The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for coronavirus disease 2019 (COVID-19), which has been associated with maternal and perinatal morbidity and mortality.1 , 2 Almost all patients with COVID-19 have a positive result for antiviral immunoglobulin G (IgG) within approximately 10–20 days after symptom onset (Figure 1 ), but the clinical value of antibody testing has not yet been completely elucidated, either in nonpregnant or particularly in pregnant patients.3
Figure 1.
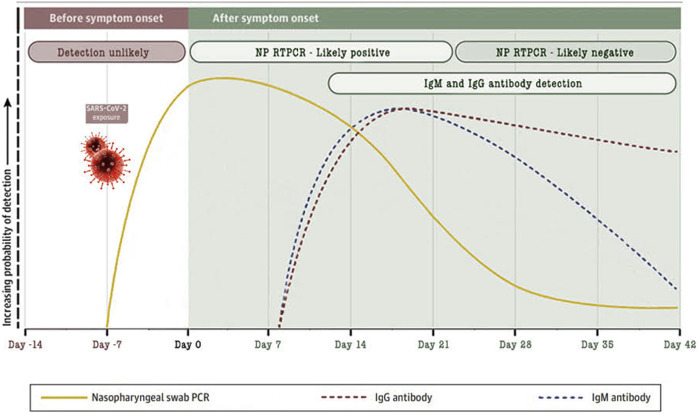
Antibody response against SARS-COV-2 based on data from several published reports
Modified from Sethuraman et al.3
NP, nasopharyngeal; RTPCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Zullo. Antibody response to COVID-19. AJOG MFM 2020.
There are different ways to test for antibodies against SARS-CoV-2. The 3 most commonly used methods are IgM and IgG titer measured by either chemiluminescence immunoassay analysis or enzyme-linked immunosorbent assay and a rapid (results within 15 minutes) IgM-IgG combined antibody test.3 Their sensitivities and specificities vary and are still being studied but have been reported to be approximately 48%, 89%, and 89%, respectively, and 100%, 91%, and 91%, respectively.3, 4, 5
Testing pregnant women for antibody response to COVID-19 may have different advantages, such as identifying (1) possibly “healed” women (eg, IgG positive) who were never tested with real-time reverse transcription polymerase chain reaction (RT-PCR) assay using nasopharyngeal (NP) swab specimens and (2) women who are still at risk for COVID-19 infection (eg, IgM and IgG negative).
Women who do know their infectious status represent a potential threat to others, including healthcare workers (HCWs) and other patients. Indeed, some governments have suggested that the detection of antibodies to SARS-CoV-2 could serve as the basis for an “immunity passport” or “risk-free certificate” (digital or physical documents that certify an individual has been infected and is purportedly immune to SARS-CoV-2) that would enable individuals to, for example, return to work or travel assuming that they are protected against reinfection.6
The use of the point-of-care rapid combined antibody test can be of paramount importance in obstetrical healthcare settings and may be particularly helpful in testing women before outpatient (Figure 2 , A) and inpatient (Figure 2, B) visits. After the rapid test, the following results can be reported:
-
•
IgG negative and IgM negative
-
•
IgG positive and IgM negative. There are limited data on when IgM disappears.3 , 4 Therefore, even if there is a high likelihood that the tested person is not contagious anymore, an NP swab should be offered.
-
•
IgG positive, IgM positive or IgG negative, IgM positive. The presence of IgM increases the possibility that the tested person is still contagious.4
Figure 2.
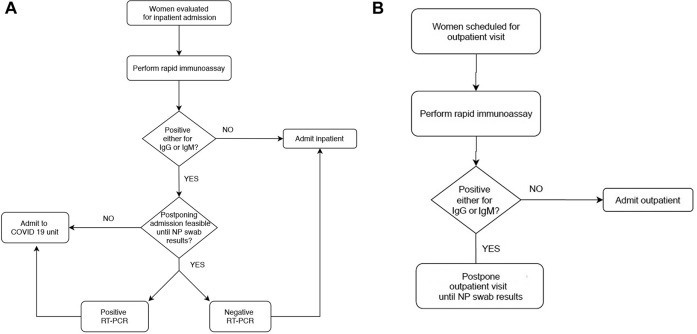
Algorithm for rapid combined antibody test used at the University of Naples Federico II (Naples, Italy)
A, For women before admission to inpatient monitoring. B, For women scheduled for outpatient appointment.
COVID-19, coronavirus disease 2019; GP, general practitioner; NP, nasopharyngeal; RT-PCR, reverse transcription polymerase chain reaction.
Zullo. Antibody response to COVID-19. AJOG MFM 2020.
We suggest that if rapid antibody testing and personnel are available, algorithms for care before outpatient and inpatient care of pregnant women be implemented (Figure 2). At our institution, the Department of Obstetrics and Gynecology at University of Naples Federico II (Naples, Italy), all pregnant women are tested with rapid combined antibody test before hospital admission. If the rapid antibody test is positive to either SARS-CoV-2 IgM or IgG, we do offer the NP swab and consider these women COVID-19 positive until the result of the NP swab is available. Patients with a positive result to rapid combined antibody test are isolated, and inpatient admission is postponed while awaiting the results of the NP swab, if feasible (eg, planned cesarean delivery, induction of labor, surgical procedure). If it is not feasible to postpone the admission (eg, laboring or bleeding pregnant women), the patient is admitted to the COVID unit and managed as COVID-19 positive (Figure 2, A). For example, in our department, recently 2 pregnant women with positive IgG and IgM had admission to the hospital postponed for 24 hours while awaiting the NP swab test result. In the case of another patient who had a positive result for IgM, postponed hospital admission was not feasible because of heavy bleeding in a first-trimester spontaneous abortion. The patient underwent an NP swab and was admitted to the COVID unit where she received dilation and curettage. The result of the RT-PCR assay, available the day after, showed positivity for SARS-CoV-2.
Furthermore, we are now testing women scheduled for outpatient visits. Those who had a positive result for either IgM or IgG at the rapid combined antibody test are offered with NP swab, and the outpatient appointment is postponed (Figure 2, B). Women with previous infection and “certified” recovered because of 2 negative NP swabs 24 hours apart are not tested for antibody response to COVID-19.
It would also be helpful to test visitors and HCWs, if feasible. In our department, we have mandatory rapid antibody testing for all HCWs every 7 days. HCWs who have a positive result for either IgM or IgG self-isolate at home while awaiting the results of the NP swab.
In summary, we recommend testing for antibody response to SARS-CoV-2 for pregnant women before receiving care in both inpatient and outpatient settings, as feasible (Figure 2). Those who have a positive result to either IgM or IgG rapid immunoassay should undergo an NP swab, and admission or appointment should be postponed, if feasible, until NP swab test results are available, and they should be considered COVID-19 positive in the meanwhile (Figure 2).
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
No financial support was received for this study.
References
- 1.Pierce-Williams R.A.M., Burd J., Felder L., et al. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.8259. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Jin Y., Wang M., Zuo Z., et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z., Yi Y., Luo X., et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall M.A., Studdert D.M. Privileges and immunity certification during the COVID-19 pandemic. JAMA. 2020 doi: 10.1001/jama.2020.7712. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


