Abstract
In this study, we aimed to evaluate the diagnostic value of serological assay for SARS-CoV-2. A newly-developed ELISA assay for IgM and IgG antibodies against N protein of SARS-CoV-2 was used to screen the serums of 238 admitted hospital patients between February 6 and February 14, 2020 with confirmed or suspected SARS-CoV-2. SARS-CoV-2 RNA was detected on pharyngeal swab specimens using real time RT-PCR. 194 (81.5%) of the serums were detected to be antibody (IgM and/or IgG) positive, significantly higher than the positive rate of viral RNA (64.3%). There was no difference in the positive rate of antibodies between the confirmed patients (83.0%, 127/153) and the suspected patients (78.8%, 67/85), whose nucleic acid tests were negative. The antibody positive rates were very low in the first five days after initial onset of symptoms, and then rapidly increased as the disease progressed. After 10 days, the antibody positive rates jumped from below 50% to over 80%. However, the positive rates of viral RNA maintained above 60% in the first 11 days after initial onset of symptoms, and then rapidly decreased. Overall, the suspected patients were most likely infected by SARS-CoV-2. Before the 11th day after initial onset of symptoms, nucleic acid test is key for confirmation of viral infection. The combination of serological assay can greatly improve the diagnostic efficacy. After the 11th day post-disease onset, the diagnosis for viral infection should be majorly dependent on serological assay.
Keywords: SARS-CoV-2, Diagnosis, Serological assay, Nucleic acid test
A novel betacoronavirus [1], named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), caused a number of respiratory illness cases, known as corona virus disease 2019 (COVID-19), in various regions of the world. This lead to a public health emergency, most severely in Wuhan city, Hubei province, China from December 2019 [2]. By April 19, 2020, more than two million confirmed cases were identified globally. There is evidence that SARS-CoV-2 can transmit rapidly from person to person as was the case in hospital and family settings [[3], [4], [5]].
SARS-CoV-2 is the seventh member of the enveloped RNA coronaviruses (CoVs) [[6], [7], [8]]. The sequence and phylogenetic tree of CoVs analysis indicates that SARS-CoV-2 is genetically distinct from SARS-CoV and is more closely related to bat-SL-CoV ZC45 and bat-SL-CoV ZXC21 [1]. SARS-CoV-2 has a similar receptor-binding domain structure to that of SARS-CoV [1]. A typical CoV contains four main structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). The S protein homotrimers are required for attachment to host receptors [9], and both the M protein and the E protein play important roles in virus assembly [10,11]. The N protein is responsible for packaging the encapsidated genome into virions [12,13], and acts as a viral RNA silencing suppressor that is beneficial for the viral replication [14]. Furthermore, the N protein has high immunogenic activity and is profusely overexpressed during infection [15]. Thus, the N protein is a potential source of diagnostic antigen for detecting the SARS-CoV-2 infection. Many diagnostic methods based on the N protein have been developed for detecting SARS-CoV [[16], [17], [18]]. In addition, different CoVs possess special structural and accessory proteins, such as the HE protein, 3a/b protein, and 4a/b protein [19].
Both nucleic acid tests and serological assays are commonly used for infectious disease screening and diagnosis. In the present case of SARS-CoV-2 in China, nucleic acid tests are routinely used to detect causative viruses from respiratory secretions using real-time RT-PCR. However, the nucleic acid tests appear to have a high false negative rate due to various unavoidable factors, including the sensitivity of the detection kits, and the sampling location and technique [20]. A large number of clinically-suspected patients, whose nucleic acid tests are negative, are unable to get timely hospitalization, potentially promoting the spread of SARS-CoV-2 and disease progression of suspected patients.
In this study, a newly-developed IgM and IgG antibody detecting Enzyme-linked immunosorbent assays (ELISA) based on a recombinant fragment of the SARS-CoV-2 N protein was used to detect IgM and IgG against SARS-CoV-2 in the serums of 238 admitted hospital patients with confirmed or suspected SARS-CoV-2 infections. The results strongly indicate that the suspected patients were in fact infected. We also analyzed the diagnostic value of the IgM and IgG testing in COVID-19, even in the early stages of the disease.
