Introduction
Vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), is still a controversial issue and studies on transplacental transmission correlations are still limited. We report on our experience with placental SARS-CoV-2 markers of infection in a series of mothers who received a diagnosis of COVID-19 in their third trimester of pregnancy.
Methods
Patients
All pregnant women who received a diagnosis of COVID-19 and delivered at the Papa Giovanni XXIII Hospital in Bergamo, Italy, between March 5, 2020, and April 21, 2020, were included in this study. The maternal and neonatal charts were reviewed retrospectively. The institutional review board approved the study, and informed consent was obtained from all the patients.
Placentas
All the placentas were collected at birth and sampled and analyzed at the Papa Giovanni XXIII Hospital. Formalin-fixed, paraffin-embedded (FFPE) placental sections were incubated with hematoxylin and eosin (Dako, Glostrup, Denmark), and anti-CD68 antibody (mouse origin, Clone KP1) (Dako, Glostrup, Denmark) for the detection of macrophages.
Real-time reverse transcription-polymerase chain reaction
We collected nasopharyngeal (NP) swabs (FLOQSwab, Copan Italia, Brescia, Italy) in Universal Transport Medium (Copan Italia, Brescia, Italy) from each mother and newborn, and obtained a placental biopsy sample that was stored at –80°C in the Biobank after treatment with RNAlater–ICE (ThermoFisher Scientific, Waltham, MA). A small piece of the placenta (about 3 mm3) was subsequently digested with 50 μL of proteinase K (QIAGEN, Hilden, Germany) in 200 μL of Tris-EDTA buffer solution (Sigma-Aldrich, Germany) for an hour.
Single-molecule RNA in situ hybridization
SARS-CoV-2 RNA was detected by using RNAscope technology (Advanced Cell Diagnostics, Newark, CA), an RNA in situ hybridization (ISH) technique described previously.1 Paired double Z oligonucleotide probes were designed for hybridization to the target RNA by using custom software. The RNAscope 2.5 LS Probe V-nCoV2019-S (catalog number 848568; Advanced Cell Diagnostics, Newark, CA) was used. The RNAscope 2.5 LSx Reagent Kit-Brown (Advanced Cell Diagnostics) in combination with a BOND-III Automated stainer (Leica Biosystems, Buffalo Gorve, IL) was used to process the samples according to the manufacturer’s instructions. The FFPE tissue section samples were prepared according to manufacturer’s recommendations. The RNA integrity of each sample was evaluated with a probe designed for hybridization specifically to the ubiquitin C and cyclophilin B housekeeping genes. The negative control background staining was evaluated using a probe specific to the bacterial dapB gene. Each punctate dot signal representing a single target RNA molecule could be detected with standard light microscopic analysis.
Results
Between March 5, 2020, and April 21, 2020, 22 women who received a diagnosis of COVID-19 gave birth at the Papa Giovanni XXIII Hospital, Bergamo, Italy.
Of the 22 neonates born from COVID-19–positive mothers, 2 tested positive for COVID-19 based on the polymerase chain reaction (PCR) results of an NP swab.
Case 1
The first neonate was vaginally delivered on March 27 after spontaneous labor of the mother who presented with a fever and a cough, and had a positive result for COVID-19 from an NP swab at 37.6 weeks of gestation. The neonatal weight was 2660 g, the Apgar scores were 9 and 10 at 1 and 5 minutes, respectively, and the umbilical artery pH was 7.28. The mother wore a surgical mask during labor and at the time of delivery, but skin-to-skin contact was not permitted. Rooming-in and breastfeeding with a mask, however, were allowed. The newborn had a positive result for COVID-19 from NP swabs that were obtained immediately after birth, 24 hours later, and after 7 days. The neonate remained asymptomatic, except for mild initial feeding difficulties, and was discharged from the hospital at 10 days of life after being hospitalized for observation owing to this being the first positive neonatal case recorded at the facility.
Case 2
The second newborn was delivered by cesarean delivery at 35.1 weeks of gestation from a mother with a fever, cough, and a positive COVID-19 NP swab test. The emergency cesarean delivery was performed owing to a nonreassuring fetal status. The neonate was female, weighing 2686 g, with Apgar scores of 9 and 10 at 1 and 5 minutes, respectively, and an umbilical artery pH of 7.32; after birth, she was immediately separated from the mother and admitted to the neonatal intensive care unit. The NP swab obtained at birth from the neonate was negative for SARS-CoV-2, but a follow-up test of an NP swab that was obtained on day 7 was positive for SARS-CoV-2, without contact between the mother and the neonate during that period. No neonatal complication were observed, except for some feeding difficulties that were reported during the first few days of life. The neonate was discharged on day 20 of life after hospitalization for routine late preterm care.
