Abstract
Neuregulin-1β (NRG-1) is a membrane-bound or secreted growth and differentiation factor that mediates its action by binding to ErbB receptors. Circulating levels of NRG-1 are characterized by large inter-individual variability with the range of absolute values covering two orders of magnitude, from hundreds to tens of thousands of picograms per milliliter of blood. NRG-1 signaling via ErbB receptors contributes to the cell survival and downregulation of the inflammatory response. A higher level of circulating NRG-1 may indicate increased shedding of membrane-bound NRG-1, which in turn can contribute to better protection against cardiovascular stress or injury. However, it is unknown whether circulating NRG-1 can induce activation of ErbB receptors.
In the current study, we performed an analysis of circulating NRG-1 functional activity using a cell-based ELISA measuring phosphorylation of ErbB3 induced by blood plasma obtained from healthy donors. We found high levels of ErbB3 activating activity in human plasma. No correlations were found between the levels of circulating NRG-1 and plasma ErbB3 activating activity. To determine the direct effect of circulating NRG-1, we incubated plasma with neutralizing antibody, which prevented the stimulatory effect of recombinant NRG-1 on activation of ErbB3. No effect of the neutralizing antibody was found on plasma-induced phosphorylation of ErbB3. We also found that a significant portion of circulating NRG-1 is comprised of full-length NRG-1 associated with large extracellular vesicles. Our results demonstrate that circulating NRG-1 does not contribute to plasma-induced ErbB3 activating activity and emphasizes the importance of functional testing of NRG-1 proteins in biological samples.
INTRODUCTION
Neuregulin-1β (NRG-1) plays an essential role in cardiac development 1–4 as well as in the regulation of tissue-protective and pro-survival processes in response to tissue injury in the adult cardiovascular system 5, 6. NRG-1 is expressed as a transmembrane growth factor 7. Full-length NRG-1 is not active; activation requires cleavage of an extracellular domain of NRG-1 by matrix metalloproteinases which can then bind to ErbB3 or ErbB4 receptors. Binding of NRG-1 to ErbB3 or ErbB4 receptors induces homo- and- heterodimer formation with each other or with ErbB2. Dimerization followed by tyrosine phosphorylation results in subsequent activation of downstream intracellular signaling which leads to cell protection and stimulation of proliferation 8–11.
NRG-1 is present in the circulation. The level of circulating NRG-1 is characterized by large inter-individual variability 12, 13. We and others have found that levels of NRG-1 protein vary over a wide range of concentrations, from hundreds to tens of thousands of picograms per milliliter of blood plasma or serum 14–17. However, the functional importance of NRG-1 variability in the circulation is not well understood. The expression of ErbB2, ErbB3 and ErbB4 receptors on human endothelial cells 11, 18, 19, human monocytes and lymphocytes 20–22, suggests that circulating NRG-1 may be involved in the regulation of vascular homeostasis. The level of circulating NRG-1 positively associates with disease severity and adverse outcomes in heart failure patients 23, suggesting that circulating NRG-1 is produced by diseased myocardium and may serve as a biomarker of myocardial stress or ischemia 17. Serum NRG-1 levels are positively correlated with vascular endothelial growth factor and angiopoietin-1 levels in patients with diabetes and unstable angina pectoris 24. Recombinant NRG-1 can induce the synthesis of vascular endothelial growth factor 25 and angiopoietin-1 26, suggesting that circulating NRG-1 may be involved in the upregulation of those factors in blood leukocytes. However, the functional activity of circulating NRG-1 has not been determined.
The goal of the current study was to determine the functional activity of circulating NRG-1 in blood plasma samples obtained from healthy donors. We employed a cell-based ELISA assay to measure phosphorylation of ErbB3 receptors expressed in MCF-7 cells in response to stimulation with human plasma. We also examined the effect of NRG-1 neutralizing antibody on plasma-induced ErbB3 activation and demonstrated the association of full-length NRG-1 with large extracellular vesicles in the circulation.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Human subjects.
Research was performed in accordance with study protocols approved by Maine Medical Center Institutional Review Board, which is accredited by the Association for the Accreditation of Human Research Protection Programs (AAHRPP). Blood samples were drawn from ten healthy donors after individuals had signed an informed consent form agreeing to be a voluntary donor. The healthy donors were a random group of male (n=5) and female (n=5) individuals between the ages of 39 and 69 years.
