Abstract
Objectives
Studies reported associations of inflammatory markers with the severity of COVID-19, but conclusions were inconsistent. We aimed to provide an overview of the association of inflammatory markers with the severity of COVID-19.
Methods
We searched PubMed, Embase, Cochrane Library, Wanfang and China National Knowledge Infrastructure (CNKI) database until March 20, 2020. Weighted mean difference (WMD) and 95% confidence intervals (CIs) were pooled using random or fixed-effects models.
Results
A total of 16 studies comprising 3962 patients with COVID-19 were included in our analysis. Random-effect results demonstrated that patients with COVID-19 in the nonsevere group had lower levels for CRP (WMD = −41.78 mg/l, 95% CI = [−52.43, −31.13], P < 0.001), PCT (WMD = −0.13 ng/ml, 95% CI = [−0.20, −0.05], P < 0.001), IL-6 (WMD = −21.32 ng/l, 95% CI = [−28.34, −14.31], P < 0.001), ESR (WMD = −8 mm/h, 95% CI = [−14, −2], P = 0.005), SAA (WMD = −43.35 μg/ml, 95% CI = [−80.85, −5.85], P = 0.020) and serum ferritin (WMD = −398.80 mg/l, 95% CI = [−625.89, −171.71], P < 0.001), compared with those in the severe group. Moreover, survivors had a lower level of IL-6 than non-survivors (WMD = −4.80 ng/ml, 95% CI = [−5.87, −3.73], P < 0.001). These results were consistent through sensitivity analysis and publication bias assessment.
Conclusions
The meta-analysis highlights the association of inflammatory markers with the severity of COVID-19. Measurement of inflammatory markers might assist clinicians to monitor and evaluate the severity and prognosis of COVID-19.
Keywords: COVID-19, SARS-CoV-2, Inflammatory markers, Severity, Meta-analysis
Introduction
The ongoing worldwide Coronavirus Disease 2019 (COVID-19) pandemic has posed a huge threat to global public health (WHO, 2020a). The pathogen has been identified as a novel single-stranded ribonucleic acid (RNA) betacoronavirus named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which shares an approximately 79% similarity at nucleotide level with severe acute respiratory syndrome coronavirus (SARS-CoV) (Zhang and Holmes, 2020). As of March, 21, 2020, a total of 266, 073 confirmed cases from 150 countries and territories were reported, including 11,183 deaths (WHO, 2020b). COVID-19 represents a spectrum of clinical severity ranged from asymptomatic to critical pneumonia, acute respiratory distress syndrome (ARDS) and even death (Guan et al., 2020). Therefore, full monitoring the severity of COVID-19 and effective early intervention are the fundamental measures for reducing mortality.
Accumulating evidence has suggested that inflammatory responses play a critical role in the progression of COVID-19 (Mehta et al., 2020, Stebbing et al., 2020). Inflammatory responses triggered by rapid viral replication of SARS-CoV-2 and cellular destruction can recruit macrophages and monocytes and induce the release of cytokines and chemokines (Tay et al., 2020). These cytokines and chemokines then attract immune cells and activate immune responses, leading to cytokine storms and aggravations (Xu et al., 2020). Several inflammatory markers have some tracing and detecting accuracy for disease severity and fatality (Wu et al., 2020). Inflammatory markers such as procalcitonin (PCT), serum ferritin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and interleukin-6 (IL-6) have been reported to be significantly associated with the high risks of the development of severe COVID-19 (Cheng et al., 2020, Gao et al., 2020, Qin et al., 2020). Moreover, increased levels of serum amyloid A (SAA) are shown to be involved in COVID-19 pathogenesis and may serve as a potential biomarker for monitoring disease progression (Cheng et al., 2020, Xiang et al., 2020). However, these results remain controversial due to no observed difference in the levels of IL-6, SAA, ESR and CRP by other studies (Chen et al., 2020b, Wu et al., 2020, Zhang et al., 2020).
To the best of our knowledge, the overall inflammatory profile is missing to date due to the insufficient sample size. Here we performed a meta-analysis based on the current scientific literature to compare the levels of inflammatory markers between severe patients and nonsevere patients with COVID-19. Our study will highlight the association of inflammatory markers with the severity of COVID-19 and assist clinicians to monitor and evaluate the severity and prognosis of COVID-19.
