Abstract
Evidence of exposure to enteric pathogens through the air and associated risk of infection is scarce in the literature outside of animal- or human-waste handling settings. Cities with poor sanitation are important locations to investigate this aerial exposure pathway as their rapid growth will pose unprecedented challenges in waste management. To address this issue, simple surveillance methods are needed. Therefore, the objectives of this study were to optimize a community exposure bioaerosol surveillance strategy for urban outdoor locations with poor sanitation, and to determine which bioaerosols could contribute to exposure. Passive and active bioaerosol sampling methods were used to characterize the fate and transport of sanitation-related bioaerosols during the rainy and dry seasons in La Paz, Bolivia. Median coliform bacteria fluxes were 71 CFU/(m2 × h) during the rainy season and 64 CFU/(m2 × h) during the dry season, with 38% of the dry season samples testing positive for E. coli. Wind speed, relative humidity and UVB irradiance were identified as significant covariates to consider in bioaerosol transport models in La Paz. Active sampling yielded one positive sample (10%) for human adenovirus (HadV) and one sample (10%) for influenza A virus during the rainy season. HadV was detected at the site with the highest bacterial flux. Four samples (8%) were positive for influenza A virus in the dry season. These findings suggest that aerosols can contribute to community exposure to potentially pathogenic microorganisms in cities with poor sanitation. The use of passive sampling, despite its limitations, can provide quantitative data on microorganisms' viability within realistic timeframes of personal exposure.
Keywords: Urban, Sanitation, Bioaerosols, Exposure, Pathogens
Graphical abstract
Highlights
-
•
Bioaerosols were characterized near a river in a city with poor sanitation.
-
•
Passive and active sampling methods were used to detect fecal pathogens.
-
•
Coliforms, E. coli, Influenza A virus and Adenovirus were detected.
-
•
A Gaussian plume model could be used to estimate transport from the source.
-
•
The sampling strategy can be optimized for bioaerosol surveillance in urban centers.
1. Introduction
Current estimates suggest that 70% of the world population will be urbanized by 2050 (UN, 2014). Many of these growing cities are in low- and middle-income countries, which, according to the World Health Organization (WHO), do not have broad access to safely managed sanitation services (UNICEF and WHO, 2017). The lack of safe water, sanitation and hygiene (WASH) is linked to gastro-intestinal infectious diseases, which caused 1.38 million deaths in 2016 (with 60% attributed to WASH) (Prüss-Ustün et al., 2019) and being one of the leading causes of mortality in children under five years of age (Troeger et al., 2019). In addition, while poor sanitation is not directly related to acute respiratory infections, a 2009 study found that 26% of acute lower respiratory infections in children under 5 years old were linked with recent diarrheal disease events (Schmidt et al., 2009). These results suggest that reducing the incidence of diarrheal disease by increasing access to safely managed sanitation services, could reduce acute lower respiratory infections (Mara et al., 2010). These, and the recent coronavirus pandemic and evidence of SARS-CoV-2 viral excretion through the gastrointestinal tract (Xu et al., 2020) suggests that a better understanding of the aeromicrobiological route of exposure and the above mentioned possible linkages are warranted (Clasen and Smith, 2019). Our study helps with bridging this knowledge gap.
Plenty of research has been done on the fecal-oral infection route from unsafe water consumption, poor sanitation and hygiene. Even so, the exposure to enteric pathogens through the air and associated risk of infection is scarce in the literature outside of narrowly defined settings. It has been shown that concentrated sources of fecal matter can release bioaerosols through different mechanisms, e.g., wind erosion or mechanical disturbance (Farling et al., 2019; Delort and Amato, 2018; Paez-Rubio et al., 2005). These bioaerosols may be transported, pose an exposure risk, and cause infectious diseases. However, most of the bioaerosol research has been conducted in developed nations and in relatively isolated contexts, such as wastewater treatment plants, agricultural use of biosolids or in animal farms, e.g., places where personnel work close to animal and human waste (Schaeffer et al., 2017; Jahne et al., 2015; Uhrbrand et al., 2011; Baertsch et al., 2007). Recent research has focused among others on developing real-time bioaerosol sensors using fluorescence spectra to monitor bioaerosol emissions (Tian et al., 2020), characterizing the effect of aeration on bioaerosol generation during wastewater treatment (Wang et al., 2019) and chemical and molecular fingerprinting of outdoor bioaerosols to track their source and transport in different meteorological conditions (Garcia-Alcega et al., 2020). As these novel methods and findings keep arising, it is important to use existing methods and instruments to understand bioaerosol dynamics and exposure risks in low-resource settings.
