Highlights
-
•
Atypical clinical features make it hard to identify VL in HLH in non-epidemic area.
-
•
VL is difficult to diagnose when traditional tests are ineffective, while mNGS is.
-
•
mNGS can be used as a detection method of HLH in children caused by rare pathogens.
Abbreviations: VL, visceral leishmaniasis; HLH, hemophagocytic lymphohistiocytosis; VL-HLH, visceral leishmaniasis-related hemophagocytic lymphohistiocytosis; mNGS, next-generation meta-genome sequencing; BMA, bone marrow aspiration; AMB, libosomal amphotericin B
Keywords: Visceral leishmaniasis-related hemophagocytic lymphohistiocytosis, Next-generation meta-genome sequencing, Bone marrow aspiration
Abstract
Background
Visceral leishmaniasis-related hemophagocytic lymphohistiocytosis (VL-HLH) is a secondary hemophagocytic syndrome, which can be life-threatening, caused by leishmania and transmitted by infected sandflies. Rapid and accurate identification of leishmania is crucial for clinical strategies.
Case report
Here, we report an infantile infection in a non-epidemic area of China. The infant was a 9.5-month-old girl with fever, pancytopenia and hepatosplenomegaly, which meet the HLH-2004 standard, and the negative gene results exclude congenital HLH. However, chemotherapy is ineffective and is accompanied by severe infection. Fortunately, she is diagnosed with VL-HLH (visceral leishmaniasis-related hemophagocytic lymphohistiocytosis), as leishmania is detected by next-generation meta-genome sequencing (mNGS) and quickly relieved after treatment with libosomal amphotericin B (L-AMB).
Conclusion
mNGS can detect leishmania in pediatric HLH, and should be performed as a new detection for VL-HLH, particularly for infants, who may not respond to HLH-2004 regimen.
Background
Hemophagocytic lymphohistiocytosis (HLH), also known as hemophagocytic syndrome, is a disease caused by uncontrollable activation and proliferation of lymphocytes and macrophages (Janka, 2012). HLH is classified as primary and secondary, and infection is the most common cause of secondary HLH (Janka et al., 1998), while leishmania is the most common protozoan triggering HLH (Colomba et al., 2016). Visceral leishmaniasis (VL) is caused by infection with leishmania. VL-HLH is very rare in childhood, and the mortality rate is as high as 100% (Gradoni et al., 2017) without early diagnosis and treatment.
Here, we report a case of VL-HLH in a 9.5-month-old girl, diagnosed by next-generation meta-genome sequencing (mNGS), while routine tests were negative.
Case report
In November 2019, a 9.5-month-old girl from handan city of hebei province, who had a fever for 8 days, was admitted to the Hematology Department of Hebei Children’s Hospital. The patient had been treated with cefoperazone sulbactam and acyclovir for 5 days in the local hospital due to high fever, but her symptoms did not relieve.
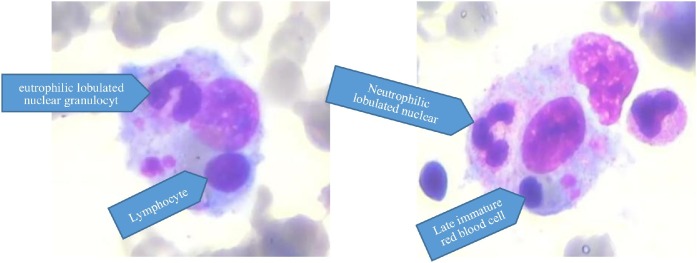
Upon admission, the baby’s parents complained of high fever, accompanied by hepatosplenomegaly, without rash, cough, jaundice or vomiting. Multiple hematological examinations confirmed leukopenia, anemia and thrombocytopenia. The laboratory testing revealed hypertriglyceridemia (4.1 mmol/L), hyperferritinemia (5254.5 ug/L-158,600 ug/L), and hypofibrinogenemia (0.71 g/L). Massive splenomegaly and hepatomegaly were tested by abdominal sonography. Hemophagocytosis was detected by bone marrow aspiration (BMA), but no leishmane-donovan bodies were found under microscopic examination of bone marrow (Wright-Giemsa, 1000×) (Figure 1 ). Serological tests for Epstein-Barr Virus (EBV), Cytomegalovirus (CMV), hepatitis viruses and HIV were performed and proved to be negative.