Methods
Search strategy
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). Original studies reporting COVID-19 were searched until March 20, 2020 through PubMed, Embase, Cochrane Library, Wanfang and China National Knowledge Infrastructure (CNKI) database. The following combined search terms were used in PubMed, Embase and Cochrane: (“Novel coronavirus” OR “Coronavirus disease 2019” OR “Coronavirus 2019” OR “nCoV-2019” OR “2019-nCoV” OR “COVID-19” OR “SARS-CoV-2”). The Chinese translation of the search terms was used in Wanfang and CNKI database. We did not apply any restriction on language or date or study design. All eligible articles were retrieved, and their references to identified publications were searched for further potentially relevant articles (Gao et al., 2020, Zhou et al., 2020a).
Selection criteria
English-language or Chinese-language publications reporting levels of inflammation markers in patients with COVID-19 were included if they met the following criteria: (1) patients were diagnosed with COVID-19 and had positive results of SARS-CoV-2 RNA; (2) patients could be grouped into severe group and nonsevere group, or intensive care unit (ICU) group and non-ICU group, or survivors group and non-survivors group; (3) literature sources and levels of inflammatory markers were available. The diagnosis and severity classification of COVID-19 was based on the New Coronavirus Pneumonia Prevention and Control Program in China (National Health Commission of China, 2020), which classified COVID-19 into four types including mild, moderate, severe, and critical pneumonia. In our meta-analysis, severe or critical COVID-19 patients were grouped into the severe group, and mild or moderate COVID-19 patients were grouped into the nonsevere group. If there were two or more studies from the same authors or institutions, only the study with the largest sample size was chosen. Studies were excluded if patients were asymptomatic carriers and did not fulfil the inclusion criteria.
Data extraction and quality assessment
The records from the initial search were scanned by two authors to exclude any duplicate and irrelevant studies. The following data were extracted: first authors, publication date, country of origin, grouping situation, cases, age, sex and levels of inflammatory markers in different groups. Stratified data or interquartile range (IQR) were converted to mean (±SD) using mathematical formulas for meta-analysis (Luo et al., 2018, Wan et al., 2014). Any discrepancies were resolved by discussion. Quality assessments of all potentially eligible studies were conducted using the Newcastle-Ottawa Scale (NOS). There are eight items in three aspects: selection, comparability and exposure. The full score was 9 stars. Four to six stars was regarded as a moderate-quality study, and seven to nine stars as a high-quality study (Ga Wells et al., 2014). Studies with NOS scores lower than 7 were recognized to be of inferior quality and therefore excluded.
Statistical analysis
All the statistical analyses were carried out by STATA (Version 12.0; STATA Corporation, College Station, TX, USA) software. Statistical heterogeneity was assessed with I2 and P-value. A fixed effects model was adopted without significant heterogeneity (I2 ≤ 50% and P ≥ 0.1), while a random effects model was employed in all other instances (I2 > 50% or P < 0.1) (Zeng et al., 2019, Zeng et al., 2020). Weighted mean difference (WMD) with 95% confidence interval (95% CI) was calculated for inflammatory markers. Sensitivity analysis was performed by omitting one study each time through influence analysis to assess the stability of results. Additionally, standard mean difference (SMD) was used to explore the consistency of the conclusion. Publication bias was evaluated by Egger’s test. If publication bias was conformed, the Duval’s trim and fill method was implemented to adjust for this bias. P < 0.05 was considered statistically significant.
