Abstract
Despite their comparatively low abundance in biological membranes, phosphoinositides are key to the regulation of a diverse array of signaling pathways and direct membrane traffic. The role of phosphoinositides in the initiation and progression of endocytic pathways has been studied in considerable depth. Recent advances have revealed that distinct phosphoinositide species feature prominently in clathrin-dependent and -independent endocytosis as well as in phagocytosis and macropinocytosis. Moreover, a variety of intracellular and cell-associated pathogens have developed strategies to commandeer host cell phosphoinositide metabolism to gain entry and/or metabolic advantage, thereby promoting their survival and proliferation. Here, we briefly survey the current knowledge on the involvement of phosphoinositides in endocytosis, phagocytosis, and macropinocytosis and highlight several examples of molecular mimicry employed by pathogens to either “hitch a ride” on endocytic pathways endogenous to the host or create an entry path of their own.
Keywords: endocytosis, phagocytosis, macropinocytosis, phosphoinositides, inositides, signaling, traffic, pathogen
Introduction: phosphoinositides and the internalization of the extracellular milieu
The seven phosphoinositides, which form through combinatory phosphorylation of the inositol ring at positions D3, D4, and D5 ( Figure 1A), are present primarily on the cytosolic surface of biological membranes. By influencing the net charge of cellular endomembranes and directing the binding of ligands, phosphoinositides control the traffic and identity of organelles. Phosphoinositides are dynamic; their abundance and subcellular distribution are regulated in both time and space by active phosphorylation and dephosphorylation reactions as well as by transport between organelles via both vesicular and non-vesicular traffic. The resulting distinct—often inhomogeneous—accumulation of inositides can recruit proteins to specific organelles and even to subdomains therein. Such recruitment, which is often accompanied by allosteric activation, is made possible by specific protein domains that stereospecifically recognize defined phosphoinositide headgroups 1– 3.
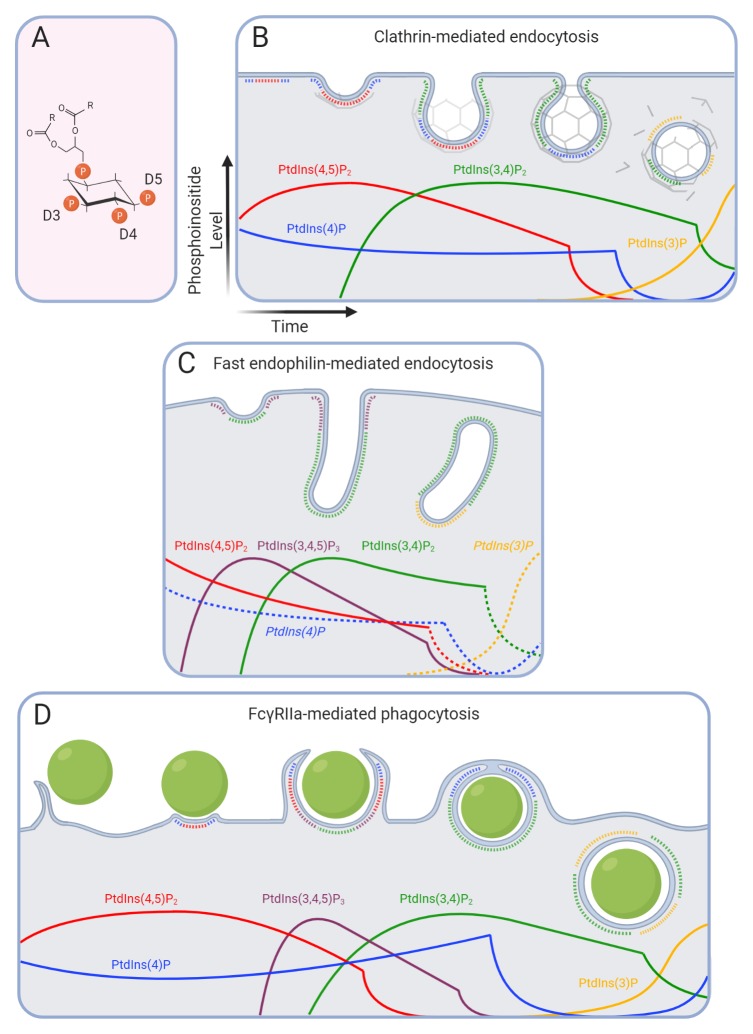
Figure 1. Phosphoinositide transitions during endocytic processes.
( A) The seven phosphoinositide species are derived from the same backbone through combinatory phosphorylation at positions D3, D4, and D5 of the inositol ring. Fatty acyl chains are abbreviated as (R) for simplicity. Phosphoinositides control and identify distinct stages of clathrin-mediated endocytosis ( B), fast endophilin-mediated endocytosis ( C), and Fcγ receptor-mediated phagocytosis ( D). Transitions that are speculative, i.e. not currently supported by experimental data, are labeled in italics and shown with dotted lines; they are predicted based on other endocytic pathways or on the presence of their precursor and/or the product of their hydrolysis. Although lipids intermix, phosphoinositides are drawn as single non-overlapping domains for simplicity. PtdIns, phosphatidylinositol.
In resting cells, phosphatidylinositol 4,5- bisphosphate—hereafter PtdIns(4,5)P 2—predominates on the inner leaflet of the plasma membrane (PM), where it regulates ion transport and cytoskeleton anchorage and is a source of multiple second messengers 2, 3. PtdIns(4)P is abundant in the Golgi complex and is also found on the inner leaflet of the PM, where together with PtdIns(4,5)P 2 it controls the non-vesicular counter-transport of phosphatidylserine to the PM 4– 6. The 3-phosphorylated species PtdIns(3,4,5)P 3 and PtdIns(3,4)P 2 are much less abundant in the PM of resting cells 3, 7. However, in response to a variety of cellular ligands including hormones, growth factors, and cytokines, the concentration of these rarer inositide species can be amplified drastically (by as much as 100-fold) to regulate key cellular processes. That dysregulated phosphoinositide metabolism underlies numerous human pathologies 1– 3 is a testament to the paramount importance of these lipids in cellular homeostasis.
Endocytosis is one of many key functions influenced by phosphoinositides. In virtually all cells, endocytosis is required for nutrient acquisition, cell-surface receptor internalization, and signaling regulation. In addition, some specialized cell types employ endocytosis for the surveillance and removal of foreign threats 8– 12. Strikingly, the endocytic machinery of the host cell can also be hijacked by some pathogens, which use molecular mimicry and deploy sophisticated toxins to gain entry to the intracellular environment 13– 15. Because they play critical roles in the formation and maturation of endosomes, phosphoinositides are often targeted by pathogens in their efforts to subvert host endocytic pathways.
Here, we review the initial events of a selection of endocytic pathways and their regulation by inositides, taking note of upstream regulators and downstream effectors. Finally, we highlight several examples of pathogenic organisms that have evolved ways to “hitch a ride” on endogenous endocytic pathways or have cleverly constructed uptake mechanisms by mimicking host pathways.
Receptor-mediated endocytosis
Clathrin-mediated endocytosis
In many cell types, clathrin-mediated endocytosis (CME) is the dominant endocytic pathway supporting housekeeping functions 16. The hallmark of CME and its distinction from other endocytic pathways is the formation of the clathrin triskelion lattice ( Figure 1B), which functions in concert with the large GTPase dynamin that mediates fission from the PM 17. Clathrin relies on the organized recruitment of over 50 adaptor and scaffolding proteins to form the clathrin-coated pit (CCP) 8. Initiation occurs at sites of low curvature enriched in PtdIns(4,5)P 2 synthesized mainly by type I phosphatidylinositol 4-phosphate 5-kinases 18– 22. Adaptors such as the heterotetrameric AP-2 complex, CALM, FCHo1/2, and epsin bind this lipid at the PM, recruit clathrin, and bridge and cluster to cargo molecules 23– 30. Specific cargoes destined for endocytosis therefore become enriched at the PM with clathrin and PtdIns(4,5)P 2.
Following cargo capture and clustering, its structural resistance must be overcome for the membrane to invaginate and form a spherical CCP ( Figure 1B). Such remodeling is elicited by cooperation between the clathrin lattice coat and scaffold proteins of the Bin, Amphiphysin and Rvs (BAR) domain family 8. BAR domains are dimeric membrane-binding modules that sense and induce membrane curvature/tubulation through their oligomerization 31, 32. Interestingly, BAR domains differ in their curvature and are recruited to CCPs in a sequential manner to promote neck constriction: F-BAR proteins with shallow curvature—such as FCH01/2—are recruited early, BAR domain proteins with intermediate curvature—like sorting nexin 9 (SNX9)—are recruited midway, and highly curved N-BAR proteins—such as endophilin and amphiphysin—are recruited at late stages 23, 30, 32– 35.
The hierarchical recruitment of BAR domain proteins is controlled through not only increasing membrane curvature but also changes in phosphoinositides. Maturation of the CCP requires the activity of the class II phosphatidylinositol 3-kinase (PI3K) C2α, which is activated at CCPs 36, 37 to locally generate PtdIns(3,4)P 2 from PtdIns(4)P 34. PtdIns(3,4)P 2 recruits the BAR domain-containing proteins SNX9 and SNX18 via their PX domains, which, together with AP-2, trigger their oligomerization and the constriction of the CCP neck 38 ( Figure 1B). Depletion of either PI3K-C2α or SNX9/18 leads to stalling of CCP necks in a U-shaped conformation 34. Actin polymerization also contributes to the shaping of the maturing CCP. The Arp2/3 complex stimulates branched F-actin assembly downstream of the nucleation-promoting factor (NPF) neural Wiskott-Aldrich syndrome protein (N-WASP) 39, 40. Both SNX9 41 and FCHSD1/2, in the presence of PtdIns(4,5)P 2/PtdIns(3,4)P 2 42, can activate N-WASP. Following constriction of the neck to an Ω-configuration, multiple BAR domain proteins recruit the fission executioner, dynamin 31.
Throughout maturation and fission, the levels of PtdIns(4,5)P 2 appear to be controlled by 5-phosphatases of the synaptojanin (Synj) family. The p170 isoform of Synj-1 is recruited early during maturation 43, while the p145 isoform is recruited shortly before dynamin via the N-BAR protein endophilin 33, 43– 45. Interestingly, the dephosphorylation of PtdIns(4,5)P 2 occurs preferentially in highly curved membranes; thus, Synj may aid in dynamin-mediated fission of the CCP neck 46. The clearance of PtdIns(4,5)P 2 following scission is also necessary for vesicle uncoating 47, 48 and is supported in non-neuronal cells by other 5-phosphatases, including OCRL 49. Its dephosphorylated product, PtdIns(4)P, may directly support clathrin coat disassembly by recruiting auxilin2 and the ATPase HSC70 50, 51. Following fission, PtdIns(4)P is hydrolyzed by the Sac2 phosphatase 52, 53, while remaining PtdIns(3,4)P 2 persists until its hydrolysis by the early endosome-localized INPP4A/B phosphatase 54, 55. On early endosomes, PtdIns(3)P is synthesized mainly by Vps34, a class III PI3K, but contributions from class II PI3Ks have been noted 56, 57. PtdIns(3)P is also posited to support clathrin coat disassembly by recruiting auxilin1 and HSC70 50, 51.
Clathrin-independent endocytosis
Here we discuss fast endophilin-mediated endocytosis (FEME), a form of clathrin-independent endocytosis (CIE). We refer the reader to several recent reviews on other important CIE pathways which occur in various tissue types 9, 58– 60. These include clathrin-independent carriers/glycosylphosphatidylinositol-anchored protein-enriched endocytic compartments (or CLIC/GEEC), ultrafast endocytosis, generalized interleukin-2 receptor endocytosis, and caveolae.