Figure 1.
High-power microscopic images (Wright-Giemsa, 1000×): histiocytes with hemophagocytosis and leukophagocytosis.
In view of the presence of pancytopenia, hypertriglyceridemia, hypofibrinogenemia, hyperferremia, splenomegaly and phagocytosis of BMA, the diagnosis of HLH was clear. Genetic examination was also performed and the result was negative, which denied congenital HLH.
According to HLH-2004 chemotherapy regimen (Henter et al., 2007): ① Dexamethasone: from Day 2 after admission, 10 mg/(m2 d) for 2 weeks, 5 mg/(m2 d) for 4 days; ② Etoposide: from Day 6 after admission, twice a week, 5 mg/kg each time, a total of 4 times; ③ Other support treatment: granisetron, hydration, alkalized urine, recombinant human granulocyte stimulating factor, immunoglobulin and blood transfusion.
Instead of getting better, the initial symptoms were followed by coughing, wheezing and dyspnea, which led to her being transferred to Pediatric Intensive Care Unit (PICU) on Day 22 after admission (Day 30 with illness).The second BMA was performed due to the deterioration, with no other findings except the disappearance of phagocytosis. We speculated that the secondary HLH caused by infection might exist, while corresponding tests for tumors and autoimmune diseases were negative. However, the negative PCR excluded the presence of streptococcus pneumoniae, staphylococcus aureus, methicillin-resistant staphylococcus, escherichia coli, klebsiella pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, stenotrophomonas maltophilia, haemophilus influenzae, legionella pneumophila, mycobacterium tuberculosis complex, mycoplasma pneumoniae, chlamydia pneumoniae, influenza virus, adenovirus, boca virus, metapneumovirus and coronavirus. Besides that, blood and sputum culture did not give any hints. Rk39 was performed, in view of their family's previous sojourn in VL-prone Shanxi province 6 months earlier, which is located in the southwest of Hebei province, but the results were negative.
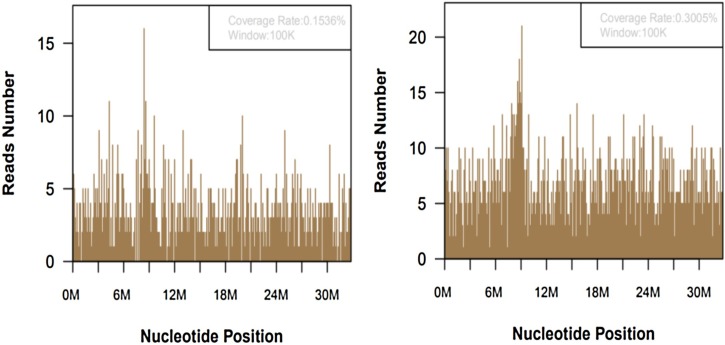
Considering that it might be a rare pathogen, mNGS tests were performed, with the consent of the family. Peripheral blood (EDTA anticoagulant) and sputum of the infant were collected to extract nucleic acid, which was used for library construction and high-throughput sequencing.The identification of pathogenic microorganisms can be realized by comparing and annotating the obtained sequences with the human genome sequence library and the pathogen microbial genome sequence library.Two days later, 2144 and 4159 sequence readings of leishmania were detected in blood and sputum respectively, accounting for 0.1536% and 0.3005% of nucleotide sequence coverage separately (Figure 2 ).
Figure 2.
A total of 2144 and 4159 DNA readings of leishmania in blood (left) and sputum samples (right), with coverage of 0.1536% and 0.3005% respectively.
