Abstract
Pigs exposed to elevated ambient temperatures exhibit reduced daily gain, alterations in muscle and fat deposition, and decreased health. Negative aspects of gastrointestinal (GI) function, integrity, and permeability also occur. High-intensity sweeteners can ameliorate the negative effects of heat stress (HS) by increasing GI glucagon-like peptide-2 production while capsicum oleoresin has been shown to reduce inflammatory response. The effects of an artificial high-intensity sweetener and capsicum oleoresin (CAPS-SUC; TakTik X-Hit, Pancosma, Switzerland) on growth performance of pigs were examined. Forty-eight pigs (12 wk of age, 43.2 ± 4.3 kg) were assigned to six treatments: thermoneutral conditions (21 ± 1.1 °C; 40% to 70% relative humidity) fed ad libitum with (TN+) or without supplement (TN−), heat stress (35 ± 1 °C; 20% to 40% relative humidity) fed ad libitum with (HS+) or without supplement (HS−), and thermoneutral conditions pair-fed to HS intake with (PFTN+) or without supplement (PFTN−). Supplementation (0.1 g/kg feed) began 2 d prior to the 3-d environmental treatment period. Body weights (BWs) and blood samples were collected on days −1 and 3. Rectal temperature (RT) and respiration rate (RR) were measured thrice daily and the feed intake (FI) was recorded daily. Intestinal sections were collected for histology. Pigs in HS conditions exhibited increased RT (~1.2 °C) and RR (~2.7-fold) compared with TN and PFTN groups (P < 0.01). HS+ animals had increased RR when compared with HS− animals (P < 0.02). Heat stress decreased FI compared with TN. HS and PFTN decreased (P < 0.05) average daily gain compared with TN. Supplement did not alter the BW gain. HS and PFTN decreased (P < 0.05) Gain:Feed compared with TN during environmental treatment. Supplementation with CAPS–SUC increased Gain:Feed by 0.12 (P < 0.05). Circulating glucose concentrations tended to decrease in CAPS–SUC vs. non-supplemented HS and PFTN animals (P ≤ 0.1). Circulating insulin concentrations as well as monocyte count increased in HS compared with PFTN (P < 0.04) but did not differ from TN and likely linked to altered FI. CAPS–SUC increased basophil count (P < 0.02), irrespective of environment. Ileal villus height tended to decrease during HS and PFTN compared with TN (P < 0.08), indicating an effect of intake. Overall, CAPS–SUC supplementation increased pig feed efficiency and may improve immune response.
Keywords: artificial sweetener, capsicum oleoresin, heat stress, immune response, intestinal morphology, insulin sensitivity
Introduction
Temperatures across the globe have been increasing over the past few decades and are predicted to continue to do so (Intergovernmental Panel on Climate change (IPCC), 2014; EPA, 2016). This affects both human and animal health and has a large impact on the production and quality of food sources (Renaudeau et al., 2012). Moreover, increases in global population have increased the demand for meat and milk, thereby increasing the production of livestock in hot and humid tropical and subtropical climates and raising the frequency of heat-related decreases in livestock productivity (Renaudeau et al., 2012).
Swine are particularly susceptible to heat stress (HS) due to their lack of functional sweat glands and relatively thick layer of subcutaneous fat (Mount, 1979; Curtis, 1983). Genetic selection for higher lean muscle growth may also play a role by influencing an increased metabolic rate and subsequent heat production (Baumgard and Rhoads, 2013). In the most recent estimation of U.S. economic losses to the livestock industry due to HS, it was calculated that the swine industry experiences about US$300 million annually due to decreases in sow productivity, market hog growth and development, and overall carcass quality (St-Pierre et al., 2003). Environmental heat abatement strategies include increased ventilation, air conditioning, shading, cooling misters, fogging systems, or a combination thereof. Economic effectiveness of these methods is typically small, however, as gains in productivity are negated by start-up and maintenance costs (St-Pierre et al., 2003).
Many responses to HS are relatively conserved across species. An increase in respiration rate is seen, which is a mechanism utilized to dispel excess heat through evaporative cooling through the mouth (Curtis, 1983; Patience et al., 2005). Feed intake (FI) is decreased, which is presumably a method of decreasing metabolism and metabolic heat production within the body (Collin et al., 2001; Renaudeau et al., 2008, 2012). Blood is redirected from splanchnic organs to peripheral veins and arteries to aid in heat dissipation through the skin (Lambert, 2009). Although these mechanisms are relatively effective in reducing core body temperature, they themselves can have negative impacts on animal health. Muscle degradation is seen during HS, and post-absorptive nutrient metabolism is shifted, resulting in reduced carcass quality and carcass price (Baumgard and Rhoads, 2013). Limited blood flow to the visceral organs results in damage to the gastrointestinal (GI) tract, disruption in proper function of the GI tract, and increased gut permeability to luminal bacteria and toxins can occur (Pearce et al., 2013c). Therefore, there is increasing interest in the potential to use functional feed additives as a strategy to decrease the negative impact of HS on animal performance and intestinal health. This is critical not only for restored nutrient absorption but also for the prevention of pathogen invasion/enteric disease that can have a double negative impact on animal performance and economic losses.
Supplementation with high-intensity artificial sweetener activates the sweet taste receptor Taste receptor type 1 member 2 (T1R2/3) in the intestine, which stimulates the release of glucagon-like peptide (GLP)-2 from the enteroendocrine cells of the gut (Drucker, 1996). GLP-2 is a potent GI protective agent that increases blood flow, stimulates mucosal growth, and prevents cell death during challenging conditions (Stephens et al., 2006; Burrin et al., 2007; Moran et al., 2010; Vegge et al., 2013). The response of the intestine to artificial sweetener—which is associated with increased villus height, increased nutrient absorption, and improved tight junction strength—has been observed in piglets (Moran et al., 2010), calves (Moran et al., 2014), and adult ruminants (Moran et al., 2014). In addition, supplementation of calves with high-intensity artificial sweetener had a protective effect against challenge with Cryptosporidium parvum (Connor et al., 2017).
Another functional feed additive is capsicum oleoresin, which is the pungent component of chili peppers. Capsicum oleoresin is a very potent anti-inflammatory and this response is mediated at the transcriptional level within the lymphocytes of the gut mucosa (Murai et al., 2008; Nevius et al., 2012). For example, when capsicum was fed to weaned piglets, the intestinal expression of inflammatory genes was decreased and this was associated with a decrease in the inflammatory response of the intestine and this was translated into improved performance (Liu et al., 2013). Recently, it was reported that supplementation of rabbits with a combination of high-intensity natural sweetener and capsicum oleoresin reduced disease symptoms during a colibacillosis challenge (Moran et al., 2020).
The objective of this study was to determine the effects of a feed supplement containing intense artificial sweetener and capsaicin oleoresin on production parameters and gut morphology in heat-stressed growing pigs. We hypothesized that feeding this supplement to growing pigs under HS will partly mitigate the detrimental effect of HS on production outcomes.
Materials and Methods
Animals and treatment
Virginia Polytechnic Institute and State University Institutional Animal Care and Use Committee approved all procedures involving animals. Forty-eight crossbred pigs (n = 24 gilts, n = 24 barrows; 43.2 ± 4.3 kg) were brought to Litton Reaves Hall at about 12 wk of age and housed in individual pens (with individual feeders and waterers) in either one of four rooms. All rooms were kept in a 12:12 (L:D) h cycle where light was provided between 6:00 a.m. and 6:00 p.m. All animals were given a 6- to 7-d acclimation period in which rooms were maintained in thermoneutral conditions with ad libitum access to an antibiotic-free commercial diet based on the NRC requirements (Table 1). Prior to arrival, animals (n = 48) were weighed and randomly assigned to one of six treatment groups (n = 8 per group) based on average body weights (BWs). Treatments consisted of 1) thermoneutral conditions (TN−; 21 ± 1.1 °C; 40% to 70% relative humidity), 2) thermoneutral + supplement conditions (TN+), 3) heat stress conditions (HS−, 35 ± 1 °C; 20% to 40% relative humidity), 4) heat stress + supplement conditions (HS+), 5) thermoneutral conditions pair-fed to HS groups (PFTN−), and 6) thermoneutral pair-fed to HS group + supplement (PFTN+).
Table 1.