Results
Literature search and studies characteristics
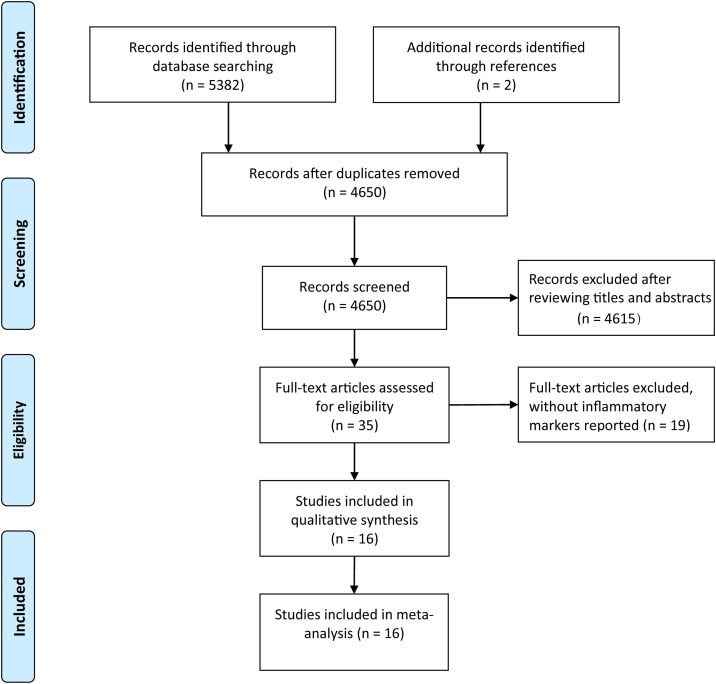
The initial literature search generated altogether 5384 records with 734 studies subsequently excluded due to duplication (Figure 1 ). After a review of the titles and abstracts, we obtained 35 studies by excluding an additional 4615 studies. We further excluded 19 studies by scanning the full text which did not report inflammatory markers. Finally, 16 studies were included in our analysis (Chang et al., 2020, Chen et al., 2020a, Chen et al., 2020b, Cheng et al., 2020, Fang et al., 2020, Gao et al., 2020, Huang et al., 2020, Li et al., 2020, Peng et al., 2020, Qin et al., 2020, Ruan et al., 2020, Wu et al., 2020, Xiang et al., 2020, Xiao et al., 2020, Zhang et al., 2020, Zhou et al., 2020a). Characteristics of 16 eligible studies are presented in Table 1 . All of these studies were published in 2020 and from China, involving 3962 patients. Eight studies were written in Chinese and the others in English. Twelve studies were grouped by nonsevere and severe groups, 2 studies grouped by non-ICU and ICU groups, and 2 studies grouped by survivors and non-survivors with COVID-19. Obviously, patients in the severe group, ICU group or non-survivors group were older than those in the corresponding control group. There was no obvious difference in the sex distribution of patients for each study. All studies were deemed of high quality with 7 or more NOS scores and details can be found in Table 2 .
Figure 1.
Literature search and filtering of studies.
Table 1.
Characteristics of enrolled studies in the meta-analysis.
| First author | Year | Country | Groups | Cases | Age | Sex (male, %) | Inflammatory markers | Quality |
|---|---|---|---|---|---|---|---|---|
| Chang Z | 2020 | China | Nonsevere | 93 | – | – | CRP, PCT, IL-6 | 8 |
| Severe | 57 | |||||||
| Chen C | 2020 | China | Nonsevere | 126 | 57.0 ± 15.6 | 66 (52.3) | CRP | 7 |
| Severe | 24 | 68.5 ± 13.6 | 18 (75.0) | |||||
| Chen L | 2020 | China | Nonsevere | 15 | – | – | IL-6, CRP | 7 |
| Severe | 14 | |||||||
| Cheng K | 2020 | China | Nonsevere | 282 | 49.7 ± 11.9 | 145 (51.4) | IL-6, CRP, PCT, ESR, SAA, Serum ferritin | 8 |
| Severe | 181 | 54.7 ± 13.5 | 99 (54.7) | |||||
| Fang X | 2020 | China | Nonsevere | 55 | 39.9 ± 14.9 | 27 (49.1) | CRP | 7 |
| Severe | 24 | 56.7 ± 14.4 | 18 (75.0) | |||||
| Gao Y | 2020 | China | Nonsevere | 28 | 43.0 ± 14.0 | 17 (60.7) | IL-6, CRP, PCT, fibrinogen | 9 |
| Severe | 15 | 45.2 ± 7.7 | 9 (60.0) | |||||
| Jin Z | 2020 | China | Nonsevere | 82 | 51.6 ± 10.7 | 38 (46.3) | CRP, PCT, SAA | 7 |
| Severe | 56 | 62.7 ± 13.6 | 33 (56.9) | |||||
| Li K | 2020 | China | Nonsevere | 58 | 41.9 ± 10.6 | 29 (50.0) | CRP, PCT | 7 |
| Severe | 25 | 53.7 ± 12.3 | 15 (60.0) | |||||
| Peng Y | 2020 | China | Nonsevere | 96 | 61.5 ± 9.4 | 44 (45.8) | CRP, PCT | 8 |
| Severe | 16 | 58.2 ± 7.3 | 9 (56.3) | |||||
| Qin C | 2020 | China | Nonsevere | 166 | 52.0 ± 15.5 | 80 (48.2) | IL-6, CRP, PCT, ESR, Serum ferritin | 7 |
| Severe | 286 | 60.3 ± 13.4 | 155 (54.2) | |||||
| Wu C | 2020 | China | Nonsevere | 117 | 47.3 ± 10.5 | 68 (58.1) | IL-6, CRP, ESR, Serum ferritin | 7 |
