Abstract
While SARS-CoV-2 infection has spread rapidly worldwide, data remains scarce about the natural history of infection in pregnant women and the risk of mother-to-fetal transmission. Current data indicates that viral RNA levels in maternal blood are low and there is no evidence of placental infection with SARS-CoV-2. Published reports to date suggest that perinatal transmission of SARSCoV- 2 can occur but is rare. Among 179 newborns tested for SARS-CoV2 at birth from mothers with COVID-19, transmission was suspected in 8 cases, 5 with positive nasopharyngeal SARS-CoV-2 RT-PCR and 3 with SARS-CoV-2 IgM. However, these cases arise from maternal infection close to childbirth and there are no information about exposition during first or second trimester of pregnancy. Welldesigned prospective cohort studies with rigorous judgement criteria are needed to determine the incidence and risk factors for perinatal transmission of SARS-CoV-2.
Keywords: Maternal-infant infection, Fetus, Newborn, Trans-placental passage, Cellular tropism, Severe acute respiratory syndrome, COVID-19
1. Introduction
The pandemic due to the emerging coronavirus named SARS-CoV-2 started in China in late 2019 and quickly spread around the world. This virus follows two previous epidemics of severe acute pneumonitis associated with the coronavirus SARS-CoV-1 and MERS-CoV [1]. The purpose of this review is to report and comment from virologists’ and obstetricians’ points of view the existing data concerning a possible mother to child transmission of SARS-CoV-2 and its potential consequences on the perinatal and subsequent outcomes.
2. Maternal viremia
During a primary infection, the passage of virus in the blood even for a short time, is an essential prerequisite for maternal-fetal transmission to occur by the trans-placental route.
In previous epidemics of severe acute respiratory syndrome associated with SARS-CoV-1, ± 78% of patients had detectable viral RNA in blood within the week of onset of symptoms. The viremia was determined using a quantitative PCR test specific for the SARS-CoV-1 genome, with a detection limit of 74 copies / ml in plasma. The plasma viral load found in patients with so-called “moderate” symptoms was low with an average concentration of 140 copies / mL, close to the detection threshold [2,3]. These SARS-1 studies were carried out with optimized methods, while the optimization of methods to detect viremia is still underway for the SARS-CoV-2 studies [4].
In patients with COVID-19, the SARS-CoV-2 virus may be undetectable by PCR tests on oropharyngeal samples. In two cohort studies of 205 and 40 patients, the presence of plasma viral RNA was detected in only 1% and 15% of patients, respectively [5,6]. Finally, German researchers recently reported the failure to isolate infectious viruses from the blood of infected patients [7]. The use of more sensitive SARS-CoV-2 PCR tests such as like a recently described assay that detected positive viremia in 11/80 samples from 15 previously negative (0/80) patients should increase the detection rate of the virus in blood as was observed in nasopharyngeal samples [8]. This new test allowed to establish in vitro the relationship between infectious virus and number of copies that is now estimated to be 1.8 TCID50 (approximately 4 infectious particles for 11 copies (95% confidence interval: 7.2–52.6 copies). A very recent submitted article shown that in a cohort of 50 COVID patient, but 78% male, the detection of viremia, using the WHO RT-qPCR protocol, rise significantly from 60% to 88% according to the severity of the disease [9]. In this article, the median of plasma viral load was from 100 to 500 copies/mL respectively. However, one patient shown viral load upper than 104 copies/mL. These plasma viral load data will be invaluable in assessing the risk of in utero virus transmission when the test becomes available and can be applied to pregnant women blood samples.
Of note, using deep sequencing methods the presence of viral RNA has been recently been shown in the mononuclear cells of the bronchoalveolar lavage, but not in those of peripheral blood mononuclear cell (PBMC), in 2 of 3 patients studied [10]. Furthermore, it has been shown in lymphoid lines (MT2 and A3.01) that the virus was able to enter lymphocytes, but these infections were not productive [11].
Thus, with regards to SARS-CoV-2, the presence of viral RNA in the blood therefore does exist, but at low levels, and its ability to transmit infection is still uncertain. Another study failed to demonstrate viral production following direct infection of blood monocytes with high infectious doses of SARS-CoV-2 virus (MOI 10, i.e. 10 infectious particles / cells) [12,11]. Conversely, the authors described a gradual decrease in the intracellular amount of RNA over time. These results should however be put into perspective if we consider the experience of Chikungunya for which there is no infection of PBMC in vitro but for which we have been able to repeatedly show the transient presence in monocytes and lymphocytes B in the blood of patients and during ex vivo infection of whole blood in humans, as well as in the macaque model [13]. These data suggest that the frequency and titers of RNAemia in SARS-CoV-2 infected patients may be lower or at most equivalent to that seen in SARS-CoV-1 infected patients. In addition, the virus does not seem capable of developing a productive infection in the circulating monocytes, or at a very low level, in line with previous observations on SARS-CoV-1 [14]. These results remain to be confirmed once the detection tests in plasma have been optimized.