Blood sample collection
Venous blood (10 ml) was collected using BD Vacutainer ACD tubes. Blood plasma was prepared at room temperature using a two-step centrifugation at 2,000g for 20 minutes. After preparation plasma was stored at - 800C until further analysis.
Cells
A human leukemia monocytic cell line, THP-1, human microvascular endothelial cells, HMEC-1, and MCF-7 breast cancer cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured according to ATCC recommendations.
Reagents
An extracellular domain of recombinant human neuregulin-1 (377-HB/CF), recombinant human epidermal growth factor (EGF, 236-EG), recombinant human betacellulin (BTC, 261-CE), and recombinant human heparin-binding epidermal growth factor (HB-EGF, 259-HE) were purchased from Bio-techne/R&D Systems and reconstituted in phosphate-buffered saline. ErbB receptor inhibitors, including AG-1478, TAK-165 (mubritinib), AZD-8931 and AST-1306 were obtained from Selleck Chemicals (Houston, TX). Stock solutions of ErbB inhibitors were prepared in dimethyl sulfoxide. When used in experiments with cells, the final concentration of dimethyl sulfoxide did not exceed 0.1%
Real-time polymerase chain reaction
Total RNA was isolated from peripheral blood mononuclear cells using an RNeasy Mini kit (Qiagen, Germantown, MD). One microgram of total DNase-treated RNA was used to generate cDNA with Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) and random hexamers (Thermo Fisher Scientific). RT-PCR was performed using the ABI PRISM 7900HT Sequence Detection System (Thermo Fisher Scientific), as previously described elsewhere (48). Primer sequences for human ErbB1-4 receptors are shown in Table 1.
Table 1.
Primers for RT-PCR used in this study
| Target | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| ErbB1/EGFR(ref) | GGACGACGTGGTGGATGCCG | GGCGCCTGTGGGGTCTGAGC |
| ErbB2 | CCAGCCCTCTGACGTCCATC | TTGATGAGGATCCCAAAGACC |
| ErbB3 | GCCAGAGCCTTTTAAGTCCAT | CCGTGGCATTGGGTGTAGAGA |
| ErbB4 | GTGAAATTGGACACAGCCCT | GCCATTACAGCAGGAGTCAT |
| β-Actin | CGCCCCAGGCACCAGGGC | GGCTGGGGTGTTGAAGGT |
Western-blotting analysis and immunoprecipitation of plasma NRG-1
MCF-7 lysates were separated on a 4-12% gradient SDS-PAGE gel and processed for Western blotting. Rabbit polyclonal phospho-HER3/ErbB3 (Tyr1289) (21D3, Cell Signaling Technologies) was used as a primary antibody, and horseradish peroxidase-conjugated anti-rabbit IgG (111-035-003, Jackson ImmunoResearch Laboratories) was used as a second antibody at a dilution of 1:25,000.
Immunoprecipitation of NRG-1 was performed using mouse anti-NRG1 monoclonal antibody (MA5-12895, ThermoFisher Scientific). Mouse IgG2a was used as a control. Both anti-NRG-1 and IgG2a were biotinylated using EZ-Link™ Sulfo-NHS-LC-Biotinylation Kit (21435, ThermoFisher Scientific). Blood plasma (1.5 ml) was incubated with biotinylated anti-NRG-1 or IgG2a for overnight at 40C, followed by a 60-minute incubation with streptavidin MagneSphere® Paramagnetic Particles (Z5481, Promega). Paramagnetic particles were captured using a magnetic particle separator (Roche) and washed 5 times with RIPA buffer. Immunoprecipitated plasma proteins were eluted using Laemmli buffer. Polyclonal goat anti-NRG-1 extracellular domain antibody (AF377, Bio-Techne/R&D Systems) was used to analyze immunoprecipitates.