Fast endophilin-mediated endocytosis
Occurring predominantly at the leading edge of cells, FEME is an actin- and dynamin-dependent pathway that mediates the ligand-triggered uptake of several families of surface receptors 61. This includes G-protein-coupled receptors (α2a, β1 but not β2-adrenergic, dopaminergic D3 and D4, muscarinic acetylcholine receptor 4), receptor tyrosine kinases ([RTKs] EGFR, HGFR, VEGF, and PDGF among others), tyrosine receptor kinase B, and the interleukin-2 receptor (2Rα, 2Rβ, and γ c) in lymphocytes 61– 65. The N-BAR protein endophilin acts as a critical node in FEME, utilizing both its BAR domain as a scaffold for oligomerization on membranes and its numerous SH3 domain interactions to coordinate the capture of activated cargo with membrane bending and fission 9. To capture cargo, endophilin binds directly to proline-rich motifs present in cytosolic loops of many cargoes 61 or relies on intermediate adaptors in the case of RTKs 62, 63 and the tyrosine receptor kinase B 64.
Prior to receptor activation, endophilin forms prominent assemblies at lamellipodia through interaction with the protein lamellipodin and PtdIns(3,4)P 2 ( Figure 1C) 66. The Pleckstrin Homology (PH) domain of lamellipodin associates with PtdIns(3,4)P 2-rich regions of the membrane, and endophilin, in turn, is scaffolded onto lamellipodin through at least 10 binding sites 66, 67. In contrast to CME, PtdIns(3,4)P 2 is generated at lamellipodia and during FEME through the production of PtdIns(3,4,5)P 3 by class I PI3Ks 68 and its subsequent dephosphorylation by the phosphatases SHIP1/2 69. Consistent with this phosphoinositide transition, RNA interference or pharmacological inhibition of SHIP1/2 decreases endophilin assembly and FEME, while PTEN reduction, which hydrolyzes position D3 of PtdIns(3,4,5)P 3 and PtdIns(3,4)P 2 70, 71, increases endophilin assembly 61. PtdIns(4,5)P 2 levels are controlled during FEME by the 5-phosphatase Synj, which is recruited to lamellipodia via endophilin 47, 72.
FEME promotes the fission of long tubular endosomes containing activated receptors. Indeed, endophilin can mediate extensive tubulation and vesicle formation when it attains high local concentrations 44, 73. However, a recent screen identified the F-BAR proteins FBP17 and CIP4 as being necessary for FEME initiation through the recruitment of SHIP2 and synthesis of PtdIns(3,4)P 2 74, 75. Interestingly, Cdc42 (a Rho-family GTPase) can recruit FB17 and CIP4 to the membrane when GTP bound, but GDP-bound Cdc42 (which is formed by the GAP activity of N-BAR proteins SH3BP1 and RICH1) terminates this cycle. After fission, PtdIns(3,4)P 2 is cleared from tubular carriers by INPP4A/4B, releasing machinery for subsequent FEME cycles 61. In such a way, Cdc42 cycling together with inositides control the sequential recruitment of BAR and SH3 domain proteins for constriction and ultimately fission 75.
Phagocytosis and macropinocytosis
Phagocytosis
Innate immunity relies on phagocytosis to recognize, internalize, and inactivate potential pathogens such as fungi and bacteria. Phagocytosis is a receptor-mediated, actin-dependent endocytic pathway that internalizes cargo larger than 0.5 μm into a membrane-bound compartment termed the phagosome. The nascent phagosomal membrane, initially derived from the PM, is transformed (matures) through a highly regulated cascade of fusion and fission events to create an acidic, lytic luminal environment that is hostile to pathogens. Professional phagocytes of the myeloid lineage not only kill invading microorganisms but also present antigens generated upon their digestion to lymphocytes, coupling the innate and adaptive immune responses. Phagocytes also maintain tissue homeostasis by clearing endogenous debris and apoptotic cells. Such functional diversity necessitates an arsenal of phagocytic receptors capable of recognizing pathogen- or danger-associated determinants; these can be intrinsic to the target or the result of deposition of serum factors termed opsonins (e.g. immunoglobulin G [IgG] and complement component iC3b) 10, 12. Here we discuss primarily phagocytosis mediated by Fcγ receptors (FcγR) that recognize IgG. Not only is this type of phagocytosis the best studied to date but it also has the additional advantage that it can be reconstituted in non-phagocytic cells (fibroblasts, epithelial cells) by heterologous expression of myeloid FcγR 76– 78.
The cytosolic domain of FcγRs encodes immunoreceptor tyrosine-based activation motifs (ITAMs), which are substrates of tyrosine phosphorylation by Src-family kinases and Syk. The simultaneous engagement of multiple FcγRs by IgG coating the target particles triggers lateral receptor clustering and exclusion of cytosolic tyrosine phosphatases, steps that are absolutely necessary for the sustained signaling required for engulfment 10, 12, 79, 80. In the initial stages, PtdIns(4,5)P 2 is present and modestly enriched at the site of particle engagement and in the actin-rich membrane pseudopods that zipper around the phagocytic target ( Figure 1D) 81. Multiple PIP5K isoforms that synthesize PtdIns(4,5)P 2 from PtdIns(4)P localize to the phagocytic cup; their genetic perturbation has severe effects on particle engagement and uptake, altering actin remodeling in the nascent cup 81– 83. The Arp2/3 complex, activated by the NPFs WASP, N-WASP, and presumably also WASP-family verprolin homologous protein (WAVE) complexes, mediate the initial burst of actin polymerization associated with the extension of phagocytic pseudopodia. Recruitment of the NPFs occurs in response to Cdc42 and Rac stimulation via multiple adaptors, including Nck 84 and Grb2/Gab2 85, downstream of activated ITAMs. PtdIns(4,5)P 2 coordinates the activation of these NPFs in the extending pseudopods 86– 91.
While abundant in the pseudopods, PtdIns(4,5)P 2 is rapidly cleared from the base of the phagocytic cup, becoming undetectable in the nascent phagosome ( Figure 1D). The local loss of the inositide marks a critical transition for particle internalization, as it demarcates the regional disassembly of F-actin that is seemingly essential for phagosome closure 81, 92. Indeed, artificially elevating PtdIns(4,5)P 2 at the cup by overexpression of PIP5Ks sustains F-actin at the base of the cup and precludes particle uptake 92. The clearance of PtdIns(4,5)P 2 from the nascent cup occurs via multiple pathways: phospholipase C (PLC)-mediated hydrolysis is thought to be the predominant mechanism 81, but PI3K-mediated phosphorylation to PtdIns(3,4,5)P 3 93, 94 (see below), focal exocytic insertion of endomembranes devoid of the inositide at the base of the cup 95, 96, and dephosphorylation by the phosphatases OCRL and INPP5B 97 also contribute. As a result of such phosphatase activity, the concentration of PtdIns(4)P spikes in the membrane of the nascent phagosome ( Figure 1D). PtdIns(4)P then declines abruptly after phagosome sealing, an effect attributed mainly to Sac2, although PLC may be partly responsible 98.
The 3-phosphorylated inositides also feature prominently in phagocytosis. PtdIns(3,4,5)P 3 and PtdIns(3,4)P 2 accumulate robustly in pseudopods and in the forming phagosomal cup 94, 99, 100 ( Figure 1D). The p85 regulatory subunit of class I PI3K can be recruited directly by activated ITAMs 101 or by other adaptor proteins 85, 102, 103 to mediate the synthesis of PtdIns(3,4,5)P 3. The 5-phosphatases SHIP1 and SHIP2 are recruited and activated by both ITAMs and immunoreceptor tyrosine-based inhibitory motif domains (ITIMs) found in FcγRIIB and mediate the dephosphorylation of PtdIns(3,4,5)P 3 to PtdIns(3,4)P 2 100, 104– 108. Analysis of the dependence of phagocytosis on PI3K signaling has revealed a peculiar disparity in the literature: although actin polymerization persists at sites of phagocytosis despite pharmacological inhibition of PI3Ks, the uptake of large (≥5 μm) but not small particles is inhibited 99, 109– 111. Several explanations for the phenomenon have been offered, the most compelling being that PI3K products signal the termination of Rho-GTPase signaling that is required for progression of actin polymerization around large targets. Following pseudopod extension, GTP hydrolysis by Rac and Cdc42 GTPases is necessary for termination of F-actin assembly at the base of the phagocytic cup, an event that is critical for the engulfment of large 110, 112 but not small particles 92. Accordingly, a recent study identified the RhoGAPs ARHGAP12, ARHGAP25, and SH3BP1 as being recruited to the phagocytic cup in a PI3K-dependent manner and established that they are required for large, but not small, particle internalization 110. Consistent with this interpretation, the overexpression of SHIP1—which is predicted to reduce PtdIns(3,4,5)P 3—inhibits FcγR-mediated phagocytosis of large particles while its 5-phosphatase-dead counterpart (that exerts a dominant-negative inhibitory effect) or knockout of SHIP1 enhances phagocytosis of large particles 106, 113. Thus, products of class I PI3K activation signal the de-activation of Rho-family GTPases and actin disassembly 110, 112. Why is the conversion of phosphoinositides paramount to phagosome sealing? The continued de novo polymerization of actin along extending pseudopods is likely to exhaust one or more cytoskeletal factors. The clearance of PtdIns(4,5)P 2 and synthesis of PtdIns(3,4,5)P 3 likely orchestrate both the termination of actin polymerization and the disassembly of existing actin filaments at the base of the cup, which likely facilitate the recycling of limiting machinery components to pseudopods 12, 100, 110.
During phagocytosis, actin polymerization does not only occur at advancing pseudopods. Arp2/3 also induces the assembly of actin in discrete podosome-like structures that exert perpendicular pressure on the PM, promoting receptor engagement and zippering around the target 114. Podosome initiation in the nascent phagosome requires class I PI3K activity while their eventual disassembly depends on PtdIns(4,5)P 2 hydrolysis.
To accommodate the protruding actin network and to envelop the targets, the PM needs to expand; this occurs by concomitant delivery and fusion of endomembranes to the phagocytic cup 95, 115– 119. The disruption of such focal exocytosis hampers pseudopod extension and impairs engulfment, especially that of large particles. Interestingly, this exocytic pathway is also dependent on PI3K activity 96, 109, possibly accounting in part for the preferential inhibition of large particle uptake by PI3K inhibitors. Although not yet demonstrated experimentally, by removing a physical barrier, the clearance of F-actin at the base of the cup may facilitate the fusion of exocytic vesicles; alternatively, PI3K products may directly stimulate the exocytic machinery.
PtdIns(3,4,5)P 3 and PtdIns(3,4)P 2 disappear from nascent phagosomes after a few minutes. PtdIns(3,4,5)P 3 is converted to PtdIns(3,4)P 2 by SHIP1/2 and the latter subsequently to PtdIns(3)P by INPP4A following closure of the phagosome 100, 108, 120, 121. Throughout closure and fission, phosphoinositides are likely to recruit and maintain membrane curvature-stabilizing/tubulating proteins of the BAR family such as amphiphysin 122, OPHN1, SH3BP1 110, FBP17 123, and SNX9 124. In contrast to other endocytic pathways, the role of BAR proteins in promoting scission of the phagosome from the PM is not known. Finally, PtdIns(3)P is acquired by the phagosomal membrane soon after sealing and is obligatory for maturation to the phagolysosome stage ( Figure 1D). PtdIns(3)P acquisition is due in part to fusion with early endosomes, but de novo synthesis of PtdIns(3)P occurs via the PI3K Vps34 on the early phagosomal membrane 120, 125.