The placentas of these 2 women who delivered neonates with SARS-CoV-2–positive NP swabs (cases 1 and 2) showed chronic intervillositis, which was accompanied by the presence of macrophages both in the intervillous and the villous space. The immunohistochemical study demonstrated chronic intervillositis with CD68+ macrophage infiltration. (Figures 1, A and B and 2, A and B ).
Figure 1.
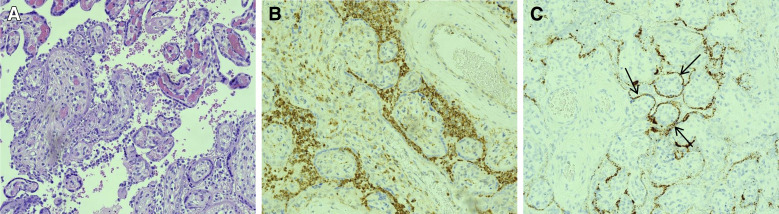
COVID-19–positive mother and neonate (case 1) with SARS-CoV-2 antigens seen in the villous syncytiotrophoblasts on the fetal side of the placenta
A, Chorionic villi showing chronic intervillositis with macrophages in formalin-fixed, paraffin-embedded placental sections using standard brightfield microscopy at 20× magnification. The tissue sections were stained with hematoxylin and eosin (20×). B, Macrophages in the intervillous spaces highlighted by anti-CD68 immunohistochemistry. C, In situ hybridization for SARS-CoV-2 spike protein mRNA, highlighting the presence of SARS-CoV-2 spike antigens in villous syncytiotrophoblasts. The brown dots are positive signals for the presence of SARS-CoV-2 (indicated by black arrows) inside a syncytiotrophoblast of a chorionic villi cross section.
COVID-19, coronavirus 2019; SARS-CoV-2, severe acute respiratory coronavirus 2.
Patanè. SARS-CoV-2 vertical transmission. AJOG MFM 2020.
Figure 2.

COVID-19–positive mother and neonate (case 2), with SARS-CoV-2 antigens seen in the villous syncytiotrophoblasts on the fetal side of the placenta
A, Chorionic villi showing chronic intervillositis with macrophages in formalin-fixed, paraffin-embedded placental sections using standard brightfield microscopy at 20× magnification. The tissue sections were stained with hematoxylin and eosin (20×). B, Macrophages in the intervillous spaces highlighted by anti-CD68 immunohistochemistry. C, In situ hybridization for SARS-CoV-2 spike protein mRNA, highlighting the presence of SARS-CoV-2 spike antigens in villous syncytiotrophoblasts. The brown dots are positive signals for the presence of SARS-CoV-2 (indicated by black arrows) inside a syncytiotrophoblast of a chorionic villi cross section.
COVID-19, coronavirus 2019; SARS-CoV-2, severe acute respiratory coronavirus 2.
Patanè. SARS-CoV-2 vertical transmission. AJOG MFM 2020.
After the purification of the viral RNA from 200 μL aliquots of each clinical sample, RNA-dependent RNA polymerase, envelope, and nucleocapsid viral genes were detected through real-time PCR using the GeneFinder COVID-19 Plus RealAmp Kit (ELITech Group, Puteaux, France) in combination with the ELITe InGenius platform (ELITech Group) according to the World Health Organization’s protocol.2 We performed an ISH using RNAscope technology, a method that enabled the detection of the SARS-CoV-2 spike protein mRNA by using the V-nCoV2019-S probe. In addition to testing the samples from cases 1 and 2 for the presence of SARS-CoV-2 using RNAscope technology, 2 negative controls were also evaluated: a sample from a COVID-19–positive mother with a COVID-19–negative neonate (case 3) and a sample from a COVID-19–negative mother and neonate dyad (case 4). Individual and clustered brown chromogenic dots were observed, using a standard brightfield microscope, in the syncytiocytotrophoblast of both placentas of mothers who delivered COVID-19–positive neonates (Figures 1, C and 2, C). No evidence of positive chromogenic dots was found in either case 3 (Figure 3 , C) or case 4. Positive control probes were well expressed in all the tested tissues, and the negative control probe verified the absence of background staining related to the assay and that the tissue specimens were prepared adequately. No significant alterations were detected in the other placental histologic examinations of all women positive for COVID-19 who delivered infants with a negative test result.
