Abstract
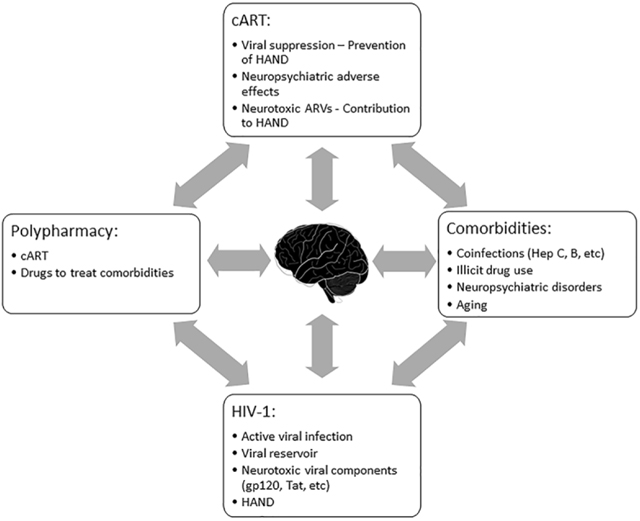
HIV-associated neurocognitive disorders (HAND) persist despite the successful introduction of combination antiretroviral therapy (cART). While insufficient concentration of certain antiretrovirals (ARV) may lead to incomplete viral suppression in the brain, many ARVs are found to cause neuropsychiatric adverse effects, indicating their penetration into the central nervous system (CNS). Several lines of evidence suggest shared critical roles of oxidative and endoplasmic reticulum stress, compromised neuronal energy homeostasis, and autophagy in the promotion of neuronal dysfunction associated with both HIV-1 infection and long-term cART or ARV use. As the lifespans of HIV patients are increased, unique challenges have surfaced. Longer lives convey prolonged exposure of the CNS to viral toxins, neurotoxic ARVs, polypharmacy with prescribed or illicit drug use, and age-related diseases. All of these factors can contribute to increased risks for the development of neuropsychiatric conditions and cognitive impairment, which can significantly impact patient well-being, cART adherence, and overall health outcome. Strategies to increase the penetration of cART into the brain to lower viral toxicity may detrimentally increase ARV neurotoxicity and neuropsychiatric adverse effects. As clinicians attempt to control peripheral viremia in an aging population of HIV-infected patients, they must navigate an increasingly complex myriad of comorbidities, pharmacogenetics, drug-drug interactions, and psychiatric and cognitive dysfunction. Here we review in comparison to the neuropathological effects of HIV-1; the available information on neuropsychiatric adverse effects and neurotoxicity of clinically used ARV and cART. It appears altogether that future cART aiming at controlling HIV-1 in the CNS and preventing HAND will require an intricate balancing act of suppressing viral replication while minimizing neurotoxicity, impairment of neurocognition, and neuropsychiatric adverse effects.
Keywords: HIV-1, infection, AIDS, NeuroHIV, HAND, antiretroviral, cART, brain, behavior, neurocognition, stress, toxicity
Graphical Abstract
Introduction
In the early 1980s, infection with human immunodeficiency virus (HIV-1) and acquired immunodeficiency syndrome (AIDS) developed into an acute epidemic (Fauci, 1999; Piot et al., 2001). The first drug for treatment of HIV-1 infection, azidothymidine (AZT)/zidovudine (ZDV) was reported in 1985 and approved by the Food and Drug Administration (FDA) in the United States in 1987. The discovery of additional, new classes of antiretrovirals and the introduction of combination antiretroviral therapy (cART), also known as highly active antiretroviral therapy (HAART), in the mid-1990s eventually changed the course of HIV-1 infection into a chronic but manageable disease, if treatment is available (Vella et al., 2012) (http://www.unaids.org/en/resources/fact-sheet). Nevertheless, 2.1 million people become newly infected by HIV-1 each year and more than 1 million people die from AIDS-related causes. As of 2017, UNAIDS estimates that the number of people living with HIV has grown globally to 36.9 million with 21.7 million receiving cART (UNAIDS, 2018). More than 1.2 million people live with HIV in the USA alone (http://www.cdc.gov/hiv/) (UNAIDS, 2013, 2015). HIV affects all organs that are infiltrated by permissive cells and thus enable the formation of viral reservoirs (Folks et al., 1988; Rothenberger et al., 2015; Sung and Margolis, 2018). HIV-infection of the central nervous system (CNS) frequently causes neurological problems, including neuropathic pain, and in about 50% of patients it leads to HIV-associated neurocognitive disorder (HAND) (Tozzi et al., 2001; Simpson et al., 2002; Cysique et al., 2004; Antinori et al., 2007; Heaton et al., 2010a; Saylor et al., 2016). Despite the use of antiretrovirals (ARVs) and effective virological control, HAND persists and the contributing neuropathological mechanisms remain to be fully elucidated (Kaul et al., 2001; Antinori et al., 2007; Kaul, 2008; Heaton et al., 2010a; McArthur et al., 2010; Simioni et al., 2010; Saylor et al., 2016).
The advent and availability of cART together with early intervention has lengthened patient lifespans to near normal ages (Antiretroviral Therapy Cohort, 2017) and reduced the incidence of HIV-associated dementia (HAD), the most severe form of HAND (Eisfeld et al., 2013; Heaton et al., 2015). However, it has become evident over time that most ARVs have neuropsychiatric adverse effects (DHHS, 2018), and the prevalence of cognitive impairment milder than dementia remains high in individuals living with HIV and receiving cART (McArthur et al., 2010; Heaton et al., 2011; Saylor et al., 2016). Moreover, recent studies found that a temporary interruption of cART in virologically controlled HIV patients gave rise to significant improvements of neurocognitive function (Robertson et al., 2010; Evans et al., 2011; Evans et al., 2012; Underwood et al., 2014). Meanwhile, several lines of experimental evidence suggest that at least some ARV compounds can themselves exert neurotoxicity (Lewin et al., 1995; Carr, 2003; Keswani et al., 2003; Robertson et al., 2012a; Akay et al., 2014; Sanchez et al., 2016; Sanchez and Kaul, 2017). All these observations suggest that HIV patients are at risk of exposure to a combination of potential contributors to neurotoxicity and HAND: namely HIV and its components and certain ARVs and their combinations. In this review we will discuss current information on HIV-1 and ARV effects on the CNS, the combination of which is encountered in the clinical setting as a chronic situation. The neurotoxic and neuropsychiatric effects of both the treatment and the disease itself requires a better understanding of the pathological and pharmacological mechanisms at the organismal and cellular level.
HIV-1 Infection
HIV-1 infection can be transmitted through certain body fluids including blood, semen, pre-seminal fluids, rectal fluids, vaginal fluids, and breast milk. Consequently, HIV-1 can be contracted through sexual contact, blood transfusion, needle-sharing, and from an infected mother to a child. Human CD4 and coreceptors, in particular chemokine receptors CXCR4 (CD184) and CCR5 (CD195), are the sites of host-virus interaction that mediate infection. The envelope protein of HIV-1, gp120, binds to CD4 receptors exclusively expressed on specific immune cells, to engage chemokine coreceptors. This action initiates infection of the virus’ primary target cells: macrophages and T-lymphocytes (Alkhatib et al., 1996; Bleul et al., 1996; Dragic et al., 1996; Oberlin et al., 1996; Trkola et al., 1996). Presumably within hours of infection in the periphery, the virus arrives in the brain where CCR3, besides CCR5, seems to facilitate HIV infection of microglia (He et al., 1997; Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005). Although CCR5-preferring (R5) HIV-1 are generally considered as being macrophage-tropic (M-tropic) and CXCR4-preferring virus strains (X4) as infecting T-lymphocytes (T-tropic), the reality appears less clear-cut. It has been discovered that syncytia-inducing viruses initially thought to be only X4-tropic can in fact be dual-tropic and infect via CXCR4 or CCR5 (Simmons et al., 1996). Additionally, M- and T-tropic SIV strains can enter target cells using CCR5 (Edinger et al., 1997). Overall, HIV-infected CD4+ T-lymphocytes seem to be highly efficient propagators of the virus, but also rapidly succumb to apoptosis, with the exception of a distinct number of memory cells that form a quiescent, latent reservoir (Bukrinsky et al., 1991; Pantaleo and Fauci, 1995; Chun and Fauci, 1999; Alexaki et al., 2008). In contrast, HIV-1 infected macrophages, which appear to be less efficient virus producers, can be a comparably long-lived reservoir that presumably carries the virus into the brain and passes it on to local macrophages and microglia (Ho et al., 1985; Koenig et al., 1986; Kaul et al., 2001; Collman et al., 2003; Gonzalez-Scarano and Martin-Garcia, 2005). ARVs have variable penetration across the blood-brain barrier (BBB), let alone the ability to reach therapeutic drug concentrations in the CNS. Several studies have reported a discordance between HIV RNA levels found in the cerebral spinal fluid (CSF) versus the plasma. Patients receiving cART and achieving suppression of plasma viremia (<50 copies/mL) have presented with neurological symptoms and were found to have higher levels of HIV RNA in the CSF (>200 copies/mL). In some patients, genotyping revealed resistance-associated mutations toward ARVs in the CSF viral population; suggesting that treatment was failing specifically in the CNS (Canestri et al., 2010; Peluso et al., 2012). Consequently, the CNS constitutes an HIV-1 reservoir which poses a major challenge for viral eradication (Nath, 2015; Ellis and Letendre, 2016; Gray et al., 2016).
HIV-1 Associated Neurocognitive Disorders (HAND) and Neuropathology
HIV-1 infected adults and children of all ages are at risk of developing neurological symptoms that comprise motor and cognitive dysfunction, termed HIV-associated neurocognitive disorders (HAND) (Navia et al., 1986; Price et al., 1988; Kaul et al., 2001; Antinori et al., 2007). Based on a panel of standardized measures, HAND is classified into three categories of disorders with increasing severity of dysfunction: i) asymptomatic neurocognitive impairment (ANI), ii) mild neurocognitive disorder (MND) and iii) HAD. The introduction of cART lowered the incidence of HAND’s most severe form, dementia, indicating a beneficial effect on cognitive function (Sacktor et al., 2001; Eisfeld et al., 2013; Heaton et al., 2015). Nevertheless, HAND/HAD remains a significant independent risk factor for death due to AIDS, and the prevalence of milder cognitive impairment (ANI, MND) continues to be high in HIV patients on cART (McArthur et al., 1993; Ellis et al., 1997; Wright et al., 2008; Heaton et al., 2010b; McArthur et al., 2010; Heaton et al., 2011; Saylor et al., 2016). Improved control of viral replication in the periphery and efficient therapy of opportunistic infections succeed in prolonging survival, but current cART regimens largely fail to protect from HAND or to reverse the disease (Cunningham et al., 2000; McArthur et al., 2003; Cysique et al., 2006; Giancola et al., 2006; Nath and Sacktor, 2006; Brew et al., 2009; Saylor et al., 2016). More than 90% of a group of 669 HIV patients receiving the first iterations of cART and who passed away between 1996 and 2001, developed HAD in the last 12 months of life as an AIDS-defining condition (Welch and Morse, 2002). Also, the proportion of new HAND/HAD cases with a CD4+ T cell count above 200 μl−1 is growing (Sacktor et al., 2002; McArthur et al., 2003). Thus, as people live longer with HIV-1 infection the prevalence of dementia could continue to rise despite cART (Lipton, 1997; Cunningham et al., 2000; Kaul et al., 2001; McArthur et al., 2003; Kaul et al., 2005; Kramer-Hammerle et al., 2005; Jones and Power, 2006; Saylor et al., 2016).
The neuropathology of HIV-1 infection is often described as HIV encephalitis (HIVE); displaying activated resident microglia, microglial nodules, multinucleated giant cells, infiltration predominantly by monocytoid cells, including blood-derived macrophages, and decreased synaptic and dendritic density, combined with selective neuronal loss, widespread reactive astrocytosis, and myelin pallor (Petito et al., 1986; Masliah et al., 1997). A subset of those pathological features, namely the increased numbers of microglia (Glass et al., 1995), decreased synaptic and dendritic density, selective neuronal loss (Achim et al., 1994; Wiley et al., 1994; Masliah et al., 1997), elevated tumor necrosis factor (TNF)-α mRNA in microglia and astrocytes (Wesselingh et al., 1997), and evidence of excitatory neurotoxins in cerebrospinal fluid (CSF) and serum (Heyes et al., 1991) present the best correlates of ante mortem signs of cognitive impairment. Additionally, two reports have also suggested an important role for myeloid cells in the outcome of HIV-1 infection in the CNS, showing that the risk of developing HAD correlated better with the amount of proviral HIV DNA in circulating monocytes and macrophages than with viral load (Shiramizu et al., 2005; Shiramizu et al., 2006).
Distinct brain regions suffer neuronal damage and loss in association with HIV infection; including the frontal cortex (Ketzler et al., 1990; Everall et al., 1991), substantia nigra (Reyes et al., 1991), cerebellum (Graus et al., 1990), and putamen (Everall et al., 1993). Signs of neuronal apoptosis have also been observed in brains of HAD patients (Petito and Roberts, 1995; Adle-Biassette et al., 1999; Rostasy et al., 2000). Especially within subcortical deep gray structures, apoptotic neurons were localized and correlated with signs of structural damage and evidence of microglial activation (Adle-Biassette et al., 1999).