Considering people living in cities with poor sanitation are broadly exposed to a variety of fecal pathogens through multiple pathways, e.g., fluids, fingers, flies, floors or food (Wagner and Lanoix, 1958), it is paramount to better understand fecal pathogen transport and exposure routes in order to minimize disease transmission. Moreover, cities with rapid urban growth will face unprecedented challenges in waste management, potentially causing an increase in diarrheal diseases that could create lifetime health deficits (Neiderud, 2015). Recent modelling efforts have found high risks of infection and illness from airborne Rotavirus and Norovirus emitted from wastewater treatment plants in Iran (Pasalari et al., 2019), identifying key areas in these facilities that increase the risk of illness from bioaerosol exposure (Carducci et al., 2018). Hence, we are conducting a series of studies looking at sanitation-related bioaerosols in developing countries to better understand their potential health impacts. The main goals of our study were 1) to optimize a robust and practical community exposure bioaerosol surveillance strategy in outdoor environments with poor sanitation and 2) explore if these bioaerosols are relevant at personal exposure levels (~3–13 Lair/min (EPA, 2011)) in such contexts. We conducted this study testing different sampling methods to characterize the fate and transport of sanitation-related bioaerosols during both the rainy and dry seasons in La Paz, Bolivia in 2019, as seasonal effects on aerosolized pathogenic microorganisms have been observed (Fan et al., 2019; Lu et al., 2019). We evaluated multiple environmental factors to explore their impact upon bioaerosols in real-world scenarios using low-cost instrumentation, identifying the key parameters to be included in future context-specific risk assessments and surveillance efforts.
2. Methods
2.1. Study site
La Paz is located at 3600 m above sea level in the Andean region of Bolivia. It is a rapidly growing city with 800,000 people (1.9 million in the metropolitan area) (Bolivian National Institute of Statistics, 2017). With a unique geography, the city lies in a canyon with poor urban planning. Industrial wastewater, hospital sewage and domestic sewage are discharged into the Choqueyapu River that crosses downtown La Paz and is fed by several tributaries also serving as sewers. Traversing steep slopes, the Choqueyapu River forms several waterfalls, creating an environment conducive to sewage aerosolization.
2.2. Bioaerosol sampling
We sampled bioaerosols during the rainy (September–April) and dry (May–August) seasons of 2019 during a 4-week field campaign split in two visits in March and June, respectively. Typical for La Paz, the rainy season (during the summer time) is characterized by very cloudy skies whereas the dry season (during winter) has more clear skies. As a results, the daily UVB irradiation is relatively uniform throughout the year (see Table A2 in SI). Five spatially distributed sites (~1.6 km from each other) adjacent to the Choqueyapu River were selected for their proximity to the river and waterfalls and five additional sampling locations were selected at 100–1000 m away from each site in the rainy season (n = 10, 6 replicates). Only three sites adjacent to the river were selected in the dry season, to increase our sample size (beginning of open-sewer, mid-way point and city exit; sites a, b and c in Appendix A – Fig. A1) and two concurrent transect samples were taken at 10–100 m downwind (n = 21, 3 replicates, not shown in Fig. A1). All other sites were only sampled in the rainy season. Three to six 100 mm settle Petri dishes (replicates) were set for 2 h at 1 m from the ground and 1 m from any obstacle, based on published methods for passive sampling (Haig et al., 2016; Pasquarella et al., 2000). We calculated the fluxes (CFU/(m2 × h)) by dividing the CFU counts by the area of the Petri dish (7.854 × 10−3 m2) and the time at each site (2 h). We used the open-source Aquatest (AT) (Bain et al., 2015) selective growth medium, Difco™ MI Agar (BD Biosciences, San Diego, CA, USA) and Compact-Dry-EC plates (Hardy Diagnostics, Santa Maria, CA, USA) for sampling and enumeration of viable fecal coliforms and E. coli.
We conducted active sampling using the National Institute for Occupational Safety and Health (NIOSH) BC 251 Personal Aerosol Samplers (Cao et al., 2011) in parallel to passive sampling, for 2 h in each sampling event (n = 10 in rainy season, n = 25 in dry season). The NIOSH sampler was selected because it had been used for personal exposure studies (Coleman et al., 2018; Choi et al., 2018; Bailey et al., 2018). It uses a sampling rate of 3.5 L/min which simulates human breathing and thus is relevant to personal exposure. For the experiments reported herein, the sampler was located ≥2 m next to the passive sampling setup. The sampling flow-rate was calibrated at 3.5 L/min before each sampling event. The NIOSH device sorts organisms by size in three compartments: >4 μm, 1–4 μm, and <1 μm. These particles were collected in a 15 mL falcon tube, a 1.5 mL centrifuge tube, and a polytetrafluoroethylene (PTFE) back-up filter (0.3 μm pore, 37 mm), respectively (Choi et al., 2018). All samples were taken between 8:00 am and 6:00 pm.