| Severe | 84 | 59.2 ± 14.3 | 60 (71.4) | |||||
| Xiang T | 2020 | China | Nonsevere | 40 | 40.6 ± 14.3 | 25 (63.5) | CRP, PCT, ESR, SAA | 8 |
| Severe | 9 | 53.0 ± 14.0 | 8 (88.9) | |||||
| Xiao K | 2020 | China | Nonsevere | 107 | 43.1 ± 1.1 | 52 (48.6) | IL-6, CRP, PCT | 7 |
| Severe | 36 | 51.3 ± 5.6 | 20 (55.6) | |||||
| Huang C | 2020 | China | Non-ICU | 28 | 49.2 ± 12.9 | 19 (68.0) | PCT | 9 |
| ICU | 13 | 50.5 ± 16.6 | 11 (85.0) | |||||
| Ruan Q | 2020 | China | Survivors | 82 | 51.6 ± 7.6 | 53 (65.0) | IL-6, CRP | 7 |
| Non-survivors | 68 | 64.3 ± 14.0 | 49 (72.0) | |||||
| Zhou F | 2020 | China | Survivors | 137 | 51.6 ± 9.7 | 81 (59.0) | IL-6, PCT, Serum ferritin | 7 |
| Non-survivors | 54 | 69.4 ± 9.9 | 38 (70.0) |
CRP: C-reactive protein; PCT: procalcitonin; IL-6: interleukin-6; ESR: erythrocyte sedimentation rate; SAA: serum amyloid A.
Table 2.
Methodological quality of enrolled studies based on Newcastle-Ottawa Scale (NOS).
| Included studies | Year | Is the definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of both groups | Ascertainment of diagnosis | Same ascertainment method for both groups | Nonresponse rate | Total scores |
|---|---|---|---|---|---|---|---|---|---|---|
| Chang Z | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 8 |
| Chen C | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Chen L | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Cheng K | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 8 |
| Fang X | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Gao Y | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐⭐ | ⭐ | ⭐ | ⭐ | 9 |
| Jin Z | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Li K | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Peng Y | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 8 |
| Qin C | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Wu C | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Xiang T | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 8 |
| Xiao K | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Huang C | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐⭐ | ⭐ | ⭐ | ⭐ | 9 |
| Ruan Q | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
| Zhou F | 2020 | ⭐ | ⭐ | ⭐ | ⭐ | – | ⭐ | ⭐ | ⭐ | 7 |
Association of inflammatory markers with the severity of COVID-19
For the patients stratified by severity of COVID-19, random-effect results demonstrated that compared with patients in the severe group, patients in the nonsevere group had lower levels for CRP (WMD = −41.78 mg/l, 95% CI = −52.43, −31.13], P < 0.001), PCT (WMD = −0.13 ng/ml, 95% CI = [−0.20, −0.05], P < 0.001), IL-6 (WMD = −21.32 ng/l, 95% CI = [−28.34, −14.31], P < 0.001), ESR (WMD = −8 mm/h, 95% CI = [−14, −2], P = 0.005), SAA (WMD = −43.35 μg/ml, 95% CI = [−80.85, −5.85], P = 0.020) and serum ferritin (WMD = −398.80 mg/l, 95% CI = [−625.89, −171.71], P < 0.001) (Figure 2 A–F). Additionally, there are two studies grouped by survivors and non-survivors with COVID-19 reporting the level of IL-6, and fixed-effect results arrived at a similar conclusion that survivors had lower levels for IL-6 than non-survivors with COVID-19 (WMD = −4.80 ng/ml, 95% CI = [−5.87, −3.73], P < 0.001) (Figure 2G).
