Abstract
Background
Coronavirus disease 2019 (COVID-19) can manifest as a viral-induced hyperinflammation with multiorgan involvement. Such patients often experience rapid deterioration and need for mechanical ventilation. Currently, no prospectively validated biomarker of impending respiratory failure is available.
Objective
We aimed to identify and prospectively validate biomarkers that allow the identification of patients in need of impending mechanical ventilation.
Methods
Patients with COVID-19 who were hospitalized from February 29 to April 9, 2020, were analyzed for baseline clinical and laboratory findings at admission and during the disease. Data from 89 evaluable patients were available for the purpose of analysis comprising an initial evaluation cohort (n = 40) followed by a temporally separated validation cohort (n = 49).
Results
We identified markers of inflammation, lactate dehydrogenase, and creatinine as the variables most predictive of respiratory failure in the evaluation cohort. Maximal IL-6 level before intubation showed the strongest association with the need for mechanical ventilation, followed by maximal CRP level. The respective AUC values for IL-6 and CRP levels in the evaluation cohort were 0.97 and 0.86, and they were similar in the validation cohort (0.90 and 0.83, respectively). The calculated optimal cutoff values during the course of disease from the evaluation cohort (IL-6 level > 80 pg/mL and CRP level > 97 mg/L) both correctly classified 80% of patients in the validation cohort regarding their risk of respiratory failure.
Conclusion
The maximal level of IL-6, followed by CRP level, was highly predictive of the need for mechanical ventilation. This suggests the possibility of using IL-6 or CRP level to guide escalation of treatment in patients with COVID-19–related hyperinflammatory syndrome.
Key words: IL-6, CRP, COVID-19, respiratory failure, mechanical ventilation, prediction, hyperinflammation
Abbreviations used: AUC, Area under the curve; BAL, Bronchoalveolar lavage; CORKUM, COVID-19 Registry of the Ludwig Maximilian University Hospital Munich; COVID-19, Coronavirus disease 2019; CRP, C-Reactive protein; NPV, Negative predictive value; PPV, Positive predictive value; SARS, Severe acute respiratory syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
The pandemic coronavirus disease 19 (COVID-19) is characterized by a highly variable course. While most patients experience only mild symptoms, a relevant proportion of patients develop severe disease progression up to respiratory failure. Interestingly, many patients do not show signs of respiratory distress, despite severe hypoxemia in blood gas analysis.1 About 5% of patients require intensive care, including mechanical ventilation.2 , 3
Recently published large retrospective analyses provide a detailed characterization of COVID-19 and identify variables associated with disease severity and high mortality.4 , 5 One of the largest studies so far shows that age, Quick Sequential Organ Failure Assessment (qSOFA) score, and D-dimer level were correlated with in-hospital death in a multivariate analysis.2 Another group showed a correlation of obesity and increased levels of inflammatory markers in the blood with respiratory failure.6
In many aspects, severe COVID-19 may be regarded as a viral-induced hyperinflammatory condition with multiorgan involvement due to a cytokine cascade.7 Of these various cytokines, the presence of raised circulating levels of IL-6 appears to be key and is closely connected to disease severity not only in COVID-198 but also in avian-origin H7N9 influenza infections9 and the common seasonal H1N1 influenza A.10
Although these studies identify the correlation of parameters with disease severity, prospective factors predicting impending deterioration of patients are not yet established. The broad spectrum of the disease courses and silent hypoxia make identification of patients at risk difficult. We aimed to identify variables that allow the prediction of patients with COVID-19 with a high risk of respiratory failure.
Methods
Patients and study design
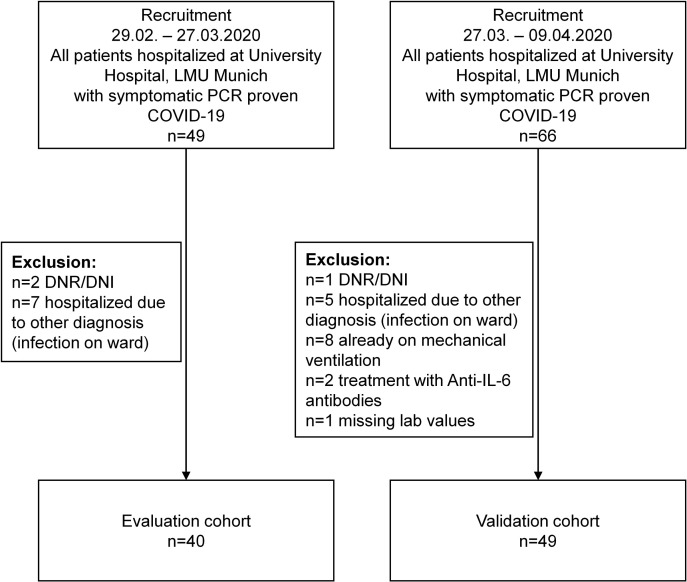
All patients with PCR-proven COVID-19 who were hospitalized at our institution from February 29 to April 9, 2020 (n = 115), were screened and analyzed for baseline clinical and laboratory findings. In total, 26 patients were excluded from the study and the depicted cohort consisted of 89 patients (Table I 11, 12, 13). Patients with palliative treatment (n = 3) or hospitalization for other medical reasons and nosocomial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on the ward (n = 13) were excluded from this study. Additionally, patients who were already mechanically ventilated at admission (n = 8) and those receiving anti–IL-6 antibody treatment (n = 2) were excluded (Fig 1 ).
Table I.