1. Methods
1.1. Patients and samples
All consecutive patients (n = 238) with confirmed or suspected SARS-CoV-2 infections who have been tested using real-time RT-PCR for viral infections and were treated in General Hospital of Central Theater Command of PLA between February 6 to February 14, 2020, were enrolled. The general information (age, sex, vital signs, coexisting disorders), clinical data, laboratory data, and radiological characteristics data of the patients were extracted from electronic medical records. Among the 238 recruited patients, 153 patients were laboratory-confirmed cases, who were tested positive for viral RNA using real time RT-PCR assay on pharyngeal swab specimens. The remaining 85 patients, who had negative results from the real time RT-PCR assay, were clinically diagnosed as highly-suspected cases according to the notice on the issuance of strategic guidelines for diagnosis and treatment of COVID-19 [21]. Suspected cases were diagnosed as long as they had one of the following epidemiological history: (a) travel or residence history in Wuhan or other areas where local cases continue to spread within 14 days before onset; (b) having contacted with patients with fever or respiratory symptoms from Wuhan or other areas where local cases continue to spread within 14 days before onset; (c) epidemiologically relevant to cluster disease or people infected with SARS-CoV-2, and two of the following clinical manifestations: (a) fever; (b) chest imaging features; (c) normal or decreased white blood cell count in the early stage of onset, or decreased lymphocyte count. The serum samples were collected once from each recruited patient. Meanwhile, the control consisted of serum samples randomly selected from 70 ordinary patients and 50 healthy blood donors. The study was approved by the Hospital Ethics Committee and written informed consent was waived for emerging infectious diseases.
1.2. Real time reverse transcription polymerase chain reaction (RT-PCR) assay
Pharyngeal swab specimens were collected from patients and placed into a collection tube with 200 μL of virus preservation solution. Total RNA was extracted using the respiratory sample RNA isolation kit (Shuoshi, Shanghai, China). After vortex, 50 μL of cell lysates were transferred into another collection tube. The collection tube was centrifugated at 1000 rpm/min for 5 min after standing at room temperature for 10 min 5 μL RNA was prepared and used for real time RT-PCR.
Real time RT-PCR was performed using the nucleic acid testing kit (Daan, Guangzhou, China) for SARS-CoV-2 detection. The open reading frame 1 ab (ORF1ab) and nucleocapsid protein (N) were simultaneously selected as the two target genes. The human GAPDH gene was used as an internal control. The specific primers and probes set for ORF1ab and N were as follows: ORF1ab-forward primer 5′-ACCTTCTCTTGCCACTGTAGC-3′, ORF1ab-reverse primer 5′-AGTATCAACCATATCCAACCATGTC-3′, probe 5′-FAM-ACGCATCACCCAACTAGCAGGCATAT-BHQ1-3′, N-forward primer 5′-TTCAAGAAATTCAACTCCAG-3, N-reverse primer 5′-AGCAGCAAAGCAAGAGCAGCATC-3′, and probe.
5′-VIC-TCCTGCTAGAATGGCTGGCAATGGCG-BHQ1-3’. The real time RT-PCR experiment was thoroughly performed according to the kit’s instructions. The reaction mixture contains 17 μL of reaction buffer A, 3 μL of reaction buffer B, and 5 μL RNA template. The real time RT-PCR assay was performed under the following conditions: incubation at 50 °C for 15 min and 95 °C for 15 min, 45 cycles of denaturation at 94 °C for 15 s, and extension and collection of fluorescence signal at 55 °C for 45 s. A cycle threshold value (Ct-value) ≤ 40 was defined as a positive test result, and a Ct-value > 40 was defined as a negative test.
1.3. Enzyme-linked immunosorbent assay (ELISA)
Serological assay was performed using an Enzyme-Linked Immunosorbent Assays kit (Lizhu, Zhuhai, China), which was developed for detecting IgM or IgG antibody against N proteins of SARS-CoV-2. For IgM detection, ELISA plates were previously coated with mouse anti-human IgM (μ chain) monoclonal antibody. 100 μL of diluted (1:100) serum sample was added to the pre-coated plates with three replicating wells for each sample and incubated at 37 °C for 1 h. The heat-inactivated positive and negative serums were included on each plate. After washing, 100 μL of horse radish peroxidase (HRP) conjugated recombinant (rN) protein of SARS-CoV-2 were added. The plate was incubated at 37 °C for 30 min and then washed. 50 μL of TMB substrate solution and 50 μL of the corresponding buffer were added and incubated at 37 °C for 15 min. The reaction was terminated by adding 50 μL of 2 M sulfuric acid, and the absorbance value at 450 nm (A450) was determined. The cut off value was calculated using the sum of 0.100 and average A450 of the negative control replicates. A450 less than cut off value was defined as a negative test, and A450 greater than or equal to cut off value was defined as a positive test.