Ingredients (DM basis) and chemical composition of commercial diet
| Items | Content |
|---|---|
| Ingredients | % of air-dried feed |
| Corn | 59.609 |
| Soybean meal | 28.300 |
| Distiller dried grains | 5.00 |
| Lard | 1.695 |
| Blood meal | 1.500 |
| Ground limestone | 1.207 |
| Dicalcium phosphate | 1.183 |
| Lysine 78.8% | 0.360 |
| Salt (plain) | 0.350 |
| Vitamin/mineral premix1 | 0.150 |
| dl-Methionine | 0.150 |
| l-Threonine 98.5% | 0.100 |
| Selenium premix (0.06%) | 0.037 |
| Choline chloride (60%) | 0.075 |
| Maxi-MIL HP Binder2 | 0.050 |
| Ronozyme P-CT3 | 0.025 |
| Formulated nutrients | % of air-dried feed |
| ME, kcal/kg | 2,961 |
| CP | 21.29 |
| Crude fat | 5.0 |
| Ca | 0.85 |
| Total P | 0.57 |
| Lysine | 1.43 |
| Methionine | 0.47 |
| Methionine + cystine | 0.79 |
| Threonine | 0.87 |
1Provided per kilogram of diet AF: 348 mg Zn, 397 mg Fe, 54 mg Cu, 1.3 mg I, 157 mg Mn, 11,015 IU vitamin A, 1,764 IU vitamin D, 122 IU vitamin E, 70 mg niacin, 30 mg pantothenic acid, 0.40 mg biotin, 1.6 g choline, 2.1 mg folic acid, and 4.4 mg pyridoxine.
2Anatox, Lawrenceville, GA.
3Novozymes, Bagsværd, Denmark.
Dietary supplement containing >90% sodium saccharide and 0.18% capsaicinoids (CAPS–SUC; TakTik X-Hit, Pancosma, Switzerland) was mixed homogenously into the feed at an inclusion rate of 0.1 g/kg feed for groups receiving it 2 d prior to the onset of environmental treatment. FI was recorded daily for all animals using a weigh-back method. Empty feeder weight was determined before start of the trial. Feeders with feed were weighed each morning (7:00 a.m.) and empty feeder weight subtracted to determine the refused feed. Refused feed was then subtracted from the amount given to each animal the previous day to determine FI. After the onset of environmental treatment, percent reduction in FI in HS pigs (with and without supplement, respectively) was calculated daily based on animal’s average FI prior to HS. The amount offered to PFTN groups (with and without supplement) was then reduced by that amount. Similarly, daily supplement intake was calculated for HS+ animals after the onset of heat, and that amount was added to feed in TN+ groups (supplement was mixed with a small amount of feed initially daily to ensure consumption, then more feed was added). PFTN and TN+ groups were lagged 1 d behind HS and TN− groups in treatment to allow for feed and supplement calculations. HS and TN− pigs were sacrificed 3 d after the initiation of environmental treatment and PFTN and TN+ groups 1 d after that.
Each room’s temperature and humidity were measured (Acurite model Thermo Hygro, Bellingham, WA) and recorded at 6:00 a.m. and 5:00 p.m. An electric forced air heater 147 (model F3F551QT, TPI Corporation) with constant circulation maintained the HS room temperatures and humidity was not governed. Room temperature was maintained under the control of a computer system (Siemens Building Automation System) and recorded in 30-min intervals. Rectal temperatures and respiration rates were taken twice daily (6:00 a.m. and 5:00 p.m.) and three times daily (6:00 a.m., 12:00 p.m., and 5:00 p.m.) during HS. Rectal temperatures were measured using a GLA M700 digital thermometer (San Luis Obispo, CA) and respiration rates (breaths/min [bpm]) were taken via visual observation and stopwatch. BWs were recorded for each pig on arrival to Litton Reaves, 1 d prior to environmental treatments, and immediately before sacrifice using a Raytec AH300 Way Pig market hog scale (Ephrata, PA).
Blood was collected 1 d prior to the onset of environmental treatments and immediately before sacrifice. Jugular vein blood was obtained (one 10-mL BD vacutainer containing either lithium heparin or silicone coat and two 10-mL vacutainers containing liquid K2 Ethylenediaminetetraacetic acid (EDTA), Franklin Lakes, NJ) and centrifuged at 4 °C for 20 min at 1,425 × g (IEC Centra-8R Centrifuge, Needham Heights, MA). One K2EDTA vacutainer was sent to the Virginia Regional College of Veterinary Medicine Animal Laboratory Services for complete blood count (CBC) analysis. After blood collections, whole blood in 10 mL silicone-coated BD vacutainers was allowed to coagulate at room temperature for approximately 1 h before centrifuging at 4 °C for 20 min at 1,425 × g. Serum was then aliquoted into three 1.5-mL microcentrifuge tubes and stored at −80 °C. After blood collection on the day of sacrifice, pigs were euthanized via captive bolt followed by exsanguination.
Tissue samples were collected within 10 min of death and included sections of the duodenum and ileum. Duodenum samples were taken by measuring 12 cm distal to the pyloric sphincter. Ileum samples were taken by measuring 18 cm proximal to the start of the ileal–cecal junction. Intestinal samples were flushed gently with ice-cold phosphate-buffered saline to remove waste. One-inch sections of each intestinal sample were fixed in 10% neutral buffered formalin for sectioning and hematoxylin and eosin staining. The remainder of the intestinal samples were snap frozen in liquid nitrogen and stored at −80 °C until analysis.
Metabolite and CBC analyses
One 10-mL BD vacutainer tube with lithium heparin in which whole blood was collected and centrifuged at 4 °C for 20 min at 1,425 × g, after which plasma was aliquoted into two 1.5-mL microcentrifuge tubes with one stored at −80 °C and the other one was sent to the Virginia-Maryland Regional College of Veterinary Medicine Animal Laboratory Services for analysis with a large animal blood panel. This panel included measurements for glucose, urea nitrogen, creatinine, and creatine kinase (CK). All plasma samples were analyzed with a Beckman Coulter AU480 (Brea, CA). A K2EDTA vacutainer was sent to the Virginia-Maryland Regional College of Veterinary Medicine Animal Laboratory Services for CBC analysis. Blood samples were analyzed using a Siemens Advia 2120 (Malvern, PA). Measurements included white blood cell (WBC) count, lymphocyte count, monocyte count, and basophil count.
Morphometry
Samples (duodenum and ileum) were fixed in 10% neutral buffered formalin in 50-mL conical tubes after collection and washing. Tubes were sealed with parafilm and sent to the Histology & Pathology Research Lab (Mt. Jackson, VA) where they were sectioned and stained with hematoxylin and eosin. Mean villus height and crypt depth were measured in 12 well-oriented villus crypt columns per intestinal section per pig. Stained intestinal sections were visualized with an Eclipse Ti-E inverted microscope, and images were captured using a DS-L3 digital camera, and measurements were made and recorded with NIS-Elements Software (Nikon Instruments). Analysis was performed by an individual blinded to treatment assignments.
Enzyme-linked immunosorbent assay
Insulin enzyme-linked immunosorbent assay (ELISA) was performed using Mercodia Porcine Insulin ELISA kit (Mercodia AB, Uppsala, Sweden). Serum samples were transferred from −80 °C freezer to ice to thaw. Thawed samples were vortexed briefly to mix, then centrifuged at 12,000 × g for 3 min to remove particulate matter. The 96-well plates coated with mouse monoclonal anti-insulin provided in the kit were used. All samples and calibrators were run in duplicate; 25 μL each of provided calibrators (six total) and samples were pipetted into appropriate wells; 100 μL of 1× enzyme conjugate was added to each well, and plate was incubated on a plate shaker at 800 rpm for 2 h at room temperature (22 °C). Plates were then washed with provided wash buffer (diluted to 1× concentration) six times using a Bio-Tek ELx50 plate washer (Bio-Tek Instruments Inc., Winooski, VT). Plates were inverted and tapped firm after washing to ensure the removal of excess liquid; 200 μL of substrate 3,3’,5,5’-Tetramethylbenzidine was added to each well and plate was incubated at room temperature for 15 min, followed by the addition of 50 μL of stop solution to each well. Plates were placed on a shaker for 5 s to ensure proper mixing of stop solution. Plates were read immediately at optical density (450 nm) using a Bio-Tek PowerWave XS. Concentrations were calculated by computerized data reduction of the absorbance for calibrators (except calibrator 0) vs. sample concentration using cubic spline regression. The intra-assay coefficient of variation (CV) and inter-assay CV are 3.1% and 8.7%, respectively.