Macropinocytosis
Evolutionarily conserved from protozoans to metazoans, macropinocytosis is an actin-based process utilized by innate immune cells to internalize bulk extracellular milieu, as well as membrane-bound structures, to survey for antigens and microbial components 11, 126, 127. It is also activated in cancer cells to drive elevated nutrient acquisition and support growth 128. Macropinocytosis is intimately dependent on membrane ruffling, driven by expansion of cortical actin networks underlying the PM. Membrane sheets must extend, curve, fuse at their margins, and ultimately undergo fission from the PM to enclose a large (>0.2–5 μm) macropinocytic vacuole 129; as such, not all ruffling leads to macropinocytosis 130. While dendritic cells and macrophages perform constitutive macropinocytosis 127, 131, here we focus on macropinocytosis induced in response to growth factors, chemokines, and Toll-like receptor agonists.
Much of the actin rearrangement in macropinocytosis revolves around PtdIns(4,5)P 2 and signaling patches of PtdIns(3,4,5)P 3/PtdIns(3,4)P 2, which we discuss sequentially. PtdIns(4,5)P 2 at the macropinocytic cup undergoes biphasic changes: increasing during the extension of F-actin-rich membrane sheets but then decreasing during sealing and internalization of the vacuole 132. The mechanism of the initial rise in PtdIns(4,5)P 2 is unknown but is likely a consequence of activation of PIP5K isoforms, as described in other settings 133. Accordingly, PIP5K activators 134 such as phosphatidic acid, Rac1, and Arf6 are present and activated at macropinocytic cups 135– 137, and the activation of Rac1 can stimulate local PtdIns(4,5)P 2 synthesis in ruffles 138. The elevation in PtdIns(4,5)P 2 is consistent with the observed initial burst of F-actin at the base of the macropinocytic cup 132. The inositide could favor net actin polymerization by inhibiting barbed-end capping and/or by severing actin networks 139. PtdIns(4,5)P 2-binding proteins such as profilin, cofilin, gelsolin, or capping protein could potentially mediate these effects. Additionally, PtdIns(4,5)P 2 can activate the NPFs WASP and N-WASP to promote Arp2/3 activity 140, 141. At least four mechanisms are likely to contribute to the subsequent local decrease in PtdIns(4,5)P 2 that accompanies macropinosome closure and fission: 1) decreased synthesis by inactivation or membrane detachment of PIP5K; 2) PLC-mediated hydrolysis that generates diacylglycerol and Ins(1,4,5)P 3; 3) phosphorylation to PtdIns(3,4,5)P 3 via class I PI3Ks (see below); and 4) dilution of the inositide upon focal exocytosis of endomembranes devoid of PtdIns(4,5)P 2. Hydrolysis by 5-phosphatases is also conceivable 142.
Following ligand binding, G-protein-coupled receptors and RTKs together with Ras GTPases recruit class I PI3Ks to the PM 143, generating patches of PtdIns(3,4,5)P 3 where macropinocytic cups form 132, 144– 146. The means whereby the localization of PtdIns(3,4,5)P 3 is spatially restricted is not clear; cytoskeletal structures could confine its diffusion, but differential distribution of kinases and phosphatases could generate a standing gradient of diffusible phosphoinositide. Although PI3K activity is not required for ruffling, both genetic and pharmacological approaches point to an essential role of class I PI3Ks in completing macropinosome closure 99, 147– 149. Modulation of small GTPase activity is likely to mediate the effects of PtdIns(3,4,5)P 3; a variety of GAPs and GEFs specific to GTPases of the Arf and Rho families are regulated by the inositide 150– 152. By activating Rac1 and its effector Pak1 153, PtdIns(3,4,5)P 3 coordinates the formation of rings of the SCAR/WAVE complex that promote the extension of branched actin along the macropinocytic cup walls by stimulating Arp2/3 146, 154. Indeed, the Arp2/3 complex delineates the border of forming macropinocytic cups 154, and interfering with SCAR/WAVE activity impairs macropinocytosis 155. Of note, members of the Ras- and Rho-family are recruited/retained at the PM by electrostatic means: the negative surface charge of the inner leaflet attracts the polycationic C-terminus of the GTPases. Phosphoinositides contribute markedly to this effect by virtue of their polyvalency 91. It is conceivable that neutralization of this interaction by accumulation of submembranous H + accounts for the effects of amiloride, an inhibitor of Na +/H + exchange that is commonly used to block macropinocytosis 156.
The mechanisms underlying closure of the macropinocytic cup and fission from the PM remain poorly understood but likely share some features with other endocytic pathways. Closure requires disassembly of submembranous actin, and this is effected, in part, by the hydrolysis of PtdIns(4,5)P 2 catalyzed by PLC. PtdIns(3,4,5)P 3 is required for the recruitment and activation of PLC 137, 149, specifically PLCγ1 157– 159 and PLCβ 160. In addition, PtdIns(3,4,5)P 3 can aid in scission directly through the recruitment of myosin proteins and indirectly through its hydrolysis products. In this regard, the disappearance of PtdIns(3,4,5)P 3 during macropinocytosis coincides with the appearance of PtdIns(3,4)P 2 132, 145, 161, 162. In mammalian cells, SHIP2 is responsible for the dephosphorylation of PtdIns(3,4,5)P 3 to PtdIns(3,4)P 2 161, and its depletion abrogates fluid-phase uptake of dextran 163, a reliable measure of macropinocytosis. The OCRL-like protein Dd5P4 performs the equivalent dephosphorylation reaction in Dictyostelium and is similarly required to support cup closure 164. Why this phosphoinositide conversion is required is not completely clear. A recent screen identified SNX9-family members (SNX9, SNX18, SNX33) as positive regulators of macropinocytosis 165. It is noteworthy that these are PX-BAR domain-containing proteins whose recruitment is triggered by PtdIns(3,4)P 2 in other settings 35. It is unclear, however, whether BAR domains contribute to the shaping of membrane ruffles or promote fission in macropinocytosis, as they do in FEME and CME. It is also remarkable that not only is the production of PtdIns(3,4)P 2 important for macropinocytosis but so too is its hydrolysis to PtdIns(3)P by INPP4B 161. What role PtdIns(3)P plays in the process is not yet understood.
The internalization of pathogens
It is becoming increasingly evident that many pathogens harness or mimic intrinsic machinery of host cells for their benefit. By commandeering the hosts’ endocytic pathways, a variety of microorganisms have evolved means of gaining entry to cells and surviving within them. For example, numerous viruses and protozoa utilize macropinocytosis to mediate their cellular entry 166, 167. Other pathogens such as Listeria monocytogenes enter host cells by engaging surface receptors that can undergo CME and CIE by FEME 14. Here we highlight three selected pathogens that enter cells by diverse means and nestle into distinct host cell niches: Salmonella enterica drives its own cellular entry to reside in an endocytic compartment, Legionella pneumophila “hitches a ride” into alveolar macrophages by phagocytosis and generates a unique intracellular compartment, and enteropathogenic Escherichia coli (EPEC) manipulates the submembranous cytoskeleton to prevent its internalization, attaching firmly to the outer surface of host epithelial cells. While differing vastly in their survival strategies, these pathogens share a key aspect of their survival strategy: the subversion of the host’s phosphoinositide metabolism.
Salmonella enterica
Salmonella enterica spp. are a major worldwide cause of food-borne gastroenteritis (serovar Typhimurium) and Typhoid fever (serovar Typhi and Paratyphi) 168– 171. Salmonella gains entry into host cells and survives therein by virtue of effector proteins that are translocated into the host cytosol by type III secretion systems (T3SS) that are encoded in defined genomic pathogenicity islands 172, 173. Salmonella pathogenicity island-1 promotes the invasion of epithelial cells and the formation of Salmonella-containing vacuoles (SCV), while Salmonella pathogenicity island-2 promotes intracellular bacterial growth 174. Phosphoinositides play a key role in these processes.
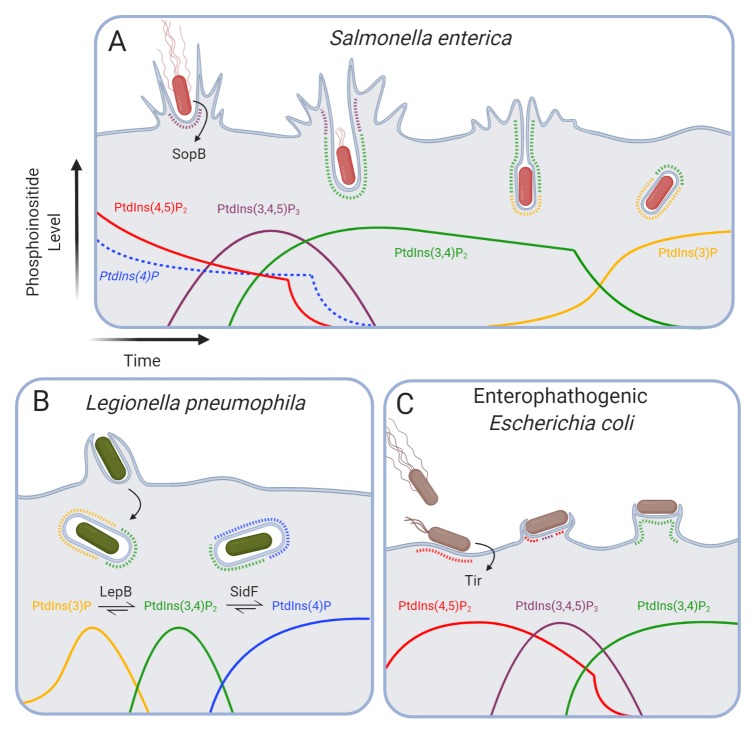
One effector secreted during invasion, SopB (also known as SigD), acts at least in part by co-opting the inositide metabolism of host cells. It is an important determinant of Salmonella virulence, contributing to inflammation and fluid secretion from infected ileum 175, 176. SopB shares homology with the mammalian phosphatases INPP4A/4B 176 and Synj 177. This predicted activity is borne out experimentally: in vitro SopB functions as a phosphatase of broad substrate specificity 176, 177. In vivo, however, only the 4-phosphatase activity of SopB has been demonstrated ( Figure 2A). Inside mammalian cells, it hydrolyzes plasmalemmal PtdIns(4,5)P 2 178, 179, generating PtdIns(5)P 179. SopB has also been suggested to possess 5-phosphatase activity 177, 180, but this requires further investigation. Regardless of the specific sites it can dephosphorylate, deletion of SopB results in the failure to clear PtdIns(4,5)P 2 from invaginating regions of the membrane where invasion normally occurs, severely delaying scission of the SCV from the PM 178, 181. As a result, the invasion efficiency is markedly decreased 180, 182. The phosphoinositide changes brought about by SopB likely facilitate scission by reducing the rigidity of the cytoskeleton underlying the PM, in the process stimulating endocytosis 178, 183. Of note, Shigella flexneri 184—the causative agent of Shigellosis 185—secretes an effector known as IpgD that is structurally homologous to SopB and seemingly shares its hydrolytic activity towards phosphoinositides.
Figure 2. Bacterial effectors alter early endocytic traffic by subverting phosphoinositide metabolism.
( A) The Salmonella enterica effector SopB promotes efficient invasion of host cells by reducing PtdIns(4,5)P 2 levels in invasion pockets while stimulating the production of PtdIns(3,4,5)P 3, PtdIns(3,4)P 2, and PtdIns(3)P. ( B) Following phagocytosis, Legionella pneumophila secretes multiple host effectors that modify phosphoinositides and disrupt early phagosome maturation. LepB functions as a phosphatidylinositide 4-kinase to generate PtdIns(3,4)P 2 from PtdIns(3)P, and SidF is a 3-phosphatase that hydrolyzes PtdIns(3,4)P 2 to produce PtdIns(4)P. ( C) Following contact with the host membrane, enteropathogenic Escherichia coli stimulates a transient increase in local PtdIns(4,5)P 2 levels. The secreted bacterial effector Tir mediates the activation of host phosphatidylinositide 3-kinases to generate PtdIns(3,4,5)P 3 and PtdIns(3,4)P 2. PtdIns, phosphatidylinositol; Tir, translocated intimin receptor.