In comparison, another virus of interest in obstetrics, ZIKV is detectable repeatedly or even continuously in the blood of pregnant women (or in animal models) while its presence can only be detected in an acute manner (less than one week) in a non-pregnant woman [15].
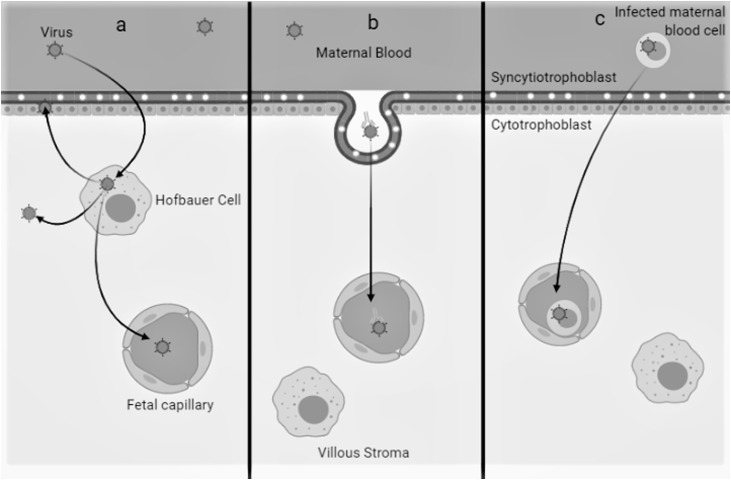
3. Does placental transmission of SARS-CoV-2 occur? (Fig. 1)
Fig. 1.
Different mechanism of viral infection and vertical transmission in placenta.
a) Placental tropism (as Hofbauer cells) and replication
b) transcytosis of opsonized or free virus
c)virus carried by an infected blood cell.
The second element that might be necessary for a maternal-fetal infection is a placental tropism of the virus i.e. the virus will infect the placental cells and thus be transmitted to the fetal side. To date, no case of placental infection with SARS-CoV-2, has been reported in published study. In five publications, a total of 7 placentas delivered from COVID-19 patients were studied using RT-PCR ; SARS-CoV-2 was not found in any of them [16,17,12,18,19]. Furthermore, histopathological analysis of three placentas did not reveal any significant lesion [16].
The hypothesis of a lack of placental infection is reinforced by the fact that the receptor for SARS-CoV-2, the angiotensin 2 converting enzyme (ACE2) necessary for its cell integration, is present only at very low levels in the human placenta during the first third trimester of pregnancy [20], while there are no data on the expression of this receptor in 2nd and 3rd trimester) placentas. However, in a hypertensive rat model induced by a saline diet, expression (mRNA) and significant enzymatic activity of the ACE2 receptor was observed in the uterus and the placenta in late gestation (day 19–20). Thus, the possibility of placental infection near delivery and therefore a potential passage to the fetus infection required further investigation. [21].
Therefore, two studies reported detection of the virus within the placenta membranes of critical cases within the third trimester by PCR [22] and more interestingly by electron microscopy [23] but any of the babies were found infected during the first week of life.
Another way for the virus to cross the barrier is to be carried by an infected blood cell. However, SARS-CoV-2, if able to enter into PBMCs does not seem to be replicative in these cells, like SARS-CoV-1 [24]. On the other hand, the resident macrophages of the lymph nodes or the spleen would express the ACE2 receptor (ACE2 +, CD169 + or CD68 + cells) and in terminally ill patients, the virus is found in these cells (Immunohistochemistry, nucleocapsid) but not in T or B lymphocytes [25]. SARS-CoV-1, which also uses the ACE2 receptor, is also found in alveolar macrophages [26].
Although no replication or transport of infectious viruses by macrophage monocytes has been demonstrated, nonetheless lymph node and spleen macrophages can harbor the virus. This underlines the need to analyze Hofbauer cells, the macrophages residing in the decidua and the placenta.
Another mechanism for viral transmission through the placenta is transcytosis of opsonized or free virus as has been shown for HIV, but this remains very hypothetical in view of the low viremia mentioned above [27].
Finally, transmission of some viruses, such as herpes simplex virus, HPV and HIV, may occur via the ascending route, from virus or infected cells in the cervicovaginal compartment. This type of transmission concerns sexually transmitted infections in particular. Only one study evaluate the presence of SARS-CoV-2 by RT-PCR in the vaginal fluid from 10 women, and all samples tested negative for the virus [28].
4. Fetal and neonatal infection: direct (PCR) and indirect (serology) detection
Very little data is available yet on neonatal infection with SARS-CoV-2, with conflicting results. The studies published to date have very small numbers. While some teams do not find an infected newborn by testing for the virus in samples of placenta, amniotic fluid, cord blood and neonatal throat swabs. Other publications suggest a possible vertical transmission due to the presence of IgM in certain newborns born to mothers infected with SARS-CoV-2.
There are 179 cases of newborns tested for SARS-CoV-2 at birth from pregnant women infected in the third trimester of pregnancy described in the literature [17,12,18,19,29,[30], [31], [32], [33], [34], [35], [36], [37]]. All of the patients were infected in late pregnancy and delivered within a few days of infection (mean: 3 days, range: 0 to 25 days). PCRs were performed on amniotic fluid and on cord blood during respectively 37 and 48 of these deliveries, all of which were negative (Table 1 ).