For IgG detection, ELISA plates were previously coated with rN protein. 5 μL of serum sample diluted with 100 μL of dilution buffer were added to the plates. After incubation and washing, HRP-conjugated mouse anti-human IgG monoclonal antibody was added to the plates for detection. The other operational steps were performed as described in the above IgM detection. The cut off value was calculated using the sum of 0.130 and average A450 of the negative control replicates. A450 less than cut off value was defined as a negative test, and A450 greater than or equal to cut off value was defined as a positive test.
1.4. Statistical analysis
Continuous variables were described in the form of means and standard deviations or medians and interquartile ranges (IQR). Categorical variables were expressed as counts and percentages. Independent group t tests were applied to continuous variables that were normally distributed; otherwise, the Mann–Whitney test was used. Categorical variables were compared using the chi-square tests, while the Fisher exact test was used when data was limited. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 22.0 software. A two-sided α of less than 0.05 was considered statistically significant.
2. Results
2.1. Demographics and patient characteristics
Serum samples were collected from 238 admitted hospital patients with confirmed or suspected SARS-CoV-2 infection in General Hospital of Central Theater Command of PLA between February 6 to February 14, 2020. The clinical characteristics of the patients are shown in Table 1 . The median age was 55 years (IQR, 38.3–65), and 138 (58.0%) of the patients were men. Hypertension (63 [26.5%]), diabetes (25 [10.5%]), and cardiovascular disease (24 [10.1%]) were the most common coexisting disorders. The most common symptoms at illness onset were fever (206 [86.6%]), dry cough (128 [53.8%]), and fatigue (78 [32.8%]). A small number of patients experienced abdominal pain (1 [0.4%]), vomiting (3 [1.3%]), and dizziness (4 [1.7%]). According to the positive or negative results of real time RT-PCR assay for pharyngeal swab specimens, the enrolled patients were divided into two groups: the confirmed group and the suspected group. There were no statistical differences between the baseline characteristics of the two patient groups.
Table 1.
Demographic and baseline characteristics of 238 enrolled patients.
| Characteristics | All patients (N = 238) | Confirmed (N = 153) | Suspected (N = 85) | P value |
|---|---|---|---|---|
| Age, Median (IQR) – y | 55.0 (38.3–65.0) | 54.0 (39.0–64.0) | 55.0 (38.0–65.0) | 0.656 |
| Sex | ||||
| Male | 138 (58.0%) | 93 (60.8%) | 45 (52.3%) | 0.240 |
| Female | 100 (42.0%) | 60 (39.2%) | 40 (47.6%) | |
| Vital signs | ||||
| Heart rate | 89 (80–100) | 89 (80–99) | 90 (80–104) | 0.496 |
| Respiratory rate | 18 (18–20) | 19 (18–20) | 18 (18–20) | 0.059 |
| Oxygen saturation | 96% (94%–98%) | 96% (94%–98%) | 97% (95%–98%) | 0.292 |
| CT findings of ground-glass opacity and/or patchy shadowing | 235/238 (98.7) | 151/153 (98.7) | 84/85 (98.8) | 0.931 |
| Leukocytes ( × 109 per L; normal range 3.5–9.5) | ||||