The manner whereby SopB (and presumably also IpgD) manipulates the host cell cytoskeleton to enable invasion is beginning to be understood. Through its phosphoinositide phosphatase activity, SopB can activate RhoG 186 and also acts as a guanine nucleotide-dissociation inhibitor towards Cdc42 187. These effects act in concert with those triggered by separate effectors, like SopE and SopE2, that operate as Rho-family GTPase GEFs for Cdc42, Rac1, RhoA, and RhoG 188– 190, the antagonizing GAP SptP 191, 192, and SipA and SipC, which are actin-bundling proteins 193– 195. Together, these Salmonella effectors induce the formation of formin-mediated actin bundles, followed by Arp2/3-driven branched actin waves that jointly promote ruffle formation and closure, resulting in encapsulation of the bacterium 194, 196– 199.
Intriguingly, despite acting as a phosphatase, SopB mediates the formation of the 3-phosphorylated species PtdIns(3,4,5)P 3 and PtdIns(3,4)P 2 at invasion ruffles 200, 201 ( Figure 2A). These phosphoinositides recruit and activate AKT through its PH domain, thereby promoting host cell survival following infection 202, 203. In the same manner described for endogenous endocytosis, the 3-phosphorylated inositides produced by SopB support the recruitment of the PX-BAR domain-containing proteins SNX9 180 and SNX18 204, likely facilitating dynamin-mediated scission of the SCV from the PM.
The mechanism responsible for the formation of 3-phosphorylated species by SopB is not obvious; it is noteworthy, however, that it is insensitive to classical inhibitors of class I PI3Ks 200, 201. A possible target of the effector is PI3K-C2α, a class II PI3K resistant to conventional class I inhibitors 34, 205. In this regard, it is interesting that PI3K-C2α is co-opted by Shigella flexneri to promote its cell-to-cell spread 206 and that IpgD may be involved in the spreading process 207, 208. These observations raise the possibility that the homologous SopB may activate this enzyme during invasion. Finally, SopB contributes to the generation of PtdIns(3)P on the nascent SCV 200 by recruiting Rab5 and its effector, the class III PI3K Vps34.
Legionella pneumophila
Legionella pneumophila, the causative agent of Legionnaire’s disease, is another pathogen able to commandeer the phosphoinositide metabolism of its host cells. Legionella is internalized by alveolar macrophages following inhalation of aerosolized bacteria 209– 211. Once ensconced within the macrophage, Legionella utilizes a defective in organelle trafficking/intracellular multiplication (Dot/Icm) type IV secretion system to inject effectors across the phagosomal membrane—which becomes the Legionella-containing vacuole or LCV—to manipulate host cell pathways 212, 213. One such effector, LepB, alters the levels of cellular PtdIns(3)P and PtdIns(3,4)P 2 70, 214 ( Figure 2B). In vitro kinase assays using purified LepB found that LepB functions as a phosphatidylinositide 4-kinase, generating PtdIns(3,4)P 2 from PtdIns(3)P, using ATP as a phosphate source 214.
PtdIns(3,4)P 2 formed during Legionella infection is eliminated by another bacterial effector, the lipid phosphatase SidF, which hydrolyzes the D3 phosphate of PtdIns(3,4)P 2 to produce PtdIns(4)P on the LCV 215 ( Figure 2B). The net result of these coordinated effector activities is to deplete PtdIns(3)P from the LCV by converting it first to PtdIns(3,4)P 2, which is in turn hydrolyzed to PtdIns(4)P. By depleting PtdIns(3)P, Legionella appears to benefit from arresting maturation at an early stage. Moreover, the sustained production of PtdIns(4)P contributes to the maintenance on the LCV of effectors that specifically bind this inositide 215– 217 and enable fusion with secretory vesicles derived from the ER 215.
Through its ability to generate PtdIns(3,4)P 2, LepB would be predicted to additionally activate AKT-dependent pro-survival host pathways 55. However, the dephosphorylation by SidF antagonizes this effect. Moreover, the supply of PtdIns(3)P to the LCV may be limited by VipD by blocking Rab5-dependent recruitment of Vps34 and/or through its phospholipase activity 218, 219. More detailed studies will be required to better establish the source and dynamics of these phosphoinositides on the LCV and their consequences on the effectors that determine bacterial virulence.
Enteropathogenic Escherichia coli
In developing countries, EPEC present in contaminated food and water is a major cause of infant diarrhea and fatality 220, 221. Unlike the bacterial species discussed above, EPEC is predominantly an extracellular pathogen that, in fact, actively inhibits its own endocytosis. Instead, it creates a niche on the surface of the gastrointestinal epithelium by adhering tightly and replicating on actin-rich cell surface structures termed “pedestals”. EPEC utilize a T3SS to deliver effectors across the bacterial cell wall and host cell membrane, which promote adherence to epithelial cells lining the gastrointestinal tract, the loss of their microvilli (lesioning), and altered ion homeostasis, ultimately compromising barrier function 222. One such effector, translocated intimin receptor (Tir), integrates into the host PM and also couples to the bacterium by interacting with the intimin receptor on its outer membrane ( Figure 2C) 223. The Tir:intimin receptor complex is critical to dock EPEC onto the epithelial cell surface and in addition initiates signaling cascades. Like Fcγ phagocytic receptors, the cytosolic domain of Tir bears ITAM/ITIM-like motifs that can be phosphorylated on tyrosine residues by several host kinases 224– 226. Following its lateral clustering upon binding to intimin, Tir is phosphorylated and mediates the formation of the actin pedestal on which the adherent bacteria rest 224, 227, 228.
During infection, PtdIns(4,5)P 2 transiently accumulates at the initial contact point between bacteria and the PM. The temporary increase occurs just after bacterial adherence and correlates with an accumulation of type I PIP5K and F-actin beneath the adhering bacteria 229, 230. The accumulation of PtdIns(4,5)P 2 at the forming pedestal is largely dependent on a functional T3SS 230, although the effectors driving the recruitment of PIP5K remain unknown. Tir clustering, via the adaptor Nck, then recruits N-WASP, which activates the polymerization of branched actin by the Arp2/3 complex 227, 228, 231. It is worth noting that several other EPEC effectors play a part in modulating and polarizing the actin cytoskeleton: EspF co-opts SNX9 to further activate N-WASP 232, while EspH inactivates several RhoGEFs 233 and EspG interferes with activation of the WAVE complex 234. Regardless of the specific bacterial and cellular effectors engaged, the importance of PtdIns(4,5)P 2 in the process is undeniable: the artificial enzymatic depletion of the inositide from the PM reduces bacterial adherence and pedestal formation 230.
Interestingly, Tir stimulates the production of PtdIns(3,4,5)P 3 at least in part by binding and recruiting the p85 regulatory subunit to the membrane to activate class I PI3Ks 230. In accordance with this observation, Tir activates AKT pro-survival signaling 230; consequently, pharmacological inhibition of PI3Ks increases host cell death in response to EPEC 235, likely by inhibiting the production of the inositides necessary for AKT activation. It is likely that both the conversion of PtdIns(4,5)P 2 to PtdIns(3,4,5)P 3 and its hydrolysis by PLC—which is activated by Tir 236—contribute to the biphasic nature of PtdIns(4,5)P 2 accumulation during pedestal formation ( Figure 2C).
More recently, the phosphoinositide phosphatase SHIP2 was identified as a host factor that is recruited to EPEC pedestals; the SH2 domain of SHIP2 associates with phosphorylated tyrosine residues on Tir. This interaction is functionally significant, since mutation of the tyrosine residues in the cytosolic domain of Tir or the depletion of SHIP2 led to the formation of disordered pedestals consisting of discrete actin-rich protrusions 237. How does SHIP2 regulate the organization of the pedestal? Rather than favoring sustained accumulation of PtdIns(3,4,5)P 3, the phosphatase activity of SHIP2 generates a local platform rich in PtdIns(3,4)P 2 in the pedestal ( Figure 2C). The latter inositide is sensed by the PH domain of lamellipodin, which is recruited to modulate F-actin polymerization in the pedestal 67, 237.
Concluding remarks
The involvement of phosphoinositides in the generation of endocytic compartments and in directing their fate is now widely recognized, though not yet fully elucidated. The purpose of this review was to provide a bird’s-eye view of the current knowledge of the field. It is important to note that our survey of the literature was limited to the entry and early maturation stages. The role of inositides in late endosomes and lysosomes and in the equivalent stages of maturation of phagosomes and macropinosomes has only begun to be studied recently and should become the subject of a comprehensive and integrated view in the next few years as more information accumulates.