2.3. Sample processing and molecular assays
We took the samples collected on Petri dishes to the Universidad Católica Boliviana's (UCB) laboratories within 3 h of collection and incubated them at 37 °C for 20–24 h. Samples collected with the NIOSH device were rinsed with 1–1.5 mL PBS (0.5% BSA), combining the filter eluent with the 1–4 μm compartment's eluent. This was to increase the concentration of targets, and reduce the probability of false negatives. The two resulting eluents were mixed with DNA/RNA Shield™ reagent (Zymo Research, Irvine, CA, USA) for molecular analyses back in the USA. Viral nucleic acids were extracted using the Quick-DNA/RNA Viral Kit (Zymo Research, Irvine, CA, USA). We screened for 10 viruses (Influenza virus A/B/C/D Coronavirus NL63/OC43/HKU1/229E, Human adenovirus and Human enterovirus) using a RT-PCR method previously described (Bailey et al., 2018). Given the sampling methodology, the results for those viruses are expressed in presence/absence, with our limit of detection being approximately 7 copies per m3 air.
2.4. Environmental conditions monitoring
We used low-cost sensors on-site to collect minute-interval measurements of PM2.5, temperature and relative humidity (RH). These sensors have been described and tested previously (Barkjohn et al., in press; Zheng et al., 2018). We also collected wind speed data on-site (Vernier Software and Technology, Beaverton, OR, USA). We obtained solar UV irradiance (UVB, 280–320 nm) data from a stationary radiometer (Yankee Environmental Systems, Turners Falls, MA, USA) located at approximately 3 km away from the sampling site at Universidad Mayor de San Andrés.
3. Results and discussion
3.1. Passive and active sampling findings
The median flux of total coliforms in the rainy season was 71 CFU/(m2 × h) [range: 0–5411] while the median flux in the dry season was 64 CFU/(m2 × h) [range: 0–3374] with 38% of the dry season samples being positive for E. coli. The percentage of positive samples for E. coli during the rainy season is unknown due to growth medium presumably being damaged by sunlight, making it difficult to reliably differentiate E. coli from total coliforms (only 1 out 60 replicates had noticeable pink CFUs). CFU differentiation was possible during the dry season but we still noticed changes on the agar, shown in the positive controls in Fig. B1 (see SI). The sampling site located at the beginning of the open sewer (location a on Fig. A1) had the highest fluxes, with a mean flux of 4064 ± 1184 CFU/(m2 × h) in the rainy season and 2706 ± 388 CFU/(m2 × h) in the dry season. We note here that this site is at the starting point of the open sewer, and has a 2–3 m waterfall, coming off a tunnel with 1–2 m of headspace. We observed higher fluxes next to the river in both seasons and our concurrent transect samples taken at 10–100 m downwind of the open sewer at two different locations showed a reduction in fluxes as the distance from the river increased (Fig. 1 ). The deposition of aerosolized pathogens on food, water or fomites is known to be a potential source for exposure (de Man et al., 2014). For example, it was the suspected cause of an E. coli O157:H7 outbreak at a county fair in Oregon, USA (Keene et al., 2004). Continuous passive sampling with a highly selective medium such as AT could allow rapid and low-cost monitoring of bioaerosols to better understand the prevalence of such events, without needing highly trained personnel or high-tech equipment.
Fig. 1.
Total coliform fluxes at three sampling sites during the rainy season and dry season.