Cytokine ELISAs were performed using Neo Scientific Porcine Interleukin (IL)-1β and IL-6 kits (Cambridge, MA). Serum samples were transferred from −80 °C freezer to ice to thaw. Thawed samples were vortexed briefly to mix, then centrifuged at 12,000 × g for 3 min to remove particulate matter; 200 μL of serum was aliquoted into new 1.5-mL centrifuge tubes, to which 20 μL of balance solution was added. The 96-well plates coated with either IL-1β or IL-6 antibodies provided in the kits were used. All samples and standards were run in duplicate; 100 μL of each standard (six total), sample, or phosphate-buffered saline (PBS) were added to appropriate wells; 50 μL of enzyme conjugate was added to each well (excluding wells containing PBS), mixed well, and incubated at 37 °C in a humid chamber for 1 h. After incubation, wells were washed using the provided wash solution (diluted to 1× concentration) five times using the Bio-Tek ELx50 plate washer. Plates were inverted and tapped to ensure removal of excess liquid; 50 μL of substrate A was added to each well, followed by 50 μL of substrate B. Plates were then covered with foil to protect the light-sensitive substrates and incubated at room temperature for 10 to 15 min; 50 μL of stop solution was then added to each well and mixed using a plate shaker. Optical density was read immediately at 450 nm, and mean blank values were subtracted from each standard and sample before the mean of duplicates was calculated.
Competitive cytokine ELISA concentrations were calculated by creating a standard curve. Nonzero raw standard values were divided by standard 0 and multiplied by 100 and used as x values, while the log10 of the concentration of the nonzero standards was used for y values. Standard x and y values were plotted using Microsoft Excel, and a linear fit line was applied to obtain a slope equation. x-Values were obtained for raw sample values by dividing by the standard 0 and multiplying by 100 and used in the slope equation to calculate y values for the samples. Ten to the power of this y value was calculated as the concentration for each sample. The IL-6 intra-assay CV and inter-assay CV are 2.9% and 12.8%, respectively. The IL-1β intra-assay CV and inter-assay CV are 3.4% and 10.8%, respectively.
Data analysis
All data were statistically analyzed using a MIXED procedure in SAS (SAS Inst. Inc., Cary, NC). Data are reported as LSmeans and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10. Daily measurements (rectal temperature, respiration rates, and FI), and blood samples for each animal’s metabolic parameter were analyzed as repeated measures with day as the repeated effect. The compound symmetry, unstructured, toeplitz, variance components, and autoregressive 1 and heterogeneous versions of covariance structures were tested and the most appropriate (lowest Akaike’s information criterion, Akaike’s information criterion with correction, and Bayesian information criterion values) was used for each analysis. Litter and gender were included as random variables; no effect of these variables was seen. Model included treatment, supplement, day, and interactions. Analysis also tested for differences between groups based on treatment and supplement for single measurements.
Results
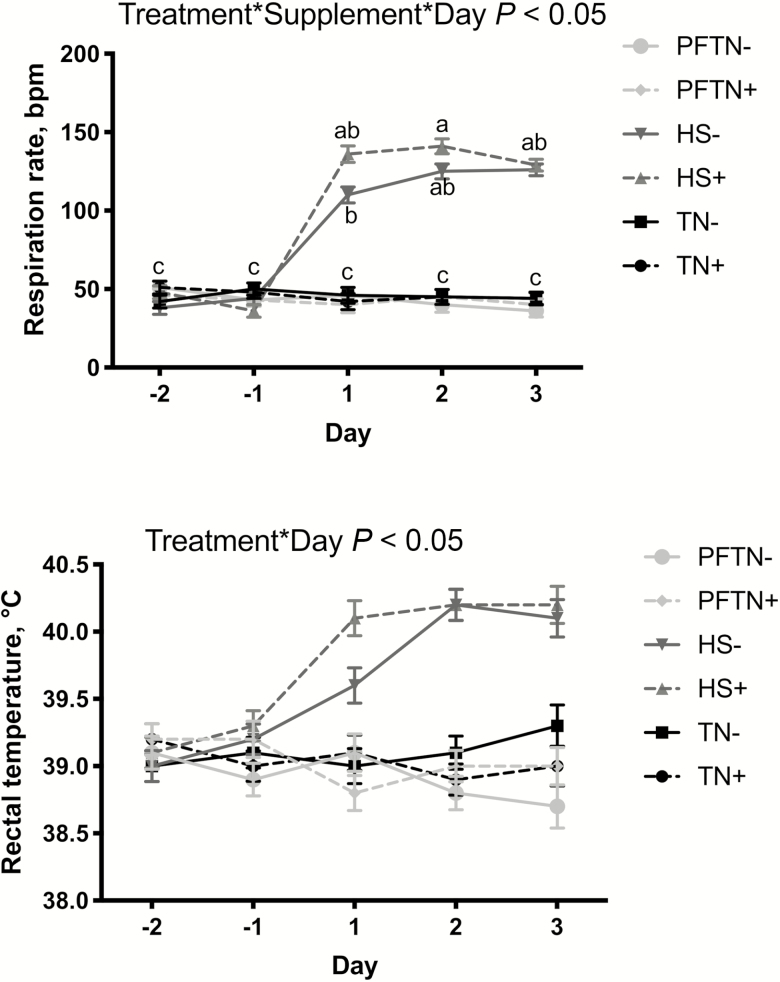
Pigs exposed to HS had increased (P < 0.05) rectal temperatures on day 1 when compared with TN and PFTN groups. This increase was maintained throughout the remaining 2 d of the experiment (Figure 1). Supplementation did not have a significant effect on rectal temperature (Figure 1). An increase (P < 0.05) in respiration rate was observed in HS groups when compared with TN and PFTN groups during the environmental treatment period (Figure 1). HS+ animals had increased (P < 0.05) respiration rates on day 2 when compared with HS− animals on day 1 (140 vs. 109 bpm; Figure 1).
Figure 1.
Effects of HS and CAPS–SUC supplementation on daily respiration rate and rectal temperature in growing pigs. Pigs (n = 8/treatment) were exposed to treatments consisting of TN+/TN− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/HS− (35 ± 1 °C; 20% to 40% relative humidity), and PFTN+/PFTN−for 3 d. Data are reported as LSmeans and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
FI in HS groups with and without supplement decreased (P < 0.05) on day 1 of HS when compared with TN groups, and this decrease continued throughout the duration of environmental treatment (Figure 2; Table 2). FI between HS and PFTN groups was similar by experimental design. HS and pair feeding decreased (P < 0.05) average daily gain (ADG) when compared with TN groups (Table 2). Average BW of groups increased in all groups during the 3 d of environmental treatment, with TN+/TN− groups gaining 4.45 and 3.75 kg, HS+/HS− groups gaining 1.21 and 1.29 kg, and PFTN+/PFTN− groups gaining 1.41 and 0.35 kg, respectively (Table 2). TN groups gained more (P < 0.05) than HS and PFTN groups during the treatment period. Supplement did not have a significant effect on BW gain. HS and pair feeding decreased (P < 0.05) Gain:Feed when compared with TN groups during environmental treatment (Table 2). Regardless of treatment, supplement significantly increased (P < 0.05) Gain:Feed by 0.12 (Table 2).
Figure 2.
Effects of HS and CAPS–SUC supplementation on daily FI (A) and average FI (B) in growing pigs. Pigs (n = 8/treatment) were exposed to treatments consisting of TN+/TN− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/HS− (35 ± 1 °C; 20% to 40% relative humidity), and PFTN+/PFTN−for 3 d. Data are reported as LSmeans and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
Table 2.