Lastly, it is important to note that the realization that inositides participate in membrane invagination, scission, and maturation was made possible primarily by the development and implementation of phosphoinositide-specific fluorescent probes that enabled real-time visualization of the individual lipid species with sufficient spatial and temporal resolution. In this regard, it is noteworthy that of the seven phosphoinositide species, suitable specific probes exist for only five of them 238– 244; to our knowledge, no satisfactory reagents are currently available to detect PtdIns5P or PtdIns(3,5)P 2. Whether and how these lipids participate in membrane internalization and pathogen invasion remains to be studied as we await the development of suitable analytical tools.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Joel Swanson, Department of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, MI, 48109-0620, USA
Tamas Balla, Section on Molecular Signal Transduction, Program for Developmental Neuroscience, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Volker Haucke, Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP), Berlin, Germany
Funding Statement
G.F.W.W. is supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR) and an MD/PhD Studentship from the University of Toronto. S.G. is supported by grant FDN-143202 from the CIHR.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Balla T: Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93(3):1019–1137. 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Hammond GRV, Burke JE: Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr Opin Cell Biol. 2020;63:57–67. 10.1016/j.ceb.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Dickson EJ, Hille B: Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem J. 2019;476(1):1–23. 10.1042/BCJ20180022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Sohn M, Korzeniowski M, Zewe JP, et al. : PI(4,5)P 2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites. J Cell Biol. 2018;217(5):1797–1813. 10.1083/jcb.201710095 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Chung J, Torta F, Masai K, et al. : INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349(6246):428–32. 10.1126/science.aab1370 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Moser von Filseck J, Čopič A, Delfosse V, et al. : INTRACELLULAR TRANSPORT. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349(6246):432–6. 10.1126/science.aab1346 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Stephens LR, Jackson TR, Hawkins PT: Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta. 1993;1179(1):27–75. 10.1016/0167-4889(93)90072-w [DOI] [PubMed] [Google Scholar]
- 8. Kaksonen M, Roux A: Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–326. 10.1038/nrm.2017.132 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Ferreira APA, Boucrot E: Mechanisms of Carrier Formation during Clathrin-Independent Endocytosis. Trends Cell Biol. 2018;28(3):188–200. 10.1016/j.tcb.2017.11.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Flannagan RS, Jaumouillé V, Grinstein S: The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. 10.1146/annurev-pathol-011811-132445 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Marques PE, Grinstein S, Freeman SA: SnapShot:Macropinocytosis. Cell. 2017;169(4):766–766.e1. 10.1016/j.cell.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 12. Freeman SA, Grinstein S: Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev. 2014;262(1):193–215. 10.1111/imr.12212 [DOI] [PubMed] [Google Scholar]
- 13. Walpole GFW, Grinstein S, Westman J: The role of lipids in host-pathogen interactions. IUBMB Life. 2018;70(5):384–392. 10.1002/iub.1737 [DOI] [PubMed] [Google Scholar]
- 14. Pizarro-Cerdá J, Kühbacher A, Cossart P: Phosphoinositides and host-pathogen interactions. Biochim Biophys Acta. 2015;1851(6):911–8. 10.1016/j.bbalip.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 15. Kumar Y, Valdivia RH: Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5(6):593–601. 10.1016/j.chom.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bitsikas V, Corrêa IR, Jr, Nichols BJ: Clathrin-independent pathways do not contribute significantly to endocytic flux. eLife. 2014;3:e03970. 10.7554/eLife.03970 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Marks B, Stowell MH, Vallis Y, et al. : GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410(6825):231–5. 10.1038/35065645 [DOI] [PubMed] [Google Scholar]
- 18. Di Paolo G, Moskowitz HS, Gipson K, et al. : Impaired PtdIns(4,5)P 2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431(7007):415–22. 10.1038/nature02896 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Antonescu CN, Aguet F, Danuser G, et al. : Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol Biol Cell. 2011;22(14):2588–600. 10.1091/mbc.E11-04-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zoncu R, Perera RM, Sebastian R, et al. : Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2007;104(10):3793–8. 10.1073/pnas.0611733104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nunez D, Antonescu C, Mettlen M, et al. : Hotspots organize clathrin-mediated endocytosis by efficient recruitment and retention of nucleating resources. Traffic. 2011;12(12):1868–78. 10.1111/j.1600-0854.2011.01273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He K, Marsland R, III, Upadhyayula S, et al. : Dynamics of phosphoinositide conversion in clathrin-mediated endocytic traffic. Nature. 2017;552(7685):410–414. 10.1038/nature25146 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Cocucci E, Aguet F, Boulant S, et al. : The first five seconds in the life of a clathrin-coated pit. Cell. 2012;150(3):495–507. 10.1016/j.cell.2012.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Ford MG, Mills IG, Peter BJ, et al. : Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419(6905):361–366. 10.1038/nature01020 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Kadlecova Z, Spielman SJ, Loerke D, et al. : Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J Cell Biol. 2017;216(1):167–179. 10.1083/jcb.201608071 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Kelly BT, Graham SC, Liska N, et al. : Clathrin adaptors. AP2 controls clathrin polymerization with a membrane-activated switch. Science. 2014;345(6195):459–63. 10.1126/science.1254836 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Messa M, Fernández-Busnadiego R, Sun EW, et al. : Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits. eLife. 2014;3:e03311. 10.7554/eLife.03311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller SE, Mathiasen S, Bright NA, et al. : CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev Cell. 2015;33(2):163–75. 10.1016/j.devcel.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaidarov I, Keen JH: Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol. 1999;146(4):755–64. 10.1083/jcb.146.4.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Henne WM, Boucrot E, Meinecke M, et al. : FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328(5983):1281–1284. 10.1126/science.1188462 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Daumke O, Roux A, Haucke V: BAR domain scaffolds in dynamin-mediated membrane fission. Cell. 2014;156(5):882–92. 10.1016/j.cell.2014.02.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Peter BJ, Kent HM, Mills IG, et al. : BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303(5657):495–9. 10.1126/science.1092586 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Taylor MJ, Perrais D, Merrifield CJ: A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9(3):e1000604. 10.1371/journal.pbio.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Posor Y, Eichhorn-Gruenig M, Puchkov D, et al. : Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature. 2013;499(7457):233–7. 10.1038/nature12360 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Schöneberg J, Lehmann M, Ullrich A, et al. : Lipid-mediated PX-BAR domain recruitment couples local membrane constriction to endocytic vesicle fission. Nat Commun. 2017;8:15873. 10.1038/ncomms15873 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Gaidarov I, Smith ME, Domin J, et al. : The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell. 2001;7(2):443–9. 10.1016/s1097-2765(01)00191-5 [DOI] [PubMed] [Google Scholar]
- 37. Wang H, Lo WT, Vujičić Žagar A, et al. : Autoregulation of Class II Alpha PI3K Activity by Its Lipid-Binding PX-C2 Domain Module. Mol Cell. 2018;71(2):343–351.e4. 10.1016/j.molcel.2018.06.042 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Lo WT, Vujičić Žagar A, Gerth F, et al. : A Coincidence Detection Mechanism Controls PX-BAR Domain-Mediated Endocytic Membrane Remodeling via an Allosteric Structural Switch. Dev Cell. 2017;43(4):522–529.e4. 10.1016/j.devcel.2017.10.019 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Collins A, Warrington A, Taylor KA, et al. : Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Curr Biol. 2011;21(14):1167–75. 10.1016/j.cub.2011.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Merrifield CJ, Qualmann B, Kessels MM, et al. : Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur J Cell Biol. 2004;83(1):13–8. 10.1078/0171-9335-00356 [DOI] [PubMed] [Google Scholar]
- 41. Yarar D, Waterman-Storer CM, Schmid SL: SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev Cell. 2007;13(1):43–56. 10.1016/j.devcel.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 42. Almeida-Souza L, Frank R, García-Nafría J, et al. : A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits. Cell. 2018;174(2):325–337.e14. 10.1016/j.cell.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Perera RM, Zoncu R, Lucast L, et al. : Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci U S A. 2006;103(51):19332–7. 10.1073/pnas.0609795104 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Gallop JL, Jao CC, Kent HM, et al. : Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25(12):2898–910. 10.1038/sj.emboj.7601174 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Verstreken P, Koh TW, Schulze KL, et al. : Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40(4):733–48. 10.1016/s0896-6273(03)00644-5 [DOI] [PubMed] [Google Scholar]
- 46. Chang-Ileto B, Frere SG, Chan RB, et al. : Synaptojanin 1-mediated PI(4,5)P 2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev Cell. 2011;20(2):206–18. 10.1016/j.devcel.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Cremona O, Di Paolo G, Wenk MR, et al. : Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99(2):179–88. 10.1016/s0092-8674(00)81649-9 [DOI] [PubMed] [Google Scholar]
- 48. Milosevic I, Giovedi S, Lou X, et al. : Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72(4):587–601. 10.1016/j.neuron.2011.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Nández R, Balkin DM, Messa M, et al. : A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. eLife. 2014;3:1–27. 10.7554/eLife.02975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He K, Song E, Upadhyayula S, et al. : Dynamics of Auxilin 1 and GAK in clathrin-mediated traffic. J Cell Biol. 2020;219(3): pii: e201908142. 10.1083/jcb.201908142 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Massol RH, Boll W, Griffin AM, et al. : A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A. 2006;103(27):10265–10270. 10.1073/pnas.0603369103 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Nakatsu F, Messa M, Nández R, et al. : Sac2/INPP5F is an inositol 4-phosphatase that functions in the endocytic pathway. J Cell Biol. 2015;209(1):85–95. 10.1083/jcb.201409064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsu F, Hu F, Mao Y: Spatiotemporal control of phosphatidylinositol 4-phosphate by Sac2 regulates endocytic recycling. J Cell Biol. 2015;209(1):97–110. 10.1083/jcb.201408027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gewinner C, Wang ZC, Richardson A, et al. : Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16(2):115–25. 10.1016/j.ccr.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Wang H, Loerke D, Bruns C, et al. : Phosphatidylinositol 3,4-bisphosphate synthesis and turnover are spatially segregated in the endocytic pathway. J Biol Chem. 2019;295(4):1091–1104. 10.1074/jbc.RA119.011774 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Devereaux K, Dall’Armi C, Alcazar-Roman A, et al. : Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One. 2013;8(10):e76405. 10.1371/journal.pone.0076405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Campa CC, Margaria JP, Derle A, et al. : Rab11 activity and PtdIns(3)P turnover removes recycling cargo from endosomes. Nat Chem Biol. 2018;14(8):801–810. 10.1038/s41589-018-0086-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Johannes L, Parton RG, Bassereau P, et al. : Building endocytic pits without clathrin. Nat Rev Mol Cell Biol. 2015;16(5):311–21. 10.1038/nrm3968 [DOI] [PubMed] [Google Scholar]