We conducted molecular analyses (RT-PCR) of actively sampled aerosols (using the NIOSH sampler) for influenza viruses, HadV, coronaviruses, and enteroviruses to test for potential presence of respiratory pathogens with aerosolized enteric bacteria. One sample (10%) was positive for HadV (positive hit on >4 μm compartment and in the <4 μm combined eluent) and one sample (10%) was positive for influenza A virus during the rainy season (positive hit on >4 μm compartment only). The HadV positive sample was from the site with the highest bacterial flux. Four samples (8%) were positive for influenza A virus in the dry season (all in the <4 μm compartment). HadV is commonly found in faecally-contaminated water and can cause enteric and respiratory infections through ingestion and inhalation, typically resulting in mild illness (WHO, 2005). A 2018 risk assessment study found high risks of illness from aerosolized HadV at wastewater treatment plants (WWTPs) (Carducci et al., 2018) supporting previous findings of adverse occupational-health effects in WWTPs (Thorn and Kerekes, 2001; Douwes et al., 2001). Influenza A virus, on the other hand, is well known to infect animals and humans alike through aerosols, causing respiratory illnesses with the potential to result in epidemics (Tellier, 2009; Mubareka et al., 2019) and detecting it through environmental sampling would enable local authorities to respond adequately. We did not detect any of the coronaviruses we tested for (NL63/OC43/HKU1/229E), and we did not test for SARS-CoV-2, the coronavirus responsible for the 2020 pandemic, as the study took place 5 months before the first known case of COVID-19 was identified (Andersen et al., 2020). The detection of viruses at low-flow rates (3.5 L/min) in short sampling periods (2 h) is of interest as viruses cause ~60% of infection cases, and to date, there are limited vaccines and antiviral medications (Boone and Gerba, 2007). In this light, it is likely that open sewers could be contributing to community exposure to viruses in places with poor sanitation, but the magnitude of the exposure needs further characterization (i.e., concentration measurements and infectivity assays to determine if the virus remains infectious). The recent coronavirus pandemic highlights the susceptibility of our society to viral infection and the needs for surveillance systems for early detection of disease outbreaks.
Our efforts to sequence aerosolized viruses to identify the sub-types were unsuccessful because the concentration of DNA was too low. However, our detection of viruses at sites with high enteric bacterial fluxes indicates that open sewers may be associated with enteric virus detection through aerosolization of contaminated water and sewage. Enteric viruses are known to remain viable for weeks to months, while respiratory viruses can do so for hours to multiple days (Boone and Gerba, 2007). Nevertheless, the contamination of non-porous fomites, such as the several playgrounds of La Paz located near our sampling sites, could harbor enteric viruses, potentially remaining infectious. At minimum, fomite sampling at those location is needed to begin understanding personal exposure.
3.2. Context-specific environmental co-variates identification
The effects of co-variates were observed by using a zero-inflated mixed effects regression model to assess the impact of the monitored environmental conditions on bacterial fluxes. This regression model addressed the fact that 35% and 33% of our passive samples were below detection limit (<1 CFU per plate) in the rainy and dry seasons, respectively. We fitted a negative binomial distribution to these data to account for its overdispersion (residual variance ≫ predicted variance). We found that wind speed had a significant positive, and UVB irradiance a significant negative effect on fluxes (p < 0.05 and p < 0.001, respectively) in the rainy season while positive effects from RH and UVB (p < 0.05 for both) were found in the dry season. While the trends during the rainy season are as expected, the positive effect of UV during the dry season is puzzling. We suspect that it could be due to i) higher bacterial flux rates compared to UVB induced death rates during the study (Tong and Lighthart, 1997). ii) Light shielding effects by large particles attached to the bacteria (Tong and Lighthart, 1998), or iii) a reduction in inactivation potential due to an effect of low RH on UVB-induced bacterial decay (Pepper and Dowd, 2009), suggesting that the average spherical irradiance may not be independent of UV dose at RH < 40% (Peccia et al., 2001). Overall, our results suggest that sanitation-related bioaerosols' viability and transport are likely to be most affected by UV radiation and wind speed. These results agree with previous bioaerosol studies which found that solar radiation reduced bioaerosol viability (Tong and Lighthart, 1997; Paez-Rubio and Peccia, 2005). Wind speed has also been found to plays a key role in bioaerosol dispersion, by affecting mechanical turbulence and particle deposition. RH has shown both positive and negative effects on inactivation rates (Van Leuken et al., 2016). Further investigations are needed to better quantify these effects.
We found a significant negative effect from distance in the dry season (p < 0.05) when sampling distances from the river were <100 m and samples were taken concurrently. We did not find a significant effect of distance in the rainy season when distances ranged from 10 m to several kilometers away from the open sewer. Samples were not taken concurrently in this season. We did observe higher fluxes next to the open sewer, implicating it as the likely source (58% of the samples taken >100 m away were below detection limit). The CFUs recovered at sites at distances >100 m could be attributed to other contamination sources. Tools such as microbial source tracking could be used in future studies to identify the origin of these bioaerosols.
We could not assess the seasonal effects statistically as our sampling strategies changed between sampling campaigns, but we observed slightly lower CFU fluxes in the dry season (Table A2), while the flow-rate of the Choqueyapu river decreased noticeably. A 2018 study in Beijing suggested that bioaerosols may represent a greater health hazard during the winter (dry season), after finding that microbial compositions of PM2.5 had more pathogenic bacteria and fungi (Du et al., 2018). In contrast, Masclaux and collaborators found aerosolized HadV in 100% of their samples taken from 31 WWTPs in the summer and 97% in the winter in Switzerland (Masclaux et al., 2014). The latter findings indicate a more uniform emission of bioaerosols from well-managed systems, commonly found in the developed world. Places with poor sanitation face a bigger challenge as open sewers or animal and human waste are not restricted spatially, increasing the possibility of community exposure.