Figure 2.
Forest plot of inflammatory markers. (A–F) Forest plot between nonsevere and severe groups for levels of CRP (A), PCT (B), IL-6 (C), ESR (D), SAA (E), and serum ferritin (F); G. Forest plot between survivors group and non-survivors group for levels of IL-6.
Additionally, one study on the level of fibrinogen between the nonsevere group and severe group, one study on the level of PCT between the non-ICU group and ICU group, and one study on the level of CRP and PCT between non-survivors and survivors, were not included in the meta-analysis due to their inadequate data; however, the results reported by these studies were consistent with the pooled results of our meta-analysis.
Investigation of heterogeneity
Strong evidence of heterogeneity was found in all the comparisons (Figure 2). Sensitivity analyses indicated that the results were not influenced by excluding any one specific study in CRP, PCT, IL-6 and ESR between the nonsevere and severe groups (Figure 3 A–D). As for SAA and serum ferritin, the conclusions changed when deleting Cheng K’s study and Qin C’s study, separately, while the heterogeneity became larger, suggesting that it is better to keep these studies in the meta-analysis (Figure 3E and F). Egger’s test was conducted to evaluate the publication bias (Figure 4 ). No significant publication bias was detected in most of the studies except for CRP (P = 0.012) and PCT (P = 0.036). When applying the trim-and-fill method, there were no trials trimmed and filled in CRP. Regarding PCT, after filling one trial, the revised result was still consistent using a random model (WMD = −0.17 ng/ml, 95% CI = [−0.26, −0.08], P < 0.001) or fixed model (WMD = −0.06 ng/ml, 95% CI = [−0.07, −0.05], P < 0.001). Additionally, using standard mean difference (SMD) for the meta-analysis still did not change the conclusions (Table 3 ).
Figure 3.
Sensitivity analyses. (A–F) Sensitivity analyses between nonsevere and severe groups for levels of CRP (A), PCT (B), IL-6 (C), ESR (D), SAA (E), and serum ferritin (F).
Figure 4.
Publication bias by Egger’s test. (A–F) Publication bias by Egger’s test between nonsevere and severe groups in CRP (A), PCT (B), IL-6 (C), ESR (D), SAA (E), and serum ferritin (F).
Table 3.
The results of the meta-analysis based on standard mean difference (SMD).
| Outcome | Studies | Participants | Heterogeneity |
Model | SMD | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||
| Nonsevere vs. Severe | ||||||||
| 1. CRP | 13 | 2092 | 95% | <0.001 | Random | −1.48 | [−1.95, −1.00] | <0.001 |
| 2. PCT | 9 | 1633 | 94% | <0.001 | Random | −1.11 | [−1.58, −0.63] | <0.001 |
| 3. IL-6 | 7 | 1481 | 98% | <0.001 | Random | −1.54 | [−2.38, −0.71] | <0.001 |
| 4. ESR | 4 | 1165 | 67% | 0.03 | Random | −0.34 | [−0.57, −0.10] | 0.005 |
| 5. SAA | 3 | 650 | 73% | 0.03 | Random | −0.41 | [−0.82, −0.00] | 0.050 |
| 6. Serum ferritin | 3 | 1116 | 80% | 0.007 | Random | −0.62 | [−0.90, −0.34] | <0.001 |
| Non-survivors vs. Survivors | ||||||||
| IL-6 | 2 | 341 | 9% | 0.30 | Fixed | −1.23 | [−1.47, −0.98] | <0.001 |
CRP: C-reactive protein; PCT: procalcitonin; IL-6: interleukin-6; ESR: erythrocyte sedimentation rate; SAA: serum amyloid.
Discussion
COVID-19, caused by SARS-CoV-2, is rapidly expanding worldwide. Despite the fact that most cases have mild symptoms and a good prognosis, COVID-19 can develop into ARDS and even death. To date, there is no effective therapy for COVID-19 (Li and De Clercq, 2020, Russell et al., 2020). Therefore, it is imperative to identify the markers monitoring the progression of disease and treat patients early.