Combined cohort
| Variable | Evaluable, n | Value | Mechanical ventilation |
P value | q value | |
|---|---|---|---|---|---|---|
| No (n = 57) | Yes (n = 32) | |||||
| Baseline characteristics∗ | ||||||
| Median age, y (range) | 89 | 61 (18-84) | 58 (18-84) | 65 (45-81) | .031 | 0.067 |
| Median respiratory rate, breaths/min (range) | 74 | 18 (11-40) | 17 (13-39) | 25 (11-40) | .0024 | 0.0073 |
| Median heart rate, beats/min (range) | 66 | 86 (54-130) | 85 (54-130) | 89 (64-112) | .32 | 0.47 |
| Median BMI, kg/m2 (range) | 71 | 26.9 (18.1-45.7) | 26.0 (18.1-36.2) | 27.6 (18.3-45.7) | .074 | 0.15 |
| Male sex, n (%) | 89 | 62 (70) | 33 (58) | 29 (91) | .0029 | 0.0073 |
| Any comorbidity, n (%) | 87 | 70 (80) | 43 (77) | 27 (87) | .38 | 0.53 |
| Hypertension, n (%) | 86 | 45 (52) | 25 (45) | 20 (65) | .14 | 0.25 |
| Diabetes mellitus, n (%) | 86 | 13 (15) | 7 (13) | 6 (19) | .61 | 0.68 |
| Coronary artery disease, n (%) | 85 | 7 (8) | 4 (7) | 3 (10) | >.99 | >0.99 |
| Chronic obstructive lung disease, n (%) | 86 | 9 (10) | 7 (13) | 2 (6) | .54 | 0.67 |
| Computed tomography† | ||||||
| Consolidation, n (%) | 78 | 46 (59) | 30 (59) | 16 (59) | >.99 | >0.99 |
| Ground glass opacity, n (%) | 78 | 72 (92) | 47 (92) | 25 (93) | >.99 | >0.99 |
| Bilateral infiltration, n (%) | 78 | 70 (90) | 44 (86) | 26 (96) | .32 | 0.47 |
| Scores‡ | ||||||
| qSOFA score, n (%)11 | 71 | 30 (42) | 13 (28) | 17 (68) | .0028 | 0.0073 |
| CURB-65 score ≥ 1, n (%)12 | 47 | 22 (47) | 11 (41) | 11 (55) | .50 | 0.67 |
| Median MuLBSTA score (range)13 | 68 | 11 (0-15) | 9 (0-15) | 11 (5-15) | .090 | 0.17 |
| Laboratory parameters† | ||||||
| Median lymphocyte count, G/L (range) | 67 | 0.92 (0.20-2.84) | 0.85 (0.31-2.36) | 0.94 (0.20-2.84) | .60 | 0.68 |
| Median CRP level, mg/L (range) | 89 | 36 (0-369) | 20 (0-315) | 93 (16-369) | 1.9 × 10–7 | 2.6 × 10–6 |
| Median bilirubin level, mg/dL (range) | 84 | 0.5 (0.2-1.9) | 0.5 (0.2-1.2) | 0.6 (0.2-1.9) | .19 | 0.32 |
| Median WBC count, G/L (range) | 89 | 5.86 (0.15-308) | 5 (1.92-12.4) | 7.26 (0.15-308) | .0024 | 0.0073 |
| Median LDH level, U/L (range) | 88 | 311 (153-1121) | 278 (153-619) | 462 (240-1121) | 1.5 × 10–6 | 0.000010 |
| Median PCT level, ng/mL (range) | 87 | 0 (0-5) | 0 (0-0.6) | 0.2 (0-5) | 8.7 × 10–7 | 8.1 × 10–6 |
| Median IL-6 level, pg/mL (range) | 86 | 34 (0-430) | 23.2 (0-209) | 95.4 (14.2-430) | 2.3 × 10–9 | 6.5 × 10–8 |
| Median thrombocyte count, G/L (range) | 89 | 194 (0.12-450) | 194 (0.27-383) | 202 (0.12-450) | .55 | 0.67 |
| Median troponin T level, ng/mL (range) | 78 | 0 (0-0.178) | 0 (0-0.143) | 0 (0-0.178) | .00010 | 0.00047 |
| Median creatinine level, mg/dL (range) | 89 | 0.9 (0.4-7) | 0.9 (0.4-5.6) | 1.1 (0.8-7) | 5.2 × 10–6 | 0.000029 |
| Median D-dimer level, ng/mL (range) | 76 | 0.7 (0-35.2) | 0.6 (0-35) | 0.9 (0-35.2) | .0079 | 0.018 |
| Median ferritin level, ng/mL (range) | 79 | 703 (30-3577) | 545 (30-2578) | 1392 (237-3577) | .00023 | 0.00092 |
q Values represent the Benjamini-Hochberg adjusted P values. Boldface indicates statistical significance.
BMI, Body mass index; LDH, lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cell count.
Respiratory rate, heart rate, and BMI were measured at admission; existing comorbidities were evaluated by patient history at admission.
CT-scans and laboratory parameters at admission.
Scores were calculated at admission. CURB-65 score predicts mortality in community-acquired pneumonia; qSOFA score predicts mortality in sepsis; and MuLBSTA score predicts mortality in patients with viral pneumonia.
Fig 1.
Consolidated Standards of Reporting Trials diagram. DNR/DNI, Do-not-resuscitate and do-not-intubate order; LMU, Ludwig Maximilian University.
Of the 89 evaluable patients, 40 were part of an initial evaluation cohort hospitalized from February 29 to March 27, 2020 (see Table E1 in this article’s Online Repository at www.jacionline.org). This cohort was used to identify predictive markers of respiratory failure.
Following an interim analysis of the initial evaluation cohort,14 we performed a power analysis to estimate the number of patients needed to validate our findings. Assuming the need for mechanical ventilation to be 20% in the validation cohort and the level of risk for mechanical ventilation to be 70% and 20% in the high-risk and low-risk groups, respectively, the total sample size for a 2-sided test was determined to be 40. We defined an additional safety margin of 10%. This subsequent validation cohort consisted of patients hospitalized from March 27 to April 9, 2020 (n = 49) (see Table E2 in this article’s Online Repository at www.jacionline.org). Follow-up for all patients was complete through April 12, 2020. A comparison of both cohorts is shown in Table E3 (in this article’s Online Repository at www.jacionline.org).
Use of compassionate medication in the study cohort before mechanical ventilation was low (5 patients received lopinavir/ritonavir and 8 patients received hydroxychloroquine).
The decision on endotracheal intubation was made following internationally accepted recommendations (PaO2/FiO2 ratio <150 mm Hg or <200 mm Hg in the case of anticipated difficult airway).15
The patients were part of the COVID-19 Registry of the Ludwig Maximilian University Hospital Munich (CORKUM). Patient data were anonymized for analysis, and the study was approved by the local ethics committee (ethics committee of Ludwig Maximilian University Munich, No. 20-245).
IL-6 and CRP measures
The fully automated Elecsys system on a cobas e801 platform (Roche Diagnostics, Basel, Switzerland) was used to measure single levels of IL-6, as described previously.16 , 17 The Elecsys IL-6 immunoassay has been standardized against the National Institute for Biological Standards and Control first international standard 89/548. C-reactive protein (CRP) levels were measured on a cobas c702 platform by using the Tina-quant C-Reactive Protein assay (Roche Diagnostics, Switzerland).
Statistical analysis
All variables with less than 50% missing data in the initial cohort were tested for the association with respiratory failure. Categoric variables were tested with the χ2 test, and numeric variables with the Mann-Whitney U test. When appropriate, a paired test was performed. All tests were 2 sided. The P values were adjusted for multiple testing with the Benjamini-Hochberg method to avoid inflating the alpha error. An adjusted P value (q value) of .05 or less was considered significant. We constructed receiver operating characteristic curves and calculated the area under the curve (AUC) to compare the predictive ability of continuous variables. The AUC can be interpreted as the probability that the predictor’s value for a randomly chosen patient requiring intubation will be higher than its value for a randomly chosen patient not requiring intubation. The optimal cutoff was defined as the value maximizing the Youden index.18 Statistical analyses were performed by using the R software package (version 3.6.2). Figures were drawn by using GraphPad Prism software, version 6.0 (GraphPad Software, La Jolla, Calif).
Results
Initial identification of IL-6 and CRP as the strongest predictors of respiratory failure
To initially evaluate predictors of respiratory failure, 40 patients with confirmed COVID-19 were recruited from February 29 to March 27, 2020, and served as an evaluation cohort (Fig 1). The condition of 13 of these patients (32.5%) deteriorated during hospitalization, requiring mechanical ventilation. The time from hospital admission to intubation varied from less than 2 hours to 9 days (median 2 days). Patients requiring mechanical ventilation did not differ in terms of age, comorbiditiy, radiologic findings, respiratory rate, or qSOFA score (see Table E1).