| Decreased | 41/238 (17.2) | 25/153 (16.3) | 16/85 (18.8) | 0.627 |
| Increased | 21/238 (8.8) | 16/153 (10.5) | 5/85 (5.9) | 0.233 |
| Lymphocytes ( × 109 per L; normal range 1.1–3.2) | ||||
| Decreased | 125/238 (52.5) | 84/153 (54.9) | 41/85 (48.2) | 0.324 |
| Neutrophils ( × 109 per L; normal range 1.8–6.3) | ||||
| Decreased | 28/238 (11.8) | 17/153 (11.1) | 11/85 (12.9) | 0.675 |
| Increased | 32/238 (13.4) | 22/153 (14.4) | 10/85 (11.8) | 0.571 |
| Signs and symptoms | ||||
| Fever | 206/238 (86.6) | 134/153 (87.6) | 72/85 (84.7) | 0.533 |
| Dry cough | 128/238 (53.8) | 86/153 (56.2) | 42/85 (49.4) | 0.314 |
| Fatigue | 78/238 (32.8) | 47/153 (30.7) | 31/85 (36.5) | 0.365 |
| Myalgia | 46/238 (19.3) | 29/153 (19.0) | 17/85 (20.0) | 0.845 |
| Dyspnea | 44/238 (18.5) | 31/153 (20.3) | 13/85 (15.3) | 0.344 |
| Chill | 31/238 (13.0) | 20/153 (13.1) | 11/85 (12.9) | 0.977 |
| Anorexia | 29/238 (12.2) | 14/153 (9.2) | 15/85 (17.6) | 0.055 |
| Diarrhea | 24/238 (10.1) | 14/153 (9.2) | 10/85 (11.8) | 0.521 |
| Expectoration | 21/238 (8.8) | 14/153 (9.2) | 7/85 (8.2) | 0.812 |
| Headache | 15/238 (6.3) | 12/153 (7.8) | 3/85 (3.5) | 0.189 |
| Pharyngalgia | 14/238 (5.9) | 9/153 (5.9) | 6/85 (7.1) | 0.720 |
| Palpitation | 9/238 (3.8) | 8/153 (5.2) | 1/85 (1.2) | 0.120 |
| Chest pain | 6/238 (2.5) | 4/153 (2.6) | 2/85 (2.4) | 0.902 |
| Nausea | 4/238 (1.7) | 2/153 (1.3) | 2/85 (2.4) | 0.548 |
| Dizziness | 4/238 (1.7) | 4/153 (2.6) | 0/85 (0.0) | 0.133 |
| Vomiting | 3/238 (1.3) | 3/153 (2.0) | 0/85 (0.0) | 0.194 |
| Abdominal pain | 1/238 (0.4) | 1/153 (0.7) | 0/85 (0.0) | 0.455 |
| Coexisting disorders | ||||
| Hypertension | 63/238 (26.5) | 42/153 (27.5) | 21/85 (24.7) | 0.646 |
| Diabetes | 25/238 (10.5) | 15/153 (9.8) | 10/85 (11.8) | 0.636 |
| Cardiovascular disease | 24/238 (10.1) | 16/153 (10.5) | 8/85 (9.4) | 0.797 |
| Malignancy | 12/238 (5.0) | 6/153 (3.9) | 6/85 (7.1) | 0.289 |
| Cerebrovascular disease | 8/238 (3.4) | 5/153 (3.3) | 3/85 (3.5) | 0.915 |
| COPD | 3/238 (1.3) | 1/153 (0.7) | 2/85 (2.4) | 0.260 |
Data are median (IQR), n (%), or n/N (%), where N is the total number of patients with available data. P values comparing the confirmed patients and the suspected patients are from χ2 test, Fisher’s exact test, or Mann–Whitney U test. Confirmed = confirmed patients. Suspected = suspected patients.
2.2. Performance and validation of ELISA assays for viral specific IgM and IgG antibodies
Each serum sample of 238 patients was tested for IgM and IgG antibodies against SARS-CoV-2 using newly developed ELISA kits based on SARS-CoV-2 N protein. IgM and/or IgG were detected in 194 serum samples. The positive rate (81.5%) was significantly higher than that of SARS-CoV-2 RNA detected using real time RT-PCR, which was 64.3% (153/238) (Fig. 1 A). More importantly, there were no significant differences in the positive rates of IgM and/or IgG between the confirmed patients (83.0%, 127/153) and the suspected patients (78.8%, 67/85) (Fig. 1B), suggesting that the clinically suspected patients with negative viral RNA tests were also mostly infected.
Fig. 1.