Flow cytometric analysis
MCF-7 cells were detached using Accutase solution, centrifuged and resuspended at the concentration of 106 cells per ml in a FACS buffer (PBS, 5% BSA and 2 mM EDTA). Cells were treated with Human TruStain FcX™ (Biolegend, San Diego, CA) to prevent non-specific binding followed by incubation with relevant antibodies for 25 min at 4°C. Cell-surface antigen expression of ErbB receptors was examined using PE-conjugated anti-human ErbB2 (Fab1129P) and IgG2b isotype-matched control (IC0041P), ErbB3 (Fab3481P) and IgG1 control (IC002P), ErbB4 (Fab11311P) and IgG2a (IC003P) isotype control. Data acquisition was performed on a MacsQuant Analyzer 10 (Miltenyi Biotec., Inc.) and the data were analyzed using WinList 5.0 software. Viable and non-viable cells were distinguished using DAPI.
Plasma ErbB3 activating activity.
MCF-7 cells were seeded at an initial density of 8 x104 cells per well into a 96-well plate. Cells were kept in growth medium, DMEM containing 10% FBS, in a CO2 incubator overnight to allow cell attachment. The medium was changed to serum-free DMEM the following day, and cells were incubated for an additional 24 hours. The reaction was initiated by the addition of the extracellular domain of recombinant NRG-1, EGF, BTC, HB-EGF or plasma samples. After a 15 minute incubation, the reaction was stopped with the addition of formaldehyde to a final concentration of 4%. Cells were permeabilized using 0.1% Triton X-100. Non-specific binding was blocked with 3% bovine serum albumin, and cells were incubated with primary antibody, phospho-HER3/ErbB3 (Tyr1289) (21D3, Cell Signaling Technologies), followed by incubation with secondary anti-rabbit IgG conjugated with horseradish peroxidase (111-035-003, Jackson ImmunoResearch. Colorimetric reaction proportional to the level of ErbB3 phosphorylation was developed using TMB substrate (N301, ThermoFisher Scientific). The absorbance was measured at 450 nm within 30 minutes of stopping the reaction. The level of plasma ErbB3 activating activity was determined from a calibration curve created with recombinant NRG-1 (377-HB-050, Bio-Techne/ RD Systems) and expressed as an amount of NRG-1 inducing a similar level of ErbB3 phosphorylation. To determine the number of cells per well and confirm a confluent MCF-7 cell monolayer, cells were incubated with DAPI solution for an additional 30 minutes and fluorescence was measured with 365nm excitation/ 415-445nm emission. The number of cells was calculated from a calibration curve created by seeding different numbers of cells per well (20-100 x 104 cells per well).
Analysis of circulating NRG-1 positive extracellular vesicles.
Extracellular vesicles were analyzed using a MACSQuant 10 analyzer (Miltenyi Biotec, Inc). Size-calibrated FITC labeled microbeads with sizes of 0.2 μm, 0.5 μm and 1 μm (F13839, Life Technologies) were used to establish a gate in a side scatter (SSC-H) and FITC-H settings using log scaling. Then FITC-labeled microbeads were backdated to a forward (FSC-H) and side scatter (SSC-H) setting to establish a gate for extracellular vesicles. The upper limit of the extracellular vesicles gate was established at the size of the 1 μm beads. In order to detect 0.2 μm beads, an absolute minimum threshold was lowered to 2.20 (from 5.0) at the SSC, resulting in a high background noise. No microbeads with size below 0.2 μm were detected. To distinguish between background noise and extracellular vesicles, plasma samples were filtered through filters with pore size of 0.1 μm (UFC30VV00, Sigma). Samples were analyzed at the lowest acquisition speed.
NRG-1 positive extracellular vesicles were identified using anti-NRG-1 antibody (AF377, Bio-Techne/RD Systems) conjugated to CF™488A (excitation/emission: 490/515 nm) using Mix-n-Stain™CF™488A Antibody Labeling kit (MIX488AS100, Sigma). Number of NRG-1 positive extracellular vesicles was calculated using a known number, 5 x 104, of 2 μm non-fluorescent microbeads (80177, Sigma) added to filtered and unfiltered plasma samples.
Statistical analysis
Normally distributed variables are expressed as mean ± SEM. Data are expressed as median values when distributions are skewed. Comparisons between two groups were performed using two-tailed unpaired t-tests or Mann Whitney test for normal or skewed distribution, respectively. Comparisons between three or more groups were performed using one-way ANOVA test with Tukey’s multiple comparisons post-test. For continuous variables, correlation analysis was performed using Spearman’s rank test (skewed distribution correlation). A P-value < 0.05 was considered significant.