3.3. Bioaerosol transport estimation model
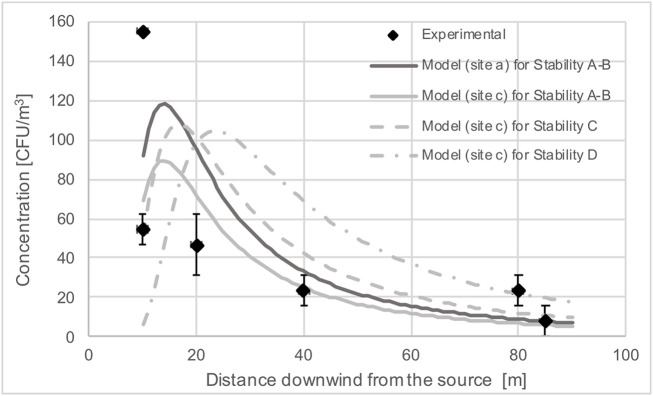
As a proof of concept, we applied a Gaussian plume model to estimate how far the bioaerosols emitted from an open sewer could travel (Fig. 2 ). We experimentally cross-validated the deposition velocity by dividing our mean flux [155 CFU/(m2 × h)] by the mean concentration from the rainy season measured by Ginn et al. (2020) [54 CFU/m3] (not published) during the rainy season, as sampling events coincided in time and location. The experimental deposition velocity was in the same order of magnitude of the theoretical deposition velocity (10−4 m/s), calculated using the Stokes settling velocity equation corrected by the Cunningham factor (Seinfeld and Pandis, 2016). A summary of the collected data can be found in the SI, Appendix A - Table A2. The concentration at 10 m downwind from the river was found using the Gaussian plume model (Eq. (2)) and with the following assumptions: i) Bioaerosols only traveled in the wind direction and estimates were for ground level concentrations only (z = 0, y = 0) ii) Concentrations were back-calculated using bacterial fluxes and theoretical particle deposition velocities. iii) The stack height was fixed at 4.68 m above the sewer, incorporating the height (3.68 m) from the water level to the ground and adding 1 m above ground at which the measurements were made. iv) Wind speed was constant for each site. v) One outlier data point was removed for the model. vi) The theoretical deposition velocity was estimated to be 1.37 m/h for spherical particles with a diameter range of 2–5 μm and a density of 1000 kg/m3.
| (1) |
| (2) |
where Q is the rate of bacteria emission per time [CFU/h]; H is the effective stack height [m]; Ws is the wind speed [m/h]; σy and σz are the standard deviation coefficients of dispersion [m] using Briggs formulas for Pasquill's atmospheric stability category A-B, C and D (Wark et al., 1998); and π is 3.14. These stability categories are semiquantitative; A and B are characterized for having wind speeds <2 m/s and slight, moderate or strong solar radiation (Wark et al., 1998), which best corresponded with the conditions during sampling.
Fig. 2.
Gaussian plume model fitted to experimental measurements taken during the dry season. The atmospheric stability category during the sampling campaigns was A-B. C and D are shown for illustration (see text for details).
This simple model shows that bioaerosol surveillance and transport modelling could be a starting point for public health officials to establish a threshold distance at which e.g., playgrounds or food stands should be located to reduce exposure to potential hazards. The variability observed highlights the importance of using this transport model with discretion when using passive sampling data. Comprehensive guidelines for model development and modelling software are available, e.g., through the Unites States EPA Support Center for Regulatory Atmospheric Modelling (EPA, 2020). Due to the inherent variability of the sampling method, we recommend a minimum of 30 samples (180 replicates) per site before drawing any conclusions. This would also allow the incorporation of environmental co-variates and their effect on the detected bioaerosols in the transport models. We did not include these here to avoid increasing the model complexity in an already limited data set.