Positive rate of viral RNA and antibody in different samples. A) The positive rate of viral RNA (black column) and antibody (white column) in 238 enrolled patients (two columns on the left), as well as the positive rate of antibody in ordinary patients and healthy donors (two columns on the right). B) Comparison of positive rate of antibody between the laboratory-confirmed (left) and highly-suspected patients (right). Results were compared by chi-square tests.
To verify the specificity of the ELISA assays, the serum samples from 70 randomly selected ordinary patients and 50 healthy blood donors were simultaneously tested. Four samples from the ordinary patients were identified as antibody positive (including one dual positive sample, two IgM-positive samples, and one IgG-positive sample) and no positives were found in the samples from healthy blood donors (Fig. 1A). These four patients had signs of pneumonia in chest imaging. One patient had a negative viral nucleic acid test, and the other three patients were not tested for viral nucleic acid. The specificity of antibody detection is 100% in healthy people and 94.3% in ordinary patients.
2.3. Dynamic analysis of ELISA and RT-PCR assays
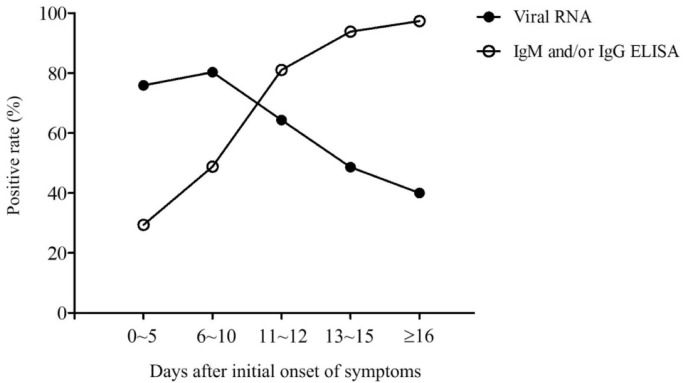
To study the diagnostic value of ELISA assay for virus-specific antibodies, especially in the early stages of the disease, we tried to analyze the positive rates of ELISA and RT-PCR assays across the different stages of the disease. To this end, each patient was assigned to different days after initial onset of symptoms based on the time of blood collection for ELISA assays. For viral nucleic acid test, each patient was assigned to different days after initial onset of symptoms based on the time when the pharyngeal swab specimen was detected to be positive or the last recoded detection was still negative. The positive rates of viral RNA, IgM, and/or IgG were compared every day after initial onset of symptoms (Table 2 ). Due to having a low number of samples for each individual day, we pooled the samples in which the positive rates were similar in consecutive days. Thus, the disease process was divided into five phases of 0–5 days, 6–10 days, 11–12 days, 13–15 days and more than 16 days after initial onset of symptoms (Table 3 & Fig. 2 ). The data show that the positive rates of IgM and/or IgG are extremely low in the first five days after initial onset of symptoms because no antibodies are produced in the majority of patients at this early stage, and rapidly increase as the disease progresses. Day 11 after initial onset of symptoms is a key point because the positive rates of IgM and/or IgG jump to over 80% from less than 50%. The sensitivity of antibody detection reaches to more than 93% since day 11 after initial onset of symptoms. This dynamic trend is consistent with SARS-CoV infection [22]. In comparison, the real-time RT-PCR was more effective for detecting SARS-CoV-2 infection than ELISA in the early stage of the disease. The positive rate of viral RNA detected by RT-PCR was maintained above 60% in the first 11 days after initial onset of symptoms, and rapidly decreased with the rapid increase of antibodies. These results demonstrate that ELISA-based IgM and/or IgG detection should be used as a major viral diagnostic test for patients with symptoms for more than 10 days. Given that 50% of clinically suspected patients with symptoms for 6–10 days were detected to be positive by ELISA-based IgM and/or IgG detection (Table 4 ), the combination of ELISA and RT-PCR assays will greatly improve the detection efficacy, even in the early stage of the COVID-19 infection.
Table 2.