Introduction of cART changed HIV neuropathology. Although opportunistic infections were largely controlled or absent, two post-mortem studies found more macrophage/microglia infiltration and activation in the hippocampus and basal ganglia (Langford et al., 2003b; Anthony et al., 2005). However, whereas Anthony et al found an overall decrease in HIVE in the Edinburgh cohort post-cART, Langford et al observed an increase in HIVE in the University of California-San Diego (UCSD) cohort in the cART era. HIV patients in the UCSD cohort who had failed cART showed even more signs of encephalitis and severe leukoencephalopathy (Langford et al., 2003b). Another autopsy cohort from a population in Oslo, Norway has found that HIV-induced brain lesions increases with lengthened survival times (Maehlen et al., 1995). Interestingly, this study noted that antiretroviral treatment, particularly ZDV/AZT, reduced the incidence of brain lesions but only if continued until death; those who discontinued use presented an increase in HIVE. Another post-mortem study of 436 HIV-seropositive patients who died between 1985 and 1999 found an increase in HIVE over time (p=0.014) despite increasing efficiency of cART regimens (Neuenburg et al., 2002). Likewise, a study performed in Brazil has found that HIVE is more frequent in autopsy cases performed on patients who received cART for 3 months or more than those who had no antiretroviral treatment (Silva et al., 2012). A study in Milan, Italy, which was divided into four time periods on the basis of wide treatment availability (1984–1987 no therapy, 1988–1994 monotherapy, 1995–1996 dual combination therapy, 1997–2000 triple therapy) noted a marked decrease in HIVE over time (Vago et al., 2002). Interestingly, differences in treated and untreated patients in the last period, for whom actual drug treatments received are known, were not statistically significant. It is important to note, as Bell points out in a review, that deaths and resulting post-mortem studies among cART recipients represent a population of HIV patients who experienced treatment failure; thus, neuropathology findings may not be representative of treated patients who survive (Bell, 2004). (Anthony et al., 2006). One report of the changing neuropathology in the era of cART described various forms of severe HIVE and white matter injury with extensive perivascular lymphocytic infiltration, suggesting ‘burnt-out’ forms of HIVE(Everall et al., 2005). Additional recent reports have shown post-mortem findings of aging-related accumulation of beta-amyloid implying an Alzheimer’s Disease-like neuropathology (Green et al., 2005) and cART-treated individuals presenting a greater number of hyperphosphorylated tau-positive neurofibrillary tangles and pre-tangles when compared with age-matched controls (Anthony et al., 2006). As the guidelines for antiretroviral therapies are still evolving, the neuropathological nature of HIV patients on modern drug regimens has yet to be fully elucidated.
HIV-1 Neurotoxicity
Besides microglia and macrophages in the brain, neurons and astrocytes also express chemokine receptors, including CCR5 and CXCR4 (Asensio and Campbell, 1999; Kaul and Lipton, 1999; Miller and Meucci, 1999; Kaul et al., 2007), but the latter cell types do not obviously permit productive HIV-1 infection under in vivo conditions. However, numerous in vitro studies suggest that HIV-associated neuronal damage prominently involves CXCR4 while CCR5 appears to play a dual role by mediating either toxic or protective effects depending on the available ligands (Hesselgesser et al., 1998; Meucci et al., 1998; Kaul and Lipton, 1999; Zheng et al., 1999; Meucci et al., 2000; Kaul et al., 2007; Maung et al., 2014). Besides intact HIV-1, picomolar concentrations of isolated viral envelope gp120 suffice to trigger CXCR4 and CCR5 receptors to cause injury and death of neurons of humans and rodents (Brenneman et al., 1988; Hesselgesser et al., 1998; Meucci et al., 1998; Kaul and Lipton, 1999; Ohagen et al., 1999; Chen et al., 2002; Garden et al., 2004; Iskander et al., 2004; Walsh et al., 2004; O’Donnell et al., 2006; Kaul et al., 2007). In addition to the Env glycoprotein gp120, ample experimental evidence demonstrates that various other viral proteins such as Tat, Nef, Vpr, and gp41, the membrane anchor of gp120, have the potential to cause neuronal injury and death (Brenneman et al., 1988; Adamson et al., 1996; New et al., 1997; Piller et al., 1998; Koedel et al., 1999; Kaul et al., 2001; Kaul et al., 2005; Mattson et al., 2005; Ellis et al., 2007; Saylor et al., 2016; Langford et al., 2018).
The mechanisms remain controversial of how exactly HIV-1 infection leads to neurocognitive and motor dysfunction and to neuronal injury and death (Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005; Kaul et al., 2005; Kramer-Hammerle et al., 2005; Mattson et al., 2005; Ellis et al., 2007; Smith et al., 2016). It is generally agreed upon that HIV-1 fails to infect post-mitotic, mature neurons but we and others observed more recently that HIV-1 proteins such as gp120 and Tat can compromise neurogenesis (Krathwohl and Kaiser, 2004; Okamoto et al., 2007; Schwartz et al., 2007; Kaul, 2008; Langford et al., 2018). All the studies, especially those addressing neurotoxicity, have contributed to the development of at least two different scenarios describing how HIV-1 causes brain injury and neurocognitive dysfunction: the “direct injury” and the “indirect’ or “bystander effect” hypothesis. The hypotheses are in no way mutually exclusive, and the available data suggest a role for both. The “direct injury” hypothesis poses that viral proteins directly damage neurons absent any contribution of non-neuronal cells (microglia/macrophages and/or astrocytes). Experiments supporting this scenario show that viral envelope protein gp120, Tat, and Vpr are toxic in serum free primary neuronal cultures (Meucci et al., 1998; Meucci et al., 2000) or in neuroblastoma cell lines (Hesselgesser et al., 1998; Piller et al., 1998; Mattson et al., 2005).
However, under conditions where glial and neuronal cells are present and which recapitulate the cellular composition of the brain, the indirect neurotoxicity mediated by macrophages and microglia may predominate (Giulian et al., 1990; Genis et al., 1992; Gartner, 2000; Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005; Kaul et al., 2005; Mattson et al., 2005; Medders et al., 2010; Maung et al., 2014). Intact HIV-1, gp120, and Tat seem to induce neuronal injury and apoptotic death predominantly in an indirect manner via the induction of soluble toxins from macrophages and microglia (Brenneman et al., 1988; Giulian et al., 1990; Kaul and Lipton, 1999; Chen et al., 2002; Garden et al., 2004; Iskander et al., 2004; Walsh et al., 2004; Sui et al., 2006; Medders et al., 2010; Gill et al., 2015).
HIV-1 infected or gp120-exposed microglia and macrophages produce factors that stimulate the N-methyl-D-aspartate-type receptor (NMDAR), an ionotropic glutamate and neurotransmitter receptor (Dreyer et al., 1990; Chen et al., 2002; O’Donnell et al., 2006). Under normal physiological conditions, activation of neuronal ionotropic glutamate receptors initiates a transient depolarization and excitation that serves a crucial role in neurocognitive function (Olney et al., 1991; Doble, 1999). However, excessive and/or extended stimulation of NMDARs leads to excitotoxicity through a sustained elevation of intracellular Ca2+ concentration, which subsequently compromises mitochondrial function and cellular energy metabolism and eventually results in the excessive production of free radicals (Olney, 1969; Doble, 1999; Kaul et al., 2001; Gill et al., 2014). A mild but sustained insult, such as macrophage toxins exert in HIV infection, eventually triggers programmed cell death (apoptosis) of neurons for which evidence has been found in post-mortem brains of HIVE/HAD patients (Petito and Roberts, 1995; Adle-Biassette et al., 1999; Kaul and Lipton, 1999; Ohagen et al., 1999). Neuronal apoptosis resulting from toxicity of HIV-1 or gp120 or Tat or a direct excitotoxic insult involves neuronal, intracellular Ca2+ overload, activation of p38 MAPK and p53, mitochondrial functional impairment with release of cytochrome c, activation of caspases, cell cycle protein and other molecules, such as apoptosis-inducing factor (AIF) from mitochondria, free radical formation, lipid release and peroxidation, and chromatin condensation (Tenneti et al., 1998; Asensio and Campbell, 1999; Kaul and Lipton, 1999; Garden et al., 2002; Haughey and Mattson, 2002; Jordan-Sciutto et al., 2002; Garden et al., 2004; Jana and Pahan, 2004; Kaul et al., 2007; Medders et al., 2010). Moreover, the activation of the unfolded protein response (UPR), amyloid precursor protein processing and changes of cellular lipid metabolism, including an increase in ceramide, sphingomyelin and hydroxynonenal have been linked to oxidative damage and cellular distress occurring in the pathways of HIV neurotoxicity (Haughey et al., 2004; Mattson et al., 2005; Lindl et al., 2007; Saylor et al., 2016; Stern et al., 2018b).
Antiretroviral drugs, neuropsychiatric adverse effects and HAND
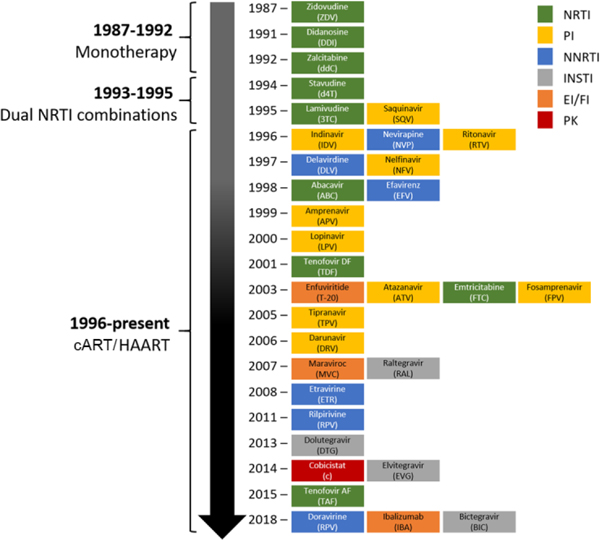
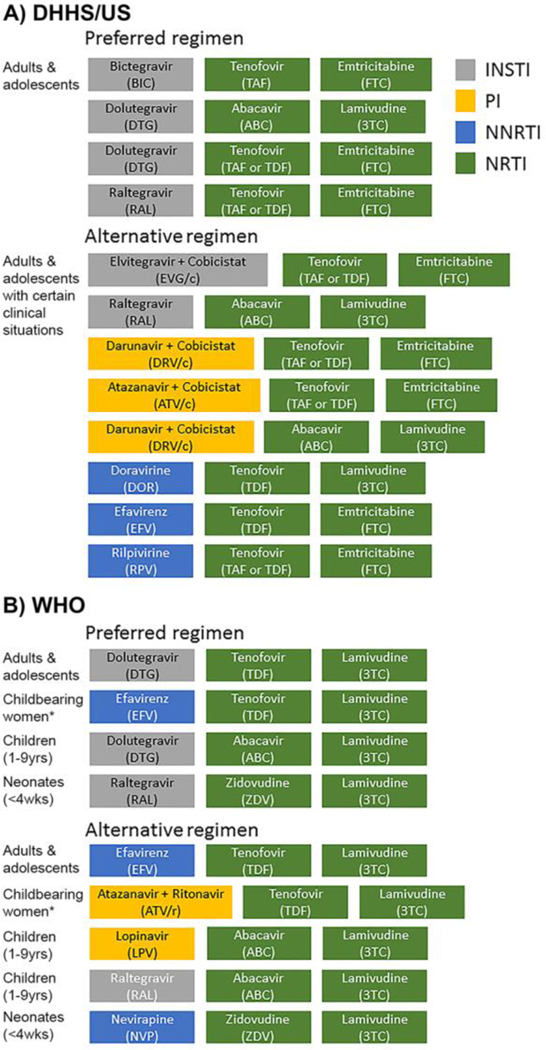
There are currently over 30 US FDA-approved ARVs utilized for the treatment of HIV infection that have become available since 1987 (Fig. 1). ARV drugs are classified based on the mechanism of action and currently include i) nucleoside/nucleotide reverse transcriptase inhibitors (NRTI), ii) non-nucleoside reverse transcriptase inhibitors (NNRTI), iii) protease inhibitors (PI), iv) integrase strand transfer inhibitors (INSTI), v) a fusion inhibitor (FI), and vi) two entry inhibitors (EI), one blocking chemokine receptor CCR5, the other inhibiting viral interaction with CD4. In addition, two drugs, ritonavir (RTV) and cobicistat (COBI) are used as pharmacokinetic enhancers (reviewed in (Badowski et al., 2016; Shah et al., 2016)). The first ARV was discovered in 1985 when a compound synthesized as a potential anti-cancer agent, 30-azido-20, 30-dideoxythymidine, now known under the name azidothymidine (AZT), showed in vitro inhibition of HIV replication (Mitsuya et al., 1985). AZT was eventually approved by the Food and Drug Administration (FDA) in the United States in 1987. Several more drugs of the same pharmacological class were developed and used as monotherapy and later in dual combination, however these treatment strategies frequently led to mutations of HIV which rendered the virus resistant to the drugs. The discovery of new classes of antiretrovirals and the introduction of cART in the mid-1990s eventually changed the course of HIV-1 infection/AIDS into a chronic but manageable disease when treatment is available (Vella et al., 2012). The Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents (a working group of the office of AIDS research) continues to update the combination regimens, utilizing more than 25 antiretroviral drugs in order to guide towards the best therapy for HIV+ individuals (http://aidsinfo.nih.gov/guidelines). Currently both the National Institutes of Health (NIH) DHHS Guidelines and the World Health Organization (WHO) recommend a cART regimen consisting of two NRTIs (usually abacavir/lamivudine (ABC/3TC), tenofovir alafenamide/emtricitabine (TAF/FTC) or tenofovir disoproxil fumarate/emtricitabine (TDF/FTC)) in combination with either an INSTI such as bictegravir (BIC), dolutegravir (DTG), or raltegravir (RAL) or an NNRTI, such as efavirenz (EFV) (DHHS, 2018; 2018). Whereas the DHHS has created separate guidelines targeting i) adults and adolescents, ii) perinatal, and iii) pediatric populations; the WHO has a single set of abridged guidelines directed towards all of these groups. The DHHS guidelines for adults and adolescents contain multiple preferred regimens for adults and adolescents with multiple alternative regimens in certain clinical situations, while the WHO guidelines have preferred first-line regimens, alternative first-line regimens, and first-line regimens for special situations. These recommendations are for patients first initiating ART or ARV-naïve patients as other factors must be considered in the management of treatment-experienced patients. The DHHS suggests alternative recommended regimens for adults and adolescents and the WHO proposes preferred first-line and alternative first-line regimens for multiple populations as illustrated in Figure 2A and 2B, respectively. ARV combinations and/or regimens are chosen considering primarily antiviral efficacy, potential adverse effects (toxicity), pill burden, drug-drug interaction, comorbid conditions, and cost. Efficient cART has transformed HIV-1 infection into a chronic condition, and infected individuals are approaching a near normal life span leading to aging with HIV as a new clinical phenomenon (Brew et al., 2009; Ciccarelli et al., 2011; Canizares et al., 2014; DeVaughn et al., 2015).
Fig. 1. Timeline of FDA approval of ARVs.
See main text for additional information on the various compounds.
Fig. 2. Antiretroviral treatment regimen.