3.4. Optimization of passive sampling
We found that only Aquatest (AT) medium could withstand the field conditions for passive sampling, compared to MI agar and Compact Dry plates (Appendix B). The E. coli staining chromogen was damaged after extended sunlight exposure (BD Diagnostics, 2009), preventing its identification and quantification vs. total coliforms. The dry environmental conditions also affected MI agar, resulting in dehydration of the medium and loss of surface area coverage (Appendix B – Fig. B1). Finally, we followed a published method to enhance AT's use for bioaerosol passive sampling, (Xu et al., 2013) and spread 0.1 mL mineral oil onto AT plates and tested them against regular AT plates during the rainy season, in triplicates. We did not find a significant difference in the CFU fluxes observed after 24 h of incubation between plates with or without mineral oil (p = 0.9055, Wilcoxon signed-rank test, n = 60). Our findings suggest that mineral oil does not increase CFU recoverability in outdoor bioaerosol passive sampling.
3.5. Study limitations
We optimized a simple, yet effective strategy of sampling bioaerosols in low-income settings for surveillance efforts, but as any study, it had its limitations. First, our sample size was limited due to time constraints for both sampling events (2 h at each site per sample) and the campaign (10 days for sample collection in each season). Continuous surveillance efforts would allow further hypothesis testing and improvement of bioaerosol transport modelling. Second, the damage that happened to the growth media may have resulted in viable but non culturable organisms, leading to an underestimation of fluxes or misidentification of E. coli. Third, the low volumes sampled for molecular analysis, while realistic from an exposure perspective, resulted in a low number of positive hits. Increased sampling flow-rates or longer sampling periods would permit better quantification of pathogens and microbial source tracking.
4. Conclusions
Our findings suggest that aerosols could play a role in the exposure to enteric microorganisms in cities with poor sanitation. The use of passive sampling, despite its limitations, can provide quantitative data on microorganisms' viability within realistic timeframes of personal exposure. Parallel active sampling at higher flowrates combined with current molecular methods could further identify and quantify pathogens of interest, including bacterial and viral species given the limitations of indicator microorganisms in passive sampling. Our future work will involve additional sampling and the development of Quantitative Microbial Risk Assessment (QMRA) frameworks to better understand the risk associated with the aeromicrobiological route of pathogen exposure of populations living in poor sanitation conditions. This will enable a better characterization of pathogen's fate and transport and the estimation of disease risks posed by these organisms, providing both technical and analytical surveillance tools.
CRediT authorship contribution statement
Lucas Rocha-Melogno:Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition.Olivia Ginn:Methodology, Investigation.Emily S. Bailey:Methodology, Writing - review & editing.Freddy Soria:Resources.Marcos Andrade:Resources, Writing - review & editing.Michael H. Bergin:Methodology, Formal analysis, Writing - review & editing.Joe Brown:Methodology, Resources, Writing - review & editing.Gregory C. Gray:Methodology, Resources, Writing - review & editing.Marc A. Deshusses:Conceptualization, Methodology, Resources, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
This study was supported by the Duke Global Health Institute funding for Global Health Doctoral Scholars and discretionary funding from Marc Deshusses and Gregory Gray at Duke University. We thank Mariana Chambi, Miriam Ajnota and Susan Aguilar for their support in field sampling and Natalie Alarja for her help in sample processing at Duke's One Health laboratory. We acknowledge Carolina Garvizu and Ethan Hicks for their thoughtful insights on transport models and Alyssa Platt, Kim Bourne and Bill Pan for their feedback on our statistical analyses. Lastly, many thanks to Ines Melogno who provided transportation and food during the sampling campaigns and to Nestor Ureña for providing lodging.
Editor: Frederic Coulon
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.139495.