RESULTS
Expression of ErbB receptors in MCF-7 cells.
To determine functional activity of circulating NRG-1, we measured phosphorylation of ErbB receptors, one of the early steps which follows NRG-1 binding and ErbB receptors dimerization. It is well-known that while the EGFR/ErbB1 receptor does not bind NRG-1, it may dimerize and induce phosphorylation of other ErbB receptors 27–29. EGFR/ErbB1 ligands, such as epidermal growth factor and transforming growth factor-alpha, are present in the circulation 30, 31. To exclude the potential contribution of EGFR/ErbB1-dependent signaling and identify cells that do not express EGFR/ErbB1, we first performed an analysis of ErbB receptors gene expression in several cell lines, including HMEC-1, THP-1, and MCF-7 cells, using RT-PCR. We found that MCF-7 cells are characterized by the absence of EGFR/ErbB1 gene expression accompanied by the highest expression of ErbB3 among tested cell lines (Figure 1A). mRNA expression of ErbB2 and ErbB4 were also detected in MCF-7 cells. Data from RT-PCR were validated using flow cytometric analysis of ErbB receptor expression, showing cell surface expression of ErbB2, ErbB3 and ErbB4 but not EGFR/ErbB1 receptors in MCF-7 cells (Figure 1B).
Figure 1. MCF-7 cells are characterized by high expression of functionally active ErbB3.
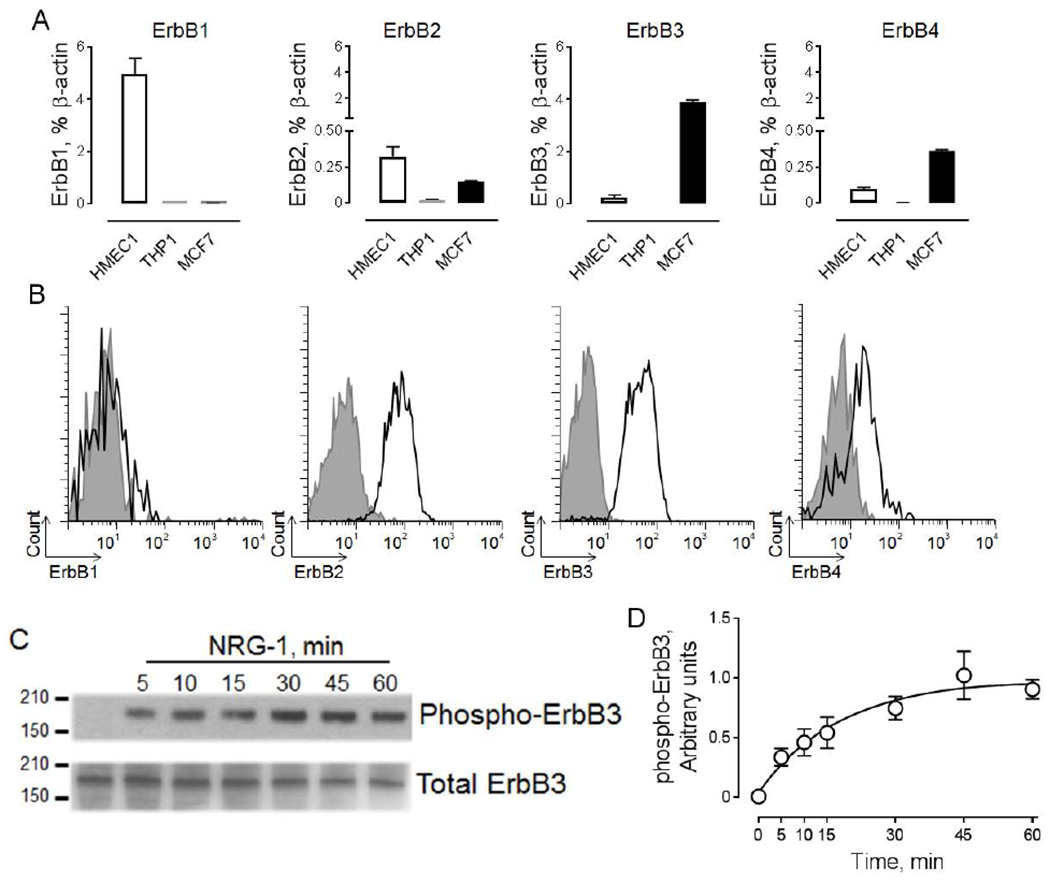
A. Graphical representation of ErbB1-4 receptors expression in a human microvascular endothelial cell line (HMEC1, positive control), a human monocytic cell line (THP1, negative control) and a human breast cancer cell line (MCF-7). B. Flow cytometric analysis of cell surface expression of ErbB receptors in MCF-7 cells. The open histogram depicts the specific ErbB antibody and the gray histogram represents the isotype-matched control. The Y-axis shows cell count; the X-axis indicates fluorescence intensity. C. Effect of 10 ng/ml neuregulin-1(NRG-1) on phosphorylation of ErbB3 in MCF-7 cells. The horizontal lines on the left are the molecular weight ladder. The ErbB3 molecular weight is 180kD. D. Graphical representation of data from western blot analysis of NRG-1 induced phosphorylation of ErbB3. Data are shown as mean±SEM; n=3.