A) DHHS guidelines, the representation is based on the Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed [4/23/2019] [F-5, Table 6a]; B) WHO recommendations, the representation is based on updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2018 (WHO/CDS/HIV/18.51). Licence: CC BY-NC-SA 3.0 IGO. *For recommendations for childbearing women see neuropsychiatric and developmental effects of ARV in tables 1 and 2. Modified from (Reust, 2011)
The life-saving effect of cART is indisputable. However, the incidence of cognitive impairment milder than HAD persists in HIV patients on cART (Robertson et al., 2007; Tozzi et al., 2007; McArthur et al., 2010; Simioni et al., 2010; Heaton et al., 2011; Saylor et al., 2016). This situation and distinct patterns of viral drug resistance in plasma and cerebrospinal fluid (CSF) compartments might at least in part be explained by limited penetration of HIV protease inhibitors and several of the nucleoside analogues into the brain (Cunningham et al., 2000; Kaul et al., 2005; Kramer-Hammerle et al., 2005). The development of the CNS penetration effectiveness (CPE) rank score by Letendre et al was largely based on available pharmacokinetic rather than pharmacodynamic data of ARVs (Letendre et al., 2008). This score has been well correlated to suppression of CSF viral loads. However, there have been conflicting reports on the effect of ARV CNS penetration on patient cognition. Some studies report improved cognition with higher CPE scores (Tozzi et al., 2009; Smurzynski et al., 2011; Carvalhal et al., 2016) while others observe cognitive benefits from lower CPE regimens (Marra et al., 2009; Kahouadji et al., 2013; Caniglia et al., 2014). The disparate results of these studies are confounded further by other studies which have found no significant relationship between CPE score and cognitive functioning (Robertson et al., 2012b; Ellis et al., 2014).
Multiple reports have meanwhile provided evidence that many, if not the majority of approved ARVs and clinically prescribed cART regimens have neuropsychiatric adverse effects; ranging from headache to insomnia, to depression and anxiety to suicidal ideation among other symptoms. Neuropsychiatric adverse effects reported by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents and CPE scores (if available) are summarized in Table 1 (DHHS, 2018). Among patients with HIV or AIDS, prevalence of major depression is reported to be between 22% to 45% (Atkinson et al., 1988; Maj et al., 1994; Perkins et al., 1994; McDaniel et al., 1995; Kelly et al., 1998) yet it is prospectively underdiagnosed (Evans et al., 1996; Halman, 2001). Therefore it is possible that pre-existing conditions can confound, exacerbate, or mask neuropsychiatric symptoms caused by ARVs. Mania, psychosis, and major depression are associated with HIV infection (Treisman et al., 1998). Furthermore, the impact of depression on HIV patient health can be significant and has been correlated with a more pronounced decline in the number of CD4+ cells, disease progression, and increased mortality (Safren et al., 2009). In an aging population of HIV patients, social isolation and depression are particularly common and may contribute to morbidity, mortality, poor medication adherence and retention in care (Kalichman et al., 2000; Grov et al., 2010). A summary of issues surrounding ART use specifically in the elderly population has been recently reviewed (Guaraldi et al., 2018).
Table 1. Neuropsychiatric adverse effects of ARV.
Summary of adverse effects reported by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents (DHHS, 2018) and CPE scores (if available) based on available clinical observations (Letendre et al., 2008). Other references mentioned in table: (Maxwell et al., 1988; Finlayson and Laing, 1998; de la Garza et al., 2001; Peyriere et al., 2001; Colebunders and Florence, 2002; Colebunders et al., 2002; Morlese et al., 2002; Puzantian, 2002; Wise et al., 2002; Foster et al., 2003; Lalezari, 2003; Moreno et al., 2003; Shah and Balderson, 2003; Foster et al., 2004; Hasse et al., 2005; Letendre et al., 2008; Toby, 2013; DHHS, 2018; Schomaker et al., 2018; Zash et al., 2018).
| Drug class | Drug | Acronym | CNS symptoms (DHHS, 2018) | CPE ranking (Letendre et al., 2008) |
|---|---|---|---|---|
| NRTI | Abacavir | ABC | Case reports include headache (Colebunders et al., 2002; Foster et al., 2004), depression, anxiety, auditory hallucinations (Colebunders et al., 2002), mutism, catatonia, homicidal behavior, persecutory delusions (Foster et al., 2003), night terrors (Foster et al., 2004) | 3 |
| NRTI | Emtricitabine | FTC | 3 | |
| NRTI | Lamivudi ne | 3TC | 2 | |
| NRTI | Stavudine | d4T | Peripheral neuropathy | 2 |
| NRTI | Tenofovir alafenami de | TAF | Headache | N/A |
| NRTI | Tenofovir disoproxil fumarate | TDF | Headache | 1 |
| NRTI | Zidovudine | ZDV | Headache, insomnia, confusion, agitation, mania, depression, myalgia. Case reports include delusions, auditory hallucinations (Maxwell et al., 1988), agitation, bizarre behavior, psychosis (1988), and mania (1988) | 4 |
| NNRTI | Doravirine | DOR | Sleep disorders and disturbances, dizziness, altered sensorium; depression and suicidality/self-harm | N/A |
| NNRTI | Efavirenz | EFV | Dizziness, abnormal dreams, headache,
confusion, stupor, impaired concentration, agitation, amnesia,
depersonalization, hallucinations, depression, suicidality, somnolence,
insomnia, neural tube birth defects, convulsions Case reports include irritability, aggression, antisocial behavior (Peyriere et al., 2001), excitability, anxiety (Peyriere et al., 2001), mental confusion (Peyriere et al., 2001), mania (Blanch et al., 2001; Shah and Balderson, 2003), disinhibition, grandiosity (Shah and Balderson, 2003), PTSD, intrusive recollections (Moreno et al., 2003), severe psychosis (Hasse et al., 2005), disorientation, paranoid delusions, violent behavior (de la Garza et al., 2001), anhedonia (Puzantian, 2002) |
3 |
| NNRTI | Nevirapine | NVP | Case reports of cognitive impairment, impulsive suicide attempts, persecutory delusions (Wise et al., 2002), and depression (Colebunders and Florence, 2002), vivid dreams (Morlese et al., 2002) | 4 |
| NNRTI | Rilpivirine | RPV | Depression, headache, suicidality, sleep disturbances | N/A |
| INSTI | Bictegravir | BIC | Headache, insomnia, depression, anxiety, suicidal ideation | N/A |
| INSTI | Dolutegravir | DTG | Headache, insomnia, depression, anxiety, suicidal ideation, neural tube birth defects (Schomaker et al., 2018; Zash et al., 2018) | N/A |
| INSTI | Elvitegravir | EVG | Headache, insomnia, depression, anxiety, suicidal ideation | N/A |
| INSTI | Raltegravir | RAL | Headache, insomnia, depression, anxiety, suicidal ideation | 3 |
| PI | Atazanavir | ATV | Case reports of headache, agitation, difficulty sleeping, disorientation, dizziness, tremulousness (Toby, 2013) | 2 |
| Atazanavir + ritonavir | ATV/r | 2 | ||
| PI | Darunavir | DRV | Headache | N/A |
| Darunavir + ritonavir | DRV/r | 3 | ||
| PI | Fosamprenavir | FPV | Headache | 2 |
| Fosamprenavir + ritonavir | FPV/r | 3 | ||
| PI | Indinavir | IDV | Headache, dizziness | 3 |
| Indinavir + ritonavir | IDV/r | 4 | ||
| PI | Lopinavir | LPV | N/A | |
| Lopinavir + ritonavir | LPV/r | 3 | ||
| PI | Saquinavir | SQV | Headache Case report of acute paranoid psychosis (Finlayson and Laing, 1998) |
1 |
| Saquinavir + ritonavir | SQV/r | 1 | ||
| PI | Tipranavir | TPV | Intracranial hemorrhage | N/A |
| Tipranavir + ritonavir | TPV/r | 1 | ||
| EI | Enfuvirtide | T-20 | Case reports of higher prevalence of neuropathy (Lalezari, 2003) | 1 |
| EI | Ibalizumab | IBA | Dizziness | N/A |
| EI | Maraviroc | MVC | Dizziness | 3 |
| PK | Cobicistat | c | N/A | |
| PI/PK | Ritonavir | RTV or r | 1 |
Neurotoxicity of antiretroviral drugs
Several studies observed that discontinuation of ARV use in HIV patients with controlled suppression of viral loads and neurocognitive impairment, resulted in significant improvement of cognitive function (Robertson et al., 2010; Evans et al., 2011; Evans et al., 2012; Underwood et al., 2015). In addition, clinical and experimental evidence is accumulating that at least some ARV compounds or combinations thereof (cART) have themselves neurotoxic effects and therefore possibly contribute to the development of HAND (Lewin et al., 1995; Carr, 2003; Keswani et al., 2003; Robertson et al., 2012a; Akay et al., 2014; Ma et al., 2016; Shah et al., 2016). Table 2 summarizes reports of neurotoxicity observed for ARV compounds.
Table 2. Neurotoxicity of ARV.
Summary of studies investigating neurotoxicity of clinically used ARV compounds. References mentioned in table: (O’Mahony et al., 2005; Pettersen et al., 2006; Grigorian et al., 2008; Manda et al., 2010; Giunta et al., 2011; Lisi et al., 2012; Robertson et al., 2012a; Tovar et al., 2012; Akay et al., 2014; Blas-Garcia et al., 2014; Brown et al., 2014; Purnell and Fox, 2014; Apostolova et al., 2015; Bertrand and Toborek, 2015; Demir and Laywell, 2015; Funes et al., 2015; Jin et al., 2016; Tricarico et al., 2016; Vivithanaporn et al., 2016; Ciavatta et al., 2017; Stern et al., 2018a; Lu et al., 2019)
| Drug class | Drug | Acronym | CNS symptoms | System | References |
|---|---|---|---|---|---|
| NRTI | Abacavir | ABC | ↓ MAP-2 at CSF concentrations, ↓ MAP-2 at therapeutic concentrations | Primary cultures rat forebrain | (Robertson et al., 2012a) |
| ↑ Neuronal beta-amyloid production | SweAPP N2a cells | (Giunta et al., 2011) | |||
| NRTI | Emtricitabine | FTC | (Robertson et al., 2012a) | ||
| NRTI | Lamivudine | 3TC | ↓ MAP-2 at therapeutic concentrations, ↓ MAP-2 at CSF concentrations | Primary cultures rat forebrain | (Robertson et al., 2012a) |
| ↑ Neuronal beta-amyloid production | SweAPP N2a cells | (Giunta et al., 2011) | |||
| NRTI | Stavudine | d4T | ↓ Neuronal viability at 100 x plasma concentrations | Primary rat cortical neuron culture | (Ciavatta et al., 2017) |
| NRTI | Tenofovir alafenamide | TAF | |||
| NRTI | Tenofovir disoproxil fumarate | TDF | ↓ MAP-2 at therapeutic concentrations | Primary cultures rat forebrain | (Robertson et al., 2012a) |
| ↓ Mitochondrial membrane potential, ↓ Cellular proliferation, ↑ Cellular apoptosis | PC-12 cell line | (Lu et al., 2019) | |||
| ↓ Dendritic length and branching at 3x plasma concentrations | Primary rat cortical neuron culture | (Ciavatta et al., 2017) | |||
| NRTI | Zidovudine Azidothymidine | ZDV AZT | Primary cultures rat forebrain | (Robertson et al., 2012a) | |
| primary rat cortical neuroglial cultures | (Akay et al., 2014) | ||||
| Primary rat mixed neuronal-glial cerebrocortic al cultures | (Sanchez et al., 2016) | ||||
| ↑ Neuronal beta-amyloid production | SweAPP N2a cells | (Giunta et al., 2011) | |||
| ↓ Proliferating NSCs | C57 BL/6 mice | (Demir and Laywell, 2015) | |||
| NNRTI | Doravirine | DOR | |||
| NNRTI | Efavirenz | EFV | ↓ MAP-2 at therapeutic concentrations, ↓ Mitochondrial membrane potential, ↓ Proliferating NSCs, ↓ Neuronal viability at 7x plasma concentrations, ↓ Neuronal respiration at 3x plasma concentrations, ↓ Neuronal excitability at 3x plasma concentrations, ↓ Mitochondrial respiration at 3x plasma concentrations, ↓ Dendritic length and branching at 3x plasma concentrations, ↓ Dendritic spines using EFV metabolites at CSF concentrations, ↑ Intraneuronal Ca2+ using EFV metabolites at CSF concentrations, ↓ Neuronal survival at CSF concentrations, ↓ Neuronal survival using EFV metabolites at CSF concentrations | Primary cultures rat forebrain | (Robertson et al., 2012a) |
| ↓ Proliferating NSCs, ↓ ATP stores, ↓ Mitochondrial membrane potential, ↑ Ph-p38 MAPK, ↑ Bax | Rat NSCs | (Jin et al., 2016) | |||
| ↓ BrdU+ area in SVZ, ↑ Caspase-3+ area in SVZ | C57BL/6J mice | ||||
| ↓ Neuronal viability at 7x plasma concentrations, ↓ Neuronal respiration at 3x plasma concentrations, ↓ Neuronal excitability at 3x plasma concentrations, ↓ Mitochondrial respiration at 3x plasma concentrations, ↓ Dendritic length and branching at 3x plasma concentrations | Primary rat cortical neuron culture | (Ciavatta et al., 2017), | |||
| ↓ Dendritic spines using EFV metabolites at CSF concentrations, ↑ Intraneuronal Ca2+ using EFV metabolites at CSF concentrations, ↓ Neuronal survival at CSF concentrations, ↓ Neuronal survival using EFV metabolites at CSF concentrations | Primary rat hippocampal neuronal cultures | (Tovar-y- Romo et al., 2012) | |||
| ↑ TNF-α in blood, ↑ IL-1β in blood, Spatial memory deficits | Wistar rats | (O’Mahony et al., 2005) | |||
| ↓ ATP production, ↑ Autophagy, ↑ Mitochondrial fragmentation, ↑ Mitochondrial depolarization, Changes in mitochondrial morphology | SH-SY5Y cells | (Purnell and Fox, 2014) | |||
| ↓ ATP production, ↑ Autophagy, Changes in mitochondrial morphology | Primary ratstriatal neurons | ||||
| ↑ LC3-II, ↑ CHOP, ↓ Mitochondrial membrane potential, ↑ Mitochondrial ROS | Primary rat cerebral cortex neurons | (Blas-Garcia et al., 2014) | |||
| ↑ Aβ1–42 protein, ↑ BACE-1 protein, ↑ ROS, ↓ ATP levels | SweAPP N2a cells | (Brown et al., 2014) | |||
| ↓ Microglial Aß1–42 phagocytosis | Primary mouse microglia | ||||
| ↑ Aβ1–42 protein in brain homogenate, ↑ BACE-1 protein | Tg2576 mice | ||||
| ↓ Mitochondrial respiration | SH-SY5Y cells | (Funes et al., 2015) | |||
| U-251MG cells | |||||
| ↑ NOS activation in glial cells, ↓ Mitochondrial respiration,↑ Mitochondrial ROS, ↓ Mitochondrial membrane potential, ↓ Activity of components in mitochondrial electron transport chain | SH-SY5Y cells | (Apostolova et al., 2015) | |||
| U-251MG cells | |||||
| ↓ IRElα protein, ↓ LC3bII protein, ↑ Intracellular Ca2+, ↓ Cytosolic PI3P levels, ↓ Association of Atg2a with Atg9, ↑ Sqstm1/p62 levels, Dysregulation of ER stress and autophagy | hCMEC/D3 cell line | (Bertrand and Toborek, 2015) | |||
| ↓ Autophagy | Primary human brain endothelial cells | ||||
| ↓ Autophagy | SVGP12 cells | ||||
| ↑ BiP protein,↓ LC3b protein | HIV transgenic mice | ||||