- 59. Posor Y, Eichhorn-Grünig M, Haucke V, et al. : Phosphoinositides in endocytosis. Biochim Biophys Acta. 2015;1851(6):794–804. 10.1016/j.bbalip.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 60. Hinze C, Boucrot E: Local actin polymerization during endocytic carrier formation. Biochem Soc Trans. 2018;46(3):565–576. 10.1042/BST20170355 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Boucrot E, Ferreira AP, Almeida-Souza L, et al. : Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517(7535):460–465. 10.1038/nature14067 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Petrelli A, Gilestro GF, Lanzardo S, et al. : The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416(6877):187–90. 10.1038/416187a [DOI] [PubMed] [Google Scholar]
- 63. Soubeyran P, Kowanetz K, Szymkiewicz I, et al. : Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416(6877):183–7. 10.1038/416183a [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Fu X, Yang Y, Xu C, et al. : Retrolinkin cooperates with endophilin A1 to mediate BDNF-TrkB early endocytic trafficking and signaling from early endosomes. Mol Biol Cell. 2011;22(19):3684–98. 10.1091/mbc.E11-04-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tang Y, Hu LA, Miller WE, et al. : Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the beta1-adrenergic receptor. Proc Natl Acad Sci U S A. 1999;96(22):12559–64. 10.1073/pnas.96.22.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vehlow A, Soong D, Vizcay-Barrena G, et al. : Endophilin, Lamellipodin, and Mena cooperate to regulate F-actin-dependent EGF-receptor endocytosis. EMBO J. 2013;32(20):2722–2734. 10.1038/emboj.2013.212 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Krause M, Leslie M, Stewart M, et al. : Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004;7(4):571–83. 10.1016/j.devcel.2004.07.024 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Servant G, Weiner OD, Herzmark P, et al. : Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287(5455):1037–1040. 10.1126/science.287.5455.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xie J, Erneux C, Pirson I: How does SHIP1/2 balance PtdIns(3,4)P 2 and does it signal independently of its phosphatase activity? Bioessays. 2013;35(8):733–43. 10.1002/bies.201200168 [DOI] [PubMed] [Google Scholar]
- 70. Goulden BD, Pacheco J, Dull A, et al. : A high-avidity biosensor reveals plasma membrane PI(3,4)P 2 is predominantly a class I PI3K signaling product. J Cell Biol. 2019;218(3):1066–1079. 10.1083/jcb.201809026 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Lee YR, Chen M, Pandolfi PP: The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19(9):547–562. 10.1038/s41580-018-0015-0 [DOI] [PubMed] [Google Scholar]
- 72. Ringstad N, Nemoto Y, De Camilli P: The SH3p4/Sh3p8/SH3p13 protein family: Binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci U S A. 1997;94(16):8569–74. 10.1073/pnas.94.16.8569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boucrot E, Pick A, Çamdere G, et al. : Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell. 2012;149(1):124–36. 10.1016/j.cell.2012.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Xiong D, Xiao S, Guo S, et al. : Frequency and amplitude control of cortical oscillations by phosphoinositide waves. Nat Chem Biol. 2016;12(3):159–66. 10.1038/nchembio.2000 [DOI] [PubMed] [Google Scholar]
- 75. Chan Wah Hak L, Khan S, Di Meglio I, et al. : FBP17 and CIP4 recruit SHIP2 and lamellipodin to prime the plasma membrane for fast endophilin-mediated endocytosis. Nat Cell Biol. 2018;20(9):1023–1031. 10.1038/s41556-018-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Tuijnman WB, Capel PJ, van de Winkel JG: Human low-affinity IgG receptor Fc gamma RIIa (CD32) introduced into mouse fibroblasts mediates phagocytosis of sensitized erythrocytes. Blood. 1992;79(7):1651–6. [PubMed] [Google Scholar]
- 77. Indik ZK, Pan XQ, Huang MM, et al. : Insertion of cytoplasmic tyrosine sequences into the nonphagocytic receptor FcγRIIB establishes phagocytic function. Blood. 1994;83(8):2072–80. [PubMed] [Google Scholar]
- 78. Downey GP, Botelho RJ, Butler JR, et al. : Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors. J Biol Chem. 1999;274(40):28436–44. 10.1074/jbc.274.40.28436 [DOI] [PubMed] [Google Scholar]
- 79. Freeman SA, Goyette J, Furuya W, et al. : Integrins Form an Expanding Diffusional Barrier that Coordinates Phagocytosis. Cell. 2016;164(1–2):128–140. 10.1016/j.cell.2015.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Bakalar MH, Joffe AM, Schmid EM, et al. : Size-Dependent Segregation Controls Macrophage Phagocytosis of Antibody-Opsonized Targets. Cell. 2018;174(1):131–142.e13. 10.1016/j.cell.2018.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Botelho RJ, Teruel M, Dierckman R, et al. : Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151(7):1353–1368. 10.1083/jcb.151.7.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mao YS, Yamaga M, Zhu X, et al. : Essential and unique roles of PIP5K-gamma and -alpha in Fcgamma receptor-mediated phagocytosis. J Cell Biol. 2009;184(2):281–96. 10.1083/jcb.200806121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Coppolino MG, Dierckman R, Loijens J, et al. : Inhibition of phosphatidylinositol-4-phosphate 5-kinase Ialpha impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem. 2002;277(46):43849–57. 10.1074/jbc.M209046200 [DOI] [PubMed] [Google Scholar]
- 84. Dart AE, Donnelly SK, Holden DW, et al. : Nck and Cdc42 co-operate to recruit N-WASP to promote FcγR-mediated phagocytosis. J Cell Sci. 2012;125(Pt 12):2825–30. 10.1242/jcs.106583 [DOI] [PubMed] [Google Scholar]
- 85. Gu H, Botelho RJ, Yu M, et al. : Critical role for scaffolding adapter Gab2 in Fc gamma R-mediated phagocytosis. J Cell Biol. 2003;161(6):1151–61. 10.1083/jcb.200212158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Caron E, Hall A: Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282(5394):1717–1721. 10.1126/science.282.5394.1717 [DOI] [PubMed] [Google Scholar]
- 87. May RC, Caron E, Hall A, et al. : Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat Cell Biol. 2000;2(4):246–8. 10.1038/35008673 [DOI] [PubMed] [Google Scholar]
- 88. Hoppe AD, Swanson JA: Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell. 2004;15(8):3509–19. 10.1091/mbc.e03-11-0847 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Lorenzi R, Brickell PM, Katz DR, et al. : Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95(9):2943–2946. 10.1182/blood.V95.9.2943.009k17_2943_2946 [DOI] [PubMed] [Google Scholar]
- 90. Park H, Cox D: Cdc42 regulates Fc gamma receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol Biol Cell. 2009;20(21):4500–8. 10.1091/mbc.e09-03-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yeung T, Terebiznik M, Yu L, et al. : Receptor activation alters inner surface potential during phagocytosis. Science. 2006;313(5785):347–51. 10.1126/science.1129551 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Scott CC, Dobson W, Botelho, RJ, et al. : Phosphatidylinositol-4,5- bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol. 2005;169(1):139–49. 10.1083/jcb.200412162 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Fitzer-Attas CJ, Lowry M, Crowley MT, et al. : Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191(4):669–682. 10.1084/jem.191.4.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marshall JG, Booth JW, Stambolic V, et al. : Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc gamma receptor-mediated phagocytosis. J Cell Biol. 2001;153(7):1369–80. 10.1083/jcb.153.7.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Bajno L, Peng XR, Schreiber AD, et al. : Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149(3):697–705. 10.1083/jcb.149.3.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lee WL, Mason D, Schreiber AD, et al. : Quantitative analysis of membrane remodeling at the phagocytic cup. Mol Biol Cell. 2007;18(8):2883–92. 10.1091/mbc.e06-05-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bohdanowicz M, Balkin DM, De Camilli P, et al. : Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol Biol Cell. 2012;23(1):176–87. 10.1091/mbc.E11-06-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Levin R, Hammond GR, Balla T, et al. : Multiphasic dynamics of phosphatidylinositol 4-phosphate during phagocytosis. Mol Biol Cell. 2017;28(1):128–140. 10.1091/mbc.E16-06-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Araki N, Johnson MT, Swanson JA: A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135(5):1249–1260. 10.1083/jcb.135.5.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kamen LA, Levinsohn J, Swanson JA: Differential association of phosphatidylinositol 3-kinase, SHIP-1, and PTEN With forming phagosomes. Mol Biol Cell. 2007;18(7):2463–72. 10.1091/mbc.e07-01-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chacko GW, Brandt JT, Coggeshall KM, et al. : Phosphoinositide 3-kinase and p72syk noncovalently associate with the low affinity Fc γ receptor on human platelets through an immunoreceptor tyrosine-based activation motif. Reconstitution with synthetic phosphopeptides. J Biol Chem. 1996;271(18):10775–81. 10.1074/jbc.271.18.10775 [DOI] [PubMed] [Google Scholar]
- 102. Tridandapani S, Lyden TW, Smith JL, et al. : The adapter protein LAT enhances fcgamma receptor-mediated signal transduction in myeloid cells. J Biol Chem. 2000;275(27):20480–7. 10.1074/jbc.M909462199 [DOI] [PubMed] [Google Scholar]
- 103. Moon KD, Post CB, Durden DL, et al. : Molecular basis for a direct interaction between the Syk protein-tyrosine kinase and phosphoinositide 3-kinase. J Biol Chem. 2005;280(2):1543–51. 10.1074/jbc.M407805200 [DOI] [PubMed] [Google Scholar]
- 104. Zhang Y, Wavreille AS, Kunys AR, et al. : The SH2 domains of inositol polyphosphate 5-phosphatases SHIP1 and SHIP2 have similar ligand specificity but different binding kinetics. Biochemistry. 2009;48(46):11075–83. 10.1021/bi9012462 [DOI] [PubMed] [Google Scholar]
- 105. Tridandapani S, Siefker K, Teillaud JC, et al. : Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002;277(7):5082–9. 10.1074/jbc.M110277200 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Nakamura K, Malykhin A, Coggeshall KM: The Src homology 2 domain-containing inositol 5-phosphatase negatively regulates Fcgamma receptor-mediated phagocytosis through immunoreceptor tyrosine-based activation motif-bearing phagocytic receptors. Blood. 2002;100(9):3374–82. 10.1182/blood-2002-03-0787 [DOI] [PubMed] [Google Scholar]
- 107. Maresco DL, Osborne JM, Cooney D, et al. : The SH2-containing 5'-inositol phosphatase (SHIP) is tyrosine phosphorylated after Fc gamma receptor clustering in monocytes. J Immunol. 1999;162(11):6458–65. [PubMed] [Google Scholar]
- 108. Ai J, Maturu A, Johnson W, et al. : The inositol phosphatase SHIP-2 down-regulates FcgammaR-mediated phagocytosis in murine macrophages independently of SHIP-1. Blood. 2006;107(2):813–20. 10.1182/blood-2005-05-1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cox D, Berg JS, Cammer M, et al. : Myosin X is a downstream effector of PI(3)K during phagocytosis. Nat Cell Biol. 2002;4(7):469–77. 10.1038/ncb805 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Schlam D, Bagshaw RD, Freeman SA, et al. : Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat Commun. 2015;6:8623. 10.1038/ncomms9623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cox D, Tseng CC, Bjekic G, et al. : A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274(3):1240–7. 10.1074/jbc.274.3.1240 [DOI] [PubMed] [Google Scholar]
- 112. Beemiller P, Zhang Y, Mohan S, et al. : A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis. Mol Biol Cell. 2010;21(3):470–80. 10.1091/mbc.e08-05-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cox D, Dale BM, Kashiwada M, et al. : A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18). J Exp Med. 2001;193(1):61–71. 10.1084/jem.193.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ostrowski PP, Freeman SA, Fairn G, et al. : Dynamic Podosome-Like Structures in Nascent Phagosomes Are Coordinated by Phosphoinositides. Dev Cell. 2019;50(4):397–410.e3. 10.1016/j.devcel.2019.05.028 [DOI] [PubMed] [Google Scholar]
- 115. Braun V, Fraisier V, Raposo G, et al. : TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J. 2004;23(21):4166–76. 10.1038/sj.emboj.7600427 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 116. Niedergang F, Colucci-Guyon E, Dubois T, et al. : ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J Cell Biol. 2003;161(6):1143–50. 10.1083/jcb.200210069 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 117. Czibener C, Sherer NM, Becker SM, et al. : Ca 2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol. 2006;174(7):997–1007. 10.1083/jcb.200605004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 118. Mohammadi S, Isberg RR: Cdc42 interacts with the exocyst complex to promote phagocytosis. J Cell Biol. 2013;200(1):81–93. 10.1083/jcb.201204090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cox D, Lee DJ, Dale BM, et al. : A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci U S A. 2000;97(2):680–5. 10.1073/pnas.97.2.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Vieira OV, Botelho RJ, Rameh L, et al. : Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155(1):19–25. 10.1083/jcb.200107069 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 121. Nigorikawa K, Hazeki K, Sasaki J, et al. : Inositol Polyphosphate-4-Phosphatase Type I Negatively Regulates Phagocytosis via Dephosphorylation of Phagosomal PtdIns(3,4)P 2. PLoS One. 2015;10(11):e0142091. 10.1371/journal.pone.0142091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gold ES, Morrissette NS, Underhill DM, et al. : Amphiphysin IIm, a novel amphiphysin II isoform, is required for macrophage phagocytosis. Immunity. 2000;12(3):285–92. 10.1016/s1074-7613(00)80181-8 [DOI] [PubMed] [Google Scholar]