Next, we tested the effect of recombinant NRG-1 on activation of ErbB receptors. ErbB3 receptors were chosen due to their direct binding to NRG-1 and high level of protein expression in MCF-7 cells. We found that NRG-1 induced rapid (within 5 minutes) phosphorylation of ErbB3, which plateaued after 30 minutes (Figure 1C). Based on these data, we selected a 15-minute treatment for the analysis of the functional activity of circulating NRG-1. This time point corresponded to the submaximal level of ErbB3 phosphorylation (Figure 1D).
Effect of ErbB receptors ligands and inhibitors on phosphorylation of ErbB3.
To determine the effect of ErbB ligands, we used a cell-based ELISA assay measuring ErbB3 phosphorylation after 15 minutes of MCF-7 cell stimulation. As seen in Figure 2A, NRG-1 but not epidermal growth factor, an EGFR/ErbB1 ligand, induced phosphorylation of ErbB3, consistent with the absence of EGFR/ErbB1 expression in MCF-7 cells. The effective concentration 50% (EC50) of NRG-1-dependent phosphorylation of ErbB3 was 26 ng/ml.
Figure 2. Pharmacological characterization of ErbB3 activation in MCF-7 cells.

A. Effect of NRG-1 (closed circles, n=12) and epidermal growth factor (EGF, open circles, n=3) on phosphorylation of ErbB3 in MCF-7 cells. EC50 [NRG1] = 26 ng/ml. B. Effect of TAK-165 (open circles), AG 1478 (closed circles), AST1306 (open triangles) and AZD8931 (closed triangles) on ErbB3 phosphorylation induced by 50 ng/ml rhNRG-1. The data are mean ±SEM, n=3.
Our analysis revealed that both reversible (AZD8931) and irreversible (AST1306) pan-ErbB antagonists, inhibit NRG-1 induced phosphorylation of ErbB3 with inhibitory concentration 50% (IC50) of 2.3 nM and 12.5 nM, respectively (Figure 2B). These concentrations are in agreement with previous studies 32, 33, indicating that the NRG-1 effect in MCF-7 cells is fully dependent on the activation of ErbB receptors. We also found that AG1478 inhibits phosphorylation of ErbB3 with an IC50 value of 857 nM, which is close to the previously demonstrated effect of AG1478 on ErbB3 and ErbB4 receptors 8,34, 35. This concentration is more than one hundredfold higher than IC50 concentration of AG1748, which inhibits activation of EGFR/erbB1 36, further confirming the absence of EGFR/ErbB1 expression in MCF-7 cells. We found no inhibitory effect of selective and potent ErbB2 inhibitor, TAK165, indicating that ErbB2 is not involved in phosphorylation of ErbB3. Our data indicate that this cell-based ELISA represents a quantifiable and highly reproducible method to measure phosphorylation of ErbB3.
Plasma ErbB3 activating activity and level of circulating NRG-1 protein.
We used recombinant NRG-1 to construct calibration curves in a similar manner for two assays: (1) conventional ELISA to determine the level of NRG-1 protein; and (2) cell-based ELISA to examine the effect of blood plasma on ErbB3 phosphorylation in MCF-7. This allowed us to compare the level of circulating NRG-1 protein and plasma ErbB activating activity in the same samples obtained from healthy volunteers.