| NNRTI | Nevirapine | NVP | ↓ MAP-2 at CSF concentrations, ↓ MAP-2 at therapeutic concentrations | Primary cultures rat forebrain | (Robertson et al., 2012a) |
| Primary rat mixed neuronal-glial cerebrocortic al cultures | (Sanchez et al., 2016) | ||||
| NNRTI | Rilpivirine | RPV | |||
| INSTI | Bictegravir | BIC | |||
| INSTI | Dolutegravir | DTG | Primary rat cortical neuroglial cultures | (Stern et al., 2018a) | |
| INSTI | Elvitegravir | EVG | ↓ MAP-2 at CSF concentrations | Primary rat cortical neuroglial cultures | (Stern et al., 2018a) |
| INSTI | Raltegravir | RAL | ↑ IL-8 | Primary rat cortical neuroglial cultures | (Stern et al., 2018a) |
| PI | Atazanavir | ATV | ↓ MAP-2 at therapeutic concentrations, ↓ Mitochondrial membrane potential | Primary cultures rat forebrain | (Robertson et al., 2012a) |
| PI | Darunavir | DRV | Primary rat cortical neuroglial cultures | (Stern et al., 2018a) | |
| PI | Fosamprenavir | FPV | |||
| PI | Indinavir | IDV | ↑ Neuronal beta-amyloid production | SweAPP N2a cells | (Giunta et al., 2011) |
| ↑ ROS, ↓ Notch4 protein | Human cerebral endothelial cells | (Grigorian et al., 2008) | |||
| ↓ Neurite length, ↓ Neuronal processes, and ↓ Neuronal soma diameter in combination with HIV infection | Primary rat dosal root ganglion cells | (Pettersen et al., 2006) | |||
| PI | Lopinavir | LPV | ↓ MAP-2 at CSF concentrations | Primary rat cortical neuroglial cultures | (Stern et al., 2018a) |
| ↓ EAAT2 expression in astrocytes, ↓ Intracellular glutamate levels, ↓ PNCA and Ki-67 | Primary human fetal astrocytes | (Vivithanaporn et al., 2016) | |||
| ↓ Mitochondrial activity, ↑ ROS production, ↑ Apoptosis | SH-SY5Y cell line | (Tricarico et al., 2016) | |||
| PI | Saquinavir | SQV | ↓ MAP-2 at therapeutic concentrations | Primary rat cortical neuroglial cultures | (Akay et al., 2014) |
| Primary rat mixed neuronal-glial cerebrocortic al cultures | (Sanchez et al., 2016) | ||||
| ↑ ROS, ↓ Notch4 protein | Human cerebral endothelial cells | (Grigorian et al., 2008) | |||
| ↑ ROS, ↓ Notch4 protein, ↓ ZO-1 | Primary rat brain microvascular endothelial cells | (Manda et al., 2010) | |||
| ↑ ROS, ↓ Notch4 protein | Fischer-344 rats | ||||
| PI | Tipranavir | TPV | |||
| EI | Enfuvirtide | T-20 | |||
| EI | Ibalizumab | IBA | |||
| EI | Maraviroc | MVC | Primary cultures rat forebrain | (Robertson et al., 2012a) | |
| ↑ Microglial activation with co-administration of gp120 (CN54) and IFNγ | Primary enriched cultures of rat glial cells | (Lisi et al., 2012) | |||
| PK | Cobicistat | c | |||
| PI/PK | Ritonavir | RTV or r | ↓ MAP-2 at therapeutic concentrations, ↓ Mitochondrial membrane potential | Primary cultures rat forebrain | (Robertson et al., 2012a) |
| ↓ MAP-2 at therapeutic concentrations, ↓ Synaptophysin at therapeutic concentrations, ↑ Oxidative stress | Primary rat cortical neuroglial cultures | (Akay et al., 2014) | |||
| ↓ Mitochondrial activity, ↑ ROS production | SH-SY5Y cell line | (Tricarico et al., 2016) | |||
| ↑ ROS, ↓ Notch4 protein | Human cerebral endothelial cells | (Grigorian et al., 2008) | |||
| ↑ ROS | Primary rat brain microvascular endothelial cells | (Manda et al., 2010) |
NRTIs:
Early versions of these ARVs carry a high risk to trigger peripheral neuropathy, in particular didanosine (ddI), stavudine (d4T), and zalcitabine (ddC) (Dragovic and Jevtovic, 2003). Mitochondrial polymerase γ, an enzyme required to maintain mitochondrial DNA (mtDNA) in axons and Schwann cells seems to be the major undesired ‘off target’ (Dalakas, 2001; Venhoff et al., 2010). NRTIs that appear less likely to induce peripheral neuropathy or cellular neurotoxicity are emtricitabine (FTC), lamivudine (3TC), tenofovir (TAF or TDF) and abacavir (ABC) (Robertson et al., 2012a). The earliest antiretroviral drug ZDV/AZT has also been reported to cause mitochondrial toxicity, impaired neurogenesis and to damage neuronal dendrites and presynaptic terminals (Lewis and Dalakas, 1995; Ewings et al., 2000; Haik et al., 2000; Sanchez et al., 2016). Overall, NRTIs seem to exert limited CNS neurotoxicity that is compound- and cell-specific (Robertson et al., 2012a; Shah et al., 2016).
NNRTIs:
Rilpivirine (TMC278) and delavirdine (DLV) are considered to be non-toxic in the CNS (Shah et al., 2016). Etravirine (ETR) appeared so far to be clinically safe but signs of a potentially neurotoxic effect were observed in vitro (Robertson et al., 2012a; Floris-Moore et al., 2016). However, multiple lines of evidence indicate neurotoxicity of efavirenz (EFV) and nevirapine (NVP), which are approved by the FDA and still frequently used in NNRTI regimen because they are highly efficient antiretrovirals (http://aidsinfo.nih.gov/guidelines), (Robertson et al., 2012a; Brown et al., 2014; Funes et al., 2014). In the clinic, EFV has been found to be associated with deterioration of neurocognition (Ciccarelli et al., 2011; Decloedt and Maartens, 2013; Ma et al., 2016). In human neuron-like SHSY-5Y cells and primary rat striatal neurons EFV was found to cause a loss of ATP, depolarization and fragmentation of mitochondria and increased mitophagy and autophagy in general, indicating that interference with cellular energy homeostasis is a mechanism of toxicity (Blas-Garcia et al., 2014; Purnell and Fox, 2014). This ARV was also found to cause endoplasmic reticulum (ER) stress in human brain endothelial cells and in microvessels of the brain in HIV-transgenic mice (Bertrand and Toborek, 2015). This latter study reported that EFV compromised autophagy by binding to a complex consisting of beclin 1, ATG14 and phosphatidyl inositol 3 kinase III (PI3KIII), which is necessary for assembly of an autophagosome. An enzyme of the cytochrome P450 (CYP) family, CYP2B6, influences the metabolism of EFV and therefore presumably, the concentration in the brain. Thus, CYP2B6 may influence neurocognition as the 8-hydroxy metabolite of EFV has been found to be neurotoxic (Gatanaga et al., 2007; Decloedt and Maartens, 2013).
PIs:
This category of ARVs is very effective as antiretrovirals and part of numerous treatment regimens (Fig. 2 and Table 1). Saquinavir (SQV) and nelfinavir (NFV) are apparently well tolerated in HIV patients whereas ritonavir (RTV) can be associated with adverse and toxic effects (Bonfanti et al., 2000). PI, NTRI, INSTI and the CCR5 inhibitor maraviroc are all metabolized by enzymes of the CYP450 family, which plays crucial roles in the metabolism/activation/inactivation of most pharmaceutical drugs (Ingelman-Sundberg, 2004). Many human CYP450 enzymes are membrane-associated and located in the inner membrane of the mitochondria or in the endoplasmic reticulum of cells. The subclasses CYP2 and CYP3 are particularly important in the metabolism of drugs and steroids, which explains why polypharmacy in the form of cART generates a significant risk of drug-drug interactions. However, on occasion the interactions can be harnessed for therapeutic applications. RTV, previously used as an active PI antiretroviral, has been employed at low doses to boost mono- or triple therapy due to the drug’s ability to inhibit cytochrome P450 isoenzymes (Hsu et al., 1998; Gonzalez-Baeza et al., 2014). Other PIs have also been linked to neurotoxicity, including amprenavir (APV), indinavir (IDV) and atazanavir (ATV) (James et al., 2002; Pettersen et al., 2006; Vivithanaporn et al., 2016). A recent study found that in non-human primates, rodents, and in vitro neuroglial cell cultures, the PIs SQY and ATV in combination with the NRTI tenofovir (TDF) and an INSTI and RTV and SQV separately all triggered stress in the endoplasmic reticulum (ER), activated the β-site amyloid precursor protein cleaving enzyme-1 (BACE-1), and induced neuronal damage (Gannon et al., 2017).
INSTIs:
A number of reports have implicated raltegravir (RAL) and elvitegravir (EVG) in CNS toxicity because of neuropsychiatric adverse effects (Harris et al., 2008; Cohen et al., 2011; Teppler et al., 2011). Both bictegravir (BIC) and dolutegravir (DTG)containing regimens are supposed to provide the advantages of a low pill burden, high barrier to resistance, and better tolerability than alternative ARVs, but at least DTG is well recognized to cause neuropsychiatric adverse effects, and the WHO international pharmacovigilance database has reported neuropsychiatric events with all approved INSTIs (Table 1) (Kheloufi et al., 2017).
FI:
Enfuvirtide (T-20) is the only FDA approved fusion inhibitor and has not displayed any conclusive evidence for neurotoxicity. Although, some earlier reports suggested an increased prevalence of sensory neuropathy (Lalezari, 2003; Fung and Guo, 2004), others found no indications of toxicity (Lazzarin et al., 2003; Fung and Guo, 2004; Cherry et al., 2008).
EI:
The CCR5 blocker maraviroc (MVC) is the only FDA-approved synthetic molecule in its class. There is currently no evidence of neurotoxicity, and to the contrary, several studies found evidence for a neuroprotective effect of MVC (Boesecke and Pett, 2012; Garvey et al., 2012; Robertson et al., 2012a; Kelly et al., 2013; Maung et al., 2014). Ibalizumab is the first humanized monoclonal antibody recently approved by the FDA for treatment of HIV-1 infection, in particular for highly drug-resistant virus (Markham, 2018). Ibalizumab binds CD4 and blocks HIV entry into target cells while apparently largely preserving the proteins physiological function. The antibody is applied intravenously in the periphery and it is not clear how much of it reaches the CNS. The only current suggestion of a potential neuropsychiatric effect is dizziness in treated individuals (Markham, 2018).
The risks of neuropsychiatric polypharmacy created by cART as treatment for HIV-1 infection are frequently further complicated by the recreational use of psychostimulant drugs, such as methamphetamine (METH) (Urbina and Jones, 2004; Kapadia et al., 2005; Mitchell et al., 2006; Soontornniyomkij et al., 2016). Notably, drug abuse itself, including that of METH, increases the risk of contracting HIV-1 (Urbina and Jones, 2004; Kapadia et al., 2005; Mitchell et al., 2006), and METH using cART-treated HIV positive individuals often show elevated viral loads (Ellis et al., 2003; Hinkin et al., 2007). Under these conditions, the brain of a significant proportion of HIV patients is exposed to HIV-1, combinations of ARVs and psychostimulants at the same time, and METH-use results in more neurocognitive deficits, neuropathology and neuronal injury than either virus and psychostimulant alone (Langford et al., 2003a; Langford et al., 2003b; Chana et al., 2006; Cadet and Krasnova, 2007; Pang et al., 2012; Soontornniyomkij et al., 2016; Sanchez and Kaul, 2017). While we and others have been able to recapitulate in animal models some of the combined effects (Roberts et al., 2010; Pendyala et al., 2012; Bortell et al., 2015; Hoefer et al., 2015; Soontornniyomkij et al., 2016) the interplay of virus and psychostimulant and cART regimen is incompletely understood (Langford et al., 2003a; Cadet and Krasnova, 2007; Sanchez et al., 2016; Soontornniyomkij et al., 2016).
For that reason, our group investigated recently in vitro the potential contributions of the various factors to neuronal damage and loss (Sanchez et al., 2016). We exposed mixed neuronal-glial cerebrocortical cell cultures to ARVs of four different pharmacological categories (NRTI: AZT, NNRTI: NVP, PI: SQV and INSTI 118-D-24) in the presence or absence of METH, and HIV-1 envelope gp120, an established inducer of viral neurotoxicity. In order to assess acute, short-term and long-term effects, respectively, the cerebrocortical cells were incubated with the treatments for 24 h and 7 days. We observed that ARVs caused changes to neurites and presynaptic terminals predominantly during the 7-day exposure and alterations depended on specific compounds and their combinations with and without METH. In contrast to ARVs applied as single compounds, specific drug combinations with and without METH or viral envelope gp120 as well as METH and gp120 each alone, all significantly diminished neuronal ATP levels. Loss of ATP was associated with activation of adenosine monophosphate-activated protein kinase (AMPK) and autophagy, which, however, failed to restore neuronal ATP to normal levels. In contrast, boosting autophagy with rapamycin abrogated the lasting reduction of ATP during exposure to cART in the presence or absence of METH or gp120.
The findings of our investigation speak to the complexities of the overall effects of exposure to HIV and cART in the presence and absence of the psychostimulant METH (Sanchez et al., 2016). The ARVs/cART, HIVgp120 and METH triggered each alone and in combination a comparable loss of neuronal ATP but showed no additive effect. Also, ARVs/cART, METH or HIV/gp120 could each induce neuronal damage in terms of compromising neuronal dendrites and presynaptic terminals or depleting neuronal ATP or, in the case of gp120, causing the frank loss of neurons themselves when applied separately, but not necessarily when combined with ARVs. Distinct ARV combinations lacked a detectable effect on neuronal dendrites or presynaptic terminals in the presence or absence of METH, yet significantly reduced neuronal ATP levels. Thus, neurons managed to maintain their dendrites and presynaptic terminals despite compromised neuronal energy homeostasis, a scenario that may explain, at least in part, a slow, yet progressive neurological disease, such as HAND. Notably, METH, which itself is toxic to neurons (Cadet and Krasnova, 2007), lacked any detectable damaging effect on neurites and synapses in combination with several ARVs and even counteracted a significant loss of neuronal ATP in combination with cART. In contrast, long-term exposure to the admixture of four ARVs and psychostimulant compromised neuronal dendrites and synapses, an injury that others observed too, as a result of ARV treatment (Robertson et al., 2012a). Similarly, a loss of neuronal ATP occurred when four ARVs and METH were combined with HIV-1 gp120.