Effects of heat stress and CAPS–SUC supplementation on production parameters in pigs
| Environment1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TN | HS | PFTN | P-value | ||||||
| Variable | − | + | − | + | − | + | Trmt | Supp | T × S |
| Average FI, kg/d | 1.91 (0.14) | 2.25 (0.14) | 1.44 (0.14) | 1.22 (0.13) | 1.23 (0.15) | 1.11 (0.14) | <0.0001 | 0.97 | 0.08 |
| ADG, kg/d | 1.07 (0.12) | 1.10 (0.12) | 0.34 (0.12) | 0.30 (0.12) | 0.04 (0.13) | 0.34 (0.12) | <0.0001 | 0.3 | 0.33 |
| Gain:Feed | 0.45 (0.07) | 0.48 (0.07) | 0.22 (0.07) | 0.28 (0.07) | 0.02 (0.07) | 0.30 (0.07) | <0.0002 | 0.04 | 0.18 |
| Δ in BW2, kg | 3.75 (0.53) | 4.45 (0.53) | 1.29 (0.53) | 1.21 (0.52) | 0.35 (0.53) | 1.41 (0.53) | <0.0001 | 0.17 | 0.49 |
1All pigs were fed ad libitum in thermoneutral conditions with or without CAPS–SUC supplementation 2 d prior to the onset of treatments. Pigs (n = 8/treatment) were exposed to treatments consisting of TN+/− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/− (35 ± 1 °C; 20% to 40% relative humidity), or PFTN+/− for 3 d. Data are reported as LSmeans, standard error is presented in parenthesis below respective mean value, and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
2Change in BW.
There was an effect of environmental treatment (P < 0.05) and day (P < 0.05) on circulating glucose levels (Table 3). PFTN groups had lower circulating glucose levels when compared with TN groups but were not significantly different from HS groups. HS and TN groups were not significantly different from one another. Animals had lower glucose levels on day 3 when compared with day −1. Irrespective of day, within treatment groups, HS and PFTN animals receiving supplement tended (P ≤ 0.10) to have lower glucose levels than those not receiving supplement, but TN+ animals had slightly higher glucose levels than TN− groups. Creatinine levels were elevated (P < 0.05) in HS groups on day 3 when compared with TN and PFTN groups (Table 3). Irrespective of day and treatment, supplement tended to increase (P ≤ 0.06) creatinine levels (Table 3). There was a treatment by supplement by day interaction (P < 0.05) on CK: HS+ animals had higher CK levels on day 3 when compared with TN+/TN− and PFTN+/PFTN− groups (Table 3). HS− animals had elevated CK levels when compared with baseline but were not significantly different from TN− animals (Table 3). Blood urea nitrogen (BUN) in PFTN groups was decreased (P < 0.05) when compared with TN groups, and HS groups were not significantly different from PFTN or TN (Table 3).
Table 3.
Effects of heat stress and CAPS–SUC supplementation on blood chemistry parameters in pigs
| Baseline1 | Treatment1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TN | HS | PFTN | TN | HS | PFTN | P-value | |||||||||||||
| Variable | − | + | − | + | − | + | − | + | − | + | − | + | Trmt | Supp | Day | T × S | T × D | S × D | T × S × D |
| Glucose, mg/dL | 117 (4.2) | 115 (4.2) | 118 (4.2) | 111 (4.1) | 115 (4.2) | 101 (4.2) | 110 (3.7) | 119 (3.5) | 109 (3.6) | 107 (3.4) | 105 (3.7) | 97 (3.5) | 0.003 | 0.118 | 0.004 | 0.100 | 0.412 | 0.038 | 0.775 |
| Creatinine, mg/dL | 0.89 (0.06) | 1.08 (0.05) | 1.02 (0.05) | 1.02 (0.05) | 1.01 (0.05) | 0.98 (0.05) | 0.99 (0.05) | 1.11 (0.05) | 1.26 (0.05) | 1.36 (0.05) | 1.08 (0.05) | 1.09 (0.05) | 0.001 | 0.056 | <0.0001 | 0.180 | <0.0001 | 0.508 | 0.205 |
| CK, U/L × 102 | 45.31 (17.79) | 50.63 (17.75) | 30.69 (17.67) | 24.88 (17.44) | 66.66 (17.69) | 45.58 (17.78) | 106.07 (35.95) | 39.23 (35.92) | 217.55 (38.43) | 379.63 (35.77) | 36.74 (35.90) | 30.62 (35.94) | <0.0001 | 0.454 | <0.0001 | 0.012 | <0.001 | 0.254 | 0.014 |
| BUN, mg/dL | 9.2 (0.63) | 9.3 (0.63) | 9.0 (0.63) | 8.7 (0.62) | 8.8 (0.63) | 8.5 (0.63) | 8.8 (0.56) | 9.8 (0.56) | 7.3 (0.56) | 8.4 (0.56) | 7.6 (0.56) | 7.3 (0.56) | 0.039 | 0.647 | 0.012 | 0.708 | 0.139 | 0.138 | 0.507 |
1All pigs were well fed in thermoneutral conditions with or without CAPS–SUC supplementation 2 d prior to the onset of treatments. Baseline blood samples were obtained 1 d prior to the onset of treatments. Pigs (n = 8/treatment) were exposed to treatments consisting of TN+/− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/− (35 ± 1 °C; 20% to 40% relative humidity), or PFTN+/− for 3 d, after which treatment blood samples were obtained. Data are reported as LSmeans, standard error is presented in parenthesis below respective mean value, and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
Regardless of day, lymphocyte count tended to decrease (P ≤ 0.05) in HS groups when compared with PFTN and TN groups (Table 4). Circulating monocyte count was increased (P < 0.05) in HS groups when compared with PFTN groups, but HS and TN groups were not significantly different (Table 4). Irrespective of treatment and day, basophil count was increased (P < 0.05) in groups receiving supplement when compared with those not receiving supplement (Table 4).
Table 4.
Effects of heat stress and CAPS–SUC supplementation on CBC in pigs
| Baseline1 | Treatment1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TN | HS | PFTN | TN | HS | PFTN | P-value | |||||||||||||
| Variable | − | + | − | + | − | + | − | + | − | + | − | + | Trmt | Supp | Day | T × S | T × D | S × D | T × S × D |
| Lymphocytes, cells/uL | 9.741 (1.211) | 11.427 (1.230) | 9.959 (1.353) | 7.985 (1.231) | 11.416 (1.236) | 9.778 (1.235) | 11.324 (1.204) | 12.098 (1.239) | 9.005 (1.203) | 8.663 (1.283) | 11.761 (1.262) | 11.918 (1.204) | 0.05 | 0.79 | 0.08 | 0.46 | 0.33 | 0.3 | 0.3 |
| Monocytes, cells/uL | 0.547 (0.193) | 0.878 (0.196) | 0.621 (0.234) | 1.141 (0.192) | 0.360 (0.192) | 0.324 (0.198) | 1.039 (0.172) | 0.639 (0.183) | 0.677 (0.172) | 1.347 (0.198) | 0.603 (0.183) | 0.722 (0.172) | <0.05 | 0.08 | 0.09 | <0.05 | 0.7 | 0.52 | 0.17 |
| Basophils, cells/uL | 0.102 (0.062) | 0.156 (0.062) | 0.110 (0.072) | 0.117 (0.062) | 0.000 (0.062) | 0.096 (0.062) | 0.022 (0.044) | 0.170 (0.042) | 0.015 (0.042) | 0.099 (0.048) | 0.047 (0.044) | 0.088 (0.042) | 0.35 | <0.05 | 0.47 | 0.77 | 0.61 | 0.56 | 0.58 |
| White blood Cell, cells/uL | 19.539 (1.773) | 21.813 (1.782) | 18.321 (1.917) | 19.721 (1.798) | 20.896 (1.819) | 17.272 (1.792) | 20.948 (1.645) | 20.866 (1.681) | 17.453 (1.633) | 19.021 (1.725) | 19.629 (1.721) | 20.391 (1.642) | 0.32 | 0.75 | 0.81 | 0.58 | 0.4 | 0.48 | <0.05 |
1All pigs were well fed in thermoneutral conditions with or without CAPS–SUC supplementation 2 d prior to the onset of treatments. Baseline blood samples were obtained 1 d prior to the onset of treatments. Pigs (n = 8/treatment) were exposed to treatments consisting of TN+/− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/− (35 ± 1 °C; 20% to 40% relative humidity), or PFTN+/− for 3 d, after which treatment blood samples were obtained. Data are reported as LSmeans, standard error is presented in parenthesis below respective mean value, and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
Irrespective of day and supplementation, ileal villus height tended (P ≤ 0.08) to be lower in HS and PFTN groups than in TN groups (391.63, 367.58, and 411.34 μm for HS, PFTN, and TN groups, respectively; Figure 3). No statistical differences were seen between groups in duodenal villus height or in duodenal and ileal crypt cell depth (Figure 3).
Figure 3.