Viral RNA and antibody positive rates of the patients detected each day from initial onset of symptoms.
| Day | Viral RNA+ | IgM+and/or IgG+ | IgM+ | IgG+ | IgM+and IgG+ |
|---|---|---|---|---|---|
| 0 | 100.0 (2/2) | 50.0 (1/2) | 0.0 (0/2) | 50.0 (1/2) | 0.0 (0/2) |
| 1 | 83.3 (5/6) | 33.3 (1/3) | 0.0 (0/3) | 33.3 (1/3) | 0.0 (0/3) |
| 2 | 71.4 (5/7) | 0.0 (0/3) | 0.0 (0/3) | 0.0 (0/3) | 0.0 (0/3) |
| 3 | 61.5 (8/13) | 25.0 (1/4) | 25.0 (1/4) | 0.0 (0/4) | 0.0 (0/4) |
| 4 | 69.2 (9/13) | 50.0 (2/4) | 0.0 (0/4) | 0.0 (0/4) | 50.0 (2/4) |
| 5 | 92.3 (12/13) | 0.0 (0/1) | 0.0 (0/1) | 0.0 (0/1) | 0.0 (0/1) |
| 6 | 62.5 (5/8) | 60.0 (3/5) | 0.0 (0/5) | 0.0 (0/5) | 60.0 (3/5) |
| 7 | 88.9 (8/9) | 50.0 (2/4) | 25.0 (1/4) | 0.0 (0/4) | 25.0 (1/4) |
| 8 | 81.8 (18/22) | 54.5 (6/11) | 18.2 (2/11) | 0.0 (0/11) | 36.4 (4/11) |
| 9 | 85.0 (17/20) | 42.9 (6/14) | 7.1 (1/14) | 7.1 (1/14) | 28.6 (4/14) |
| 10 | 75.0 (9/12) | 42.9 (3/7) | 14.3 (1/7) | 0.0 (0/7) | 28.6 (2/7) |
| 11 | 72.2 (13/18) | 81.8 (9/11) | 18.2 (2/11) | 27.3 (3/11) | 36.4 (4/11) |
| 12 | 50.0 (5/10) | 80.0 (8/10) | 30.0 (3/10) | 20.0 (2/10) | 30.0 (3/10) |
| 13 | 44.4 (4/9) | 90.9 (10/11) | 27.3 (3/11) | 9.1 (1/11) | 54.5 (6/11) |
| 14 | 69.2 (9/13) | 100.0 (17/17) | 29.4 (5/17) | 0.0 (0/17) | 70.6 (12/17) |
| 15 | 30.8 (4/13) | 90.0 (18/20) | 10.0 (2/20) | 15.0 (3/20) | 65.0 (13/20) |
| 16 | 50.0 (4/8) | 100.0 (16/16) | 12.5 (2/16) | 25.0 (4/16) | 62.5 (10/16) |
| 17 | 57.1 (4/7) | 88.9 (16/18) | 0.0 (0/18) | 5.6 (1/18) | 83.3 (15/18) |
| 18 | 33.3 (2/6) | 100.0 (16/16) | 0.0 (0/16) | 6.3 (1/16) | 93.8 (15/16) |
| 19 | 33.3 (3/9) | 100.0 (19/19) | 10.5 (2/19) | 21.1 (4/19) | 68.4 (13/19) |
| 20 | 0.0 (0/1) | 83.3 (5/6) | 0.0 (0/6) | 33.3 (2/6) | 50.0 (3/6) |
| >20 | 36.8 (7/19) | 97.2 (35/36) | 2.8 (1/36) | 8.3 (3/36) | 86.1 (31/36) |
Data are % (n/N). Day = the day after initial onset of symptoms. Viral RNA+ = a positive result detected by real time RT-PCR. IgM+ = a positive result detected by IgM ELISA and simultaneously a negative result detected by IgG ELISA. IgG+ = a positive result detected by IgG ELISA and simultaneously a negative result detected by IgM ELISA. IgM+ and IgG+ = a dual positive result detected by IgM and IgG ELISA. IgM+and/or IgG+ = at least a positive result detected by IgM and IgG ELISA.
Table 3.