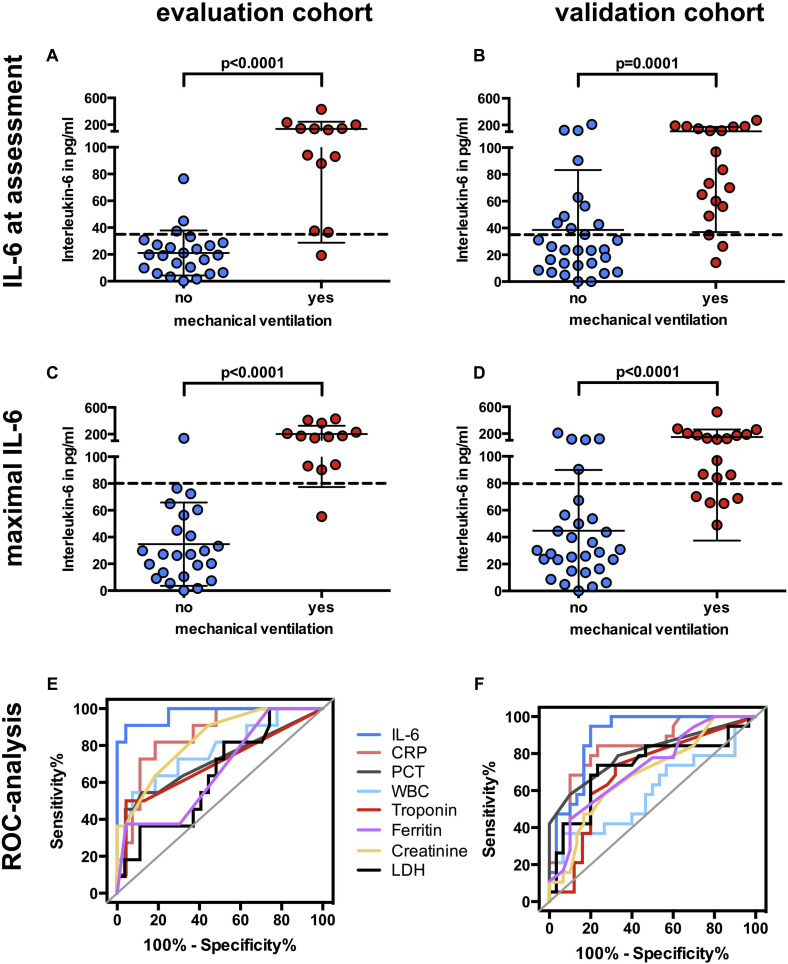
Heart rate, markers of inflammation, lactate dehydrogenase level, and creatinine level at admission were significantly associated with respiratory failure (see Table E1). Elevated IL-6 level showed the strongest association with the need for mechanical ventilation (P = 1.2 × 10–5) (Fig 2 , A).
Fig 2.
IL-6 levels at presentation, maximal IL-6 levels before mechanical ventilation, and receiver operating characteristic (ROC) analysis of different parameters in the evaluation and validation cohorts. Box plots showing IL-6 levels at first assessment (A and B) and maximal IL-6 levels before mechanical ventilation (C and D) in the evaluation cohort and in the validation cohort; dashed lines represents the cutoff calculated from the evaluation cohort (IL-6 level at initial assessment >35 pg/mL, maximal IL-6 level >80 pg/mL). Data are represented as means ± SDs. ROC curve of maximal follow-up levels before mechanical ventilation in the evaluation (E) and validation cohorts (F).
In addition to the values at first assessment, follow-up data were available for laboratory variables. These follow-up data were used to test whether there were critical laboratory values associated with respiratory failure once they had been reached during the disease course. For each patient, we assessed the maximum level of each parameter during disease (for patients requiring ventilation, only values before intubation were used). The maximal values were correlated with respiratory failure (Table II ). Maximal IL-6 level predicted respiratory failure with the highest accuracy (AUC = 0.97 [CI = 0.93-1.0]), followed by CRP level (AUC = 0.86 [CI = 0.74-0.98]) and creatinine level (AUC = 0.85 [CI = 0.74-0.97]) (Fig 2, C and E and Fig 3, C). The optimal cutoff for maximal IL-6 level was 80 pg/mL. After an IL-6 value of 80 pg/mL had been reached, the median time to mechanical ventilation was 1.5 days (range 0-4 days). The optimal cutoff for maximal CRP level was 97 mg/L, with a median time to mechanical ventilation of 0 days after the cutoff had been reached (range 0-4 days).
Table II.
P values, AUC values, and optimal cutoffs in the evaluation, validation, and combined cohorts
| Variable | Evaluation set |
Validation set |
Combined cohort |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At presentation |
Maximal |
At presentation |
Maximal |
At presentation |
Maximal |
|||||||||||||
| P value | AUC (CI) | Cutoff | P value | AUC (CI) | Cutoff | P value | AUC (CI) | Cutoff | P value | AUC (CI) | Cutoff | P value | AUC (CI) | Cutoff | P value | AUC (CI) | Cutoff | |
| IL-6 level (pg/mL) | .000012 | 0.94 (0.86-1.00) | 35 | 5.4 × 10–8 | 0.97 (0.93-1.00) | 80 | .000076 | 0.84 (0.73-0.95) | 48.9 | 4.9×10–7 | 0.90 (0.81-0.98) | 60 | 2.3 × 10–9 | 0.89 (0.81-0.96) | 48.9 | 2.6 × 10–11 | 0.93 (0.88-0.98) | 65 |
| CRP level (mg/L) | .0031 | 0.79 (0.65-0.93) | 32.5 | .00027 | 0.86 (0.74-0.98) | 97 | .000032 | 0.86 (0.75-0.96) | 32.5 | .000097 | 0.83 (0.72-0.95) | 97 | 1.9 × 10–7 | 0.83 (0.75-0.92) | 32.5 | 7.0 × 10–8 | 0.85 (0.76-0.93) | 97 |
| PCT level (ng/mL) | .0043 | 0.74 (0.58-0.90) | 0.05 | .0084 | 0.74 (0.57-0.91) | 0.25 | .000073 | 0.81 (0.69-0.93) | 0.05 | .00015 | 0.80 (0.67-0.93) | 0.25 | 8.7 × 10–7 | 0.78 (0.68-0.88) | 0.05 | 4.2 × 10–6 | 0.78 (0.67-0.88) | 0.25 |
| LDH level (U/L) | .00062 | 0.83 (0.70-0.97) | 320 | .071 | 0.68 (0.50-0.86) | 590 | .00032 | 0.81 (0.67-0.95) | 410 | .0076 | 0.73 (0.60-0.89) | 440 | 1.4 × 10–6 | 0.81 (0.72-0.91) | 410 | .0015 | 0.70 (0.59-0.82) | 380.5 |
| WBC level (G/L) | .0028 | 0.80 (0.66-0.93) | 4920 | .010 | 0.75 (0.58-0.93) | 9860 | .13 | 0.63 (0.45-0.81) | 6190 | .30 | 0.59 (0.41-0.77) | 10510 | .0024 | 0.69 (0.57-0.81) | 6190 | .015 | 0.66 (0.53-0.78) | 9860 |
| Creatinine level (mg/dL) | .00051 | 0.84 (0.72-0.96) | 0.95 | .00028 | 0.85 (0.74-0.97) | 1.05 | .0023 | 0.76 (0.63-0.89) | 0.95 | .026 | 0.69 (0.54-0.84) | 1.05 | 5.2 × 10–6 | 0.79 (0.70-0.88) | 0.95 | .000070 | 0.75 (0.65-0.86) | 1.05 |
| Troponin level (ng/mL) | .0053 | 0.72 (0.56-0.88) | 0.005 | .0079 | 0.72 (0.55-0.90) | 0.005 | .0078 | 0.72 (0.57-0.87) | 0.005 | .020 | 0.69 (0.54-0.85) | 0.005 | .00010 | 0.73 (0.62-0.83) | 0.005 | .00027 | 0.72 (0.61-0.83) | 0.005 |
| Ferritin level (ng/mL) | .064 | 0.72 (0.52-0.91) | 766 | .12 | 0.68 (0.47-0.89) | 530 | .0026 | 0.76 (0.62-0.90) | 1285 | .010 | 0.72 (0.58-0.87) | 1510 | .00023 | 0.75 (0.64-0.86) | 1285 | .0024 | 0.71 (0.59-0.83) | 1610 |
LDH, Lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cell count.