Our analysis revealed that the level of plasma-induced ErbB3 activating activity was approximately 50 fold higher compared to the NRG-1 protein level (Figure 3A). We also found that the level of ErbB3 activating activity was characterized by a lower quartile coefficient of dispersion, with a value of 0.21, versus a value of 0.85 found for NRG-1 protein.
Figure 3. NRG-1 protein level does not correlate to ErbB3 activation activity in healthy donor plasma.
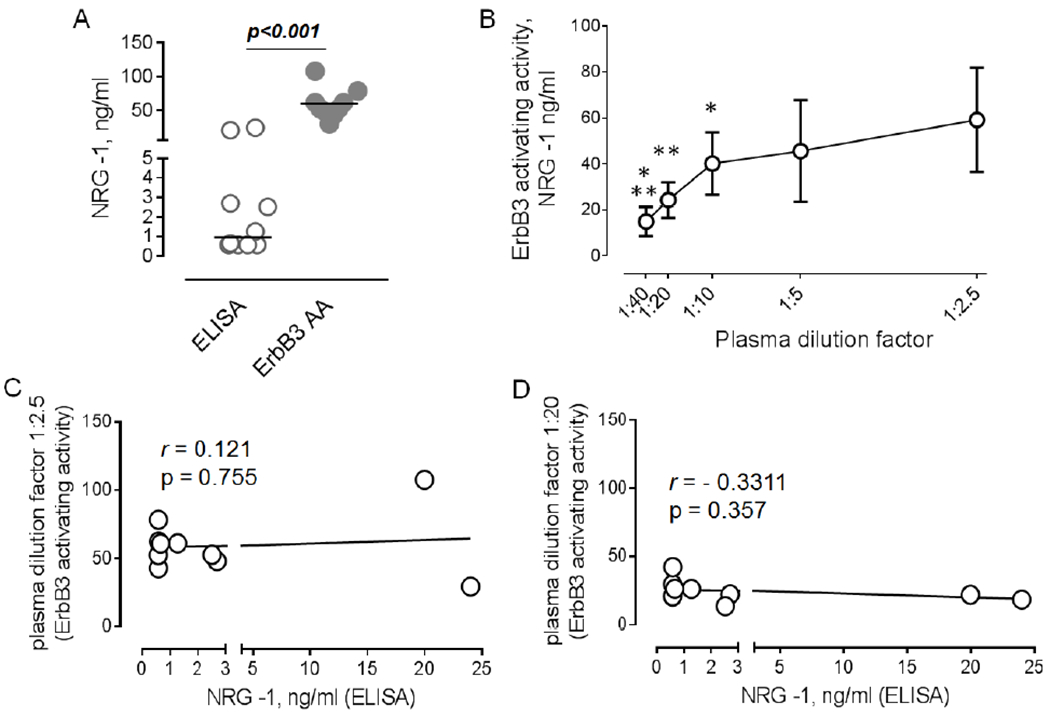
A. Graphical representation of data for circulating levels of NRG-1 protein (ELISA) and plasma ErbB3 activating activity (ErbB3 AA) in healthy donor plasma (n=10). Mann Whitney test. B. Effect of plasma dilutions (1:2.5, 1:5, 1:10, 1:20, and 1:40) on phosphorylation of ErbB3. Results become linear with dilutions between 1:10 and 1:40. Repeated measures one-way AN OVA, Tukey’s posttest, n=10. C-D. The association between levels of NRG-1 protein and ErbB3 activating activity at 1:2.5 (C) and 1:20 (D) dilutions.
To better characterize ErbB3 activating activity we performed an analysis of multiple plasma dilutions on ErbB3 phosphorylation. As seen in Figure 3B, a serial dilution curve is comprised of two parts. Within the range of plasma dilutions from 1:2.5 to 1:10, there is a very low decline in the ErbB3 activating activity, most likely due to saturation of the ErbB3 receptor pool in the presence of high concentrations of factors contributing to ErbB3 activation. We found a linear decrease in ErbB3 phosphorylation with dilution of plasma samples from 1:10 to 1:40.