Table 1.
Maternal and neonatal characteristics from published studies (only case for which newborn were tested for SARS-CoV-2 have been reported). If some samples are not mentioned, they have been considered as not performed.
| Liu et al. [16] | Wang X et al. [17] | Zhu H et al. [27] | Yu N et al. [18] | Zeng et al. [28] | Breslin et al. [29] | Wang S et al. [19] | Zeng H et al. [30] | Dong et al. [31] | Yang P et al. [32] | Liu W et al. [33] | Alzamora et al. [34] | Yan et al. [35] | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of mother infant pairs | 3 | 1 | 8 | 3 | 33 | 18 | 1 | 6 | 1 | 7 | 19 | 1 | NS | 101 |
| Gestational age at infection – Mean (WG,days) | 38 | 30 | 39,4 | 38,3 | NS | 37 | 40 | 3d trim | 34,2 | 36,4 | NS | 32,3 | 38j | 38 WG |
| Positive maternal RT-PCR in nasopharyngeal swab | 3 | 1 | 7c | 3 | 33 | 18 | 1 | 6 | 1 | 7 | 10 h | 1 | NS | – |
| Positive maternal RT-PCR in feces | 1a | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | 1/2 (50%) |
| Positive maternal RT-PCR in vaginal swab | 0a | NP | NP | NP | NP | NP | NP | NP | 0 | NP | NP | NP | 0j,k | 0/3 (0%) |
| Positive maternal RT-PCR in breast milk | 0a | NP | NP | NP | NA | NP | 0 | NP | 0 | NP | 0 i | NP | 0j,l | 0/26 (0%) |
| Gestational age at delivery (WG,days) | 394 | 31 | 35,5 | 39,2 | 37,2g | NS | 40 | NS | 37,6 | 37 | 38,6 | 33 | 38,4j | 38,3 WG |
| Infection to delivery interval days - mean (range) | 8,3 | 6 | 1,4d | 4,8 | 1g | NS | 0 | NS | 25 | 2,4 | 4 | 4 | 2,5j | 3 (0, 25) |
| Number of newborn | 3 | 1 | 9e | 3 | 33 | 18 | 1 | 6 | 1 | 7 | 19 | 1 | 86 | 179 |
| Suspected materno-foetal infection (Neonatal RT-PCR or IgM positive for SARS-Cov-2) | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 9 (5%) |
| Positive neonatal RT-PCR in amniotic fluid | NP | 0 | NP | NP | NP | NP | NP | NP | NP | 0 | 0 | NP | 0m | 0/37 (0%) |
| Positive neonatal RT-PCR in placenta | 0b | 0 | NP | 0f | NP | NP | 0 | NP | NP | NP | NP | NP | NP | 0/4 (0%) |
| Positive neonatal RT-PCR in cord blood | 0 | 0 | NP | 0f | NP | NP | 0 | NP | NP | 0 | 0 | NP | 0m | 0/48 (0%) |
| Positive neonatal RT-PCR in nasopharyngeal swab | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 6/179 (3,4%) |
| Birth to positive neonatal PCR interval (hours) | – | – | – | 36 | 48 | – | 36 | – | – | – | – | 16 | – | 38 hours |
| Positive IgM for SARS-CoV-2 in newborn | NP | NP | NP | NP | NP | NP | NP | 2 | 1 | NP | NP | 0 | 0 | 3/7 (42%) |
NP = not performed / NS = Not stated.
Only two patients were tested.
Only one placenta was tested.
The mother of the twins had typical clinical symptoms, and viral interstitial pneumonia was revealed by a CT scan of her chest. Although her nasopharyngeal swab returned a negative result, other diseases that could cause fever and lung infection were excluded. The local CDC then registered her as a confirmed 2019-nCoV case.
One prelevement was positive 3 days after delivery.
One twin pregnancy.
Only the infected newborn was tested.
Only stated for the 3 positive newborns.
Only ten patients were laboratory-confirmed with COVID-19. According to the study, a clinically diagnosed COVID-19 case was defined as a case of pneumonia that fullfilled the following four criteria – fever and/or respiratory symptoms; radiographic evidence of typical viral pneumonia (bilateral ground-glass opacities); low or normal white-cell count or low lymphocyte count; and no improvement in symptoms after antimicrobial treatment for 2 days, ruling out common virus infection like influenza with or without an epidemiologic link to the Huanan Seafood Wholesale Market or contact with other patients with similar symptoms.
Only ten breast milk samples from mothers were performed.
Clinical characteristics and laboratory findings are mentioned for 99 patients, while only 86 newborns were tested for SARS-CoV-2.
Only six mothers were tested.
Only twelve mothers’ breast milk were tested.
Only ten patients were tested.