Our findings were in accordance with earlier reports that ARVs disturb mitochondrial function by altering mitochondrial membrane components, such as transporters, and mitochondrial bioenergetics, in particular membrane potential, by affecting mitochondrial kinases, such as Thymidine kinase 2 (TK2) and deoxyguanosine kinase (dGK) (Sun et al., 2014), and by inhibiting mitochondrial polymerase γ and consequently mtDNA homeostasis (Arnaudo et al., 1991; Cherry et al., 2002; Cote et al., 2002) or by stimulating major deletions in neuronal mtDNA (Apostolova et al., 2011; Zhang et al., 2014). Moreover, our results indicated that neuronal energy homeostasis could significantly change without precipitating obvious structural neuronal damage or loss. The mechanism regulating such neuronal adaptation may serve cellular robustness and resilience and remain to be elucidated.
Approaches to balancing viral suppression with neuropsychiatric adverse effects and neurotoxicity
In advancing the 90-90-90 world treatment targets launched by the UNAIDS in 2014, speed has been emphasized in terms of scaling-up and providing early initiation of HIV treatment. Accordingly, strides have been made in providing safe, tolerable, effective ARVs with higher barriers to resistance and lower pill burden in therapy regimes (DHHS, 2018). There has been an increasing interest in simplifying drug regimens of HIV patients from three drugs to two drug regimens. However, a recent study into this strategy discovered that the majority of these patients had a decline in neurocognition. Only patients switched to an INSTI/PI combination remained neurocognitively stable (Arendt et al., 2019).
With the popularization of commercialized direct-to-consumer genetic testing kits capable of detecting single nucleotide polymorphisms (SNPs), the information available to clinicians and the general public is higher than ever. The Precision Medicine Initiative, launched in 2015, is a data-driven field of medicine that attempts to take into account individual differences in people’s genes, microbiomes, environments, and lifestyles. Pharmacogenomic testing for HIV patients can be performed on several genes to guide selection of antiretroviral therapy (ART). Genotypic co-receptor tropism assays should be used if prescription of a CCR5 antagonist is being considered. Due to the ability of HIV to develop resistance to ARVs, genotypic and tropism testing is recommended at entry into care. The genotypic assay to assess mutations in the gp41 gene is associated with resistance to the fusion inhibitor enfuvirtide while phenotypic tropism assays can determine whether the virus is susceptible to CCR5 inhibition by maraviroc (DHHS, 2018). In prescribing the NRTI abacavir, the major histocompatibility complex class I allele HLA-B*5701 confers a 60% chance of a hypersensitivity reaction to the drug and genotyping for the gene has become standard clinical practice (Hetherington et al., 2002; Mallal et al., 2002; Saag et al., 2008; Novelli, 2010). In the case of efavirenz, CYP2B6 polymorphisms can result in poor metabolism thus increasing the plasma concentration of the drug and causing more severe CNS symptoms (Marzolini et al., 2001). The pharmacogenetics of ARVs has been reviewed previously (Tozzi, 2010; Pavlos and Phillips, 2012).
Polypharmacy and comorbidity is unavoidable in an aging population of HIV patients and raises the risk of drug-drug interactions. In fact, HIV infected individuals have an increased risk for cardiovascular disease, hepatic and renal disease, osteoporosis, in addition to mental, neurological and substance-use disorders (Esser et al., 2013; Brothers et al., 2014; Guaraldi et al., 2014; Achhra et al., 2015; Nedelcovych et al., 2017; Cook et al., 2018). The risk of adverse effects and outcomes increases with the increasing number of medications and nearly 50% of older adults take one or more medications that are not medically necessary (Maher et al., 2014). Drug to drug interactions can delay, decrease, or enhance absorption of drugs, thus decreasing or increasing their efficacy and causing adverse effects. For example, the ARVs atazanavir and rilpivirine require an acidic gastric pH for effective dissolution and absorption and antacids, H2 blockers, and proton pump inhibitors are likely to lower treatment efficacy (Luber, 2005; Beique et al., 2007; Crauwels et al., 2013). In contrast, ritonavir or cobicistat potently inhibit CYP3A and cause drugs metabolized through the CYP3A pathway, such as statins and antipsychotics, to accumulate (Fichtenbaum et al., 2002; Chauvin et al., 2013; DHHS, 2018). Opioid use among HIV-infected individuals is common with prescription rates as high as 53%, due to the prevalence of chronic pain caused by HIV infection, aging, and adverse effects of medications. This high proportion of opioid use in the HIV-infected population puts them at risk for addiction (Cunningham, 2018). The pharmacokinetics of both methadone and naloxone are effected by boosted protease inhibitors and certain NNRTIs (Clarke et al., 2001a, b; McCance-Katz et al., 2003; Scholler-Gyure et al., 2008; Sekar et al., 2011; Gruber et al., 2012; Bruce et al., 2013a; Bruce et al., 2013b; Bruce et al., 2013c; Crauwels et al., 2014). These extensive pharmacological interactions can be further compounded by coinfection with hepatitis C, a common comorbidity which the CDC estimates that approximately 25% of HIV patients in the US have. Co-infected individuals require complicated multi-drug antiretroviral therapy (Cope et al., 2015; Wyles, 2015; King and Menon, 2017). Furthermore, certain ARVs have been implicated in higher rates of comorbidities as a result of drug toxicity (Chow et al., 2003; Palacios et al., 2006; Guaraldi et al., 2014; Schouten et al., 2014). Highlighting this issue further, a recent study by Molas et al surveying 1259 HIV patients has found that 70% of patients receiving ART took co-medication with 44.7% having at least one clinically relevant potential drug-drug interaction (Molas et al., 2018). Individual differences in viral resistance and tropism as well as the patient’s pharmacogenetic and pharmacoecologic background highlight the importance of developing personalized drug regimes in an era of precision medicine.
In achieving active viral suppression and providing treatments with speed and early initiation, it is important to consider not only increasing lifespan but also preservation of quality of life. In addition to direct neurotoxicity, the neuropsychiatric adverse effects related to certain ARVs can lead to poor adherence, treatment interruptions, or change of drug regimens. These factors are confounded further by polypharmacy, viral resistance, and a patient’s pharmacogenetic and pharmacoecologic situation. Discerning the safety of strategies to increase cART penetration into the CNS may be a balancing act of suppressing viral replication while minimizing neurotoxicity, impairment of neurocognition and neuropsychiatric adverse effects.
Acknowledgments:
This work was supported by National Institutes of Health grants R01 MH087332, MH104131, MH105330, R03 DA02948 and P50 DA026306 (Translational Methamphetamine AIDS Research Center (TMARC), Project 5) to M.K.. The TMARC is affiliated with the University of California, San Diego (UCSD), University of California, Riverside (UCR) and the Sanford Burnham Prebys Medical Discovery Institute (SBP). The TMARC is comprised of: Director - Igor Grant, M.D.; Co-Directors - Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager - Mariana Cherner, Ph.D.; Assistant Center Manager - Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D., Virawudh Soontornniyomkij, M.D.; Administrative Coordinating Core (ACC) - Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC - Statistics Unit: Ian Abramson, Ph.D. (Unit Chief), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC - Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D., Jared Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Erin Morgan, Ph.D. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Disclosure
The authors state that they have no conflict of interest.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- Achhra AC, Mocroft A, Ross MJ, Ryom L, Lucas GM, Furrer H, Neuhaus J, Somboonwit C, Kelly M, Gatell JM, Wyatt CM, International Network for Strategic Initiatives in Global HIVTSSG (2015) Kidney disease in antiretroviral-naive HIV-positive adults with high CD4 counts: prevalence and predictors of kidney disease at enrolment in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 16 Suppl 1:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achim CL, Wang R, Miners DK, Wiley CA (1994) Brain viral burden in HIV infection. J Neuropathol Exp Neurol 53:284–294. [DOI] [PubMed] [Google Scholar]
- Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL (1996) Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science 274:1917–1921. [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Chrétien F, Wingertsmann L, Héry C, Ereau T, Scaravilli F, Tardieu M, Gray F (1999) Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol 25:123–133. [DOI] [PubMed] [Google Scholar]
- Akay C et al. (2014) Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol 20:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki A, Liu Y, Wigdahl B (2008) Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res 6:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA (1996) CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2005) Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 64:529–536. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2006) Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 111:529–538. [DOI] [PubMed] [Google Scholar]
- Antinori A et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort C (2017) Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 4:e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova N, Blas-Garcia A, Esplugues JV (2011) Mitochondrial toxicity in HAART: an overview of in vitro evidence. Current pharmaceutical design 17:2130–2144. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Funes HA, Blas-Garcia A, Alegre F, Polo M, Esplugues JV (2015) Involvement of nitric oxide in the mitochondrial action of efavirenz: a differential effect on neurons and glial cells. The Journal of infectious diseases 211:1953–1958. [DOI] [PubMed] [Google Scholar]
- Arendt G, Schlonies S, Orhan E, Stuve O (2019) Simplification of combination antiretroviral therapy (cART) and the brain-a real-life experience. J Neurovirol 25:174–182. [DOI] [PubMed] [Google Scholar]
- Arnaudo E, Dalakas M, Shanske S, Moraes CT, DiMauro S, Schon EA (1991) Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 337:508–510. [DOI] [PubMed] [Google Scholar]
- Asensio VC, Campbell IL (1999) Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci 22:504–512. [DOI] [PubMed] [Google Scholar]
- Atkinson JH Jr., Grant I, Kennedy CJ, Richman DD, Spector SA, McCutchan JA (1988) Prevalence of psychiatric disorders among men infected with human immunodeficiency virus. A controlled study. Arch Gen Psychiatry 45:859–864. [DOI] [PubMed] [Google Scholar]
- Badowski ME, Perez SE, Biagi M, Littler JA (2016) New Antiretroviral Treatment for HIV. Infect Dis Ther 5:329–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique L, Giguere P, la Porte C, Angel J (2007) Interactions between protease inhibitors and acid-reducing agents: a systematic review. HIV Med 8:335–345. [DOI] [PubMed] [Google Scholar]
- Bell JE (2004) An update on the neuropathology of HIV in the HAART era. Histopathology 45:549–559. [DOI] [PubMed] [Google Scholar]
- Bertrand L, Toborek M (2015) Dysregulation of Endoplasmic Reticulum Stress and Autophagic Responses by the Antiretroviral Drug Efavirenz. Mol Pharmacol 88:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blas-Garcia A, Polo M, Alegre F, Funes HA, Martinez E, Apostolova N, Esplugues JV (2014) Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: a comparison with efavirenz. J Antimicrob Chemother 69:2995–3000. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA (1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829–833. [DOI] [PubMed] [Google Scholar]
- Boesecke C, Pett SL (2012) Clinical studies with chemokine receptor-5 (CCR5)-inhibitors. Curr Opin HIV AIDS 7:456–462. [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Valsecchi L, Parazzini F, Carradori S, Pusterla L, Fortuna P, Timillero L, Alessi F, Ghiselli G, Gabbuti A, Di Cintio E, Martinelli C, Faggion I, Landonio S, Quirino T (2000) Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. J Acquir Immune Defic Syndr 23:236–245. [DOI] [PubMed] [Google Scholar]
- Bortell N, Morsey B, Basova L, Fox HS, Marcondes MC (2015) Phenotypic changes in the brain of SIV-infected macaques exposed to methamphetamine parallel macrophage activation patterns induced by the common gamma-chain cytokine system. Front Microbiol 6:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB (1988) Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature 335:639–642. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G (2009) Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol 4:163–174. [DOI] [PubMed] [Google Scholar]
- Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, Rockwood K (2014) Frailty in people aging with human immunodeficiency virus (HIV) infection. The Journal of infectious diseases 210:1170–1179. [DOI] [PubMed] [Google Scholar]
- Brown LA, Jin J, Ferrell D, Sadic E, Obregon D, Smith AJ, Tan J, Giunta B (2014) Efavirenz promotes beta-secretase expression and increased Abeta1-40,42 via oxidative stress and reduced microglial phagocytosis: implications for HIV associated neurocognitive disorders (HAND). PLoS ONE 9:e95500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Moody DE, Altice FL, Gourevitch MN, Friedland GH (2013a) A review of pharmacological interactions between HIV or hepatitis C virus medications and opioid agonist therapy: implications and management for clinical practice. Expert Rev Clin Pharmacol 6:249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Winkle P, Custodio JM, Wei LX, Rhee MS, Kearney BP, Ramanathan S, Friedland GH (2013b) The pharmacokinetic and pharmacodynamic interactions between buprenorphine/naloxone and elvitegravir/cobicistat in subjects receiving chronic buprenorphine/naloxone treatment. J Acquir Immune Defic Syndr 63:480–484. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Winkle P, Custodio JM, Wei X, Rhee MS, Kearney BP, Ramanathan S, Friedland GH (2013c) Investigation of the interactions between methadone and elvitegravir-cobicistat in subjects receiving chronic methadone maintenance. Antimicrob Agents Chemother 57:6154–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M (1991) Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254:423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN (2007) Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotox Res 12:181–204. [DOI] [PubMed] [Google Scholar]
- Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50:773–778. [DOI] [PubMed] [Google Scholar]
- Caniglia EC et al. (2014) Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 83:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canizares S, Cherner M, Ellis RJ (2014) HIV and aging: effects on the central nervous system. Semin Neurol 34:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A (2003) Toxicity of antiretroviral therapy and implications for drug development. Nat Rev Drug Discov 2:624–634. [DOI] [PubMed] [Google Scholar]
- Carvalhal A, Gill MJ, Letendre SL, Rachlis A, Bekele T, Raboud J, Burchell A, Rourke SB, Centre for Brain Health in HA (2016) Central nervous system penetration effectiveness of antiretroviral drugs and neuropsychological impairment in the Ontario HIV Treatment Network Cohort Study. J Neurovirol 22:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E (2006) Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology 67:1486–1489. [DOI] [PubMed] [Google Scholar]