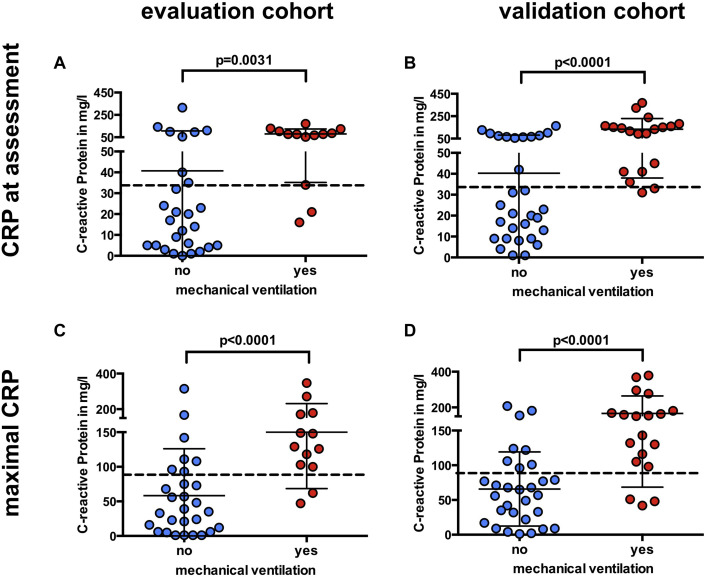
Fig 3.
CRP levels at presentation and maximal CRP levels before mechanical ventilation. Box plot showing CRP levels at first assessment (A and B) and maximal IL-6 levels before mechanical ventilation (C and D) in the evaluation cohort and in the validation cohort; dashed lines represents the cutoff calculated from the training cohort (CRP levels at assessment >32.5 mg/L, maximal CRP level >97 mg/L). Data are represented as means ± SDs.
Prospective validation of calculated cutoffs for IL-6 and CRP
A cohort of 40 patients was estimated to have an adequate power to validate our findings (see the Methods section). The validation cohort prospectively recruited 49 patients from March 27 to April 9, 2020, of whom 19 (39%) required mechanical ventilation. As in the initial cohort, creatinine level, lactate dehydrogenase level, and levels of several markers of inflammation were significantly elevated in patients requiring intubation (Table II and see Table E2). Again, IL-6 level at assessment was strongly associated with respiratory failure (Fig 2, B), and maximal IL-6 level was the best predictor of future respiratory failure among all parameters (AUC = 0.90 [CI = 0.81-0.98]) (Fig 2, D and F; Table II). CRP levels at initial assessment were significantly associated with respiratory failure (Fig 3, B) (AUC = 0.86 [CI = 0.75-0.96]). Follow-up CRP levels during the disease course did not improve the prediction of respiratory failure in the validation cohort (AUC = 0.83 [CI = 0.72-0.95]) (Fig 3, D and Table II).
To validate our findings from the initial cohort, we analyzed the number of patients correctly classified regarding their need for mechanical respiratory support by the determined cutoffs of IL-6 and CRP levels at presentation and in the course of disease (Table III ). At presentation, an IL-6 level greater than 35 pg/mL as well as a CRP level greater than 32.5 mg/L showed high rates of sensitivity to detect patients at risk for respiratory failure (84% and 95%) with moderate specificity (63% for both parameters). Measuring IL-6 and CRP values in the course of disease (cutoffs of 80 pg/mL and 97 mg/L) increased the specificity for both parameters (83% and 77%), accompanied by a decrease in sensitivity (74% vs 84%). In detail, 19 patients (39%) exceeded the calculated maximal IL-6 cutoff (>80 pg/mL) in the validation cohort, compared with 23 patients (47%) exceeding the CRP cutoff (>97 mg/L). Of these patients, 74% and 70% were correctly classified by IL-6 level and CRP level, respectively, as being at risk for respiratory failure (positive predictive value [PPV]). Of the 30 patients with values below the IL-6 cutoff, 83% did not require mechanical ventilation, whereas this was the case for 88% of the 26 patients with a CRP level remaining below the cutoff of 97 mg/L (negative predictive value [NPV]). In total, the calculated cutoffs for maximal IL-6 and CRP levels both correctly classified 80% of patients regarding their risk of respiratory failure (Table III), whereas the values at assessment showed poorer predictor properties owing to moderate specificity (correct classification of 71% for IL-6 level and 76% for CRP level)
Table III.
Contingency table for high-risk and low-risk groups as defined by IL-6 and CRP level in the validation cohort
| Variable | Value | Mechanical ventilation |
P value | |
|---|---|---|---|---|
| No | Yes | |||
| IL-6 level at presentation (pg/mL) | ≤35 | 19 | 3 | .0030 |
| >35 | 11 | 16 | ||
| Maximal IL-6 level (pg/mL) | ≤80 | 25 | 5 | .00022 |
| >80 | 5 | 14 | ||
| CRP level at presentation (mg/L) | ≤32.5 | 19 | 1 | .00019 |
| >32.5 | 11 | 18 | ||
| Maximal CRP level (mg/L) | ≤97 | 23 | 3 | .00011 |
| >97 | 7 | 16 | ||
Taken together, although both values have a strong sensitivity at assessment, specificity is gained when values are examined in the course of disease. The risk ratios for the cutoffs of IL-6 level and CRP level were 4.4 and 6.0 in the validation cohort, with corresponding P values of .00022 and .00011. The optimal cut point in the validation cohort was slightly lower for IL-6 level (60 pg/mL) and identical for CRP level (97 mg/L).
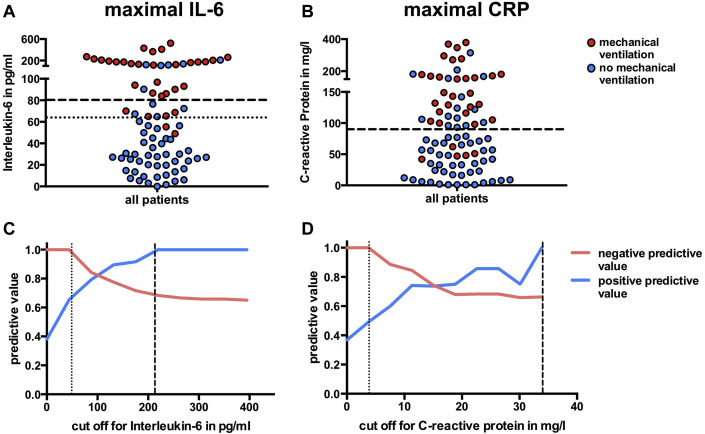
Predictive values of the combined cohort
To further evaluate the PPV and NPV of IL-6 and CRP levels, we combined the 2 cohorts (Fig 4 , A and B; Table I). We calculated predictive values across the range of all possible cutoffs. The PPV of CRP level was consistently lower than that of IL-6 level in the overall study cohort (Fig 4, C and D). In other words, increased CRP level misclassified more patients as being at risk for respiratory failure than IL-6 level did. However, the predictive values strongly depend on the selected cutoff. For cutoffs less than 50 pg/mL for IL-6 level and less than 40 mg/L for CRP level (dotted line in Fig 4, C and D), the risk of intubation for patients with subthreshold levels was roughly 0, whereas patients with levels above these values showed a dramatic increase in the risk of respiratory failure. The risk for respiratory failure in patients with IL-6 levels exceeding 210 pg/mL was 100% (dashed line). The NPVs of IL-6 and CRP parameters were comparable. In the combined cohort, the optimal threshold value (maximal Youden index18) was highest at 65 pg/mL for IL-6 (dotted line in Fig 4, A) level and at 97 mg/L for CRP level (corresponding risk ratios of 18.1 and 6.9).