Viral RNA and antibody positive rates of the patients detected in different stages of disease.
| Days | Viral RNA+ | IgM+and/or IgG+ | IgM+ | IgG+ | IgM+and IgG+ |
|---|---|---|---|---|---|
| 0–5 | 75.9 (41/54) | 29.4 (5/17) | 5.9 (1/17) | 11.8 (2/17) | 11.8 (2/17) |
| 6–10 | 80.3 (57/71) | 48.8 (20/41) | 12.2 (5/41) | 2.4 (1/41) | 34.1 (14/41) |
| 11–12 | 64.3 (18/28) | 81.0 (17/21) | 23.8 (5/21) | 23.8 (5/21) | 33.3 (7/21) |
| 13–15 | 48.6 (17/35) | 93.8 (45/48) | 20.8 (10/48) | 8.3 (4/48) | 64.6 (31/48) |
| ≥16 | 40.0 (20/50) | 96.4 (107/111) | 4.5 (5/111) | 13.5 (15/111) | 78.4 (87/111) |
| Total | 64.3 (153/238) | 81.5 (194/238) | 10.9 (26/238) | 11.3 (27/238) | 59.2 (141/238) |
Data are % (n/N). Days = the day after initial onset of symptoms. Viral RNA+ = a positive result detected by real time RT-PCR. IgM+ = a positive result detected by IgM ELISA and simultaneously a negative result detected by IgG ELISA. IgG+ = a positive result detected by IgG ELISA and simultaneously a negative result detected by IgM ELISA. IgM+ and IgG+ = a dual positive result detected by IgM and IgG ELISA. IgM+and/or IgG+ = at least a positive result detected by IgM and IgG ELISA.
Fig. 2.
Dynamics of the positive rate of viral RNA and antibody of the patients at the different stages of disease. The disease courses were divided into five phases of 0–5, 6–10,11-12, 13–15 days and more than 16 days after initial onset of symptoms. The positive rate of viral RNA (solid circle) and antibody (hollow circle) of the patients at the different phase of disease was shown.
Table 4.
Comparison of the antibody positive rates between the confirmed and suspected patients.
| 0–5 days | 6–10 days | ≥11 days | |
|---|---|---|---|
| Confirmed | 55.6 (5/9) | 44.0 (11/25) | 93.3 (111/119) |
| Suspected | 0.0 (0/8) | 56.3 (9/16) | 95.1 (58/61) |
Data are % (n/N). Confirmed = confirmed patients. Suspected = suspected patients.
3. Discussion
The outbreak of the recently emerged novel coronavirus (SARS-CoV-2) poses a challenge for public health laboratories, especially for clinical laboratories in hospitals in Wuhan, China. Although serological assay is a frequently used method for viral infection screening and diagnosis, there are few reports on serological assay in the detection of SARS-CoV-2. In this study, we evaluate the application of the SARS-CoV-2 N protein-based ELISA for detection of IgM and IgG antibodies in admitted hospital patients with confirmed or suspected SARS-CoV-2 infections. The results show that the positive rate of antibodies detected by ELISA was significantly higher than that of viral RNA detected by real-time RT-PCR across all the enrolled patients, suggesting that antibody detection is more sensitive than viral nucleic acid test to diagnose SARS-CoV2 infection. More importantly, the suspected patients exhibited nearly the same antibody detection rate as the confirmed patients. This data strongly demonstrates that the majority of suspected patients, who were tested negative for viral RNA, were in fact infected by SARS-CoV-2.
In this study, serum samples were collected in a time period of 9 days from patients in different stages of the disease. The positive rates of nucleic acid test and serological assay in total populations cannot reflect their diagnostic value in surveillance and control of the disease. In order to objectively determine the disease stage of the patients, we used the initial onset of symptoms exhibited by the patients as the starting time point. All patients were defined to different stages of disease based on when the test sample was collected. The dynamics patterns of positive rates of viral RNA and antiviral antibodies confirmed the rationality of the definition of disease stage. Our analysis identified the 11th day after initial onset of symptoms as a key time point in the disease process. This is when most infected patients produce antiviral antibodies. After the 11th day, the diagnosis of viral infection should mainly depend on serological assays. Before the 11th day, the nucleic acid test is important for confirmation of viral infection. The combination of serological assay and viral nucleic acid test can greatly improve the diagnostic efficacy. Our conclusions closely align with that of to several recently published papers [23,24] further demonstrating the importance of serological testing in the diagnosis of the SARS-CoV-2 infection.