Among the 179 newborns, SARS-CoV-2 was detected in nasopharyngeal samples from six of them, one at 16 h of life, two at 36 h of life and three at 48 h of life. Thus, the timing of transmission cannot be determined in these cases. Transmission may have occurred after birth via the inhalation of droplets produced by contaminated parents or professionals, or via breastfeeding. The authors state that the infants were delivered by cesarean section and immediately separated from their mothers and placed in isolation, suggesting that postnatal transmission by the mother was unlikely (Table 2, Table 3 ). To date, breast milk has been analyzed in 26 cases, without evidence of SARS-CoV-2 [17,19,33,35,36,37]. Thus, transplacental transmission cannot be completely excluded in these cases, and intrapartum transmission could have occurred as well, during the passage in the genital tract via maternal secretions [38].
Table 2.
Maternal and neonatal characteristics detailed for suspected materno-fetal infection with positive neonatal RT-PCR for SARS-CoV2 at birth.
| Yu N et al. [18] | Zeng et al. [28] | Wang et al. [19] | Alzamora et al. [34] | |||
|---|---|---|---|---|---|---|
| N° of patient | 1 | 1 | 2 | 3 | 1 | 1 |
| Age of patiente | 34 | NS | NS | NS | 34 | 41 |
| Gestational age at infection (WG, d) | 39,3 | 40 | 40,1 | 29,6 | 40 | 32.3 |
| Gestational age at admission (WG, d) | 39,6 | 40 | NS | NS | NS | 33 |
| Term of delivery | 40 | 40 | 40,4 | 31,2 | 40 | 33 |
| Infection to delivery interval (days) | 4 | 0 | 0 | 3 | 0 | 4 |
| Maternal clinical characteristics | This patient present common COVID-19 disease with only fever as symptom, and abdominal pain (labour). | Only fever was reported as symptom and pneumonia per computed tomography diagnosis was made. The delivery was by cesarean delivery because of meconium-stained amniotic fluid and confirmed maternal COVID-19 pneumonia | Only fever and cough are described. Cesarean section was performed because of confirmed maternal COVID-19 pneumonia | No symptoms was report for COVID-19, only a close contact wit a diagnosed patient. Cesarean section was made after premature rupture of membrane because of fetal distress and confirmed maternal COVID-19 pneumonia | The pregnant woman developed small amount of per vaginal bleeding and lower abdominal pain. Two hours later, she developed a fever (37.8 °C) and attended to medical care center. Thoracic computerized tomography scan showed ground-glass opacities in the left upper and lower lobes, indicating the possibility of viral pneumonia. Blood tests revealed lymphopenia, neutrophilia and elevated CRP level (11.5 mg/L, normal: <1 mg/L). She was hospitalized for suspected viral pneumonia. On admission, her body temperature was 37.8 °C and her blood pressure was 131/89 mmHg, with respiratory rate of 20 breaths per minute, pulse of 96 beats per minute. She had no cough or sputum. Emergency Cesarean section was performed. | The patient presented with a 4-day history of general malaise, fatigue, and low-grade fever, and later developed worsening shortness of breath, which prompted her to seek medical attention. In the emergency department, the patient’s pulse was 131 beats per minute, the respiratory rate 38 breaths per minute, and the oxygen saturation 99% with a FiO2 of approximately 90%. Her body mass index (BMI) was 35 kg/m2. Laboratory tests showed metabolic acidosis on arterial blood gases, pancytopenia, elevated C-reactive protein, elevated ferritin, and slightly elevated D-dimer and glucose. The patient was intubated and placed on mechanical ventilation due to severe respiratory insufficiency in the setting of suspected COVID-19. The patient underwent a cesarean delivery due to maternal respiratory compromised status |
| Mode of delivery | ceasarian section | ceasarian section | caesarian section | caesarian section | Caesarian section | Caesarian section |
| Maternal RT-PCR in nasopharyngeal swab | Positive | Positive | Positive | Positive | Positive | Positive |
| Maternal RT-PCR + in feces | NP | NP | NP | NP | NP | NP |
| Maternal RT-PCR + in vaginal swab | NP | NP | NP | NP | NP | NP |
| Maternal RT-PCR + in breast milk | NP | NP | NP | NP | 0 | NP |
| N° of newborn | 1 | 1 | 2 | 3 | 1 | 1 |
| Preventive mesurement | All the patients delivered infants by caesarean section, and then the neonates were transferred to the neonatology department. | Strict infection control and prevention procedures were implemented during the delivery | The mother had been wearing an N95 mask throughout the operation, and the baby had no contact with the mother after birth. The infant was transferred to neonatology department 10 minutes after birth for close observation and the mother was transferred to the fever ward for isolation after surgery. | He was immediately separated from his mother and was not exposed to family members, who were at home under strict isolation measures. Due to the maternal condition, maternal medical regimen, breastfeeding was not initiated. He was placed in the neonatal intensive care unit (NICU) with no other COVID-19 cases. | ||