Fig 4.
Cutoffs and predictive values of maximal IL-6 and CRP level values in the combined cohort. Box plots depicting the maximal values of IL-6 and CRP levels in the overall cohort (A and B); dashed line represents the validated cutoff; dotted line represents the calculated improved cutoff from all patients (applicable only for IL-6). PPV and NPV as a function of different cutoffs are shown for IL-6 (C) and CRP (D) level values (dotted line represents cutoff for perfect NPV; dashed line represents cutoff for perfect PPV).
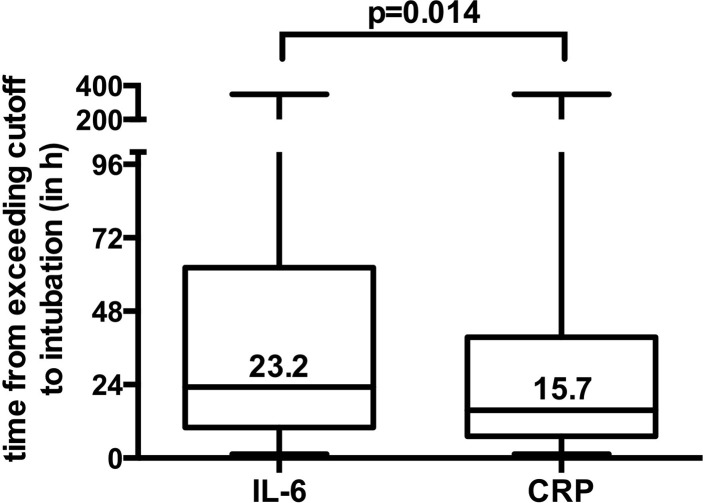
Furthermore, we analyzed the time lag from when the cutoff values were reached to intubation in the combined cohort. Patients reached the cutoff of IL-6 level (>65 ng/mL) and CRP level (>97 mg/L) at a median of 23.2 and 15.7 hours before intubation, resulting in a significant time difference between the 2 values of 7.5 hours in favor of IL-6 level (Fig 5 ) (P = .014).
Fig 5.
Time from when the maximal cutoff values of IL-6 or CRP level were exceeded to intubation in the combined cohort. Box plot depicting the time from when the IL-6 level (>65 ng/mL) and CRP level (>97 mg/L) cutoffs were exceeded to intubation in hours in the combined cohort. Data represented as median ± min/max are shown.
Discussion
Our study in hospitalized patients with COVID-19 has provided 3 key findings. First, circulating levels of IL-6 and CRP were highly predictive of the need for invasive ventilation, with corresponding AUC values of 0.97 and 0.90 for IL-6 level and 0.86 and 0.83 for CRP level in the first and the second cohorts, respectively. Second, we defined cutoffs for IL-6 level (>35 pg/mL at presentation and maximal value >80 pg/mL) and CRP (>32.5 mg/L at presentation and maximal value >97 mg/L) in the evaluation cohort. Cutoff values at assessment correctly classified 71% (for IL-6 level) and 76% (for CRP level) of patients in the validation cohort, with a further increase when measuring maximal values in the course of disease (80% for both parameters). Third, elevated IL-6 levels in the course of disease predicted respiratory failure significantly earlier than CRP did (at 23.2 vs 15.7 hours). Therefore, IL-6 level and CRP level are useful markers that predict impending respiratory failure with high accuracy and can help physicians correctly allocate patients who might benefit from early treatment escalation (eg, use of anti-cytokine strategies). We believe that having these data reproduced across the 2 separate cohorts enhances the strength of our conclusions. It is important to note that the commercial diagnostic IL-6 assay used in our study allows the measurement of Il-6 level in a time scale comparable to that with measurement of CRP level. Because it uses the broadly available cobas platform, this method can be implemented in most laboratories.
Our study also has several limitations. Whether elevated inflammatory markers merely represent an epiphenomenon or are a causal pathogenic element of severe COVID-19 is still unclear.19 It is likely that elevated IL-6 level reflects the cytokine-mediated hyperinflammatory state as evidenced by the similarly predictive values for CRP level. Further, even though IL-6 and CRP levels are significantly elevated in patients requiring ventilation, they are relatively low compared with the levels observed in patients with septic shock.20 However, earlier studies in patients with severe acute respiratory syndrome (SARS) or H7N9 influenza show that inflammatory cytokines are highly expressed in lung tissues. Autopsy reports from patients with SARS showed a high amount of inflammatory cytokines in cells expressing angiotensin-converting enzyme 2,21 the functional receptor for SARS-CoV, and in even higher affinity for SARS-CoV-2.22 Bronchoalveolar lavage (BAL) fluid in H7N9 influenza patients showed 103 times higher concentrations of different cytokines, including IL-6 compared with their plasma levels, hinting toward a massively increased local concentration of inflammatory cytokines in the diseased lung.9 Recent preprints provide detailed single-cell RNA sequencing data from immune cells in peripheral blood as well as in BAL fluid from patients with COVID-19. The authors report that PBMCs did not substantially express proinflammatory cytokines,23 although there was high expression in monocyte-derived macrophages in BAL fluid.24 Taken together, these data possibly suggest that circulating levels of IL-6 might be a putative surrogate for the burden of lung tissue damage and provide a “window” into the lung.9
IL-6 and CRP have previously been associated with severity of COVID-19 (in most cases defined by the Chinese National Health Commission) and mortality.25, 26, 27 To our knowledge, however, our study is the first to demonstrate a prospective prediction of the end point mechanical ventilation, which is of high clinical relevance not only for patient treatment but also for resource planning. Very recent publications provide additional data that strengthen the role of IL-6 and CRP in COVID-19 as predictive markers.25 , 26 Unfortunately, these studies did not include a prospective validation cohort and sometimes did not mention analysis platforms.25 A further difference between our and other studies is the dramatic discrepancy in mortality of severely diseased patients. We are not able to analyze mortality as an end point because only 2 patients had died as of April 12. This number had increased by only 1 by May 6 (overall mortality 3.4%). Although some patients are still in critical condition and the mortality rate in our cohort is likely to increase in the next weeks, it will be significantly below those reported. We can only speculate about the reasons for this huge difference, but we argue that overwhelmed hospitals and patient selection might have contributed to the increased mortality observed in other studies. As we did not perform sequential computed tomography scans after 24 to 48 hours in our patients owing to radiation hygiene, we are not able to precisely calculate severity of COVID-19 according the Chinese National Health Commission classification to compare our patient cohort with the cohorts of the aforementioned studies. However, at least 63% of the patients in our validation cohort had severe COVID-19 according to the available parameters (2% with mild and 35% with moderate symptoms), which exceeds the rates in the recently published cohorts.25 , 26
Since the start of the pandemic, hundreds of research articles on COVID-19 have been published.28 To our knowledge, we are reporting the first predictive marker for respiratory failure that was prospectively validated in an independent cohort. Although our sample sizes were small, the large difference in risk for respiratory failure between the high-risk and the low-risk group made it possible to successfully validate our findings. Interestingly, a study of 134 patients with avian-origin H7N9 influenza in 2013 also showed a strong correlation of IL-6 level and disease severity. In analogy to our findings, this study reports that IL-6 plasma levels higher than 80 pg/mL were found in all patients with a lethal outcome compared to only 8.3% of surviving patients.9 The combined cohort (n = 89) produced an only slightly lower cutoff for IL-6 level (65 pg/mL), whereas the cutoff for CRP levels remained the same at 97 mg/L when calculated from the combined cohort. However, even the combined sample size is probably too small to determine an optimal cutoff value. Furthermore, the acceptable proportion of falsely identified low-risk patients, and therefore the set threshold, is largely dictated by the availability of health care resources. Future prospective studies with larger sample sizes are needed to formally address this issue. We want to stress that IL-6 and CRP levels should be used as a predictor and not as an indication for invasive respiratory support, as mechanical ventilation per se has several unintended adverse consequences and may support inflammation of distal airways in patients with COVID-19.