Effects of HS and CAPS–SUC supplementation on villus height and crypt depth in the ileum (A, C) and duodenum (B, D) of growing pigs. Pigs (n = 8/treatment) were exposed to treatments consisting of TN+/TN− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/HS− (35 ± 1 °C; 20% to 40% relative humidity), or PFTN+/PFTN− for 3 d. Data are reported as LSmeans and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
Treatment and day both had significant effects on serum insulin levels. Animals in HS groups had significantly elevated (P < 0.05) insulin levels when compared with PFTN animals, but did not differ from TN groups (Figure 4). Insulin levels were decreased (P < 0.05) in animals on day 3 when compared to day −1 (Figure 4). There was no treatment by day interaction; however, supplement did not appear to have a significant effect on serum insulin concentration (Figure 4). Irrespective of treatment or supplementation, glucose:insulin was higher (P < 0.05) on day 3 of treatment when compared to day −1 (Figure 4). Treatment and supplement did not have a significant effect on glucose:insulin (Figure 4).
Figure 4.
Effects of HS and CAPS–SUC supplementation on insulin concentrations and glucose:insulin before and after treatment in growing pigs. Pigs (n = 8/treatment) were exposed to treatments consisting of TN+/TN− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/HS− (35 ± 1 °C; 20% to 40% relative humidity), or PFTN+/PFTN− for 3 d. Data are reported as LSmeans and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
Irrespective of treatment and supplement, serum IL-1β concentrations were lower (P < 0.05) on day 3 than on day −1 (14.3 vs. 9.0 pg/mL; Figure 5).There tended to be a treatment by day interaction in which all treatment groups, regardless of supplementation, tended to have lower circulating IL-1β levels on day 3 when compared with day −1 (Figure 5). There was a significant treatment by day interaction (P < 0.05) on serum (IL-6 concentration: there were no differences between groups on day −1 but by day 3, HS animals had lower circulating levels of IL-6 when compared with TN, although they were not significantly different from PFTN groups (Figure 5).
Figure 5.
Effects of HS and CAPS–SUC supplementation on serum IL-1β (A) and IL-6 (B) concentrations before and after treatment in growing pigs. Pigs were exposed to treatments consisting of TN+/TN− (21 ± 1.1 °C; 40% to 70% relative humidity), HS+/HS− (35 ± 1 °C; 20% to 40% relative humidity), or PFTN+/PFTN− for 3 d. Data are reported as LSmeans and statistical differences were accepted as significant at P < 0.05 or a tendency at P < 0.10.
Discussion
The goal of this study was to elucidate the efficacy of CAPS–SUC in its ability to partially attenuate the negative effects of HS on pig growth performance and intestinal morphology. We chose a heat load model based on our previous studies (Pearce et al., 2013b; Sanz Fernandez et al., 2014) where the temperature treatment is constant. We sought to prevent the animals’ body temperatures from exhibiting a diurnal rise and fall, typical of a cyclical heat load application, to allow the supplement to be tested during a high magnitude, constant HS response. As expected, an immediate effect of increased ambient temperature was an increase in rectal temperature in the HS groups. Measurable hyperthermia continued throughout the remainder of the experiment in these animals, with rectal temperatures staying approximately 1.2 °C higher than their TN counterparts. Supplementation with CAPS–SUC did not have a significant effect on the rectal temperatures of the animals in this study.
The onset of heat also led to a marked increase in respiration rate. This panting response is adopted to increase bodily heat load dissipation via evaporative cooling through moisture loss from the esophagus and lungs (Baumgard and Rhoads, 2013). Interestingly, the addition of the saccharin and capsicum oleoresin supplement to the diet of the HS+ group resulted in a 9.2% overall increase in respiration rate when compared with the non-supplemented HS− group. One explanation for the increased respiration rate in the HS+ groups may be related to the redirection of blood flow during HS. In bouts of hyperthermia, blood is directed away from the splanchnic bed to maximize perfusion of the skin for optimal heat dissipation (Lambert, 2009). This response may be counteracted, however, by the gut hormone GLP-2, which rapidly increases intestinal blood flow when released from enteroendocrine cells in the intestinal tract. Guan et al. (2006) and Stephens et al. (2006) both established this response when GLP-2 was given as an intravenous infusion in piglets. Moran et al. (2010) demonstrated that the addition of Sucram to the diets of weaning piglets resulted in a release of GLP-2 from enteroendorcine cells and a response similar to that observed when an intravenous infusion of GLP-2 was given. In the context of the current study, such a GLP-2 response could increase blood flow back to the GI tract, which may limit the animal’s ability to dispel heat from the skin. This may have led to the rise in respiration rate to increase evaporative cooling.
Reduced FI is a response that has been well documented in numerous HS studies involving various species, including pigs (Renaudeau et al., 2012; Pearce et al., 2013b). It is presumed that this decrease in nutrient ingestion is a behavioral adaptation to decrease metabolic heat production (Collin et al., 2001; Baumgard and Rhoads, 2013). As expected, environmental HS resulted in a reduction in average voluntary FI in HS groups during the treatment period when compared with TN groups, with decreases beginning immediately after the onset of heat. The PFTN groups had a similar intake pattern of the HS groups, with respect to supplementation, as part of the experimental design to lessen the confounding effects of different FI. Numerically, ad libitum-fed TN groups consumed more when their diet was supplemented with CAPS–SUC (2.25 vs. 1.91 kg/d for TN+ and TN−, respectively; overall average FI during the treatment period), indicating that the supplement may have the ability to increase FI in TN conditions. This intake response may also have been mediated by GLP-2 (Baldassano et al., 2016); however, the presence of HS or feed restriction clearly overrode the positive effect of supplementation on FI.
Following a similar pattern as that of FI, total BW gain was significantly reduced in HS and PFTN groups when compared with their TN, ad libitum-fed counterparts. Numerically, PFTN+ groups gained an average of 1.4 kg of BW, whereas the PFTN− groups gained 0.3 kg during the 3-d treatment period, suggesting that the addition of the artificial sweetener and capsicum oleoresin combination positively affected the rate at which the animals gained weight in undernourished conditions. Without taking into effect day or treatment, the results showed that supplementation of CAPS–SUC also increased feed efficiency, calculated by measuring the ratio of average weight of gain per day (kg) over average feed consumption per day (kg). On average per day, supplemented animals gained 0.12 kg more BW per kilogram of feed consumed than non-supplemented groups across the environmental treatments. Given that skeletal muscle growth is the primary driver for increased BW gain and that skeletal muscle can dramatically influence whole body fuel substrate dynamics, it is tempting to speculate that the supplement could modify metabolic flexibility. We have previously shown that HS decreases metabolic flexibility and limits the ability to use non-esterified fatty acids, whereas PFTN groups maintain metabolic flexibility and increase fatty acid oxidation (Zhao et al., 2018). Additional shifts of metabolic flexibility within skeletal muscle and/or changing the fuel substrates for use in inter-organ metabolic pathways could underlie an increase in feed efficiency with supplementation. Future studies are needed to examine the effect of supplementation on fuel substrate use and metabolic flexibility during HS and periods of undernutrition.
Overall, there was a tendency for HS and PFTN groups receiving supplement to have lower circulating glucose levels than those groups not receiving supplement. One postulation for this occurrence is that the addition of capsaicin to the diet may have the ability to decrease blood glucose. In a study conducted by Karlsson et al. (1994), mice treated with capsaicin showed an increase in early insulin secretory response and an increase in glucose clearance, but basal insulin and plasma glucose were not affected. Similarly, Tolan et al. (2001) identified capsaicin in its ability to decrease blood glucose levels in dogs when measured at the 2.5 time interval during an oral glucose tolerance test (OGTT), and that there was an increase in plasma insulin levels at this time point as well. Gram et al. (2005) also demonstrated in rats that capsaicin administration lowered mean blood glucose concentrations during an OGTT, but plasma insulin levels during the OGTT were unchanged. This study also concluded that the addition of capsaicin did not improve insulin resistance in obese Zucker rats. The pigs on a lowered plane of nutrition receiving capsaicin (as capsicum oleoresin in the CAPS–SUC supplement) in the current study followed similar trends to the ones in the above reports. On the contrary, TN+ animals tended to have higher glucose levels than their non-supplemented TN− counterparts.