According to the rapid advice guideline for the diagnosis and treatment of SARS-CoV-2 currently implemented in China [21], confirmation of COVID-19 in patients exclusively depends on the positive results of nucleic acid tests or virus gene sequencing. Despite being a preliminary ELISA assay for SARS-CoV-2, our study strongly demonstrates that serological assays are key for the surveillance and control of COVID-19, especially in epidemic areas where there is an abundance of patients in need of diagnosis confirmation.
Authors’ contributions
S.Z., S.W., and L.L. conceived the study and designed experimental procedures; W.L., Y.Z., W.N.,Y.D., W.W., S.T., X.J., J.D., Q.H., Z.H., W.X., Y.Z., B.Z., Z.T., X.Z., H.L., Z.R., H.J., and X.R. collected patients’ samples. Q.W. and L.T. performed viral RNA tests. G.K.,W.L. W.N., and Y.Z established ELISA and performed serological assays. S.W., L.L., S.Z., and W.L. wrote the paper. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
Declaration of Competing Interest
The authors declare that no conflict of interest exists.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81801984, 81830003); the National Key Research and Development Program of China (2019YFC130030); the China Postdoctoral Science Foundation (2019M664008); and the Military Medical Science and Technology Youth Cultivation Project (20QNPY092). We extend our gratitude to all healthcare workers who were involved in this study and to Wuhan Institute of Virology of Chinese Academy of Sciences and Zhuhai Lizhu Diagnostics Inc. for providing assistance in ELISA detection.
Contributor Information
Shengdian Wang, Email: sdwang@ibp.ac.cn.
Shangen Zheng, Email: sxkzsg@sina.com.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J., Chowell G., Jung E. A dynamic compartmental model for the Middle East respiratory syndrome outbreak in the Republic of Korea: a retrospective analysis on control interventions and superspreading events. J Theor Biol. 2016;408:118–126. doi: 10.1016/j.jtbi.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto-Torres J.L., DeDiego M.L., Verdia-Baguena C., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C.K., Sue S.C., Yu T.H., Hsieh C.M., Tsai C.K., Chiang Y.C. Modular organization of SARS coronavirus nucleocapsid protein. J Biomed Sci. 2006;13:59–72. doi: 10.1007/s11373-005-9035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst K.R., Koetzner C.A., Masters P.S. Identification of in vivo-interacting domains of the murine coronavirus nucleocapsid protein. J Virol. 2009;83:7221–7234. doi: 10.1128/JVI.00440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui L., Wang H., Ji Y., Yang J., Xu S., Huang X. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells. J Virol. 2015;89:9029–9043. doi: 10.1128/JVI.01331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanan K., Chen C.J., Maeda J., Makino S. Nucleocapsid-independent specific viral RNA packaging via viral envelope protein and viral RNA signal. J Virol. 2003;77:2922–2927. doi: 10.1128/JVI.77.5.2922-2927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Che X.Y., Qiu L.W., Pan Y.X., Wen K., Hao W., Zhang L.Y. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. 2004;42:2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan M., Chen H.Y., Foo S.Y., Tan Y.J., Goh P.Y., Wee S.H. Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients. Clin Diagn Lab Immunol. 2004;11:287–291. doi: 10.1128/CDLI.11.2.287-291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo P.C., Lau S.K., Wong B.H., Chan K.H., Chu C.M., Tsoi H.W. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11:665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Infantino M., Damiani A., Gobbi F.L., Grossi V., Lari B., Macchia D. Serological assays for SARS-CoV-2 infectious disease: benefits, limitations and perspectives. Isr Med Assoc J. 2020;22:203–210. [PubMed] [Google Scholar]
- 21.General Office of National Health Committee & Office of State Administration of Traditional Chinese Medicine . 2020. Notice on the issuance of strategic guidelines for diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia.http://bgs.satcm.gov.cn/zhengcewenjian/2020-02-09/12930.html (Fourth edition draft) (2020-02-09) [EB/OL] [Google Scholar]
- 22.Chen S., Lu D., Zhang M., Che J., Yin Z., Zhang S. Double-antigen sandwich ELISA for detection of antibodies to SARS-associated coronavirus in human serum. Eur J Clin Microbiol Infect Dis. 2005;24:549–553. doi: 10.1007/s10096-005-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L., Ren L., Yang S., Xiao M., Chang Yang F. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa310. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]