Supplementary data
Supplementary material
References
- Andersen K.G. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertsch C. Source tracking aerosols released from land-applied class B biosolids during high-wind events. Appl. Environ. Microbiol. 2007;73(14):4522–4531. doi: 10.1128/AEM.02387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey E. Molecular surveillance of respiratory viruses with bioaerosol sampling in an airport. Tropical diseases, travel medicine and vaccines. 2018;4:11. doi: 10.1186/s40794-018-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain R. Evaluation of an inexpensive growth medium for direct detection of Escherichia coli in temperate and sub-tropical waters. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkjohn K. Using low-cost sensors to quantify the effects of air filtration on indoor and personal exposure relevant PM2.5 concentrations in Beijing, China. Aerosol Air Qual. Res. 2020;x:1–17. doi: 10.4209/aaqr.2018.11.0394. (in press) [DOI] [Google Scholar]
- BD Diagnostics . 2009. MI Agar. Difco and BBL Manual of Microbiological Culture Media Second Edition; p. 321.https://www.trios.cz/wp-content/uploads/sites/149/2016/08/DIFCO-A-BBL-MANUAL-2.pdf [Google Scholar]
- Bolivian National Institute of Statistics 2017. https://www.ine.gob.bo/index.php/notas-de-prensa-y-monitoreo/itemlist/tag/Poblaci%C3%B3n
- Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007;73:1687 LP–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G. Development of an improved methodology to detect infectious airborne influenza virus using the NIOSH bioaerosol sampler. J. Environ. Mon. 2011;13(12):3321. doi: 10.1039/c1em10607d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A. Quantitative microbial risk assessment for workers exposed to bioaerosol in wastewater treatment plants aimed at the choice and setup of safety measures. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.Y. Aerosol sampling in a hospital emergency room setting: a complementary surveillance method for the detection of respiratory viruses. Front. Public Heal. 2018;6(June):174. doi: 10.3389/fpubh.2018.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen T., Smith K. Let the “A” in WASH stand for air: integrating research and interventions to improve household air pollution (HAP) and water, sanitation and hygiene (WaSH) in low-income settings. Environ. Health Perspect. 2019;127 doi: 10.1289/EHP4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K. Bioaerosol sampling for respiratory viruses in Singapore’s mass rapid transit network. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-35896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man H. Human exposure to endotoxins and fecal indicators originating from water features. Water Res. 2014;51:198–205. doi: 10.1016/j.watres.2013.10.057. [DOI] [PubMed] [Google Scholar]
- Delort A.-M., Amato P. 2018. Microbiology of Aerosols; Part II: Sources and Transport of Microbial Aerosols. [Google Scholar]
- Douwes J., Mannetje A., Heederik D. Work-related symptoms in sewage treatment workers. Ann. Agric. Environ. Med. 2001;8:39–45. [PubMed] [Google Scholar]
- Du P. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 2018;610–611:308–315. doi: 10.1016/j.scitotenv.2017.07.097. [DOI] [PubMed] [Google Scholar]
- EPA . 2011. Exposure Factors Handbook: Chapter 6 – Inhalation Rates; pp. 3–4. [Google Scholar]
- EPA Support Center for Regulatory Atmospheric Modeling (SCRAM) 2020. https://www.epa.gov/scram
- Fan C. Characteristics of airborne opportunistic pathogenic bacteria during autumn and winter in Xi’an, China. Sci. Total Environ. 2019;672:834–845. doi: 10.1016/j.scitotenv.2019.03.412. [DOI] [PubMed] [Google Scholar]
- Farling S. Bioaerosol emissions associated with pit latrine emptying operations. Sci. Total Environ. 2019;648:1082–1086. doi: 10.1016/j.scitotenv.2018.08.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alcega S. Fingerprinting ambient air to understand bioaerosol profiles in three different environments in the south east of England. Sci. Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2020.137542. [DOI] [PubMed] [Google Scholar]
- Ginn et al. 2020. Airborne Antimicrobial Resistance Genes in Urban India and Bolivia, and the US. (not published) [Google Scholar]
- Haig C.W. Bioaerosol sampling: sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016;93(3):242–255. doi: 10.1016/j.jhin.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahne M.A. Emission and dispersion of bioaerosols from dairy manure application sites: human health risk assessment. Environ. Sci. Technol. 2015;49(16):9842–9849. doi: 10.1021/acs.est.5b01981. [DOI] [PubMed] [Google Scholar]
- Keene W., deBroekert M., Gillette K. Programs and Abstracts of the International Conference on Emerging Infectious Diseases, Atlanta, GA. 2004. A large Escherichia coli O157:H7 outbreak at a county fair [Abstract 55:77] [Google Scholar]
- Lu J. Effect of meteorological factors on scarlet fever incidence in Guangzhou City, Southern China, 2006–2017. Sci. Total Environ. 2019;663:227–235. doi: 10.1016/j.scitotenv.2019.01.318. [DOI] [PubMed] [Google Scholar]