Although some differences in blood glucose levels were seen between groups, concentrations can vary greatly based on physiological status and plane of nutrition of the subjects and are a reflection of dietary glucose absorption, hepatic output, and glucose clearance by tissues. In this study, animals were not specifically fasted prior to blood collection, so the use of blood glucose concentration as an indicator of overall glucose dynamics is limited. Additionally, glucose levels can be affected by physical stress, such as the use of restraint during blood draws (Moberg, 2000; Theil et al., 2012), as was the case in this experiment.
A marked reduction in FI, such as that observed in bouts of hyperthermia, typically leads to a decrease in circulating insulin levels due to the lack of dietary carbohydrates. However, despite the classic reduction in nutrient intake, hyperinsulinemia is a condition that has been documented in many HS studies across a multitude of species, including pigs, dairy and beef cattle, and rats (Hall et al., 1980; Torlińska et al., 1987; O’Brien et al., 2010; Wheelock et al., 2010). In agreement with those reports, the current study resulted in a 27.2% increase in circulating insulin concentrations in HS groups during the treatment period when compared with PFTN groups on the same plane of nutrition. The role of elevated insulin during HS is not well understood but has been implicated in its ability to upregulate heat shock proteins (Li et al., 2006).
HS animals showed an approximate 12% increase in serum creatinine levels when compared with TN and PFTN groups, and supplement appeared to increase creatinine levels, regardless of environmental treatment group. Additionally, HS groups had elevated CK levels when compared with the other groups. These results coincide with other HS studies involving heat-stressed rabbits, pigs, and humans (Kachadorian and Johnson, 1972; Marder et al., 1990; Alzeer et al., 1997; Pearce et al., 2013b). Circulating BUN, creatinine, and CK in the blood and/or urine have been used in other studies to assess the extent of muscle catabolism during stress (Lynch, 1990; Zhang, 2012; Keltz et al., 2013). An increased amount of these compounds in the circulatory system of heat-stressed animals in this project may point to an increase in muscle catabolism, consistent with the above studies. In the study conducted by Pearce et al. (2013b), a distinct increase in BUN was observed on day 1 of HS, but animals had reduced plasma BUN by days 3 and 7 when compared with TN groups. The current study produced no significant changes in BUN levels when blood was sampled on day 3, but it could be that BUN was increased at the onset of HS then had decreased by day 3. The results of the current study do coincide with Pearce et al. (2013b) report of sustained increased creatinine levels, however. Together, elevation of these parameters indicates that skeletal muscle damage and/or catabolism is occurring in response to HS. Some research suggests that capsaicin has the ability to decrease levels of markers typically associated with muscle damage, such as BUN, creatinine, and CK (Zhou et al., 1999; Hsu et al., 2016). However, the HS+ group in this study showed a much greater increase in creatinine and CK than the HS− group. These data differ from the results seen by Hsu et al. (2016), in which exercised-challenged mice showed a significant decrease in circulating creatinine and CK levels when capsaicin was orally administered prior to stress. The reason for the spike in the metabolites in HS+ animals is unclear but using these metabolic markers as indicators of the physiological status of muscle tissue can be limited, as dehydration and inflammation can also lead to an increase in their concentrations (Pearce et al., 2013b; Ozkan and Ibrahim, 2016). Further research is needed to determine if supplementation could provide a protective effect to the skeletal damage observed during HS.
The elicitation of an immune response has been observed in HS situations and negatively impacts growth and productivity of livestock (Morrow-Tesch et al., 1994; Hall et al., 2001; Mashaly et al., 2004; Pearce et al., 2013a). A study conducted by Tolan et al. (2001) have shown that capsaicin-treated dogs showed a 2.4 × 10–4 reduction in monocytes when compared with control groups. This study, however, resulted in an increase in monocyte count in HS groups when compared with PFTN animals, with HS animals receiving supplement having a numerically higher count than HS animals without supplement. Supplementation also raised basophil count in all groups regardless of environmental treatment. In contrast, a reduction in lymphocyte count was observed in groups exposed to high temperatures, similar to a recent study (Seelenbinder et al., 2018) and a study conducted by Mashaly et al. (2004) in which heat-stressed commercial laying hens showed an inhibition of lymphocyte activity post-treatment. These conflicting results make it unclear whether the supplement was successful in reducing systemic inflammatory response during HS and lowered nutrition.
As mentioned previously, a physiological reaction to high ambient temperatures is a damage to the GI tract. This most likely results from the reduction in blood flow to abdominal organs as the body attempts to maximize perfusion of peripheral blood vessels to decrease bodily heat load through the skin. Lessened blood flow to the enteric cells leads to hypoxia, Adenosine triphosphate depletion, and acidosis, ultimately resulting in apoptosis of these cells (Yan et al., 2006; Pearce et al., 2013a). Some studies measuring intestinal health during restricted nutrition and bouts of HS show that significant changes can be seen by morphological evaluation of the intestinal mucosa. Santos et al. (2015) demonstrated in broiler chickens that exposure to 4 d of HS resulted in villus denudation, decreased villus height and breadth, and crypt damage in the small intestine when compared with control birds. An experiment conducted by Yu et al. (2010) reported desquamation at the tips of intestinal villi and exposure of lamina propria in the duodenum of pigs exposed to 3 d of HS.
Activation of the T1R2/3 receptor increases secretion of GLP-2 in the small intestine, which has established intestinotrophic effects in a multitude of species and acts to increase blood flow to the splanchnic organs (Moran et al., 2010, 2014; Daly et al., 2012). Our hypothesis was that an induction of GLP-2 release stimulated by the CAPS–SUC supplement would promote GI growth and blood flow and would lessen the damage to the gut due to HS or feed restriction. In the current study, both HS and pair-feeding tended to decrease ileal villus height when compared with TN groups being fed ad libitum. However, no differences were seen between groups in duodenal villus height or duodenal and ileal crypt depth, which more closely resembles the outcomes seen in a study carried out by Quinteiro-Filho et al. (2010) in broiler chickens. It could be that our histology techniques did not completely encompass the changes to villi, as villus width was not measured, as was done in the study by Santos et al. (2015). An alternative explanation is that the dietary treatments were not fed sufficiently long to elicit a measurable improvement in gut structure. More comprehensive measurements are warranted to elucidate the effects of high ambient heat and nutrient restriction, in combination with CAPS–SUC supplementation, on intestinal morphology.
Increased permeability of the GI tract markedly increases the likelihood of translocation of bacterial endotoxins from the lumen and can increase the risk of local and systemic inflammation. Endotoxins, or lipopolysaccharides (LPS), are constituents of the membrane in Gram-negative bacteria—the endotoxins interact with LPS-sensitive cells, such as monocytes and macrophages, which mediate their pathogenicity (Galanos and Freudenberg, 1993). Binding of LPS to receptors on the surface of these cells triggers an intercellular cascade resulting in the release of pro-inflammatory cytokines and an elicitation of the innate immune response. Tsuji et al. (2010) demonstrated in a murine model that capsaicin inhibited tumor necrosis factor-α and IL-1β and augmented IL-10 production. Evaluation of serum IL-1β and IL-6 levels in the animals used in this study revealed no significant differences between HS groups receiving supplement, and circulating levels of IL-6 were actually lower in HS groups on day 3 when compared with TN groups. The results from Webel et al. (1997) suggested that peak cytokine release and immune response to endotoxin exposure occur within the first 12 h, and Pearce et al. (2013b) reported a similar reduction in circulating cytokines in heat-stressed pigs when sampling at a 24-h time point after the onset on heat. It is possible that more accurate information on the immune response could have been obtained by performing more frequent blood sampling after initiating environmental treatment.
Conclusions
It is clear that HS negatively impacts pig growth performance and the overall animal health through a variety of physiological mechanisms. The efficacy of a novel combination of artificial sweeteners and capsicum oleoresin was tested in its ability to partially mitigate some negative effects of HS. Our research shows that the addition of the supplement increased pig growth performance (Gain:Feed) across environmental treatment groups, but results evaluating ability of the supplement to improve physiological response(s), attenuate damage to the cellular architecture of the GI tract and reduce inflammatory response during HS are unclear. Further experiments—including those of longer duration—are needed to determine the effectiveness of functional feed additives to maximize pig performance in hot climates or in situations of limited FI.