- Chauvin B, Drouot S, Barrail-Tran A, Taburet AM (2013) Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet 52:815–831. [DOI] [PubMed] [Google Scholar]
- Chen W, Sulcove J, Frank I, Jaffer S, Ozdener H, Kolson DL (2002) Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway. J Virol 76:9407–9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry CL, Duncan AJ, Mackie KF, Wesselingh SL, Brew BJ (2008) A report on the effect of commencing enfuvirtide on peripheral neuropathy. AIDS Res Hum Retroviruses 24:1027–1030. [DOI] [PubMed] [Google Scholar]
- Cherry CL, Gahan ME, McArthur JC, Lewin SR, Hoy JF, Wesselingh SL (2002) Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. J Acquir Immune Defic Syndr 30:271–277. [DOI] [PubMed] [Google Scholar]
- Chow DC, Souza SA, Chen R, Richmond-Crum SM, Grandinetti A, Shikuma C (2003) Elevated blood pressure in HIV-infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials 4:411–416. [DOI] [PubMed] [Google Scholar]
- Chun TW, Fauci AS (1999) Latent reservoirs of HIV: obstacles to the eradication of virus. Proc Natl Acad Sci U S A 96:10958–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavatta VT, Bichler EK, Speigel IA, Elder CC, Teng SL, Tyor WR, Garcia PS (2017) In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochem Res 42:3220–3232. [DOI] [PubMed] [Google Scholar]
- Ciccarelli N, Fabbiani M, Di GS, Fanti I, Baldonero E, Bracciale L, Tamburrini E, Cauda R, De LA, Silveri MC (2011) Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 76:1403–1409. [DOI] [PubMed] [Google Scholar]
- Clarke SM, Mulcahy FM, Tjia J, Reynolds HE, Gibbons SE, Barry MG, Back DJ (2001a) Pharmacokinetic interactions of nevirapine and methadone and guidelines for use of nevirapine to treat injection drug users. Clin Infect Dis 33:1595–1597. [DOI] [PubMed] [Google Scholar]
- Clarke SM, Mulcahy FM, Tjia J, Reynolds HE, Gibbons SE, Barry MG, Back DJ (2001b) The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol 51:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Elion R, Ruane P, Shamblaw D, DeJesus E, Rashbaum B, Chuck SL, Yale K, Liu HC, Warren DR, Ramanathan S, Kearney BP (2011) Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. AIDS 25:F7–12. [DOI] [PubMed] [Google Scholar]
- Colebunders R, Florence E (2002) Neuropsychiatric reaction induced by clarithromycin. Sex Transm Infect 78:75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R, Hilbrands R, De Roo A, Pelgrom J (2002) Neuropsychiatric reaction induced by abacavir. Am J Med 113:616. [DOI] [PubMed] [Google Scholar]
- Collman RG, Perno CF, Crowe SM, Stevenson M, Montaner LJ (2003) HIV and cells of macrophage/dendritic lineage and other non-T cell reservoirs: new answers yield new questions. J Leukoc Biol 74:631–634. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Steigman PJ, Schwartz RM, Hessol NA, Milam J, Merenstein DJ, Anastos K, Golub ET, Cohen MH (2018) Prevalence, Comorbidity, and Correlates of Psychiatric and Substance Use Disorders and Associations with HIV Risk Behaviors in a Multisite Cohort of Women Living with HIV. AIDS Behav 22:3141–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope R, Pickering A, Glowa T, Faulds S, Veldkamp P, Prasad R (2015) Majority of HIV/HCV Patients Need to Switch Antiretroviral Therapy to Accommodate Direct Acting Antivirals. AIDS Patient Care STDS 29:379–383. [DOI] [PubMed] [Google Scholar]
- Cote HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, Ting L, Wong H, Harris M, Harrigan PR, O’Shaughnessy MV, Montaner JS (2002) Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 346:811–820. [DOI] [PubMed] [Google Scholar]
- Crauwels H, van Heeswijk RP, Stevens M, Buelens A, Vanveggel S, Boven K, Hoetelmans R (2013) Clinical perspective on drug-drug interactions with the non-nucleoside reverse transcriptase inhibitor rilpivirine. AIDS Rev 15:87–101. [PubMed] [Google Scholar]
- Crauwels HM, van Heeswijk RP, Vandevoorde A, Buelens A, Stevens M, Hoetelmans RM (2014) The effect of rilpivirine on the pharmacokinetics of methadone in HIV-negative volunteers. J Clin Pharmacol 54:133–140. [DOI] [PubMed] [Google Scholar]
- Cunningham CO (2018) Opioids and HIV Infection: From Pain Management to Addiction Treatment. Top Antivir Med 25:143–146. [PMC free article] [PubMed] [Google Scholar]
- Cunningham PH, Smith DG, Satchell C, Cooper DA, Brew B (2000) Evidence for independent development of resistance to HIV-1 reverse transcriptase inhibitors in the cerebrospinal fluid. AIDS 14:1949–1954. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ (2004) Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol 10:350–357. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ (2006) Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology 66:1447–1450. [DOI] [PubMed] [Google Scholar]
- Dalakas MC (2001) Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst 6:14–20. [DOI] [PubMed] [Google Scholar]
- de la Garza CL, Paoletti-Duarte S, Garcia-Martin C, Gutierrez-Casares JR (2001) Efavirenz-induced psychosis. AIDS 15:1911–1912. [DOI] [PubMed] [Google Scholar]
- Decloedt EH, Maartens G (2013) Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf 12:841–846. [DOI] [PubMed] [Google Scholar]
- Demir M, Laywell ED (2015) Neurotoxic effects of AZT on developing and adult neurogenesis. Front Neurosci 9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVaughn S, Muller-Oehring EM, Markey B, Bronte-Stewart HM, Schulte T (2015) Aging with HIV-1 Infection: Motor Functions, Cognition, and Attention - A Comparison with Parkinson’s Disease. Neuropsychol Rev 25:424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS (2018) Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. In: (Services DoHaH, ed). [Google Scholar]
- Doble A (1999) The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther 81:163–221:. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA (1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. [DOI] [PubMed] [Google Scholar]
- Dragovic G, Jevtovic D (2003) Nucleoside reverse transcriptase inhibitor usage and the incidence of peripheral neuropathy in HIV/AIDS patients. Antivir Chem Chemother 14:281–284. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA (1990) HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science 248:364–367. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Amedee A, Miller K, Doranz BJ, Endres M, Sharron M, Samson M, Lu ZH, Clements JE, Murphey-Corb M, Peiper SC, Parmentier M, Broder CC, Doms RW (1997) Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci U S A 94:4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisfeld C, Reichelt D, Evers S, Husstedt I (2013) CSF Penetration by Antiretroviral Drugs. CNS Drugs 27:31–55. [DOI] [PubMed] [Google Scholar]
- Ellis R, Letendre SL (2016) Update and New Directions in Therapeutics for Neurological Complications of HIV Infections. Neurotherapeutics 13:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8:33–44. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I (2003) Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. The Journal of infectious diseases 188:1820–1826. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, Abramson I, Thal LJ, Atkinson JH, Wallace MR, Grant I (1997) Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol 54:416–424. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Letendre S, Vaida F, Haubrich R, Heaton RK, Sacktor N, Clifford DB, Best BM, May S, Umlauf A, Cherner M, Sanders C, Ballard C, Simpson DM, Jay C, McCutchan JA (2014) Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis 58:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser S, Gelbrich G, Brockmeyer N, Goehler A, Schadendorf D, Erbel R, Neumann T, Reinsch N (2013) Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol 102:203–213. [DOI] [PubMed] [Google Scholar]
- Evans DL, Staab J, Ward H, Leserman J, Perkins DO, Golden RN, Petitto JM (1996) Depression in the medically ill: management considerations. Depress Anxiety 4:199–208. [DOI] [PubMed] [Google Scholar]
- Evans SR, Lee AJ, Ellis RJ, Chen H, Wu K, Bosch RJ, Clifford DB (2012) HIV peripheral neuropathy progression: protection with glucose-lowering drugs? J Neurovirol 18:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB (2011) Peripheral neuropathy in HIV: prevalence and risk factors. AIDS 25:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, Luthert P, Lantos P (1993) A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol 52:561–566. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL (1991) Neuronal loss in the frontal cortex in HIV infection.Lancet 337:1119–1121 [DOI] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E (2005) The shifting patterns of HIV encephalitis neuropathology. Neurotox Res 8:51–61. [DOI] [PubMed] [Google Scholar]
- Ewings EL, Gerschenson M, St Claire MC, Nagashima K, Skopets B, Harbaugh SW, Harbaugh JW, Poirier MC (2000) Genotoxic and functional consequences of transplacental zidovudine exposure in fetal monkey brain mitochondria. J Acquir Immune Defic Syndr 24:100–105. [DOI] [PubMed] [Google Scholar]
- Fauci AS (1999) The AIDS epidemic--considerations for the 21st century. N Engl J Med 341:1046–1050. [DOI] [PubMed] [Google Scholar]
- Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, Blaschke T, Alston B, Fang F, Kosel B, Aweeka F, Group NACT (2002) Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 16:569–577. [DOI] [PubMed] [Google Scholar]
- Finlayson JA, Laing RB (1998) Acute paranoid reaction to saquinavir. Am J Health Syst Pharm 55:2016–2017. [DOI] [PubMed] [Google Scholar]
- Floris-Moore MA, Mollan K, Wilkin AM, Johnson MA, Kashuba AD, Wohl DA, Patterson KB, Francis O, Kronk C, Eron JJ (2016) Antiretroviral activity and safety of once-daily etravirine in treatment-naive HIV-infected adults: 48-week results. Antivir Ther 21:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks TM, Kessler SW, Orenstein JM, Justement JS, Jaffe ES, Fauci AS (1988) Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science 242:919–922. [DOI] [PubMed] [Google Scholar]
- Foster R, Olajide D, Everall IP (2003) Antiretroviral therapy-induced psychosis: case report and brief review of the literature. HIV Med 4:139–144. [DOI] [PubMed] [Google Scholar]
- Foster R, Taylor C, Everall IP (2004) More on abacavir-induced neuropsychiatric reactions. AIDS 18:2449. [PubMed] [Google Scholar]
- Funes HA, Blas-Garcia A, Esplugues JV, Apostolova N (2015) Efavirenz alters mitochondrial respiratory function in cultured neuron and glial cell lines. J Antimicrob Chemother. [DOI] [PubMed] [Google Scholar]
- Funes HA, Apostolova N, Alegre F, Blas-Garcia A, Alvarez A, Marti-Cabrera M, Esplugues JV (2014) Neuronal Bioenergetics and Acute Mitochondrial Dysfunction: A Clue to Understanding the Central Nervous System Side Effects of Efavirenz. The Journal of infectious diseases. [DOI] [PubMed] [Google Scholar]
- Fung HB, Guo Y (2004) Enfuvirtide: a fusion inhibitor for the treatment of HIV infection. Clin Ther 26:352–378. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Akay-Espinoza C, Yee AC, Briand LA, Erickson MA, Gelman BB, Gao Y, Haughey NJ, Zink MC, Clements JE, Kim NS, Van De Walle G, Jensen BK, Vassar R, Pierce RC, Gill AJ, Kolson DL, Diehl JA, Mankowski JL, Jordan-Sciutto KL (2017) HIV Protease Inhibitors Alter Amyloid Precursor Protein Processing via betaSite Amyloid Precursor Protein Cleaving Enzyme-1 Translational Up-Regulation. Am J Pathol 187:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS (2004) HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J 18:1141–1143. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA (2002) Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci 22:4015–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S (2000) HIV infection and dementia. Science 287:602–604. [DOI] [PubMed] [Google Scholar]
- Garvey L, Nelson M, Latch N, Erlwein OW, Allsop JM, Mitchell A, Kaye S, Watson V, Back D, Taylor-Robinson SD, Winston A (2012) CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother 67:206–212. [DOI] [PubMed] [Google Scholar]
- Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, Fujimoto K, Sato I, Ueda M, Horiba M, Hamaguchi M, Yamamoto M, Takata N, Kimura A, Koike T, Gejyo F, Matsushita S, Shirasaka T, Kimura S, Oka S (2007) Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis 45:1230–1237. [DOI] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, . (1992) Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med 176:1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola ML, Lorenzini P, Balestra P, Larussa D, Baldini F, Corpolongo A, Narciso P, Bellagamba R, Tozzi V, Antinori A (2006) Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 41:332–337. [DOI] [PubMed] [Google Scholar]
- Gill AJ, Kovacsics CE, Vance PJ, Collman RG, Kolson DL (2015) Induction of Heme Oxygenase-1 Deficiency and Associated Glutamate-Mediated Neurotoxicity Is a Highly Conserved HIV Phenotype of Chronic Macrophage Infection That Is Resistant to Antiretroviral Therapy. J Virol 89:10656–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill AJ, Kovacsics CE, Cross SA, Vance PJ, Kolson LL, Jordan-Sciutto KL, Gelman BB, Kolson DL (2014) Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest 124:4459–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA (1990) Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science 250:1593–1596. [DOI] [PubMed] [Google Scholar]
- Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, Jin J, Fernandez F, Tan J, Shytle RD (2011) Antiretroviral medications disrupt microglial phagocytosis of beta-amyloid and increase its production by neurons: Implications for HIV associated neurocognitive disorders. Mol Brain 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 38:755–762. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Baeza A, Carvajal F, Bayon C, Perez-Valero I, Estebanez M, Bernardino JI, Monge S, Lagarde M, Hernando A, Arnalich F, Arribas JR (2014) Pattern of neurocognitive function in patients receiving boosted protease inhibitor monotherapy: a detailed neuropsychological study. J Neurovirol. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81. [DOI] [PubMed] [Google Scholar]
- Graus F, Ribalta T, Abos J, Alom J, Cruz-Sanchez F, Mallolas J, Miro JM, Cardesa A, Tolosa E (1990) Subacute cerebellar syndrome as the first manifestation of AIDS dementia complex. Acta Neurol Scand 81:118–120. [DOI] [PubMed] [Google Scholar]
- Gray LR, Cowley D, Welsh C, Lu HK, Brew BJ, Lewin SR, Wesselingh SL, Gorry PR, Churchill MJ (2016) CNS-specific regulatory elements in brain-derived HIV-1 strains affect responses to latency-reversing agents with implications for cure strategies. Mol Psychiatry 21:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19:407–411. [DOI] [PubMed] [Google Scholar]
- Grigorian A, Hurford R, Chao Y, Patrick C, Langford TD (2008) Alterations in the Notch4 pathway in cerebral endothelial cells by the HIV aspartyl protease inhibitor, nelfinavir. BMC Neurosci 9:27.:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE (2010) Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care 22:630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber VA, Rainey PM, Moody DE, Morse GD, Ma Q, Prathikanti S, Pade PA, Alvanzo AA, McCance-Katz EF (2012) Interactions between buprenorphine and the protease inhibitors darunavir-ritonavir and fosamprenavir-ritonavir. Clin Infect Dis 54:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Prakash M, Moecklinghoff C, Stellbrink HJ (2014) Morbidity in older HIV-infected patients: impact of long-term antiretroviral use. AIDS Rev 16:75–89. [PubMed] [Google Scholar]
- Guaraldi G, Pintassilgo I, Milic J, Mussini C (2018) Managing antiretroviral therapy in the elderly HIV patient. Expert Rev Clin Pharmacol:1–11. [DOI] [PubMed] [Google Scholar]
- Haik S, Gauthier LR, Granotier C, Peyrin JM, Lages CS, Dormont D, Boussin FD (2000) Fibroblast growth factor 2 up regulates telomerase activity in neural precursor cells. Oncogene 19:2957–2966. [DOI] [PubMed] [Google Scholar]