- 123. Tsuboi S, Takada H, Hara T, et al. : FBP17 Mediates a Common Molecular Step in the Formation of Podosomes and Phagocytic Cups in Macrophages. J Biol Chem. 2009;284(13):8548–8556. 10.1074/jbc.M805638200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cheng S, Wang K, Zou W, et al. : PtdIns(4,5)P 2 and PtdIns3P coordinate to regulate phagosomal sealing for apoptotic cell clearance. J Cell Biol. 2015;210:485–502. 10.1083/jcb.201501038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fratti RA, Backer JM, Gruenberg J, et al. : Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154(3):631–644. 10.1083/jcb.200106049 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 126. Swanson JA: Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9(8):639–49. 10.1038/nrm2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Canton J, Schlam D, Breuer C, et al. : Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat Commun. 2016;7:11284. 10.1038/ncomms11284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. : Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–7. 10.1038/nature12138 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 129. Lim JP, Gleeson PA: Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836–43. 10.1038/icb.2011.20 [DOI] [PubMed] [Google Scholar]
- 130. Swanson JA: Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94(Pt 1):135–42. [DOI] [PubMed] [Google Scholar]
- 131. Sallusto F, Cella M, Danieli C, et al. : Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400. 10.1084/jem.182.2.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Araki N, Egami Y, Watanabe Y, et al. : Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp Cell Res. 2007;313(7):1496–507. 10.1016/j.yexcr.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 133. Tolias KF, Hartwig JH, Ishihara H, et al. : Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10(3):153–156. 10.1016/s0960-9822(00)00315-8 [DOI] [PubMed] [Google Scholar]
- 134. Bishop AL, Hall A: Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. 10.1042/0264-6021:3480241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Grimmer S, van Deurs B, Sandvig K: Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115(Pt 14):2953–62. [DOI] [PubMed] [Google Scholar]
- 136. Haga Y, Miwa N, Jahangeer S, et al. : CtBP1/BARS is an activator of phospholipase D1 necessary for agonist-induced macropinocytosis. EMBO J. 2009;28(9):1197–207. 10.1038/emboj.2009.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bohdanowicz M, Schlam D, Hermansson M, et al. : Phosphatidic acid is required for the constitutive ruffling and macropinocytosis of phagocytes. Mol Biol Cell. 2013;24(11):1700–12, S1-7. 10.1091/mbc.E12-11-0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Fujii M, Kawai K, Egami Y, et al. : Dissecting the roles of Rac1 activation and deactivation in macropinocytosis using microscopic photo-manipulation. Sci Rep. 2013;3:2385. 10.1038/srep02385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Saarikangas J, Zhao H, Lappalainen P: Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev. 2010;90(1):259–289. 10.1152/physrev.00036.2009 [DOI] [PubMed] [Google Scholar]
- 140. Rohatgi R, Nollau P, Ho HY, et al. : Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276(28):26448–52. 10.1074/jbc.M103856200 [DOI] [PubMed] [Google Scholar]
- 141. Rohatgi R, Ho HY, Kirschner MW: Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol. 2000;150(6):1299–310. 10.1083/jcb.150.6.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hoeller O, Kay RR: Chemotaxis in the absence of PIP3 gradients. Curr Biol. 2007;17(9):813–7. 10.1016/j.cub.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 143. Burke JE, Williams RL: Synergy in activating class I PI3Ks. Trends Biochem Sci. 2015;40(2):88–100. 10.1016/j.tibs.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 144. Yoshida S, Hoppe AD, Araki N, et al. : Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages. J Cell Sci. 2009;122(Pt 18):3250–61. 10.1242/jcs.053207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Welliver TP, Swanson JA: A growth factor signaling cascade confined to circular ruffles in macrophages. Biol Open. 2012;1(8):754–60. 10.1242/bio.20121784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Veltman DM, Williams TD, Bloomfield G, et al. : A plasma membrane template for macropinocytic cups. eLife. 2016;5: pii:e20085. 10.7554/eLife.20085 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 147. Hoeller O, Bolourani P, Clark J, et al. : Two distinct functions for PI3-kinases in macropinocytosis. J Cell Sci. 2013;126(Pt 18):4296–307. 10.1242/jcs.134015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 148. Salloum G, Jakubik CT, Erami Z, et al. : PI3Kβ is selectively required for growth factor-stimulated macropinocytosis. J Cell Sci. 2019;132(16): pii:jcs231639. 10.1242/jcs.231639 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 149. Amyere M, Payrastre B, Krause U, et al. : Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11(10):3453–67. 10.1091/mbc.11.10.3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ridley AJ, Paterson HF, Johnston CL, et al. : The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–10. 10.1016/0092-8674(92)90164-8 [DOI] [PubMed] [Google Scholar]
- 151. Krugmann S, Anderson KE, Ridley SH, et al. : Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell. 2002;9(1):95–108. 10.1016/s1097-2765(02)00434-3 [DOI] [PubMed] [Google Scholar]
- 152. Das B, Shu X, Day GJ, et al. : Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J Biol Chem. 2000;275(20):15074–81. 10.1074/jbc.M907269199 [DOI] [PubMed] [Google Scholar]
- 153. Dharmawardhane S, Schürmann A, Sells MA, et al. : Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11(10):3341–52. 10.1091/mbc.11.10.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Insall R, Müller-Taubenberger A, Machesky L, et al. : Dynamics of the Dictyostelium Arp2/3 complex in endocytosis, cytokinesis, and chemotaxis. Cell Motil Cytoskeleton. 2001;50(3):115–28. 10.1002/cm.10005 [DOI] [PubMed] [Google Scholar]
- 155. Seastone DJ, Harris E, Temesvari LA, et al. : The WASp-like protein scar regulates macropinocytosis, phagocytosis and endosomal membrane flow in Dictyostelium. J Cell Sci. 2001;114(Pt 14):2673–83. [DOI] [PubMed] [Google Scholar]
- 156. Koivusalo M, Welch C, Hayashi H, et al. : Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188(4):547–563. 10.1083/jcb.200908086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Barker SA, Caldwell KK, Pfeiffer JR, et al. : Wortmannin-sensitive phosphorylation, translocation, and activation of PLCgamma1, but not PLCgamma2, in antigen-stimulated RBL-2H3 mast cells. Mol Biol Cell. 1998;9(2):483–96. 10.1091/mbc.9.2.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bae YS, Cantley LG, Chen CS, et al. : Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(8):4465–9. 10.1074/jbc.273.8.4465 [DOI] [PubMed] [Google Scholar]
- 159. Falasca M, Logan SK, Lehto VP, et al. : Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17(2):414–22. 10.1093/emboj/17.2.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhang Y, Kwon SH, Vogel WK, et al. : PI(3,4,5)P 3 potentiates phospholipase C-beta activity. J Recept Signal Transduct Res. 2009;29(1):52–62. 10.1080/10799890902729449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Maekawa M, Terasaka S, Mochizuki Y, et al. : Sequential Breakdown of 3-Phosphorylated Phosphoinositides Is Essential for the Completion of Macropinocytosis. Proc Natl Acad Sci U S A. 2014;111(11):E978–87. 10.1073/pnas.1311029111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Dormann D, Weijer G, Dowler S, et al. : In Vivo Analysis of 3-phosphoinositide Dynamics During Dictyostelium Phagocytosis and Chemotaxis. J Cell Sci. 2004;117(Pt 26):6497–509. 10.1242/jcs.01579 [DOI] [PubMed] [Google Scholar]
- 163. Hasegawa J, Tokuda E, Tenno T, et al. : SH3YL1 Regulates Dorsal Ruffle Formation by a Novel Phosphoinositide-Binding Domain. J Cell Biol. 2011;193(5):901–16. 10.1083/jcb.201012161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Loovers HM, Kortholt A, de Groote H, et al. : Regulation of Phagocytosis in Dictyostelium by the Inositol 5-phosphatase OCRL Homolog Dd5P4. Traffic. 2007;8(5):618–28. 10.1111/j.1600-0854.2007.00546.x [DOI] [PubMed] [Google Scholar]
- 165. Wang JTH, Kerr MC, Karunaratne S, et al. : The SNX-PX-BAR Family in Macropinocytosis: The Regulation of Macropinosome Formation by SNX-PX-BAR Proteins. PLoS One. 2010;5(10):e13763. 10.1371/journal.pone.0013763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yamauchi Y, Helenius A: Virus Entry at a Glance. J Cell Sci. 2013;126(Pt 6):1289–1295. 10.1242/jcs.119685 [DOI] [PubMed] [Google Scholar]
- 167. de Carvalho TMU, Barrias ES, de Souza W: Macropinocytosis: A Pathway to Protozoan Infection. Front Physiol. 2015;6:106. 10.3389/fphys.2015.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Crump JA, Luby SP, Mintz ED: The Global Burden of Typhoid Fever. Bull World Health Organ. 2004;82(5):346–353. [PMC free article] [PubMed] [Google Scholar]
- 169. Crump JA, Ram PK, Gupta SK, et al. : Part I. Analysis of Data Gaps Pertaining to Salmonella Enterica Serotype Typhi Infections in Low and Medium Human Development Index Countries, 1984-2005. Epidemiol Infect. 2008;136(4):436–448. 10.1017/S0950268807009338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. House D, Bishop A, Parry C, et al. : Typhoid Fever: Pathogenesis and Disease. Curr Opin Infect Dis. 2001;14(5):573–578. 10.1097/00001432-200110000-00011 [DOI] [PubMed] [Google Scholar]
- 171. Tsolis RM, Kingsley RA, Townsend SM, et al. : Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv Exp Med Biol. 1999;473:261–274. 10.1007/978-1-4615-4143-1_28 [DOI] [PubMed] [Google Scholar]
- 172. Galán JE, Lara-Tejero M, Marlovits TC, et al. : Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol. 2014;68:415–38. 10.1146/annurev-micro-092412-155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ham H, Sreelatha A, Orth K: Manipulation of host membranes by bacterial effectors. Nat Rev Microbiol. 2011;9(9):635–646. 10.1038/nrmicro2602 [DOI] [PubMed] [Google Scholar]
- 174. Schlumberger MC, Hardt WD: Salmonella type III secretion effectors: pulling the host cell’s strings. Curr Opin Microbiol. 2006;9(1):46–54. 10.1016/j.mib.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 175. Galyov EE, Wood MW, Rosqvist PB, et al. : A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25(5):903–12. 10.1111/j.1365-2958.1997.mmi525.x [DOI] [PubMed] [Google Scholar]
- 176. Anderson Norris F, Wilson MP, Wallis TS, et al. : SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci U S A. 1998;95(24):14057–14059. 10.1073/pnas.95.24.14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Marcus SL, Wenk MR, Steele-Mortimer O, et al. : A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett. 2001;494(3):201–207. 10.1016/s0014-5793(01)02356-0 [DOI] [PubMed] [Google Scholar]
- 178. Terebiznik MR, Vieira OV, Marcus SL, et al. : Elimination of host cell PtdIns(4,5)P (2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4(10):766–773. 10.1038/ncb854 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 179. Mason D, Mallo GV, Terebiznik MR, et al. : Alteration of epithelial structure and function associated with PtdIns(4,5)P 2 degradation by a bacterial phosphatase. J Gen Physiol. 2007;129(4):267–283. 10.1085/jgp.200609656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Piscatelli HL, Li M, Zhou D: Dual 4- and 5-phosphatase activities regulate SopB-dependent phosphoinositide dynamics to promote bacterial entry. Cell Microbiol. 2016;18(5):705–719. 10.1111/cmi.12542 [DOI] [PubMed] [Google Scholar]
- 181. Bakowski MA, Braun V, Lam GY, et al. : The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe. 2010;7(6):453–462. 10.1016/j.chom.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 182. Zhou D, Chen LM, Hernandez L, et al. : A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol. 2001;39(2):248–259. 10.1046/j.1365-2958.2001.02230.x [DOI] [PubMed] [Google Scholar]
- 183. Hernandez L, Hueffer K, Wenk M, et al. : Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304(5678):1805–7. 10.1126/science.1098188 [DOI] [PubMed] [Google Scholar]