Combined with clinical symptoms, VL-HLH was diagnosed and treated with l-AMB on Day 31 after admission (Aronson et al., 2016). Fever subsided, and then the ventilator was removed successfully on Day 34 after admission. After one cycle of l-AMB treatment regimen, fever and splenomegaly were relieved, blood routine, fibrinogen, triglyceride, NK cell activity and soluble CD25 returned to normal.
Discussion
The diagnosis of secondary HLH was clear, but the pathogen was unknown. The infant fell ill in winter, and denied sandfly bites and family aggregation diseases. She lived in Handan City of Hebei Province, a non-VL epidemic area, and had spent a short time in Yangquan City of Shanxi Province, where sporadic leishmaniasis was found (Zhou, et al., 2020). It was difficult to make a biased judgment according to her symptoms, especially when routine pathogen culture, PCR and serological examination were all negative. Therefore, we chose mNGS with wide coverage and high accuracy, which found the leishmania.
VL-HLH is a systemic disease caused by leishmania, transmitted by infected sandflies, and meet the criteria of HLH-2004, with an average incubation period of 3–8 months (Gramiccia et al., 2013). Adamczick C (Adamczick et al., 2018) has reported the interval between clinical symptoms of twin children infected simultaneously was up to 9 months. Furthermore, VL had a wide range of clinical symptoms, which overlap with severe infection, hematological diseases and autoimmune diseases, such as fever, splenomegaly, and hemocytopenia, which confused the clinicians greatly.
Common tests of leishmaniasis included serology, microscopic examination of bone marrow and PCR. Rajagopala S (Rajagopala et al., 2008) found that leishmane-donovan bodies could be seen in only 35.3%of patients in the first BMA microscopic examination, and in 78% of VL-HLH patients after repeated BMAs. In addition, it should be emphasized that the identification of the leishman-donovan bodies was closely related to the experience of laboratorians. The rk39 test is the most common serological method in China, and the result depends on the chromogenic reaction, which may be unconspicuous for low titer antibody in the early stage of the infection. Moreover, different individual immune responses and the quality of test strips would also affect the results. Reports about negative rk39 in children with VL-HLH are not rare in China (Guo et al., 2011) and abroad (Scalzone et al., 2016). The PCR test with high specificity has improved detection of leishmania, but it is not universalized in China, especially in non-epidemic areas, where bone marrow needs to be sent to special testing institutions. More importantly, the test will be carried out only when leishmaniasis is highly suspected, which is difficult for inexperienced clinicians in non-epidemic areas.
To the best of our knowledge, this is the first report of direct detection of leishmania using mNGS in infants with HLH in China. mNGS refers to the direct high-throughput sequencing of nucleic acids in samples, and then compared with the pathogen sequence contained in the database, which has been an important technique for unexplained infections. Different from the traditional PCR, mNGS does not need primers and specific amplification, and it can detect multiple pathogen gene sequences simultaneously, and achieve comprehensive unbiased screening quickly and accurately (Westblade et al., 2016). Currently, mNGS can detect more than 6350 bacteria, 1798 viruses, 1064 fungi and 234 parasites.
Miao Q (Miao et al., 2018) and Zinter MS (Zinter et al., 2019) confirmed that mNGS with high sensitivity was less affected by antibiotic exposure and immune function than traditional etiological detections. However, it should be noted that the positive results must be interpreted in the clinical context, not simply identified as the pathogen (Chiu et al., 2019). In this case, leishmania is detected by mNGS, and the condition is rapidly relieved after anti-VL treatment, which confirms the accuracy of the detection results.
Conclusion
As far as we know, this is the first report that mNGS can detect leishmania in pediatric HLH, when leishmane-donovan bodies are not found under the microscope and rk39 test is negative. mNGS can provide an effective basis for early diagnosis of VL-HLH, and should be recommended in clinical practice, particularly for children who are not responsive to HLH-2004 regimen and complicated with severe infection. mNGS should be performed to determine whether there is secondary HLH caused by rare pathogens including VL.