| Neonatal clinical characteristics | After ceasarean section, a 3250 g newborn was managed without neonatal complications. The neonate had no fever and cough, with mild shortness of breath. symptoms. Chest x-ray revealed mild pulmonary infection. The shortness of breath relieved quickly under neonatal care and monitoring. The neonate was discharged after 2 weeks following two consecutive negative nucleic acid test results. | On day 2 of life, the infant experienced lethargy and fever, with unremarkable physical examination results, and was moved to the neonatal intensive care unit. A chest radiographic image showed pneumonia, but other laboratory tests. (except procalcitonin) were normal. Nasopharyngeal and anal swabs were positive for SARS-CoV-2 on days 2 and 4 of life and negative on day 6 | He presented with lethargy, vomiting, and fever. A physical examination was unremarkable. Labora- tory tests showed leukocytosis, lymphocytopenia, and an elevated creatine kinase–MB fraction. A chest radiographic image showed pneumonia. Nasopharyngeal and anal swabs were positive for SARS-CoV-2 on days 2 and 4 of life and negative on day 6 | Resuscitation was required. The infant’s Apgar scores were 3, 4, and 5 at 1, 5, and 10 minutes after birth. Neonatal respiratory distress syndrome and pneumonia confirmed by chest radiographic image on admission resolved on day 14 of life after treatment with noninvasive ventilation, caffeine, and antibiotics. He also had suspected sepsis, with an Enterobacter agglomerates– positive blood culture, leukocytosis, thrombocytopenia (11 cells × 103/μL; to convert to cells × 109/L, multiply by 1.0), and coagulopathy (prothrombin time, 21 seconds; acti- vated partial thromboplastin time, 81.9 seconds), which improved with antibiotic treatment. Nasopharyngeal and anal swabs were positive for SARS-CoV-2 on days 2 and 4 of life and negative on day 7 | a baby boy was delivered, weighted 3205 g. Apgar scores at 1 and 5 minutes were 8 and 9. The infant had no moaning or spitting after birth. The skin was ruddy and the crying was loud. Half an hour after birth, the infant vomitted once after feeding formula, which we considered to be swallowing syndrome. After gastric lavage, the infant could be fed normally. Blood tests of the neonate revealed lymphopenia, deranged liver function tests and elevated creatine kinase level. Intravenous penicillin G and vitamin K1 were given as antibiotic prophylaxis and to prevent coagulopathy, respectively. | The neonate weighed 2,970 g, with Apgar’s scores of 6 and 8 at 1 and 5 minutes, respectively. The neonate was intubated in another room due to the high level of sedation of the mother. The newborn required ventilatory support for 12 hours, after which he was extubated and placed on continuous positive airway pressure, with favorable outcome and not requiring antibiotic treatment. At the sixth day of life, the newborn presented mild respiratory difficulty and sporadic cough requiring supplemental oxygen with nasal cannula. Imaging and laboratory testing remain normal. |
| Neonatal RT-PCR in nasopharyngeal swab | Positive | Positive | Positive | Positive | Positive | Positive |
| Birth to positive neonatal PCR interval (hours) | 36 | 48 | 48 | 48 | 36 | 16 |
| Neonatal RT-PCR in amniotic fluid | NP | NP | NP | NP | NP | NP |
| Neonatal RT-PCR in placenta | Negative | NP | NP | NP | Negative | NP |
| Neonatal RT-PCR 2 in cord blood | Negative | NP | NP | NP | Negative | NP |
| IgM for SARS-CoV-2 in newborn | NP | NP | NP | NP | NP | Negative |
NP = not performed / NS= Not stated.
Table 3.
Maternal and neonatal characteristics detailed for suspected materno-fetal infection with positive neonatal IgM for SARS-CoV2 at birth.
| Zeng H et al. [30] | Dong et al. [31] | ||
|---|---|---|---|
| N° of patient | 1 | 2 | 1 |
| Age of patiente | NS | NS | 29 |
| Gestational age at infection (WG,days) | 3d trimester | 3d trimester | 34 + 2 |
| Gestational age at admission (WG, days) | NS | NS | 35 |
| Term of delivery | NS | NS | 37 + 6 |
| Infection to delivery interval (days) | NS | NS | 25 |
| Maternal clinical characteristics | All 6mothers had mild clinical manifestations. All had cesarean deliveries in their third trimester. | A primiparous woman suspected of being exposed to SARS-CoV-2 developed a temperature of 37.9 °C and nasal congestion, which progressed to respiratory difficulties. A chest CT showed patchy ground-glass opacities in the periphery of both lungs. The RT-PCR on a nasopharyngeal swab was positive. The patient was admitted to RenminHospital and received antiviral, antibiotic, corticosteroid, and oxygen therapies. | |
| Mode of delivery | Caesarian section | Caesarian section | ceasarian section |
| Maternal RT-PCR 2 in nasopharyngeal swab | Positive | Positive | Positive |
| Maternal RT-PCR in feces | NP | NP | NP |
| Maternal RT-PCR in vaginal swab | NP | NP | negative |
| Maternal RT-PCR in breast milk | NP | NP | Negative |
| N° of newborn | 1 | 2 | 1 |
| Preventive mesurement | All had deliveries in negative pressure isolation rooms. All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery | An infant girl was delivered in a negative-pressure isolation room. The mother wore an N95 mask and did not hold the infant. The infant was immediately quarantined in the neonatal intensive care unit. | |
| Neonatal clinical characteristics | All 6 infants had 1-minute Apgar scores of 8 to 9 and 5-minute Apgar scores of 9 to 10. None of the infants presented any symptoms. | 1 | Her birth weight was 3120 g and Apgar scores were 9 at 1 minute and 10 at 5 minutes. The neonate had no symptoms. |
| Neonatal RT-PCR in nasopharyngeal swab | Negative | Negative | Negative |
| Neonatal RT-PCR in amniotic fluid | NP | NP | NP |
| Neonatal RT-PCR in placenta | NA | NA | NP |
| Neonatal RT-PCR in cord blood | Negative | Negative | NP |
| IgM for SARS-CoV-2 in newborn | Positive IgG level of 125.5 AU/mL (reference range < 10) and IgM level of 39.6 AU/mL (reference range < 10) | Positive IgG level of 113.91 AU/mL (reference range < 10) and IgM level of 16.25 AU/mL (reference range < 10) | Positive At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL. Her IgM (11.75 AU/mL) and IgG (69.94 AU/mL) levels were still elevated on March 7 (reference range < 10), and she was discharged on March 18. |
NP = not performed / NS = Not stated.