- 184. Niebuhr K, Giuriato S, Pedron T, et al. : Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 2002;21(19):5069–78. 10.1093/emboj/cdf522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Killackey SA, Sorbara MT, Girardin SE: Cellular Aspects of Shigella Pathogenesis: Focus on the Manipulation of Host Cell Processes. Front Cell Infect Microbiol. 2016;6:38. 10.3389/fcimb.2016.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Patel JC, Galán JE: Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J Cell Biol. 2006;175(3):453–63. 10.1083/jcb.200605144 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 187. Burkinshaw BJ, Prehna G, Worrall LJ, et al. : Structure of Salmonella effector protein SopB N-terminal domain in complex with host Rho GTPase Cdc42. J Biol Chem. 2012;287(16):13348–13355. 10.1074/jbc.M111.331330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rudolph MG, Weise C, Mirold S, et al. : Biochemical analysis of SopE from Salmonella typhimurium, a highly efficient guanosine nucleotide exchange factor for RhoGTPases. J Biol Chem. 1999;274(43):30501–9. 10.1074/jbc.274.43.30501 [DOI] [PubMed] [Google Scholar]
- 189. Friebel A, Ilchmann H, Aepfelbacher M, et al. : SopE and SopE2 from Salmonella typhimurium Activate Different Sets of RhoGTPases of the Host Cell. J Biol Chem. 2001;276:34035–34040. 10.1074/jbc.M100609200 [DOI] [PubMed] [Google Scholar]
- 190. Stender S, Friebel A, Linder S, et al. : Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol Microbiol. 2000;36(6):1206–21. 10.1046/j.1365-2958.2000.01933.x [DOI] [PubMed] [Google Scholar]
- 191. Stebbins CE, Galán JE: Modulation of host signaling by a bacterial mimic: Structure of the Salmonella effector SptP bound to Rac1. Mol Cell. 2000;6(6):1449–60. 10.1016/s1097-2765(00)00141-6 [DOI] [PubMed] [Google Scholar]
- 192. Fu Y, Galán JE: A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401(6750):293–7. 10.1038/45829 [DOI] [PubMed] [Google Scholar]
- 193. Myeni SK, Zhou D: The C terminus of SipC binds and bundles F-actin to promote Salmonella invasion. J Biol Chem. 2010;285(18):13357–63. 10.1074/jbc.M109.094045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. McGhie EJ, Hayward RD, Koronakis V: Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J. 2001;20(9):2131–9. 10.1093/emboj/20.9.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hayward RD, Koronakis V: Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 1999;18(18):4926–34. 10.1093/emboj/18.18.4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Truong D, Brabant D, Bashkurov M, et al. : Formin-mediated actin polymerization promotes Salmonella invasion. Cell Microbiol. 2013;15(12):2051–63. 10.1111/cmi.12173 [DOI] [PubMed] [Google Scholar]
- 197. Van Engelenburg SB, Palmer AE: Quantification of real-time Salmonella effector type III secretion kinetics reveals differential secretion rates for SopE2 and SptP. Chem Biol. 2008;15(6):619–28. 10.1016/j.chembiol.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Unsworth KE, Way M, McNiven M, et al. : Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell Microbiol. 2004;6(11):1041–55. 10.1111/j.1462-5822.2004.00417.x [DOI] [PubMed] [Google Scholar]
- 199. Criss AK, Casanova JE: Coordinate regulation of Salmonella enterica serovar Typhimurium invasion of epithelial cells by the Arp2/3 complex and Rho GTPases. Infect Immun. 2003;71(5):2885–91. 10.1128/iai.71.5.2885-2891.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mallo GV, Espina M, Smith AC, et al. : SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182(4):741–52. 10.1083/jcb.200804131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Cooper KG, Winfree S, Malik-Kale P, et al. : Activation of Akt by the bacterial inositol phosphatase, SopB, is wortmannin insensitive. PLoS One. 2011;6(7):e22260. 10.1371/journal.pone.0022260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Steele-Mortimer O, Knodler LA, Marcus SL, et al. : Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J Biol Chem. 2000;275(48):37718–24. 10.1074/jbc.M008187200 [DOI] [PubMed] [Google Scholar]
- 203. Knodler LA, Finlay BB, Steele-Mortimer O: The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280(10):9058–64. 10.1074/jbc.M412588200 [DOI] [PubMed] [Google Scholar]
- 204. Liebl D, Qi X, Zhe Y, et al. : SopB-Mediated Recruitment of SNX18 Facilitates Salmonella Typhimurium Internalization by the Host Cell. Front Cell Infect Microbiol. 2017;7:257. 10.3389/fcimb.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 205. Domin J, Pages F, Volinia S, et al. : Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem J. 1997;326(Pt 1):139–47. 10.1042/bj3260139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Dragoi AM, Agaisse H: The class II phosphatidylinositol 3-phosphate kinase PIK3C2A promotes Shigella flexneri dissemination through formation of vacuole-like protrusions. Infect Immun. 2015;83(4):1695–1704. 10.1128/IAI.03138-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Koestler BJ, Fisher CR, Payne SM: Formate Promotes Shigella Intercellular Spread and Virulence Gene Expression. mBio. 2018;9(5): pii e01777-18. 10.1128/mBio.01777-18 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 208. Kuehl CJ, Dragoi AM, Talman A, et al. : Bacterial spread from cell to cell: beyond actin-based motility. Trends Microbiol. 2015;23(9):558–66. 10.1016/j.tim.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. McDade JE, Shepard CC, Fraser DW, et al. : Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297(22):1197–203. 10.1056/NEJM197712012972202 [DOI] [PubMed] [Google Scholar]
- 210. Fraser DW, Tsai TR, Orenstein W, et al. : Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297(22):1189–97. 10.1056/NEJM197712012972201 [DOI] [PubMed] [Google Scholar]
- 211. Watarai M, Derre I, Kirby J, et al. : Legionella Pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J Exp Med. 2001;194(8):1081–1095. 10.1084/jem.194.8.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Segal G, Purcell M, Shuman HA: Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci U S A. 1998;95(4):1669–1674. 10.1073/pnas.95.4.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Vogel JP, Andrews HL, Wong SK, et al. : Conjugative transfer by the virulence system of Legionella Pneumophila. Science. 1998;279(5352):873–6. 10.1126/science.279.5352.873 [DOI] [PubMed] [Google Scholar]
- 214. Dong N, Niu M, Hu L, et al. : Modulation of membrane phosphoinositide dynamics by the phosphatidylinositide 4-kinase activity of the Legionella LepB effector. Nat Microbiol. 2016;2:16236. 10.1038/nmicrobiol.2016.236 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 215. Hsu F, Zhu W, Brennan L, et al. : Structural basis for substrate recognition by a unique Legionella phosphoinositide phosphatase. Proc Natl Acad Sci U S A. 2012;109(34):13567–72. 10.1073/pnas.1207903109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Weber SS, Ragaz C, Reus K, et al. : Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2006;2(5):e46. 10.1371/journal.ppat.0020046 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 217. Brombacher E, Urwyler S, Ragaz C, et al. : Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella Pneumophila. J Biol Chem. 2009;284(8):4846–56. 10.1074/jbc.M807505200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ku B, Lee K-H, P WS, et al. : VipD of Legionella Pneumophila targets activated Rab5 and Rab22 to interfere with endosomal trafficking in macrophages. PLoS Pathog. 2012;8(12):e1003082. 10.1371/journal.ppat.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Gaspar AH, Machner MP: VipD is a Rab5-activated phospholipase A1 that protects Legionella pneumophila from endosomal fusion Proc Natl Acad Sci U S A. 2014;111(12):4560–5. 10.1073/pnas.1316376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ochoa TJ, Contreras CA: Enteropathogenic escherichia coli infection in children. Curr Opin Infect Dis. 2011;24(5):478–83. 10.1097/QCO.0b013e32834a8b8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Lanata CF, Fischer-Walker CL, Olascoaga AC, et al. : Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8(9):e72788. 10.1371/journal.pone.0072788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Singh AP, Aijaz S: Enteropathogenic E. coli: breaking the intestinal tight junction barrier. F1000Res. 2015;4:231. 10.12688/f1000research.6778.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Hayward RD, Leong JM, Koronakis V, et al. : Exploiting pathogenic Escherichia coli to model transmembrane receptor signalling. Nat Rev Microbiol. 2006;4(5):358–70. 10.1038/nrmicro1391 [DOI] [PubMed] [Google Scholar]
- 224. Phillips N, Hayward RD, Koronakis V: Phosphorylation of the enteropathogenic E. coli receptor by the Src-family kinase c-Fyn triggers actin pedestal formation. Nat Cell Biol. 2004;6(7):618–25. 10.1038/ncb1148 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 225. Swimm A, Bommarius B, Li Y, et al. : Enteropathogenic Escherichia coli use redundant tyrosine kinases to form actin pedestals. Mol Biol Cell. 2004;15:3520–9. 10.1091/mbc.e04-02-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Bommarius B, Maxwell D, Swimm A, et al. : Enteropathogenic Escherichia coli Tir is an SH2/3 ligand that recruits and activates tyrosine kinases required for pedestal formation. Mol Microbiol. 2007;63(6):1748–68. 10.1111/j.1365-2958.2007.05626.x [DOI] [PubMed] [Google Scholar]
- 227. Campellone KG, Rankin S, Pawson T, et al. : Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J Cell Biol. 2004;164(3):407–16. 10.1083/jcb.200306032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Gruenheid S, DeVinney R, Bladt F, et al. : Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3(9):856–9. 10.1038/ncb0901-856 [DOI] [PubMed] [Google Scholar]
- 229. Rescher U, Ruhe D, Ludwig C, et al. : Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J Cell Sci. 2004;117(Pt 16):3473–80. 10.1242/jcs.01208 [DOI] [PubMed] [Google Scholar]
- 230. Sason H, Milgrom M, Weiss AM, et al. : Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol Biol Cell. 2009;20(1):544–55. 10.1091/mbc.e08-05-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Lommel S, Benesch S, Rottner K, et al. : Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2001;2(9):850–7. 10.1093/embo-reports/kve197 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 232. Alto NM, Weflen AM, Rardin MJ, et al. : The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J Cell Biol. 2007;178(7):1265–78. 10.1083/jcb.200705021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Dong N, Liu L, Shao F: A bacterial effector targets host DH-PH domain RhoGEFs and antagonizes macrophage phagocytosis. EMBO J. 2010;29(8):1363–76. 10.1038/emboj.2010.33 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 234. Humphreys D, Singh V, Koronakis V: Inhibition of WAVE Regulatory Complex Activation by a Bacterial Virulence Effector Counteracts Pathogen Phagocytosis. Cell Rep. 2016;17(3):697–707. 10.1016/j.celrep.2016.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Crane JK, Majumdar S, Pickhardt DF 3rd: Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67(5):2575–2584. 10.1128/IAI.67.5.2575-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Kenny B, Finlay BB: Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-gamma1. Infect Immun. 1997;65(7):2528–36. 10.1128/IAI.65.7.2528-2536.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Smith K, Humphreys D, Hume PJ, et al. : Enteropathogenic Escherichia coli recruits the cellular inositol phosphatase SHIP2 to regulate actin-pedestal formation. Cell Host Microbe. 2010;7(1):13–24. 10.1016/j.chom.2009.12.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 238. Pemberton JG, Kim YJ, Humpolickova J, et al. : Defining the subcellular distribution and metabolic channeling of phosphatidylinositol. J Cell Biol. 2020;219(3): e201906130. 10.1083/jcb.201906130 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 239. Zewe JP, Miller AM, Sangappa S, et al. : Probing the subcellular distribution of phosphatidylinositol reveals a surprising lack at the plasma membrane. J Cell Biol. 2020;219(3): e201906127. 10.1083/jcb.201906127 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 240. Várnai P, Balla T: Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[ 3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143(2):501–10. 10.1083/jcb.143.2.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Stauffer TP, Ahn S, Meyer T: Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P 2 concentration monitored in living cells. Curr Biol. 1998;8(6):343–6. 10.1016/s0960-9822(98)70135-6 [DOI] [PubMed] [Google Scholar]
- 242. Hammond GRV, Machner MP, Balla T: A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J Cell Biol. 2014;205(1):113–26. 10.1083/jcb.201312072 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 243. Kutateladze TG, Ogburn KD, Watson WT, et al. : Phosphatidylinositol 3-phosphate recognition by the FYVE domain. Mol Cell. 1999;3(6):805–11. 10.1016/s1097-2765(01)80013-7 [DOI] [PubMed] [Google Scholar]
- 244. Gaullier JM, Ronning E, Gillooly DJ, et al. : Interaction of the EEA1 FYVE finger with phosphatidylinositol 3-phosphate and early endosomes. Role of conserved residues. J Biol Chem. 2000;275(32):24595–600. 10.1074/jbc.M906554199 [DOI] [PubMed] [Google Scholar]