Several studies have shown increased proinflammatory cytokines in serum of COVID-19 patients. Also, anti-inflammatory agents for COVID-19 therapy highlight the critical role of inflammation in the progression of COVID-19 (Mehta et al., 2020, Stebbing et al., 2020). However, the role of inflammatory markers in monitoring the severity of COVID-19 is still controversial. In this study, through analyzing the 16 retrospective studies, we concluded that inflammatory markers, especially CRP, PCT, IL-6 and ESR, were positively correlated with the severity of COVID-19.
IL-6 has been implicated in the 2003 SARS outbreak and the H5N1 avian influenza infections (Law et al., 2005, Saito et al., 2018). Recent studies showed that IL-6 and granulocyte-macrophage colony stimulating factor (GM-CSF) could be secreted by the active pathogenic T cell upon SARS-CoV-2 infection. Also, CD14+CD16+ inflammatory monocytes activated by GM-CSF could secrete more IL-6 and other inflammatory factors (Zhou et al., 2020b). According to the New Coronavirus Pneumonia Prevention and Control Program (7th edition) published by the National Health Commission of China, decreasing level of IL-6 indicates the deterioration of COVID-19. Our study firstly provided evidence-based medicine evidence through meta-analysis. Moreover, the level of IL-6 could not be routinely detected in many hospitals in China, but some inflammatory markers such as CRP, PCT and ESR usually could be detected. Our study firstly put forward that in addition to IL-6, other inflammatory markers such as CRP, PCT and ESR were also positively correlated with the severity of COVID-19. These conclusions were consistent through sensitivity analysis and publication bias assessment.
CRP is an exquisitely sensitive systemic marker of acute-phase response in inflammation, infection, and tissue damage, which could be used as indicator of inflammation (Pepys and Hirschfield, 2003). In the study by Chen et al., although no statistically significant difference was found in the level of CRP between the nonsevere and the severe group, the mean level of CRP was higher in the severe group than in the nonsevere group (Chen et al., 2020b). Other studies all reported CRP level was positively related to the severity of COVID-19. PCT is also a main inflammatory marker routinely measured in clinical practice. Among 9 studies, the levels of PCT were all higher in the severe group than the nonsevere group. ESR is a non-specific inflammatory marker, which mainly reflects the changes of plasma protein types (Wu et al., 2018). In our meta-analysis, we found a higher ESR level in the severe group than in the nonsevere group. One reason is that patients in the severe group had higher inflammation. Another possible explanation is that patients with older age in the severe group contributed to the higher level of ESR considering that the level of ESR increased with age (Piva et al., 2001).
We also found patients with COVID-19 in the severe group had higher levels of SAA and serum ferritin than those in the nonsevere group. Considering that only three studies reported their levels and sensitivity analysis changed the conclusion, we temporarily could not conclude their association with the severity of COVID-19. SAA is a sensitive acute response protein and is used as a sensitive index to reflect the control of infection and inflammation. Serum ferritin is a surrogate marker of stored iron and increases in inflammation, liver disease, and malignancy (Cohen et al., 2010, Facciorusso et al., 2014, Kowdley et al., 2012). All of these highlight that an overexuberant inflammatory response is associated with the severity of COVID-19.
To our knowledge, this is the first meta-analysis on the associations of a series of inflammatory markers with the severity of COVID-19. Admittedly, our meta-analysis had some limitations. Firstly, noticeable heterogeneity exists in most of the analyses. Sensitivity analysis and SMD were used for the meta-analysis, yet the heterogeneity could not be eliminated completely. Secondly, reporting and publication bias may result from the lack of information or unpublished negative studies although the conclusion did not change through the trim-and-fill method. Thirdly, the studies included in our meta-analysis were mainly from China and whether the conclusion is consistent in other countries needs to be further investigated. Finally, this study is underpowered to investigate the underlying mechanism of these inflammatory markers with the severity of COVID-19.
In conclusion, inflammatory markers, especially CRP, PCT, IL-6 and ESR, were positively correlated with the severity of COVID-19. The association of SAA and serum ferritin with the severity of COVID-19 needs to be further clarified. Measurement of inflammatory markers might assist clinicians to monitor and evaluate the severity and prognosis of COVID-19.
Authors contribution
GD, LX and FZ were responsible for study design; YH and YG were involved in data collection; GD and YH analyzed the data; FZ and YH wrote the manuscript. GD, LX, MY and XC revised the manuscript.
Funding
This research was funded by the grants from the National Natural Science Foundation of China, No. 62041208.
Ethics in publishing
Approval was not required.