- Halman M (2001) Management of depression and related neuropyschiatric symptoms associated with HIV/AIDS and antiretroviral therapy. Can J Infect Dis 2001:9C–19C. [Google Scholar]
- Harris M, Larsen G, Montaner JS (2008) Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS 22:1890–1892. [DOI] [PubMed] [Google Scholar]
- Hasse B, Gunthard HF, Bleiber G, Krause M (2005) Efavirenz intoxication due to slow hepatic metabolism. Clin Infect Dis 40:e22–23. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP (2002) Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr 31 Suppl 2:S55–S61. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP (2004) Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol 55:257–267. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D (1997) CCR3 and CCR5 are coreceptors for HIV-1 infection of microglia. Nature 385:645–649. [DOI] [PubMed] [Google Scholar]
- Heaton RK et al. (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 60:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2010a) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2010b) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R (1998) Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol 8:595–598. [DOI] [PubMed] [Google Scholar]
- Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, Lai E, Davies K, Handley A, Dow DJ, Fling ME, Stocum M, Bowman C, Thurmond LM, Roses AD (2002) Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 359:1121–1122. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, Yergey JA, Mouradian MM, Sadler AE, Keilp J, . (1991) Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol 29:202–209. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Myers HF, Longshore D (2007) Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav 11:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, Resnick L, Felsenstein D, Andrews CA, Hirsch MS (1985) Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med 313:1493–1497. [DOI] [PubMed] [Google Scholar]
- Hoefer MM, Sanchez AB, Maung R, de Rozieres CM, Catalan IC, Dowling CC, Thaney VE, Pina-Crespo J, Zhang D, Roberts AJ, Kaul M (2015) Combination of methamphetamine and HIV-1 gp120 causes distinct long-term alterations of behavior, gene expression, and injury in the central nervous system. Exp Neurol 263:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A, Granneman GR, Bertz RJ (1998) Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet 35:275–291. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M (2004) Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci 25:193–200. [DOI] [PubMed] [Google Scholar]
- Iskander S, Walsh KA, Hammond RR (2004) Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CW, McNelis KC, Matalia MD, Cohen DM, Szabo S (2002) Central nervous system toxicity and amprenavir oral solution. Ann Pharmacother 36:174. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K (2004) Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci 24:9531–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Grimmig B, Izzo J, Brown LAM, Hudson C, Smith AJ, Tan J, Bickford PC, Giunta B (2016) HIV Non-Nucleoside Reverse Transcriptase Inhibitor Efavirenz Reduces Neural Stem Cell Proliferation in Vitro and in Vivo. Cell Transplant 25:1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Power C (2006) Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis 21:1–17. [DOI] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA (2002) Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. J Neurosci 22:2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahouadji Y, Dumurgier J, Sellier P, Lapalus P, Delcey V, Bergmann J, Hugon J, Paquet C (2013) Cognitive function after several years of antiretroviral therapy with stable central nervous system penetration score. HIV Med 14:311–315. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Heckman T, Kochman A, Sikkema K, Bergholte J (2000) Depression and thoughts of suicide among middle-aged and older persons living with HIV-AIDS. Psychiatr Serv 51:903–907. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Donahoe RM, Friedland G (2005) The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis 41:1027–1034. [DOI] [PubMed] [Google Scholar]
- Kaul M (2008) HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci 13:2484–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA (1999) Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A 96:8212–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA (2005) HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12 Suppl 1:878–892. [DOI] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA (2007) HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ 14:296–305. [DOI] [PubMed] [Google Scholar]
- Kelly B, Raphael B, Judd F, Perdices M, Kernutt G, Burrows GD, Burnett PC, Dunne M (1998) Psychiatric disorder in HIV infection. Aust N Z J Psychiatry 32:441–453. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Beck SE, Pate KA, Queen SE, Dorsey JL, Adams RJ, Avery LB, Hubbard W, Tarwater PM, Mankowski JL (2013) Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. AIDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Chander B, Hasan C, Griffin JW, McArthur JC, Hoke A (2003) FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann Neurol 53:57–64. [DOI] [PubMed] [Google Scholar]
- Ketzler S, Weis S, Haug H, Budka H (1990) Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol (Berl) 80:92–94. [DOI] [PubMed] [Google Scholar]
- Kheloufi F, Boucherie Q, Blin O, Micallef J (2017) Neuropsychiatric events and dolutegravir in HIV patients: a worldwide issue involving a class effect. AIDS 31:1775–1777. [DOI] [PubMed] [Google Scholar]
- King JR, Menon RM (2017) Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir: Drug Interactions With Antiretroviral Agents and Drugs forSubstance Abuse. Clin Pharmacol Drug Dev 6:201–205. [DOI] [PubMed] [Google Scholar]
- Koedel U, Kohleisen B, Sporer B, Lahrtz F, Ovod V, Fontana A, Erfle V, Pfister HW (1999) HIV type 1 Nef protein is a viral factor for leukocyte recruitment into the central nervous system. J Immunol 163:1237–1245. [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS (1986) Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089–1093. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R (2005) Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res 111:194–213. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL (2004) HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis 190:216–226. [DOI] [PubMed] [Google Scholar]
- Lalezari JP (2003) Clinical safety and efficacy of enfuvirtide (T-20), a new fusion inhibitor. A review of the presentation at the satellite symposium “New hope: advancing care in HIV infection” at the 15th annual Association of Nurses in AIDS Care conference, November 2002. AIDS Read 13:S9–13. [PubMed] [Google Scholar]
- Langford D, Oh Kim B, Zou W, Fan Y, Rahimain P, Liu Y, He JJ (2018) Doxycycline-inducible and astrocyte-specific HIV-1 Tat transgenic mice (iTat) as an HIV/neuroAIDS model. J Neurovirol 24:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E (2003a) Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr 34:467–474. [DOI] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Larrea GJ, Masliah E (2003b) Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol 13:195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarin A, Clotet B, Cooper D, Reynes J, Arasteh K, Nelson M, Katlama C, Stellbrink HJ, Delfraissy JF, Lange J, Huson L, DeMasi R, Wat C, Delehanty J, Drobnes C, Salgo M, Group TS (2003) Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med 348:2186–2195. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ (2008) Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin SR, Hoy J, Crowe SM, McDonald CF (1995) The role of bronchoscopy in the diagnosis and treatment of pulmonary disease in HIV-infected patients. Aust N Z J Med 25:133–139. [DOI] [PubMed] [Google Scholar]
- Lewis W, Dalakas MC (1995) Mitochondrial toxicity of antiviral drugs. Nat Med 1:417–422. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Akay C, Wang Y, White MG, Jordan-Sciutto KL (2007) Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathol Appl Neurobiol 33:658–669. [DOI] [PubMed] [Google Scholar]
- Lipton SA (1997) Treating AIDS dementia [letter; comment]. Science 276:1629–1630:. [DOI] [PubMed] [Google Scholar]
- Lisi L, Tramutola A, De LA, Navarra P, Dello RC (2012) Modulatory effects of the CCR5 antagonist maraviroc on microglial pro-inflammatory activation elicited by gp120. J Neurochem 120:106–114. [DOI] [PubMed] [Google Scholar]
- Lu M, Dong H, Bao D, Liu B, Liu H (2019) Tenofovir disoproxil fumarate induces pheochromocytoma cells apoptosis. Eur J Pharmacol 844:139–144. [DOI] [PubMed] [Google Scholar]
- Luber AD (2005) Use of acid-reducing agents in protease inhibitor-based HAART and the potential for negative treatment outcomes. AIDS Read 15:692–695, 698–700. [PubMed] [Google Scholar]
- Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, Morgello S, Simpson DM, Heaton RK, Grant I, Letendre SL, Group C (2016) Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol 22:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlen J, Dunlop O, Liestol K, Dobloug JH, Goplen AK, Torvik A (1995) Changing incidence of HIV-induced brain lesions in Oslo, 1983–1994: effects of zidovudine treatment. AIDS 9:1165–1169. [PubMed] [Google Scholar]
- Maher RL, Hanlon J, Hajjar ER (2014) Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 13:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, Satz P, Janssen R, Zaudig M, Starace F, D’Elia L, Sughondhabirom B, Mussa M, Naber D, Ndetei D, et al. (1994) WHO Neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch Gen Psychiatry 51:5161. [DOI] [PubMed] [Google Scholar]
- Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, James I, Christiansen FT (2002) Association between presence of HLAB*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 359:727–732. [DOI] [PubMed] [Google Scholar]
- Manda VK, Mittapalli RK, Geldenhuys WJ, Lockman PR (2010) Chronic exposure to nicotine and saquinavir decreases endothelial Notch-4 expression and disrupts blood-brain barrier integrity. J Neurochem 115:515–525. [DOI] [PubMed] [Google Scholar]
- Markham A (2018) Ibalizumab: First Global Approval. Drugs 78:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, Ellis RJ, Rodriguez B, Coombs RW, Schifitto G, McArthur JC, Robertson K, Team ACTGS (2009) Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 23:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T (2001) Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71–75. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I (1997) Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV Neurobehavioral Research Center. Ann Neurol 42:963–972. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A (2005) Cell death in HIV dementia. Cell Death Differ 12 Suppl 1:893–904. [DOI] [PubMed] [Google Scholar]
- Maung R, Hoefer MM, Sanchez AB, Sejbuk NE, Medders KE, Desai MK, Catalan IC, Dowling CC, de Rozieres CM, Garden GA, Russo R, Roberts AJ, Williams R, Kaul M (2014) CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J Immunol 193:1895–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S, Scheftner WA, Kessler HA, Busch K (1988) Manic syndrome associated with zidovudine treatment. JAMA 259:3406–3407. [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 67:699–714. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N (2003) Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol 9:205–221. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. (1993) Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 43:2245–2252. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Friedland G, Jatlow P (2003) The protease inhibitor lopinavir-ritonavir may produce opiate withdrawal in methadone-maintained patients. Clin Infect Dis 37:476–482. [DOI] [PubMed] [Google Scholar]
- McDaniel JS, Fowlie E, Summerville MB, Farber EW, Cohen-Cole SA (1995) An assessment of rates of psychiatric morbidity and functioning in HIV disease. Gen Hosp Psychiatry 17:346–352. [DOI] [PubMed] [Google Scholar]
- Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M (2010) Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol 185:4883–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ (2000) Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A 97:8075–8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ (1998) Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A 95:14500–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Meucci O (1999) AIDS and the brain: is there a chemokine connection? Trends Neurosci 22:471–479. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Morris SR, Kent CK, Stansell J, Klausner JD (2006) Methamphetamine use and sexual activity among HIV-infected patients in care--San Francisco, 2004. AIDS Patient Care STDS 20:502–510. [DOI] [PubMed] [Google Scholar]
- Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S (1985) 3’-Azido-3’-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A 82:7096–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molas E, Luque S, Retamero A, Echeverria-Esnal D, Guelar A, Montero M, Guerri R, Sorli L, Lerma E, Villar J, Knobel H (2018) Frequency and severity of potential drug interactions in a cohort of HIV-infected patients Identified through a Multidisciplinary team. HIV Clin Trials 19:1–7. [DOI] [PubMed] [Google Scholar]
- Moreno A, Labelle C, Samet JH (2003) Recurrence of post-traumatic stress disorder symptoms after initiation of antiretrovirals including efavirenz: a report of two cases. HIV Med 4:302–304. [DOI] [PubMed] [Google Scholar]
- Morlese JF, Qazi NA, Gazzard BG, Nelson MR (2002) Nevirapine-induced neuropsychiatric complications, a class effect of non-nucleoside reverse transcriptase inhibitors? AIDS 16:1840–1841. [DOI] [PubMed] [Google Scholar]
- Nath A (2015) Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol 21:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Sacktor N (2006) Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Curr Opin Neurol 19:358–361. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW (1986) The AIDS dementia complex: I. Clinical features. Ann Neurol 19:517–524. [DOI] [PubMed] [Google Scholar]
- Nedelcovych MT, Manning AA, Semenova S, Gamaldo C, Haughey NJ, Slusher BS (2017) The Psychiatric Impact of HIV. ACS Chem Neurosci 8:1432–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenburg JK, Brodt HR, Herndier BG, Bickel M, Bacchetti P, Price RW, Grant RM, Schlote W (2002) HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 31:171–177. [DOI] [PubMed] [Google Scholar]
- New DR, Ma M, Epstein LG, Nath A, Gelbard HA (1997) Human immunodeficiency virus type 1 tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol 3:168–173:. [DOI] [PubMed] [Google Scholar]
- Novelli G (2010) Personalized genomic medicine. Intern Emerg Med 5 Suppl 1:S81–90. [DOI] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL (2006) Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci 26:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Myint AM, Steinbusch H, Leonard BE (2005) Efavirenz induces depressive-like behaviour, increased stress response and changes in the immune response in rats. Neuroimmunomodulation 12:293–298. [DOI] [PubMed] [Google Scholar]
- Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B (1996) The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382:833–835. [DOI] [PubMed] [Google Scholar]
- Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D (1999) Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol 73:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA (2007) HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell 1:230–236. [DOI] [PubMed] [Google Scholar]
- Olney JW (1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164:719–721. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA (1991) NMDA antagonist neurotoxicity: mechanism and prevention. Science 254:1515–1518. [DOI] [PubMed] [Google Scholar]
