 8.Ram CV. Beta-blockers in hypertension. Am J Cardiol. 2010;106:1819–25. doi: 10.1016/j.amjcard.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Christoph RA, Buchanan L, Begalla K, et al. Pain reduction in local anesthetic administration through pH buffering. Ann Emerg Med. 1988;17:117–20. doi: 10.1016/s0196-0644(88)80293-2. [DOI] [PubMed] [Google Scholar]
- 10.Faiz HR, Rahimzade P, Visnjevac O, et al. Intravenous acetaminophen is superior to ketamine for postoperative pain after abdominal hysterectomy. J Pain Res. 2014;7:65–70. doi: 10.2147/JPR.S53234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedighinejad A, Haghighi M, Naderi Nabi B, et al. Magnesium sulfate and sufentanil for patient-controlled analgesia in orthopedic surgery. Anesth Pain Med. 2014;4:e11334. doi: 10.5812/aapm.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonnell JG, Finnerty O, Laffey JG. Stellate ganglion blockade for analgesia following upper limb surgery. Anaesthesia. 2011;66:611–4. doi: 10.1111/j.1365-2044.2011.06626.x. [DOI] [PubMed] [Google Scholar]
- 13.Imani F, Hemati K, Rahimzade P, et al. Effectiveness of stellate ganglion block under fluoroscopy or ultrasound guidance in upper extremity CRPS. J Clin Diagn Res. 2016;10:UC09. doi: 10.7860/JCDR/2016/14476.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou XL, Li BL, Zhao L, et al. Effects of Xuefu Zhuyu Capsule on endothelin-1 release in myocardium and vascular endothelium and nitric oxide/nitric oxide synthase system of swines after acute myocardial infarction and reperfusion. J Chin Integr Med. 2008;6:381–6. doi: 10.3736/jcim20080411. [DOI] [PubMed] [Google Scholar]
- 15.Chen YQ, Hu GX, Fu Q, Jin XJ. Effects of stellate ganglion block on blood pressure in spontaneously hypertensive rats. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012;41:65–8. doi: 10.3785/j.issn.1008-9292.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Thapa D, Gombar S, et al. Analgesic efficacy of pre-operative stellate ganglion block on postoperative pain relief: a randomised controlled trial. Anaesthesia. 2014;69:954–60. doi: 10.1111/anae.12774. [DOI] [PubMed] [Google Scholar]
- 17.Price DD, Long S, Wilsey B, et al. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clin J Pain. 1998;14:216–26. doi: 10.1097/00002508-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Hardy PAJ, Wells JCD. Extent of sympathetic blockade after stellate ganglion block with bupivacaine. Pain. 1989;36:193–6. doi: 10.1016/0304-3959(89)90023-7. [DOI] [PubMed] [Google Scholar]
- 19.Hurley RW, Wu CL. Acute post-operative pain. In: Miller RD, Eriksson LI, Fleisher LA, et al., editors. Miller’s Anaesthesia. 7th edn. Vol. 2. Philadelphia, PA: Elsevier Churchill Livingstone; 2010. pp. 2757–82. [Google Scholar]
- 20.Bantel C, Trapp S. The role of the autonomic nervous system in acute surgical pain processing – what do we know? Anaesthesia. 2011;66:541–4. doi: 10.1111/j.1365-2044.2011.06791.x. [DOI] [PubMed] [Google Scholar]
- 21.Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clinic Proc. 2002;77:174–80. doi: 10.4065/77.2.174. [DOI] [PubMed] [Google Scholar]
- 22.De La Vega Costa KP, Perez MA, Roqueta C, et al. Effects on hemodynamic variables and echocardiographic parameters after a stellate ganglion block in 15 healthy volunteers. Auton Neurosci. 2016;197:46–55. doi: 10.1016/j.autneu.2016.04.002. [DOI] [PubMed] [Google Scholar]