Zeng et al. and Dong et al., described three cases of newborns with positive anti-SARS-CoV-2 IgM and IgG serologies at birth from mothers infected with SARS-CoV-2 [33,33]. While maternal IgG antibodies cross the placenta, IgM are of fetal origin, thus suggesting in utero exposure to the SARS-CoV-2 virus [39]. However, whether this is evidence of in utero transmission has been disputed [40]. The sensitivity / specificity of IgM detection would be 88.2% / 96.2% and 70.2% / 99% according to these same studies and thus much higher than that observed for other viral infections. Furthermore, none of this children had a positive RT-PCR in nasopharyngeal samples [32,33] nor in the blood [32]. In addition, the decrease in IgM is very rapid in the Dong L study, going from 45.83 AU / mL to 2 h of life compared to 11.75 AU / mL on the 14th day of life (for a positivity threshold of 10 AU / mL), which seems surprising in the case of a congenital infection.
Although the majority of children born to infected mothers did not seem to have any symptoms, three of them nevertheless presented with severe pneumonia linked to SARS-CoV-2 [30]. In these three children the possibility of perinatal or postnatal transmission is unlikely since they were born by cesarean section and were separated from their mother from birth. In the perinatal period, maternal SARS-CoV-2 infection can have harmful consequences on obstetric outcomes and on newborns, resulting in particular in respiratory distress, biological abnormalities, premature deliveries and even fetal death in utero [41]. The authors hypothesize that hypoxemia in the mother may be responsible for fetal hypoxia at birth and premature delivery. Finally, rare cases of very severe infection have been described in very young children [42], suggesting that infants may not be very susceptible to COVID-19.
There are still no data on a maternal SARS-CoV-2 infection in the 1 st and 2nd trimester of pregnancy, including the risk of early miscarriage, fetal death in utero and growth retardation. A single study do not find viral RNA in amniotic fluid in mid-pregnancy on two patients exposed in the first trimester [43]. A study published in 2004 during the SARS epidemic found a higher rate of miscarriage, premature delivery and stunting, but no argument for vertical transmission [44]. In addition, abnormally high mortality was not observed in pregnant women infected with SARS-CoV-2, compared to what had been observed during the epidemics SARS-CoV-1 and MERS-CoV [45]. No fetopathy has been described to date in fetuses or neonates whose mothers had COVID-19.
5. Conclusion
In summary, current data demonstrate very rare maternal-fetal transmission, but are largely incomplete. According to these data, the transmission risk is probably very low, possibly under 1% following maternal SARS-CoV-1 infection during pregnancy. However, taking in account only the severe or critical form of COVID end of pregnancy, it was shown that the virus can be found in the placenta. Thus, as shown by one of us, in a single case of vertical in utero transmission, is associated with syncitiotrophoblast then in amniotic fluid and fetal blood [46]. The available studies concerned patients infected at the end of pregnancy, and in these studies, it should be noted that the time between maternal infection and delivery was often very short (of the order of a few days), which may not be sufficient for transplacental passage to occur.
We lack a clear understanding of the natural history of SARS-CoV-2 infection in pregnant women and the risk of in utero transmission. Prospective cohort studies should be able to answer the following important questions:
-
-
What is the impact of SARS CoV-2 on maternal and pregnancy outcomes according to the period of infection in pregnancy, the severity and management, including therapies?
-
-
What are the proportions with viral replication and its duration in the nasopharyngeal tract, intestine and maternal blood?
-
-
What are the risks of mother-to-child during the pregnancy, during labor and vaginal delivery and postnatally, in children who are not separated from the mother, as is common practice in most settings outside of China.
In parallel, in vitro or ex vivo studies are needed to determine whether the virus infects and is produced by decidual or placental cells.
The answers to these questions will determine how to revise current recommendations [47] for the care of COVID-19 pregnant women and their neonates in the future.
Financial support
Charles Egloff was supported by a grant from ARS-Ile-de-France.