- Palacios R, Santos J, Garcia A, Castells E, Gonzalez M, Ruiz J, Marquez M (2006) Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients. A prospective study in a cohort of naive patients. HIV Med 7:10–15. [DOI] [PubMed] [Google Scholar]
- Pang T, Wang J, Benicky J, Sanchez-Lemus E, Saavedra JM (2012) Telmisartan directly ameliorates the neuronal inflammatory response to IL-1beta partly through the JNK/c-Jun and NADPH oxidase pathways. J Neuroinflammation 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Fauci AS (1995) Apoptosis in HIV infection. Nat Med 1:118–120. [DOI] [PubMed] [Google Scholar]
- Pavlos R, Phillips EJ (2012) Individualization of antiretroviral therapy. Pharmgenomics Pers Med 5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, Gisslen M, Angoff N, Price RW, Cinque P, Spudich S (2012) Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 26:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala G, Buescher JL, Fox HS (2012) Methamphetamine and inflammatory cytokines increase neuronal Na+/K+-ATPase isoform 3: relevance for HIV associated neurocognitive disorders. PLoS ONE 7:e37604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Stern RA, Golden RN, Murphy C, Naftolowitz D, Evans DL (1994) Mood disorders in HIV infection: prevalence and risk factors in a nonepicenter of the AIDS epidemic. Am J Psychiatry 151:233–236. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B (1995) Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol 146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Cho ES, Lemann W, Navia BA, Price RW (1986) Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol 45:635–646. [DOI] [PubMed] [Google Scholar]
- Pettersen JA, Jones G, Worthington C, Krentz HB, Keppler OT, Hoke A, Gill MJ, Power C (2006) Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol 59:816–824. [DOI] [PubMed] [Google Scholar]
- Peyriere H, Mauboussin JM, Rouanet I, Fabre J, Reynes J, Hillaire-Buys D (2001) Management of sudden psychiatric disorders related to efavirenz. AIDS 15:1323–1324. [DOI] [PubMed] [Google Scholar]
- Piller SC, Jans P, Gage PW, Jans DA (1998) Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc Natl Acad Sci U S A 95:4595–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piot P, Bartos M, Ghys PD, Walker N, Schwartlander B (2001) The global impact of HIV/AIDS. Nature 410:968–973. [DOI] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P (1988) The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 239:586–592. [DOI] [PubMed] [Google Scholar]
- Purnell PR, Fox HS (2014) Efavirenz induces neuronal autophagy and mitochondrial alterations. J Pharmacol Exp Ther 351:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzantian T (2002) Central nervous system adverse effects with efavirenz: case report and review. Pharmacotherapy 22:930–933. [DOI] [PubMed] [Google Scholar]
- Reust CE (2011) Common adverse effects of antiretroviral therapy for HIV disease. Am Fam Physician 83:1443–1451. [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R (1991) Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol (Berl) 82:39–44. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Maung R, Sejbuk NE, Ake C, Kaul M (2010) Alteration of Methamphetamine-induced stereotypic behaviour in transgenic mice expressing HIV-1 envelope protein gp120. J Neurosci Methods 186:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB (2012a) Antiretroviral neurotoxicity. J Neurovirol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K et al. (2012b) Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis 55:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, Skiest DJ (2010) Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 74:1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ (2007) The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21:1915–1921. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Monti L, Yiannoutsos C, Wu J, Bell J, Hedreen J, Navia BA (2000) NFkappaB activation, TNF-alpha expression, and apoptosis in the AIDS-Dementia-Complex. J Neurovirol 6:537–543. [DOI] [PubMed] [Google Scholar]
- Rothenberger MK et al. (2015) Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 112:E1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, Stancil B, Mosteller M, Brothers C, Wannamaker P, Hughes A, Sutherland-Phillips D, Mallal S, Shaefer M, Study of Hypersensitivity to A, Pharmacogenetic Evaluation Study T (2008) High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis 46:1111–1118. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC (2001) HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology 56:257–260. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L (2002) HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 8:136–142. [DOI] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, Mayer KH (2009) A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol 28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AB, Kaul M (2017) Neuronal Stress and Injury Caused by HIV-1, cART and Drug Abuse: Converging Contributions to HAND. Brain Sci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AB, Varano GP, de Rozieres CM, Maung R, Catalan IC, Dowling CC, Sejbuk NE, Hoefer MM, Kaul M (2016) Antiretrovirals, Methamphetamine, and HIV-1 Envelope Protein gp120 Compromise Neuronal Energy Homeostasis in Association with Various Degrees of Synaptic and Neuritic Damage. Antimicrob Agents Chemother 60:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD (1997) Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med 185:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder-pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler-Gyure M, van den Brink W, Kakuda TN, Woodfall B, De Smedt G, Vanaken H, Stevens T, Peeters M, Vandermeulen K, Hoetelmans RM (2008) Pharmacokinetic and pharmacodynamic study of the concomitant administration of methadone and TMC125 in HIV-negative volunteers. J Clin Pharmacol 48:322–329. [DOI] [PubMed] [Google Scholar]
- Schomaker M, Davies MA, Cornell M, Ford N (2018) Assessing the risk of dolutegravir for women of childbearing potential. Lancet Glob Health 6:e958–e959. [DOI] [PubMed] [Google Scholar]
- Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, Prins M, Reiss P, Group AGCS (2014) Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 59:1787–1797. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DM, Hazra R, Major EO (2007) Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol 13:274–283. [DOI] [PubMed] [Google Scholar]
- Sekar V, Tomaka F, Lefebvre E, De Pauw M, Vangeneugden T, van den Brink W, Hoetelmans R (2011) Pharmacokinetic interactions between darunavir/ritonavir and opioid maintenance therapy using methadone or buprenorphine/naloxone. J Clin Pharmacol 51:271–278. [DOI] [PubMed] [Google Scholar]
- Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A (2016) Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotox Res 30:677–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MD, Balderson K (2003) A manic episode associated with efavirenz therapy for HIV infection. AIDS 17:1713–1714. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V (2005) Circulating proviral HIV DNA and HIV-associated dementia. AIDS 19:4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Ratto-Kim S, Sithinamsuwan P, Nidhinandana S, Thitivichianlert S, Watt G, Desouza M, Chuenchitra T, Sukwit S, Chitpatima S, Robertson K, Paul R, Shikuma C, Valcour V (2006) HIV DNA and Dementia in Treatment-Naive HIV-1-Infected Individuals in Bangkok, Thailand. Int J Med Sci 4:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Rodrigues BS, Micheletti AM, Tostes S Jr., Meneses AC, Silva-Vergara ML, Adad SJ (2012) Neuropathology of AIDS: An Autopsy Review of 284 Cases from Brazil Comparing the Findings Pre- and Post-HAART (Highly Active Antiretroviral Therapy) and Pre- and Postmortem Correlation. AIDS Res Treat 2012:186850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24:1243–1250. [DOI] [PubMed] [Google Scholar]
- Simmons G, Wilkinson D, Reeves JD, Dittmar MT, Beddows S, Weber J, Carnegie G, Desselberger U, Gray PW, Weiss RA, Clapham PR (1996) Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol 70:8355–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DM, Haidich AB, Schifitto G, Yiannoutsos CT, Geraci AP, McArthur JC, Katzenstein DA (2002) Severity of HIV-associated neuropathy is associated with plasma HIV-1 RNA levels. AIDS 16:407–412. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Auwaerter P, Knowlton A, Saylor D, McArthur J (2016) Treatment of human immunodeficiency virus-related peripheral neuropathy with Scrambler Therapy: a case report. Int J STD AIDS. [DOI] [PubMed] [Google Scholar]
- Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R (2011) Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 25:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Kesby JP, Morgan EE, Bischoff-Grethe A, Minassian A, Brown GG, Grant I, Translational Methamphetamine ARCG (2016) Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. J Neuroimmune Pharmacol 11:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AL, Lee RN, Panvelker N, Li J, Harowitz J, Jordan-Sciutto KL, Akay-Espinoza C (2018a) Differential Effects of Antiretroviral Drugs on Neurons In Vitro: Roles for Oxidative Stress and Integrated Stress Response. J Neuroimmune Pharmacol 13:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AL, Ghura S, Gannon PJ, Akay-Espinoza C, Phan JM, Yee AC, Vassar R, Gelman BB, Kolson DL, Jordan-Sciutto KL (2018b) BACE1 Mediates HIV-Associated and Excitotoxic Neuronal Damage Through an APP-Dependent Mechanism. J Neurosci 38:4288–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Fan S, Sniderhan L, Reisinger E, Litzburg A, Schifitto G, Gelbard HA, Dewhurst S, Maggirwar SB (2006) Inhibition of mixed lineage kinase 3 prevents HIV-1 Tat-mediated neurotoxicity and monocyte activation. J Immunol 177:702–711. [DOI] [PubMed] [Google Scholar]
- Sun R, Eriksson S, Wang L (2014) Down-regulation of mitochondrial thymidine kinase 2 and deoxyguanosine kinase by didanosine: implication for mitochondrial toxicities of anti-HIV nucleoside analogs. Biochemical and biophysical research communications 450:1021–1026. [DOI] [PubMed] [Google Scholar]
- Sung JM, Margolis DM (2018) HIV Persistence on Antiretroviral Therapy and Barriers to a Cure. Adv Exp Med Biol 1075:165–185. [DOI] [PubMed] [Google Scholar]
- Tenneti L, D’Emilia DM, Troy CM, Lipton SA (1998) Role of caspases in N-methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem 71:946–959. [DOI] [PubMed] [Google Scholar]
- Teppler H, Brown DD, Leavitt RY, Sklar P, Wan H, Xu X, Lievano F, Lehman HP, Mast TC, Nguyen BY (2011) Long-term safety from the raltegravir clinical development program. Curr HIV Res 9:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toby M (2013) Atazanavir Causing CNS Toxicity? Unexplained Neurological Symptoms in Two Patients Recently Started on Atazanavir. J AIDS Clin Res 4:196. [Google Scholar]
- Tovar YRL, Bumpus NN, Pomerantz D, Avery LB, Sacktor N, McArthur JC, Haughey NJ (2012) Dendritic spine injury induced by the 8-hydroxy metabolite of Efavirenz. J Pharmacol Exp Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V (2010) Pharmacogenetics of antiretrovirals. Antiviral Res 85:190–200. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Galgani S, Narciso P, Sampaolesi A, Antinori A, Giulianelli M, Serraino D, Ippolito G (2001) Changes in neurocognitive performance in a cohort of patients treated with HAART for 3 years. J Acquir Immune Defic Syndr 28:19–27. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Salvatori MF, Vlassi C, Liuzzi G, Giancola ML, Giulianelli M, Narciso P, Antinori A (2009) Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr 52:56–63. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45:174–182. [DOI] [PubMed] [Google Scholar]
- Treisman G, Fishman M, Schwartz J, Hutton H, Lyketsos C (1998) Mood disorders in HIV infection. Depress Anxiety 7:178–187. [DOI] [PubMed] [Google Scholar]
- Tricarico PM, de Oliveira Franca RF, Pacor S, Ceglia V, Crovella S, Celsi F (2016) HIV Protease Inhibitors Apoptotic Effect in SH-SY5Y Neuronal Cell Line. Cell Physiol Biochem 39:1463–1470. [DOI] [PubMed] [Google Scholar]
- Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP (1996) CD4-dependent, antibody-sensitive interactions between HIV-1 and its co- receptor CCR-5. Nature 384:184–187. [DOI] [PubMed] [Google Scholar]
- UNAIDS (2013) UNAIDS report on the global AIDS epidemic 2013. [Google Scholar]
- UNAIDS (2015) Global AIDS Response Progress Reporting 2015. [Google Scholar]
- UNAIDS (2018) UNAIDS DATA 2018. https://www.unaidsorg/en/resources/documents/2018/unaids-data-2018. [Google Scholar]
- Underwood J, Robertson KR, Winston A (2014) Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS. [DOI] [PubMed] [Google Scholar]
- Underwood J, Robertson KR, Winston A (2015) Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 29:253–261. [DOI] [PubMed] [Google Scholar]
- Urbina A, Jones K (2004) Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clin Infect Dis 38:890–894. [DOI] [PubMed] [Google Scholar]
- Vago L, Bonetto S, Nebuloni M, Duca P, Carsana L, Zerbi P, D’Arminio-Monforte A (2002) Pathological findings in the central nervous system of AIDS patients on assumed antiretroviral therapeutic regimens: retrospective study of 1597 autopsies. AIDS 16:1925–1928. [DOI] [PubMed] [Google Scholar]
- Vella S, Schwartlander B, Sow SP, Eholie SP, Murphy RL (2012) The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS 26:1231–1241. [DOI] [PubMed] [Google Scholar]
- Venhoff N, Lebrecht D, Deveaud C, Beauvoit B, Bonnet J, Muller K, Kirschner J, Venhoff AC, Walker UA (2010) Oral uridine supplementation antagonizes the peripheral neuropathy and encephalopathy induced by antiretroviral nucleoside analogues. AIDS 24:345–352. [DOI] [PubMed] [Google Scholar]
- Vivithanaporn P, Asahchop EL, Acharjee S, Baker GB, Power C (2016) HIV protease inhibitors disrupt astrocytic glutamate transporter function and neurobehavioral performance. AIDS 30:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KA, Megyesi JF, Wilson JX, Crukley J, Laubach VE, Hammond RR (2004) Antioxidant protection from HIV-1 gp120-induced neuroglial toxicity. J Neuroinflammation 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K, Morse A (2002) The clinical profile of end-stage AIDS in the era of highly active antiretroviral therapy. AIDS Patient Care STDS 16:75–81. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE (1997) Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol 74:1–8. [DOI] [PubMed] [Google Scholar]
- WHO (2018) Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. . In: (Organization WH, ed). Geneva: WHO/CDS/HIV/18.51. [Google Scholar]
- Wiley CA, Masliah E, Achim CL (1994) Measurement of CNS HIV burden and its association with neurologic damage. Adv Neuroimmunol 4:319–325. [DOI] [PubMed] [Google Scholar]
- Wise ME, Mistry K, Reid S (2002) Drug points: Neuropsychiatric complications of nevirapine treatment. BMJ 324:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EJ, Nunn M, Joseph J, Robertson K, Lal L, Brew BJ (2008) NeuroAIDS in the Asia Pacific Region. J Neurovirol 14:465–473. [DOI] [PubMed] [Google Scholar]
- Wyles DL (2015) Regimens for Patients Coinfected with Human Immunodeficiency Virus. Clin Liver Dis 19:689–706, vi-vii. [DOI] [PubMed] [Google Scholar]
- Zash R, Makhema J, Shapiro RL (2018) Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N Engl J Med 379:979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Song F, Gao Z, Ding W, Qiao L, Yang S, Chen X, Jin R, Chen D (2014) Long-term exposure of mice to nucleoside analogues disrupts mitochondrial DNA maintenance in cortical neurons. PLoS ONE 9:e85637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE (1999) Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol 98:185–200. [DOI] [PubMed] [Google Scholar]