Immunologically, CRP and IL-6 are closely intertwined. IL-6 is known to induce gene expression and release of CRP from the liver29 , 30 and also from immune cells.31 A functional connection has been shown in different trials using IL-6 inhibition, in which CRP levels rapidly normalized after blocking of IL-6.32 In analogy, we found that IL-6 levels predicted respiratory failure significantly earlier than CRP levels, which is essential for a predictive marker. Although inhibition of inflammatory pathways represents a promising approach to treat hyperinflammatory patients with COVID-19, inhibition of IL-6 could be detrimental in the immune response to virus-induced pneumonias.33 , 34 Thus, our study does not facilitate any recommendations for or against IL-6 inhibition. Ongoing randomized controlled clinical trials of IL-6-antibodies in the treatment of COVID-19 will shed light on this question (eg, NCT04320615 and NCT04331795). More importantly, in times of missing established therapeutic options, best supportive care is essential.35
In summary, we were able to validate our finding that IL-6 and CRP levels serve as strong predictors of patients in need of ventilator support. In the current situation with overwhelmed intensive care units and overcrowded emergency rooms, correct identification of patients in need of intensive care is crucial. Assessing these parameters to identify patients at risk of respiratory failure at an early stage might be helpful for triage planning and timely allocation of critically ill patients as well as a guide to escalation of treatment strategies in patients with COVID-19.
Clinical implications.
IL-6 level, followed by CRP level, strongly predicted patients at risk of respiratory deterioration and might be pivotal for risk-adapted escalation of treatment.
Acknowledgments
We would like to thank all of the CORKUM investigators and staff. We would also like to thank the patients and their families for their participation in the CORKUM registry, as well as all health care workers for their outstanding service.
Footnotes
Disclosure of potential conflict of interest: B. Lipworth reports grants and personal fees from Sanofi, AstraZeneca, and Teva; personal fees from Cipla, Glenmark, and Lupin; and research grants, consulting, advisory board, personal fees from Chiesi, outside the submitted work; in addition, he reports that his son is an employee of AstraZeneca. M. von Bergwelt-Baildon is the local principal investigator of the COVACTA-Trial (A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients with Severe COVID-19 Pneumonia [NCT04320615]; sponsored by Hoffmann-La Roche), which is currently being conducted, and he has previously received honoraria and research funding from Hoffman-La Roche unrelated to this project. M. Klein has received speaker fees from BioMerieux and served on the advisory board of BioMerieux. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
Evaluation cohort
| Variable | Evaluable, n | Value | Mechanical ventilation |
P value | q value | |
|---|---|---|---|---|---|---|
| No (n = 27) | Yes (n = 13) | |||||
| Baseline characteristics∗ | ||||||
| Median age, y (range) | 40 | 57 (19-81) | 54 (19-80) | 64 (45-81) | .15 | 0.29 |
| Median respiratory rate, breaths/min (range) | 34 | 18 (14-40) | 18 (14-32) | 23 (15-40) | .066 | 0.14 |
| Median heart rate, beats/min (range) | 32 | 81 (54-112) | 77 (54-111) | 94 (80-112) | .0069 | 0.022 |
| Median BMI, kg/m2 (range) | 30 | 25.9 (19.0-45.7) | 23.7 (19.0-34.7) | 30.5 (24.8- 45.7) | .0030 | 0.014 |
| Male sex, n (%) | 40 | 29 (72) | 16 (59) | 13 (100) | .020 | 0.051 |
| Any comorbidity, n (%) | 39 | 32 (82) | 20 (77) | 12 (92) | .46 | 0.81 |
| Hypertension, n (%) | 38 | 19 (50) | 10 (40) | 9 (69) | .17 | 0.32 |
| Diabetes mellitus, n (%) | 38 | 3 (8) | 1 (4) | 2 (15) | .55 | 0.82 |
| Coronary artery disease, n (%) | 36 | 3 (8) | 3 (12) | 0 (0) | .52 | 0.82 |
| Chronic obstructive lung disease, n (%) | 37 | 3 (8) | 2 (8) | 1 (8) | >.99 | >0.99 |
| Computed tomography† | ||||||
| Consolidation, n (%) | 36 | 21 (58) | 14 (61) | 7 (54) | .95 | >0.99 |
| Ground glass opacity, n (%) | 36 | 31 (86) | 20 (87) | 11 (85) | >.99 | >0.99 |
| Bilateral infiltration, n (%) | 36 | 33 (92) | 21 (91) | 12 (92) | >.99 | >0.99 |
| Score‡ | ||||||
| qSOFA score, n (%)E1 | 32 | 12 (37) | 7 (32) | 5 (50) | .55 | 0.82 |
| CURB-65 score ≥ 1, n (%)E2 | 24 | 7 (29) | 5 (31) | 2 (25) | >.99 | >0.99 |
| Median MuLBSTA score, (%)E3 | 29 | 9 (4-15) | 9 (4-13) | 7 (5-15) | .89 | >0.99 |
| Laboratory parameters† | ||||||
| Median lymphocyte count, G/L (range) | 31 | 0.99 (0.45-2.50) | 0.99 (0.45-1.80) | 0.95 (0.57-2.50) | 0.92 | >0.99 |
| Median CRP level, mg/L (range) | 40 | 28 (0-315) | 17 (0-315) | 77 (16-171) | .0031 | 0.014 |
| Median bilirubin level, mg/dL (range) | 37 | 0.5 (0.2-1.9) | 0.5 (0.2-1.2) | 0.5 (0.4-1.9) | .78 | >0.99 |
| Median WBC count, G/L (range) | 40 | 5.04 (2.12-308) | 4.67 (2.12-10.8) | 7.38 (4.67-308) | .0028 | 0.014 |
| Median LDH level, U/L (range) | 39 | 285 (153-1078) | 258 (153- 619) | 381 (252-1078) | .00062 | 0.0058 |
| Median PCT level, ng/mL (range) | 38 | 0 (0-5) | 0 (0-0.6) | 0.1 (0-5) | .0043 | 0.017 |
| Median IL-6 level, pg/mL (range) | 37 | 27.1 (0-430) | 19.6 (0-76.5) | 121 (19.2-430) | .000012 | 0.00034 |
| Median thrombocyte count, G/L (range) | 40 | 161 (0.12-440) | 162 (0.27-334) | 160 (0.12-440) | .74 | >0.99 |
| Median troponin T level, ng/mL (range) | 34 | 0 (0-0.032) | 0 (0-0.022) | 0 (0-0.032) | .0053 | 0.019 |
| Median creatinine, mg/dL (range) | 40 | 0.9 (0.4-2.1) | 0.9 (0.4-1.3) | 1.0 (0.9-2.1) | .00051 | 0.0058 |
| Median D-dimer level, ng/mL (range) | 31 | 0.7 (0-2.9) | 0.6 (0-2.2) | 1.1 (0.6-2.9) | .019 | 0.051 |
| Median ferritin level, ng/mL (range) | 31 | 626 (46-2153) | 553 (46-1748) | 810 (431-2153) | .064 | 0.14 |
q Values represent the Benjamini-Hochberg adjusted P values. Boldface indicates statistical significance.