Summary main points
Only 180 neonates born to women with COVID-19 have been reported, among which 6 were diagnosed with SARS-CoV-2. We reviewed the potential mechanisms of perinatal SARS-CoV-2 transmission and the studies required to assess this risk.
Declaration of Competing Interest
The authors report no potential conflicts.
Acknowledgments
The authors thank Dr. Elisabeth Menu for critical reading of the manuscript.
References
- 1.Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng Enders K.O., Hui David S., Allen Chan K.C., Hung Emily C.W., Chiu Rossa W.K., Nelson Lee Alan Wu. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin. Chem. 2003;49(12):1976–1980. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant Paul R., Garson Jeremy A., Tedder Richard S., Chan Paul K.S., Tam John S., Sung Joseph J.Y. Detection of SARS coronavirus in plasma by real-time RT-PCR. N. Engl. J. Med. 2003;349(25):2468–2469. doi: 10.1056/NEJM200312183492522. [DOI] [PubMed] [Google Scholar]
- 4.Mahony James B., Richardson Susan. Molecular diagnosis of severe acute respiratory syndrome. J. Mol. Diagn. 2005;7(5):551–559. doi: 10.1016/S1525-1578(10)60587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Wenling, Xu Yanli, Gao Ruqin, Lu Roujian, Han Kai, Wu Guizhen, Wenjie Tan. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;(March) doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Chaolin, Wang Yeming, Li Xingwang, Ren Lili, Zhao Jianping, Hu Yi, Zhang Li. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wölfel Roman, Corman Victor M., Guggemos Wolfgang, Seilmaier Michael, Zange Sabine, Müller Marcel A., Niemeyer Daniela. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;(April):1–10. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 8.Chan Jasper Fuk-Woo, Yip Cyril Chik-Yan, To Kelvin Kai-Wang, Tang Tommy Hing-Cheung, Wong Sally Cheuk-Ying, Leung Kit-Hang, Fung Agnes Yim-Fong. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020;(March) doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadjadj Jerome, Yatim Nader, Barnabei Laura, Corneau Aurelien, Boussier Jeremy, Pere Helene, Charbit Bruno. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. edRxiv. 2020;(April) doi: 10.1101/2020.04.19.20068015. 2020.04.19.20068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Yong, Liu Yuan, Cao Liu, Wang Dehe, Guo Ming, Jiang Ao, Guo Dong. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Xinling, Xu Wei, Hu Gaowei, Xia Shuai, Sun Zhiping, Liu Zezhong, Xie Youhua, Zhang Rong, Jiang Shibo, Lu Lu. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020;(April):1–3. doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Xiaotong, Zhou Zhiqiang, Zhang Jianping, Zhu Fengfeng, Tang Yongyan, Shen Xinghua. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin. Infectious Diseases. 2020;(February) doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Her Zhisheng, Malleret Benoit, Chan Monica, Ong Edward K.S., Wong Siew-Cheng, Kwek Dyan J.C., Tolou Hugues. Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J. Immunol. 2010;184(10):5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 14.Castilletti Concetta, Bordi Licia, Lalle Eleonora, Rozera Gabriella, Poccia Fabrizio, Agrati Chiara, Abbate Isabella, Capobianchi Maria R. Coordinate induction of IFN-α and -γ by SARS-CoV also in the absence of virus replication. Virology. 2005;341(1):163–169. doi: 10.1016/j.virol.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen Sydney M., Antony Kathleen M., Dudley Dawn M., Kohn Sarah, Simmons Heather A., Bryce Wolfe, Shahriar Salamat M. Highly efficient maternal-fetal zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017;13(5) doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y., Nie X., Huang B.X. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49(0):E005. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 17.Liu Weiyong, Wang Qianli, Zhang Qi, Chen Ling, Chen Junbo, Zhang Bo, Lu Yanjun. 2020. Coronavirus Disease 2019 (COVID-19) During Pregnancy: A Case Series.https://www.preprints.org/manuscript/202002.0373/v1 February. [Google Scholar]
- 18.Yu Nan, Li Wei, Kang Qingling, Xiong Zhi, Wang Shaoshuai, Lin Xingguang, Liu Yanyan. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020;(March) doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Shaoshuai, Guo Lili, Chen Ling, Liu Weiyong, Cao Yong, Zhang Jingyi, Feng Ling. A case report of neonatal COVID-19 infection in China. Clin. Infectious Diseases. 2020;(March) doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng. n.d. “Single-Cell RNA Expression Profiling of ACE2 and AXL in the Human Maternal–Fetal Interface.” Accessed April 6, 2020. http://www.repdevmed.org/article.asp?issn=2096-2924;year=2020;volume=4;issue=1;spage=7;epage=10;aulast=Zheng.