Consent for publication
Written informed consent was obtained from the legal guardian of this case report.
Funding source
This report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Since the data were anonymous and no threat to patients' rights, the Ethics Committee of Hebei Children's Hospital exempts the need for ethical approval.
Competing interests
As the authors of this report, we all declare that there are no known conflicts of competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adamczick C., Dierig A., Welzel T. Double trouble: visceral leishmaniasis in twins after traveling to Tuscany – a case report. BMC Infect Dis. 2018;18(1):495. doi: 10.1186/s12879-018-3394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N., Herwaldt B.L., Libman M. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) Clin Infect Dis. 2016;63:e202–e264. doi: 10.1093/cid/ciw670. [DOI] [PubMed] [Google Scholar]
- Chiu C.Y., Miller S.A. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341. doi: 10.1038/s41576-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomba C., Di Carlo P., Scarlata F. Visceral leishmaniasis, hypertriglyceridemia and secondary hemophagocytic lymphohistiocytosis. Infection. 2016;44:391–392. doi: 10.1007/s15010-016-0881-3. [DOI] [PubMed] [Google Scholar]
- Gradoni L., López-Vélez R., Mokni M. 2017. Manual on case management and surveillance of leishmaniases in the WHO European Region.http://www.who.int/leishmaniasis/resources/978-92-89052-51-1.en [Google Scholar]
- Gramiccia M., Scalone A., Di Muccio. The burden of visceral leishmaniasis in Italy from 1982 to 2012: a retrospective analysis of the multi-annual epidemic that occurred from 1989 to 2009. Euro Surveill. 2013;18:20535. [PubMed] [Google Scholar]
- Guo X., Chen N., Wang T.Y. Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis: report of four childhood cases. Zhonghua Er Ke Za Zhi. 2011;49(7):550–553. [PubMed] [Google Scholar]
- Henter J.I., Horne A., Aricó M. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- Janka G.E. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233–246. doi: 10.1146/annurev-med-041610-134208. [DOI] [PubMed] [Google Scholar]
- Janka G., Imashuku S., Elinder G. Infection- and malignancy-associated hemophagocytic syndromes. Secondary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am. 1998;12:435–444. doi: 10.1016/s0889-8588(05)70521-9. [DOI] [PubMed] [Google Scholar]
- Miao Q., Ma Y., Wang Q. Microbiological diagnostic performance of metagenomic ext-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67:S231–S240. doi: 10.1093/cid/ciy693. [DOI] [PubMed] [Google Scholar]
- Rajagopala S., Dutta U., Chandra K.S. Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis—case report and systematic review. Infect. 2008;56(5):381–388. doi: 10.1016/j.jinf.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Scalzone Maria, Ruggiero Antonio, Mastrangelo Stefano. Hemophagocytic lymphohistiocytosis and visceral leishmaniasis in children: case report and systematic review of literature. Infect Dev Ctries. 2016;10(1):103–108. doi: 10.3855/jidc.6385. [DOI] [PubMed] [Google Scholar]
- Westblade L.F., Van Belkum A., Grundhoff A. Role of clinicogenomics in infectious disease diagnostics and public health microbiology. Clin Microbiol. 2016;54(7):1686–1693. doi: 10.1128/JCM.02664-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.B., Li Y.Y., Zhang Y. Prevalence of visceral leishmaniasis in China in 2018. Chin J Parasitol Parasit Dis. 2020;38(2) [Google Scholar]
- Zinter M.S., Dvorak C.C., Mayday M.Y. Pulmonary metagenomic sequencing suggests missed infections in immunocompromised children. Clin Infect Dis. 2019;68(11) doi: 10.1093/cid/ciy802. [DOI] [PMC free article] [PubMed] [Google Scholar]