Figure 3.
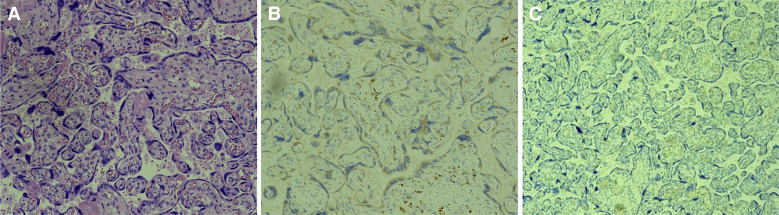
COVID-19–positive mother with a COVID-19–negative neonate (case 3) showing the control placenta from which the virus is absent
A, Normal chorionic villi of formalin-fixed, paraffin-embedded placental sections viewed using a standard brightfield microscope at 20× magnification. The tissue sections were stained with hematoxylin and eosin (20×). B, Normal placental tissues incubated with anti-CD68 antibody. C, In situ hybridization for SARS-CoV-2 spike protein mRNA in the absence of SARS-CoV-2 spike antigens in the villous syncytiotrophoblasts.
COVID-19, coronavirus 2019; SARS-CoV-2, severe acute respiratory coronavirus 2.
Patanè. SARS-CoV-2 vertical transmission. AJOG MFM 2020.
Discussion
The possibility of SARS-CoV-2 vertical transmission is still controversial. Literature with supporting evidence of vertical transmission is limited.3 Two reports described the presence of elevated SARS-CoV-2 immunoglobulin M antibodies in 3 newborns, but repeated NP samples from the infants were negative.4 Wang et al5 reported on 1 case with positive quantitative RT-PCR results from both the mother and the neonate. The neonate was delivered by cesarean delivery, transferred to neonatology, and the neonate had no contact with the mother before the neonate’s NP swab test had a positive result 36 hours after birth. In this case, the swabs from the placenta were negative, but a possible mother-to-child transmission of SARS-CoV-2 could not be excluded. Penfield et al6 reported on the presence of SARS-CoV-2 RNA in 3 of 11 placental samples from COVID-19–positive women. None of the infants tested positive or displayed symptoms.
To our knowledge, this is the first report on cases with positive PCR results for SARS-CoV-2 in the mother, neonate, and the placental tissues. The RNA ISH assay gave us the ability to visualize the virus directly by evaluating the presence of the SARS-CoV-2 spike protein mRNA as the molecular target while still retaining the tissue morphology, a feature that is lost with other methods such as PCR. The RNAscope probe detected positive staining for COVID-19 viral RNA in the infected tissues but not in the uninfected placentas, demonstrating the specificity of the RNAscope probes. The presence of SARS-CoV-2 RNA in the syncytiotrophoblasts signifies the presence of the virus on the fetal side of the placenta.
Conclusion
To our knowledge, this is the first study that describes the presence of SARS-CoV-2 RNA on the fetal side of the placenta in 2 cases of mothers who received a diagnosis of COVID-19 as well as neonates with positive test results for SARS-CoV-2 at birth. These findings support the possibility of vertical transmission of SARS-CoV-2, the virus responsible for COVID-19, from the mother to the fetus in utero.
Moreover, the direct visualization of SARS-CoV-2 RNA in the infected placentas raises the possibility of estimating the viral load in the cells with morphologic context. Further studies are required to confirm our results.
Acknowledgments
The authors thank Rebecca Linn, MD (Department of Pathology, Children's Hospital of Philadelphia, Philadelphia, PA) and Joanna Chan, MD, and John Farber, MD (Department of Pathology, Thomas Jefferson University, Philadelphia, PA) for their input with regard to the pathology.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
References
- 1.Wang F., Flanagan J., Su N., et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamouroux A., Attie-Bitach T., Martinovic J., Leruez-Ville M., Ville Y. Evidence for and against vertical transmission for SARS-CoV-2 (COVID-19) Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.039. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng H., Xu C., Fan J., et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S., Guo L., Chen L., et al. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penfield C.A., Brubaker S.G., Limaye M.A., et al. Detection of SARS-COV-2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100133. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