- 21.Levy Anat, Yagil Yoram, Bursztyn Michael, Barkalifa Ronit, Scharf Shimon, Yagil Chana. ACE2 expression and activity are enhanced during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(6):R1953–1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 22.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., Meyer J., Roman A.S. Detection of SARS-COV-2 in placental and fetal membrane samples. Am. J. Obstetrics Gynecology MFM. 2020 doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Algarroba G.N., Rekawek P., Vahanian S.A., Khullar P., Palaia T., Peltier M.R., Chavez M.R., Vintzileos A.M. Visualization of SARS-CoV-2 virus invading the human placenta using electron microscopy. Am. J. Obstetrics Gynecology. 2020 doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.To K.F., Tong Joanna H.M., Chan Paul K.S., Au Florence W.L., Chim Stephen S.C., Allen Chan K.C., Cheung Jo L.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J. Pathol. 2004;202(2):157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Yongwen, Feng Zeqing, Diao Bo, Wang Rongshuai, Wang Gang, Wang Chenhui, Tan Yingjun. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. edRxiv. 2020;(March) doi: 10.1101/2020.03.27.20045427. 2020.03.27.20045427. [DOI] [Google Scholar]
- 26.Chen Paul Chih-Hsueh, Hsiao Cheng-Hsiang. Re: to KF, tong JH, chan PK, et al. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J pathol 2004; 202: 157-163. J. Pathol. 2004;203(2):729–730. doi: 10.1002/path.1575. author reply 730-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagaye S., Derrien M., Menu E., Coïto C., Tresoldi E., Mauclère P., Scarlatti G. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J. Virol. 2001;75(10):4780–4791. doi: 10.1128/JVI.75.10.4780-4791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu Lin, Liu Xia, Xiao Meng, Xie Jing, Cao Wei, Liu Zhengyin, Morse Abraham, Xie Yuhua, Li Taisheng, Lan Zhu. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin. Infectious Diseases. 2020;(April) doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Huaping, Wang Lin, Fang Chengzhi, Peng Sicong, Zhang Lianhong, Chang Guiping, Xia Shiwen, Zhou Wenhao. Clinical analysis of 10 neonates born to mothers with 2019-NCoV pneumonia. Transl. Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Lingkong, Xia Shiwen, Yuan Wenhao, Yan Kai, Xiao Feifan, Shao Jianbo, Zhou Wenhao. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;(March) doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breslin Noelle, Baptiste Caitlin, Gyamfi-Bannerman Cynthia, Miller Russell, Martinez Rebecca, Bernstein Kyra, Ring Laurence. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City Hospitals. Am. J. Obstetrics Gynecol. MFM. 2020;(April) doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Hui, Xu Chen, Fan Junli, Tang Yueting, Deng Qiaoling, Zhang Wei, Long Xinghua. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;(March) doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Lan, Tian Jinhua, He Songming, Zhu Chuchao, Wang Jian, Liu Chen, Yang Jing. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;(March) doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Pu, Wang Xia, Liu Pin, Wei Cong, He Bingyan, Zheng Junwen, Zhao Dongchi. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J. Clin. Virol. 2020;127(April) doi: 10.1016/j.jcv.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Wei, Wang Jing, Li Wenbin, Zhou Zhaoxian, Liu Siying, Rong Zhihui. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med. 2020;(April) doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alzamora Maria Claudia, Paredes Tania, Caceres David, Webb Camille M., Valdez Luis M., La Rosa Mauricio. Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol. 2020;(April) doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Jie, Guo Juan Juan., Fan Cuifang, Juan Juan., Yu Xuechen, Li Jiafu, Feng Ling. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020;(April) doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Huijun, Guo Juanjuan, Wang Chen, Luo Fan, Yu Xuechen, Zhang Wei, Li Jiafu. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England) 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malek A., Sager R., Kuhn P., Nicolaides K.H., Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. (New York) 1996;36(5):248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 40.Kimberlin David W., Stagno Sergio. Can SARS-CoV-2 infection be acquired in utero?: More definitive evidence is needed. JAMA. 2020;(March) doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 41.Liu Yangli, Chen Haihong, Tang Kejing, Yubiao Guo. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020;(March) doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui Yuxia, Tian Maolu, Huang Dong, Wang Xike, Huang Yuying, Fan Li, Wang Liang. A 55-Day-Old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J. Infect. Dis. 2020;(March) doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Nan, Li Wei, Kang Qingling, Zeng Wanjiang, Feng Ling, Jianli Wu. No SARS-CoV-2 detected in amniotic fluid in mid-pregnancy. Lancet Infect. Dis. 2020;(April) doi: 10.1016/S1473-3099(20)30320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong Shell F., Chow Kam M., Leung Tse N., Ng Wai F., Ng Tak K., Shek Chi C., Ng Pak C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz David A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020;(March) doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 46.Vivanti Alexandre, Vauloup-Fellous Chistelle, Prevot Sophie, Zupan Veronique, Suffee Cecile, Do Cao Jeremy, Benachi Alexandra, De Luca Daniele. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020 doi: 10.1038/s41467-020-17436-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peyronnet V., Sibiude J., Deruelle P., Huissoud C., Lescure X., Lucet J.-C., Mandelbrot L. SARS-CoV-2 infection during pregnancy. Information and proposal of management care. CNGOF. Gynecol. Obstet. Fertil. Senol. 2020;(March) doi: 10.1016/j.gofs.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]