BMI, Body mass index; LDH, lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cell count.
Respiratory rate, heart rate, and BMI were measured at admission; existing comorbidities were evaluated by patient history at admission.
CT-scans and laboratory parameters at admission.
Scores were calculated at admission. CURB-65 score predicts mortality in community-acquired pneumonia; qSOFA score predicts mortality in sepsis; and MuLBSTA score predicts mortality in patients with viral pneumonia.
Table E2.
Validation cohort
| Variable | Evaluable, n | Value | Mechanical ventilation |
P value | q value | |
|---|---|---|---|---|---|---|
| No (n = 30) | Yes (n = 19) | |||||
| Baseline characteristics∗ | ||||||
| Median age, y (range) | 49 | 64 (18-84) | 61 (18-84) | 65 (46-81) | .18 | 0.31 |
| Median respiratory rate, breaths/min (range) | 34 | 18 (11-40) | 17 (13-39) | 26 (11-40) | .027 | 0.083 |
| Median heart rate, beats/min (range) | 34 | 90 (64-130) | 94 (74-130) | 86 (64-107) | .033 | 0.091 |
| Median BMI, kg/m2 (range) | 41 | 27.5 (18.1-36.2) | 27.6 (18.1-36.2) | 27.0 (18.4-34.7) | .58 | 0.71 |
| Male sex, n (%) | 49 | 33 (67) | 17 (57) | 16 (84) | .091 | 0.21 |
| Any comorbidities, n (%) | 48 | 38 (79) | 23 (77) | 15 (83) | .85 | 0.96 |
| Hypertension, n (%) | 48 | 26 (54) | 15 (50) | 11 (61) | .65 | 0.76 |
| Diabetes mellitus, n (%) | 48 | 10 (21) | 6 (20) | 4 (22) | >.99 | >0.99 |
| Coronary artery disease, n (%) | 49 | 4 (8) | 1 (3) | 3 (16) | .31 | 0.46 |
| Chronic obstructive lung, n (%) disease | 49 | 6 (12) | 5 (17) | 1 (5) | .46 | 0.61 |
| Computed tomography† | ||||||
| Consolidation, n (%) | 42 | 25 (59) | 16 (57) | 9 (64) | >.99 | 0.98 |
| Ground glass opacity, n (%) | 42 | 41 (98) | 27 (96) | 14 (100) | >.99 | >0.99 |
| Bilateral infiltration, n (%) | 42 | 37 (88) | 23 (82) | 14 (100) | .24 | 0.37 |
| Scores‡ | ||||||
| qSOFA score, n (%)E1 | 39 | 18 (46) | 6 (25) | 12 (80) | .0025 | 0.010 |
| CURB-65 score ≥ 1, n (%)E2 | 23 | 15 (65) | 6 (55) | 9 (75) | .55 | 0.71 |
| MuLBSTA score, n (%)E3 | 39 | 11 (0-15) | 10 (0-15) | 13 (9-15) | .038 | 0.096 |
| Laboratory parameters† | ||||||
| Median lymphocyte count, G/L (range) | 36 | 0.80 (0.20-2.84) | 0.73 (0.31-2.36) | 0.94 (0.20-2.84) | .43 | 0.60 |
| Median CRP level, mg/L (range) | 49 | 42 (1-369) | 22 (1-163) | 134 (31-369) | .000032 | 0.00068 |
| Median bilirubin level, mg/dL (range) | 47 | 0.5 (0.2-1.2) | 0.4 (0.2-1.2) | 0.6 (0.2-1.1) | .16 | 0.30 |
| Median WBC count, G/L (range) | 49 | 6.0 (0.15-25.8) | 5.79 (1.92-12.4) | 7.22 (0.15-25.8) | .13 | 0.26 |
| Median LDH level, U/L (range) | 49 | 336 (181-1121) | 278 (181-502) | 474 (240-1121) | .00032 | 0.0022 |
| Median PCT level, ng/mL (range) | 49 | 0 (0-2.3) | 0 (00.3) | 0.2 (02.3) | .000073 | 0.00068 |
| Median IL-6 level, pg/mL (range) | 49 | 42.7 (0-272) | 23.7 (0209) | 83.5 (14.2272) | .000072 | 0.00068 |
| Median thrombocyte count, G/L (range) | 49 | 216 (93-450) | 212 (112-383) | 220 (93-450) | .23 | 0.37 |
| Median troponin T, ng/mL (range) | 44 | 0 (0-0.178) | 0 (0-0.143) | 0.022 (0- | .0078 | 0.027 |
| Median creatinine level, mg/dL (range) | 49 | 0.9 (0.5-7.0) | 0.9 (0.5-5.6) | 1.1 (0.8-7.0) | .0023 | 0.010 |
| Median D-dimer level, ng/mL (range) | 45 | 0.8 (0-35.2) | 0.6 (0-35) | 0.9 (0-35.2) | .11 | 0.24 |
| Median ferritin, ng/mL (range) | 48 | 789 (30-3577) | 508 (30-2578) | 1692 (237-3577) | .0026 | 0.010 |
q Values represent the Benjamini-Hochberg adjusted P values. Boldface indicates statistical significance.
BMI, Body mass index; LDH, lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cell count.
Respiratory rate, heart rate, and BMI were measured at admission; existing comorbidities were evaluated by patient history at admission.
CT-scans and laboratory parameters at admission.
Scores were calculated at admission. CURB-65 score predicts mortality in community-acquired pneumonia; qSOFA score predicts mortality in sepsis; and MuLBSTA score predicts mortality in patients with viral pneumonia.
Table E3.