Glossary
Abbreviations
- ADG
average daily gain
- bpm
breaths per minute
- BUN
blood urea nitrogen
- BW
body weight
- CAPS–SUC
artificial high intensity sweetener and capsicum oleoresin
- CBC
complete blood count
- CK
creatine kinase
- EDTA
Ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- FI
feed intake
- GI
gastrointestinal
- GLP
glucagon-like peptide
- HS
heat stress
- HS−
heat stress fed ad libitum without supplement
- HS+
heat stress fed ad libitum with supplement
- IL
interleukin
- LPS
lipopolysaccharides
- OGTT
oral glucose tolerance test
- PBS
phosphate-buffered saline
- PFTN−
thermoneutral conditions pair-fed to HS intake without supplement
- PFTN+
thermoneutral conditions pair-fed to HS intake with supplement
- RR
respiration rate
- RT
rectal temperature
- T1R2/3
taste receptor type 1 member 2/3
- TN−
thermoneutral conditions fed ad libitum without supplement
- TN+
thermoneutral conditions fed ad libitum
- WBC
white blood cell
Acknowledgments
The financial support for this work was provided, in part, by Pancosma SA, by the Virginia Agricultural Experiment Station (Blacksburg), and by the Hatch Program of the National Institute of Food and Agriculture.
Conflict of interest statement
The authors declare no real or perceived conflict of interest.
Literature Cited
- Alzeer, A. H., el-Hazmi M. A., Warsy A. S., Ansari Z. A., and Yrkendi M. S... 1997. Serum enzymes in heat stroke: prognostic implication. Clin. Chem. 43:1182–1187. [PubMed] [Google Scholar]
- Baldassano, S., Amato A., and Mulè F... 2016. Influence of glucagon-like peptide 2 on energy homeostasis. Peptides 86:1–5. doi: 10.1016/j.peptides.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Baumgard, L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Burrin, D. G., Stoll B., Guan X., Cui L., Chang X., and Hadsell D... 2007. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am. J. Physiol. Endocrinol. Metab. 292:E281–E291. doi: 10.1152/ajpendo.00129.2006 [DOI] [PubMed] [Google Scholar]
- Collin, A., van Milgen J., Dubois S., and Noblet J... 2001. Effect of high temperature and feeding level on energy utilization in piglets. J. Anim. Sci. 79:1849–1857. doi: 10.2527/2001.7971849x [DOI] [PubMed] [Google Scholar]
- Connor, E. E., Wall E. H., Bravo D. M., Evock-Clover C. M., Elsasser T. H., Baldwin R. L. 6th, Santín M., Vinyard B. T., Kahl S., and Walker M. P... 2017. Reducing gut effects from Cryptosporidium parvum infection in dairy calves through prophylactic glucagon-like peptide 2 therapy or feeding of an artificial sweetener. J. Dairy Sci. 100:3004–3018. doi: 10.3168/jds.2016-11861. [DOI] [PubMed] [Google Scholar]
- Curtis, S. E. 1983. Environmental management in animal agriculture. Ames (IA): Iowa State University Press. [Google Scholar]
- Daly, K., Al-Rammahi M., Arora D. K., Moran A. W., Proudman C. J., Ninomiya Y., and Shirazi-Beechey S. P... 2012. Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303:R199–R208. doi: 10.1152/ajpregu.00031.2012 [DOI] [PubMed] [Google Scholar]
- Drucker, D. J., Erlich P., Asa S. L., and Brubaker P. L... 1996. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. U. S. A. 93:7911–7916. doi: 10.1073/pnas.93.15.7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA . 2016. Climate change indicators: U.S. and global temperature. Available from https://www.epa.gov/climate-indicators/climate-change-indicators-us-and-global-temperature [Accessed January 27, 2020]
- Galanos, C., and Freudenberg M. A... 1993. Bacterial endotoxins: biological properties and mechanisms of action. Mediators Inflamm. 2:S11–S16. doi: 10.1155/S0962935193000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram, D. X., Hansen A. J., Wilken M., Elm T., Svendsen O., Carr R. D., Ahrén B., and Brand C. L... 2005. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur. J. Endocrinol. 153:963–969. doi: 10.1530/eje.1.02046 [DOI] [PubMed] [Google Scholar]
- Guan, X., Karpen H. E., Stephens J., Bukowski J. T., Niu S., Zhang G., Stoll B., Finegold M. J., Holst J. J., Hadsell D.,. et al. 2006. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130:150–164. doi: 10.1053/j.gastro.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Hall, D. M., Buettner G. R., Oberley L. W., Xu L., Matthes R. D., and Gisolfi C. V... 2001. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509 [DOI] [PubMed] [Google Scholar]
- Hall, G. M., Lucke J. N., Lovell R., and Lister D... 1980. Porcine malignant hyperthermia. VII: hepatic metabolism. Br. J. Anaesth. 52:11–17. doi: 10.1093/bja/52.1.11 [DOI] [PubMed] [Google Scholar]
- Hsu, Y.-J., Huang W.-C., Chiu C.-C., Liu Y.-L., Chiu W.-C., Chiu C.-H., Chiu Y.-S., and Huang C.-C... 2016. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients 8:648. doi: 10.3390/nu8100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate change (IPCC). 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Core Writing Team, Pachauri, R. K., and L. A. Meyer, editors. IPCC; Geneva, Switzerland; p. 151. [Google Scholar]
- Kachadorian, W. A., and Johnson R. E... 1972. The effects of activity and thermal environment on creatinine clearance. Int. Z. Angew. Physiol. 30:161–170. doi: 10.1007/bf00699117 [DOI] [PubMed] [Google Scholar]
- Karlsson, S., Scheurink A. J., Steffens A. B., and Ahrén B... 1994. Involvement of capsaicin-sensitive nerves in regulation of insulin secretion and glucose tolerance in conscious mice. Am. J. Physiol. 267:R1071–R1077. doi: 10.1152/ajpregu.1994.267.4.R1071 [DOI] [PubMed] [Google Scholar]
- Keltz, E., Khan F. Y., and Mann G... 2013. Rhabdomyolysis. The role of diagnostic and prognostic factors. Muscles Ligaments Tendons J. 3:303–312. [PMC free article] [PubMed] [Google Scholar]
- Lambert, G. P. 2009. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 87:E101–E108. doi: 10.2527/jas.2008-1339 [DOI] [PubMed] [Google Scholar]
- Li, G., Ali I. S., and Currie R. W... 2006. Insulin induces myocardial protection and Hsp70 localization to plasma membranes in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 291:H1709–H1721. doi: 10.1152/ajpheart.00201.2006 [DOI] [PubMed] [Google Scholar]
- Liu, Y., Che T. M., Song M., Lee J. J., Almeida J. A., Bravo D., Van Alstine W. G., and Pettigrew J. E... 2013. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 91:5668–5679. doi: 10.2527/jas.2013-6495 [DOI] [PubMed] [Google Scholar]
- Lynch, E. C. 1990. Peripheral blood smear. In: Walker, H. K., Hall W. D., and Hurst J. W., editors. Clinical methods: the history, physical, and laboratory examinations. Oxford (UK):Butterworth Publishers; p. 732–734. [PubMed] [Google Scholar]
- Marder, J., Eylath U., Moskovitz E., and Sharir R... 1990. The effect of heat exposure on blood chemistry of the hyperthermic rabbit. Comp. Biochem. Physiol. A. Comp. Physiol. 97:245–247. doi: 10.1016/0300-9629(90)90179-v [DOI] [PubMed] [Google Scholar]
- Mashaly, M. M., Hendricks G. L. 3rd, Kalama M. A., Gehad A. E., Abbas A. O., and Patterson P. H... 2004. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 83:889–894. doi: 10.1093/ps/83.6.889 [DOI] [PubMed] [Google Scholar]
- Moberg G. P. 2000. Biological response to stress: implications for animal welfare. In: Moberg, G. P., and Mench J. A., editors. The biology of animal stress: basic principles and implications for animal welfare. Boston (MA):CABI Publishing; p. 1–22. [Google Scholar]
- Moran, A. W., Al-Rammahi M. A., Arora D. K., Batchelor D. J., Coulter E. A., Ionescu C., Bravo D., and Shirazi-Beechey S. P... 2010. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br. J. Nutr. 104:647–655. doi: 10.1017/S0007114510000954 [DOI] [PubMed] [Google Scholar]