- Mara D. Sanitation and health. PLoS Med. 2010;7(11) doi: 10.1371/journal.pmed.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux F.G. Assessment of airborne virus contamination in wastewater treatment plants. Environ. Res. 2014;133:260–265. doi: 10.1016/j.envres.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Mubareka S. Bioaerosols and transmission, a diverse and growing community of practice. Front. Public Heal. 2019 doi: 10.3389/fpubh.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderud C.J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015;5(1) doi: 10.3402/iee.v5.27060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Rubio T., Peccia J. Estimating solar and nonsolar inactivation rates of airborne bacteria. J. Environ. Eng. 2005;131(4):512–517. doi: 10.1061/(ASCE)0733-9372(2005)131:4(512). [DOI] [Google Scholar]
- Paez-Rubio T. Source bioaerosol concentration and rRNA gene-based identification of microorganisms aerosolized at a flood irrigation wastewater reuse site. Appl. Environ. Microbiol. 2005;71(2):804–810. doi: 10.1128/AEM.71.2.804–810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasalari H. Assessment of airborne enteric viruses emitted from wastewater treatment plant: atmospheric dispersion model, quantitative microbial risk assessment, disease burden. Environ. Pollut. 2019;253:464–473. doi: 10.1016/j.envpol.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Pasquarella C., Pitzurra O., Savino A. The index of microbial air contamination. J. Hosp. Infect. 2000;46(4):241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- Peccia J. Effects of relative humidity on the ultraviolet induced inactivation of airborne bacteria. Aerosol Sci. Technol. 2001;35:728–740. doi: 10.1080/02786820152546770. [DOI] [Google Scholar]
- Pepper I.L., Dowd S.E. Environmental Microbiology. Elsevier Inc; 2009. Aeromicrobiology; pp. 83–102. [DOI] [Google Scholar]
- Prüss-Ustün A. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: an updated analysis with a focus on low- and middle-income countries. Int. J. Hyg. Environ. Health. 2019;222:765–777. doi: 10.1016/j.ijheh.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer J.W. Size, composition, and source profiles of inhalable bioaerosols from Colorado dairies. Environ. Sci. Technol. 2017;51(11):6430–6440. doi: 10.1021/acs.est.7b00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W.P. Recent diarrheal illness and risk of lower respiratory infections in children under the age of 5 years. Int. J. Epidemiol. 2009;38(3):766–772. doi: 10.1093/ije/dyp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinfeld J., Pandis S. Third edition. Wiley; New Jersey: 2016. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. [Google Scholar]
- Tellier R. Aerosol transmission of influenza a virus: a review of new studies. J. R. Soc. Interface. 2009;6:S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn J., Kerekes E. Health effects among employees in sewage treatment plants: a literature survey. Am. J. Ind. Med. 2001;40:170–179. doi: 10.1002/ajim.1085. [DOI] [PubMed] [Google Scholar]
- Tian J. han. Real time detection and characterisation of bioaerosol emissions from wastewater treatment plants. Sci. Total Environ. 2020;721 doi: 10.1016/j.scitotenv.2020.137629. [DOI] [PubMed] [Google Scholar]
- Tong Y., Lighthart B. Solar radiation has a lethal effect on natural populations of culturable outdoor atmospheric bacteria. Atm. Environ. 1997;31(6):897–900. doi: 10.1016/S1352-2310(96)00235-X. [DOI] [Google Scholar]
- Tong Y., Lighthart B. Effect of simulated solar radiation on mixed outdoor atmospheric bacterial populations. FEMS Micro. Eco. 1998;26:311–316. doi: 10.1016/S0168-6496(98)00046-4. [DOI] [Google Scholar]
- Troeger C.E. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: an analysis of the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019 doi: 10.1016/S1473-3099(19)30401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrbrand K., Schultz A.C., Madsen A.M. Exposure to airborne noroviruses and other bioaerosol components at a wastewater treatment plant in Denmark. Food Environ. Virol. 2011;3(3–4):130–137. doi: 10.1007/s12560-011-9068-3. [DOI] [Google Scholar]
- UNICEF, WHO . 2017. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; Geneva. [Google Scholar]
- United Nations . 2014. World Urbanization Prospects; the 2014 Revision. [Google Scholar]
- Van Leuken J. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock – a review to inform risk assessment studies. Microb. Risk Analysis. 2016;1:19–39. doi: 10.1016/j.mran.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E.G., Lanoix J.N. World Health Organization; 1958. Excreta Disposal for Rural Areas and Small Communities. [PubMed] [Google Scholar]
- Wang Y. Effects of aeration on microbes and intestinal bacteria in bioaerosols from the BRT of an indoor wastewater treatment facility. Sci. Total Environ. 2019;648:1453–1461. doi: 10.1016/j.scitotenv.2018.08.244. [DOI] [PubMed] [Google Scholar]
- Wark K., Warner C., Davis W. Third edition. 1998. Air Pollution: Its Origin and Control; p. 157. [Google Scholar]
- World Health Organization (WHO) IWA Publishing; 2005. Water Recreation and Disease: Plausibility of Associated Infections: Acute Effects, Sequelae and Mortality. Chapter 6—Viruses; pp. 191–219. [Google Scholar]
- Xu Z. Enhancing bioaerosol sampling by Andersen impactors using mineral-oil-spread agar plate. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020 doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T. Field evaluation of low-cost particulate matter sensors in high- and low-concentration environments. Atmos. Meas. Tech. 2018;11:4823–4846. doi: 10.5194/amt-11-4823-2018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material