Declaration of interest
The authors declare no conflicts of interest.
References
- Chang Z., Yang W., Wang Q., Liao G. Clinical significance of serum hs-CRP, IL-16, and PCT in diagnosis and prognosis of patients with COVID-19 (In Chinese) Drugs Clin. 2020;35(3) [Google Scholar]
- Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19 (In Chinese) Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia (In Chinese) Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- Cheng K., Wei M., Shen H., Wu C., Chen D., Xiong W. Clinical characteristics of 463 patients with common and severe type coronavirus disease (In Chinese) Shanghai Med J. 2020:1–15. [Google Scholar]
- National Health Commission of China . 2020. The notice of launching guideline on diagnosis and treatment of the novel coronavirus pneumonia. (In Chinese)http://www.gov.cn/zhengce/zhengceku/2020-02/19/content_5480948.htm [Google Scholar]
- Cohen L.A., Gutierrez L., Weiss A., Leichtmann-Bardoogo Y., Zhang D.L., Crooks D.R. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116(9):1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- Facciorusso A., Del Prete V., Antonino M., Neve V., Crucinio N., Di Leo A. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29(11):1905–1910. doi: 10.1111/jgh.12618. [DOI] [PubMed] [Google Scholar]
- Fang X., Mei Q., Yang T., Zhang L., Yang Y., Wang Y. Clinical characteristics and treatment strategies of 79 patients with COVID-19 (In Chinese) Chin Pharmacol Bull. 2020;36(4) [Google Scholar]
- Wells G.A., Shea Brooke, O’connell Dianne L., Peterson Joan, Welch V., Losos Michael. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Open J Rheumatol Autoimmune Dis. 2014;4 [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowdley K.V., Belt P., Wilson L.A., Yeh M.M., Neuschwander-Tetri B.A., Chalasani N. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(1):77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law H.K., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Li K., Wu J., Wu F., Guo D., Chen L., Fang Z. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV] (In Chinese) Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva E., Sanzari M.C., Servidio G., Plebani M. Length of sedimentation reaction in undiluted blood (erythrocyte sedimentation rate): variations with sex and age and reference limits. Clin Chem Lab Med. 2001;39(5):451–454. doi: 10.1515/CCLM.2001.071. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito L.B., Diaz-Satizabal L., Evseev D., Fleming-Canepa X., Mao S., Webster R.G. IFN and cytokine responses in ducks to genetically similar H5N1 influenza A viruses of varying pathogenicity. J Gen Virol. 2018;99(4):464–474. doi: 10.1099/jgv.0.001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. WHO Virtual press conference on COVID-19 (March 11, 2020) [Accessed 16 March 2020] [Google Scholar]
- WHO . 2020. WHO Virtual press conference on COVID-19 (March 21, 2020) [Accessed 21 March 2020] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Zhou Y., Hua H.Y., Zhang Y., Zhu W.Y., Wang Z.Q. Inflammation marker ESR is effective in predicting outcome of diffuse large B-cell lymphoma. BMC Cancer. 2018;18(1):997. doi: 10.1186/s12885-018-4914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T., Liu J., Xu F., Cheng N., Liu Y., Qian K. Analysis of clinical characteristics of 49 patients with Novel Coronavirus Pneumonia in Jiangxi province (In Chinese) Chin J Respir Crit Care Med. 2020;19(2) [Google Scholar]
- Xiao K., Shui L., Pang X., Mu H., Wang J., Lang C. The clinical features of the 143 patients with COVID-19 in North-East of Chongqing (In Chinese) J Third Mil Med Univ. 2020:1–5. [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F., Chen B., Zeng J., Wang Z., Xiao L., Deng G. Preoperative neutrophil-lymphocyte ratio predicts the risk of microvascular invasion in hepatocellular carcinoma: a meta-analysis. Int J Biol Markers. 2019;34(3):213–220. doi: 10.1177/1724600819874487. [DOI] [PubMed] [Google Scholar]
- Zeng F., Chen L., Liao M., Chen B., Long J., Wu W. Laparoscopic versus open gastrectomy for gastric cancer. World J Surg Oncol. 2020;18(1):20. doi: 10.1186/s12957-020-1795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., qi Y. Vol. 2020. 2020. (Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus). 02.12.945576. [Google Scholar]