- Moran, A. W., Al-Rammahi M. A., Daly K., Grand E., Ionescu C., Bravo D. M., Wall E. H., and Shirazi-Beechey S. P... 2020. Consumption of a natural high-intensity sweetener enhances activity and expression of rabbit intestinal Na+/glucose cotransporter 1 (SGLT1) and improves colibacillosis-induced enteric disorders. J. Agric. Food Chem. 68:441–450. doi: 10.1021/acs.jafc.9b04995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, A. W., Al-Rammahi M., Zhang C., Bravo D., Calsamiglia S., and Shirazi-Beechey S. P... 2014. Sweet taste receptor expression in ruminant intestine and its activation by artificial sweeteners to regulate glucose absorption. J. Dairy Sci. 97:4955–4972. doi: 10.3168/jds.2014-8004 [DOI] [PubMed] [Google Scholar]
- Morrow-Tesch, J. L., McGlone J. J., and Salak-Johnson J. L... 1994. Heat and social stress effects on pig immune measures. J. Anim. Sci. 72:2599–2609. doi: 10.2527/1994.72102599x [DOI] [PubMed] [Google Scholar]
- Mount, L. E. 1979. Adaptation to thermal environment. Man and his productive animals. London (UK):Edward Arnold (Publishers) Ltd. [Google Scholar]
- Murai, M., Tsuji F., Nose M., Seki I., Oki K., Setoguchi C., Suhara H., Sasano M., and Aono H... 2008. SA13353 (1-[2-(1-Adamantyl)ethyl]-1-pentyl-3-[3-(4-pyridyl)propyl]urea) inhibits TNF-alpha production through the activation of capsaicin-sensitive afferent neurons mediated via transient receptor potential vanilloid 1 in vivo. Eur. J. Pharmacol. 588:309–315. doi: 10.1016/j.ejphar.2008.04.037 [DOI] [PubMed] [Google Scholar]
- Nevius, E., Srivastava P. K., and Basu S... 2012. Oral ingestion of Capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol. 5:76–86. doi: 10.1038/mi.2011.50 [DOI] [PubMed] [Google Scholar]
- O’Brien, M. D., Rhoads R. P., Sanders S. R., Duff G. C., and Baumgard L. H... 2010. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 38:86–94. doi: 10.1016/j.domaniend.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Ozkan, I., and Ibrahim C. H... 2016. Dehydration, skeletal muscle damage and inflammation before the competitions among the elite wrestlers. J. Phys. Ther. Sci. 28:162–168. doi: 10.1589/jpts.28.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patience, J. F., Umboh J. F., Chaplin R. K., and Nyachoti C. M... 2005. Nutritional and physiological responses of growing pigs exposed to a diurnal pattern of heat stress. Liv. Prod. Sci. 96:205–214. doi: 10.1016/j.livprodsci.2005.01.012 [DOI] [Google Scholar]
- Pearce, S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H... 2013b. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91(5):2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Pearce, S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., Rhoads R. P., Baumgard L. H., and Gabler N. K... 2013c. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 8:e70215. doi: 10.1371/journal.pone.0070215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, S. C., Mani V., Weber T. E., Rhoads R. P., Patience J. F., Baumgard L. H., and Gabler N. K... 2013a. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi: 10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho, W. M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M. L., Sakai M., Sá L. R., Ferreira A. J., and Palermo-Neto J... 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 89:1905–1914. doi: 10.3382/ps.2010-00812 [DOI] [PubMed] [Google Scholar]
- Renaudeau, D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J... 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Renaudeau, D., Gourdine J., Silva B., and Noblet J... 2008. Nutritional routes to attenuate heat stress in pigs. In: Rowlinson, P., Steele M., and Nefzaoui A., editors. Livestock and global climate change. Cambridge (UK): Cambridge University Press; p. 134. [Google Scholar]
- Santos, R. R., Awati A., Roubos-van den Hil P. J., Tersteeg-Zijderveld M. H., Koolmees P. A., and Fink-Gremmels J... 2015. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathol. 44:19–22. doi: 10.1080/03079457.2014.988122 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez, M. V., Pearce S. C., Gabler N. K., Patience J. F., Wilson M. E., Socha M. T., Torrison J. L., Rhoads R. P., and Baumgard L. H... 2014. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 8:43–50. doi: 10.1017/S1751731113001961 [DOI] [PubMed] [Google Scholar]
- Seelenbinder, K. M., Zhao L. D., Hanigan M. D., Hulver M. W., McMillan R. P., Baumgard L. H., Selsby J. T., Ross J. W., Gabler N. K., and Rhoads R. P... 2018. Effects of heat stress during porcine reproductive and respiratory syndrome virus infection on metabolic responses in growing pigs. J. Anim. Sci. 96:1375–1387. doi: 10.1093/jas/sky057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, J., Stoll B., Cottrell J., Chang X., Helmrath M., and Burrin D. G... 2006. Glucagon-like peptide-2 acutely increases proximal small intestinal blood flow in TPN-fed neonatal piglets. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R283–R289. doi: 10.1152/ajpregu.00588.2005 [DOI] [PubMed] [Google Scholar]
- St-Pierre, N., Cobanov B., and Schnitkey G... 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Theil P. K., Pedersen L. J., Jensen M. B., Yde C. C., Bach Knudsen K. E. 2012. Blood sampling and hemolysis affect concentration of plasma metabolites. J. Anim. Sci. 90:412–414. doi: 10.2527/jas.53968 [DOI] [PubMed] [Google Scholar]
- Tolan, I., Ragoobirsingh D., and Morrison E. Y... 2001. The effect of capsaicin on blood glucose, plasma insulin levels and insulin binding in dog models. Phytother. Res. 15:391–394. doi: 10.1002/ptr.750 [DOI] [PubMed] [Google Scholar]
- Torlińska, T., Banach R., Paluszak J., and Gryczka-Dziadecka A... 1987. Hyperthermia effect on lipolytic processes in rat blood and adipose tissue. Acta Physiol. Pol. 38:361–366. [PubMed] [Google Scholar]
- Tsuji, F., Murai M., Oki K., Seki I., Ueda K., Inoue H., Nagelkerken L., Sasano M., and Aono H... 2010. Transient receptor potential vanilloid 1 agonists as candidates for anti-inflammatory and immunomodulatory agents. Eur. J. Pharmacol. 627:332–339. doi: 10.1016/j.ejphar.2009.10.044 [DOI] [PubMed] [Google Scholar]
- Vegge, A., Thymann T., Lund P., Stoll B., Bering S. B., Hartmann B., Jelsing J., Qvist N., Burrin D. G., Jeppesen P. B.,. et al. 2013. Glucagon-like peptide-2 induces rapid digestive adaptation following intestinal resection in preterm neonates. Am. J. Physiol. Gastrointest. Liver Physiol. 305:G277–G285. doi: 10.1152/ajpgi.00064.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel, D. M., Finck B. N., Baker D. H., and Johnson R. W... 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 75:1514–1520. doi: 10.2527/1997.7561514x [DOI] [PubMed] [Google Scholar]
- Wheelock, J. B., Rhoads R. P., Vanbaale M. J., Sanders S. R., and Baumgard L. H... 2010. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 93:644–655. doi: 10.3168/jds.2009-2295 [DOI] [PubMed] [Google Scholar]
- Yan, Y. E., Zhao Y. Q., Wang H., and Fan M... 2006. Pathophysiological factors underlying heatstroke. Med. Hypotheses 67:609–617. doi: 10.1016/j.mehy.2005.12.048 [DOI] [PubMed] [Google Scholar]
- Yu, J., Yin P., Liu F., Cheng G., Guo K., Lu A., Zhu X., Luan W., and Xu J... 2010. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 156:119–128. doi: 10.1016/j.cbpa.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Zhang, M.-H. 2012. Rhabdomyolosis and its pathogenesis. World J. Emerg. Med. 3:11. doi: 10.5847/wjem.j.issn.1920-8642.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., McMillan R. P., Xie G., Giridhar S. G. L. W., Baumgard L. H., El-Kadi S., Selsby J., Ross J., Gabler N., Hulver M. W.,. et al. 2018. Heat stress decreases metabolic flexibility in skeletal muscle of growing pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315:R1096–R1106. doi: 10.1152/ajpregu.00404.2017 [DOI] [PubMed] [Google Scholar]
- Zhou, F. W., Li Y. J., and Deng H. W... 1999. Early and delayed protection by capsaicin against reperfusion injury in rat hearts. Zhongguo Yao Li Xue Bao 20:912–916. [PubMed] [Google Scholar]