Comparison of the evaluation and validation cohorts
| Variable | Cohort |
P value | |
|---|---|---|---|
| Evaluation (n = 40) | Validation (n = 49) | ||
| Baseline characteristics∗ | |||
| Median age, y (range) | 57 (19-81) | 64 (18-84) | .15 |
| Median respiratory rate, breaths/min (range) | 18 (14-40) | 18 (11-40) | .76 |
| Median heart rate (beats/min) | 81 (54-112) | 90 (64-130) | .017 |
| Median BMI, kg/m2 (range) | 25.9 (19.0-45.7) | 27.5 (18.1-36.2) | .18 |
| Male sex, n (%) | 29 (72) | 33 (67) | .77 |
| Any comorbidity, n (%) | 32 (82) | 38 (79) | .95 |
| Hypertension, n (%) | 19 (50) | 26 (54) | .87 |
| Diabetes mellitus, n (%) | 3 (8) | 10 (21) | .17 |
| Coronary artery disease, n (%) | 3 (8) | 4 (8) | >.99 |
| Chronic obstructive lung disease, n (%) | 3 (8) | 6 (12) | .79 |
| Computed tomography† | |||
| Consolidation, n (%) | 21 (58) | 25 (60) | >.99 |
| Ground glass opacity, n (%) | 31 (86) | 41 (98) | .14 |
| Bilateral infiltration, n (%) | 33 (92) | 37 (88) | .89 |
| Scores‡ | |||
| qSOFA score, n (%)E1 | 12 (37) | 18 (46) | .62 |
| CURB-65 score ≥ 1, n (%)E2 | 7 (29) | 15 (65) | .029 |
| MuLBSTA score, n (%)E3 | 9 (4-15) | 11 (0-15) | .13 |
| Median lymphocyte count, G/L (range) | 0.99 (0.45-2.5) | 0.8 (0.2-2.84) | .27 |
| Median CRP level, mg/L (range) | 28 (0-315) | 42 (1-369) | .10 |
| Median bilirubin level, mg/dL (range) | 0.5 (0.2-1.9) | 0.5 (0.2-1.2) | .71 |
| Median WBC count, G/L (range) | 5.04 (2.12-308) | 6 (0.15-25.8) | .47 |
| Median LDH level, U/L (range) | 285 (153-1078) | 336 (181-1121) | .18 |
| Median PCT level, ng/mL (range) | 0 (0-5) | 0 (0-2.3) | .32 |
| Median IL-6 level, pg/mL (range) | 27.1 (0-430) | 42.7 (0-272) | .34 |
| Median thrombocyte count, G/L (range) | 161 (0.12-440) | 216 (93-450) | .0084 |
| Median troponin T level, ng/mL (range) | 0 (0-0.032) | 0 (0-0.178) | .016 |
| Median creatinine level, mg/dL (range) | 0.9 (0.4-2.1) | 0.9 (0.5-7.0) | .82 |
| Median D-dimer level, ng/mL (range) | 0.7 (0-2.9) | 0.8 (0-35.2) | .57 |
| Median ferritin level, ng/mL (range) | 626 (46-2153) | 789 (30-3577) | .20 |
q Values represent the Benjamini-Hochberg adjusted P values. Boldface indicates statistical significance.
BMI, Body mass index; LDH, lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cell count.
Respiratory rate, heart rate, and BMI were measured at admission; existing comorbidities were evaluated by patient history at admission.
CT-scans and laboratory parameters at admission.
Scores were calculated at admission. CURB-65 score predicts mortality in community-acquired pneumonia; qSOFA score predicts mortality in sepsis; and MuLBSTA score predicts mortality in patients with viral pneumonia.
References
- 1.Xie J., Tong Z., Guan X., Du B., Qiu H., Slutsky A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [e-pub ahead of print]. JAMA https://doi.org/10.1001/jama.2020.1585. Accessed April 14, 2020. [DOI] [PMC free article] [PubMed]
- 4.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy [e-pub ahead of print]. JAMA https://doi.org/10.1001/jama.2020.5394. Accessed April 7, 2020. [DOI] [PMC free article] [PubMed]
- 5.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell LF, Chernyak Y, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City [e-pub ahead of print]. medRxiv 10.1101/2020.04.08.20057794. Accessed April 14,2020. [DOI]
- 6.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C. Charakteristik von 50 hospitalisierten COVID-19-Patienten mit und ohne ARDS. Dtsch Arztebl Int. 2020;117:271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipworth B, Chan R, Lipworth S, RuiWen Kuo C. Weathering the cytokine storm in susceptible patients with severe SARS-CoV-2 infection [e-pub ahead of print]. J Allergy Clin Immunol Pract https://doi.org/10.1016/j.jaip.2020.04.014. Accessed April 27, 2020. [DOI] [PMC free article] [PubMed]
- 8.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients [e-pub ahead of print]. Clin Infect Dis https://doi.org/10.1093/cid/ciaa449. Accessed April 7, 2020. [DOI] [PMC free article] [PubMed]
- 9.Wang Z., Zhang A., Wan Y., Liu X., Qiu C., Xi X. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc Natl Acad Sci U S A. 2014;111:769–774. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagau N., Slavcovici A., Gonganau D.N., Oltean S., Dirzu D.S., Brezoszki E.S. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care. 2010;14:R203. doi: 10.1186/cc9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L., Wei D., Zhang X., Wu Y., Li Q., Zhou M. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold T, Jurinovic V, Arnreich C, Hellmuth JC, von Bergwelt-Baildon M, Klein M, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv 10.1101/2020.04.01.20047381. Accessed April 27, 2020. [DOI]
- 15.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 16.Fischer S.K., Williams K., Wang L., Capio E., Briman M. Development of an IL-6 point-of-care assay: utility for real-time monitoring and management of cytokine release syndrome and sepsis. Bioanalysis. 2019;11:1777–1785. doi: 10.4155/bio-2019-0192. [DOI] [PubMed] [Google Scholar]
- 17.Jekarl D.W., Lee S.Y., Lee J., Park Y.J., Kim Y., Park J.H. Procalcitonin as a diagnostic marker and IL-6 as a prognostic marker for sepsis. Diagn Microbiol Infect Dis. 2013;75:342–347. doi: 10.1016/j.diagmicrobio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Hirano T, Murakami M. COVID-19: a new virus, but an old cytokine release syndrome [e-pub ahead of print]. Imunity https://doi.org/10.1016/j.immuni.2020.04.003. Accessed April 15, 2020.
- 20.Surbatovic M., Popovic N., Vojvodic D., Milosevic I., Acimovic G., Stojicic M. Cytokine profile in severe gram-positive and gram-negative abdominal sepsis. Sci Rep. 2015;5:11355. doi: 10.1038/srep11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L., Ding Y., Zhang Q., Che X., He Y., Shen H. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response to severe COVID-19 [e-pub ahead of print]. medRxiv https://doi.org/10.1038/s41586-020-2179-y. Accessed April 25, 2020. [DOI] [PMC free article] [PubMed]
- 24.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing [e-pub ahead of print]. medRxiv 10.1101/2020.02.23.20026690. Accessed April 25, 2020. [DOI]
- 25.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.London A.J., Kimmelman J. Against pandemic research exceptionalism. Science. 2020;368:476–477. doi: 10.1126/science.abc1731. [DOI] [PubMed] [Google Scholar]
- 29.Castell J.V., Gomez-Lechon M.J., David M., Andus T., Geiger T., Trullenque R. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 30.Castell J.V., Andus T., Kunz D., Heinrich P.C. Interleukin-6. The major regulator of acute-phase protein synthesis in man and rat. Ann N Y Acad Sci. 1989;557:87–99. [discussion 100-1] [PubMed] [Google Scholar]
- 31.Sehgal P.B. Interleukin-6: a regulator of plasma protein gene expression in hepatic and non-hepatic tissues. Mol Biol Med. 1990;7:117–130. [PubMed] [Google Scholar]
- 32.Nishimoto N., Terao K., Mima T., Nakahara H., Takagi N., Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 33.Yang M.L., Wang C.T., Yang S.J., Leu C.H., Chen S.H., Wu C.L. IL-6 ameliorates acute lung injury in influenza virus infection. Sci Rep. 2017;7:43829. doi: 10.1038/srep43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauder S.N., Jones E., Smart K., Bloom A., Williams A.S., Hindley J.P. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol. 2013;43:2613–2625. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization Clinical management of severe acute respiratory infection when Covid-19 is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected updated March 13, 2020. Available at: Accessed April 14, 2020.
References
- Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.S., Van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Wei D., Wu Y., Zhou M., Zhang X., Li Q. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]