To determine whether or not circulating NRG-1 contributes to plasma ErbB3 activating activity, we first performed correlation analysis between levels of NRG-1 protein and plasma-induced ErbB3 phosphorylation using two dilution factors, 2.5 and 20, corresponding to lowest dilution and those which are on the linear part of serial dilution curve, respectively. No associations were found between circulating NRG-1 protein and ErbB3 activating activity at dilution of 1 to 2.5 (Figure 3C) or 1 to 20 (Figure 3D).
Effect of NRG-1 neutralizing antibody on plasma ErbB3 activating activity.
To further characterize the functional activity of circulating NRG-1, we determined the effect of NRG-1 neutralizing antibody on plasma-induced ErbB3 phosphorylation. As seen in Figure 4A, the pre-incubation of NRG-1 with neutralizing antibody resulted in a 85% reduction of ErbB3 phosphorylation induced by 10 ng/ml of recombinant NRG-1. This concentration is ten-fold higher than the median value for the level of circulating NRG-1 in human plasma. Therefore, we expected to find a reduction in the plasma ErbB3 activating activity if circulating NRG-1 protein is functionally active and contributes to ErbB3 phosphorylation.
Figure 4. NRG-1 neutralizing antibody does not reduce the level of plasma ErbB3 activating activity.
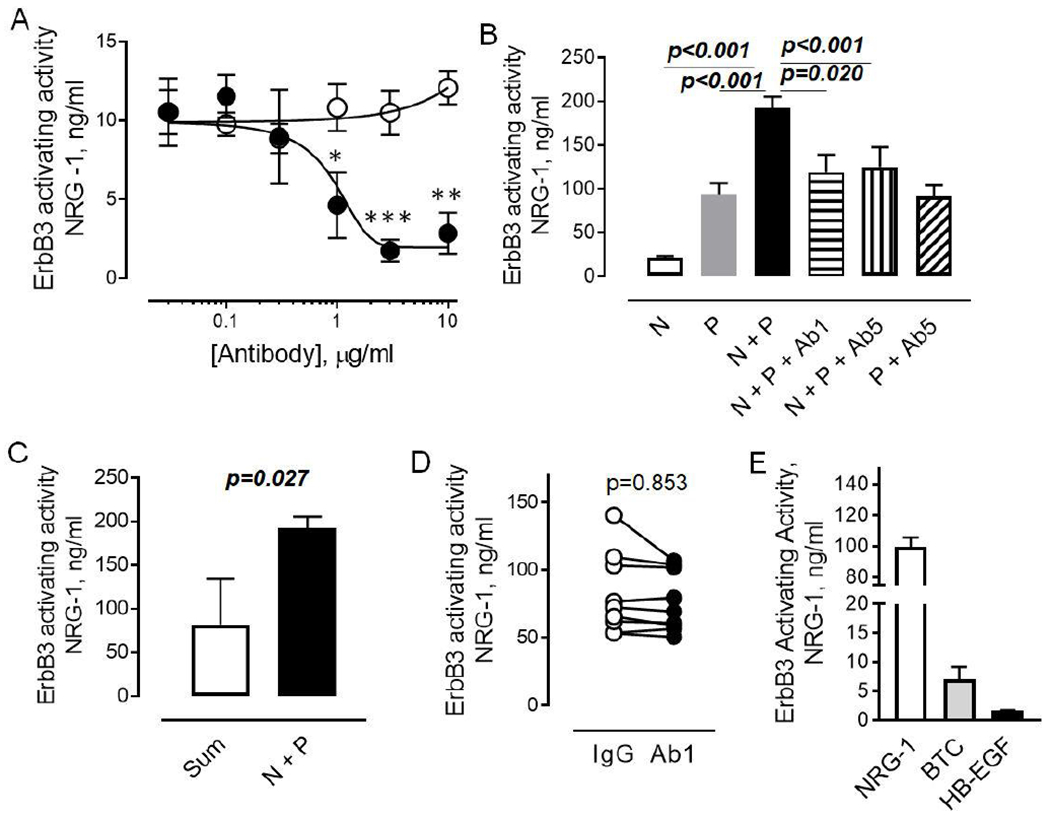
A. Effect of anti-NRG-1 antibody (AF377, closed circles) or control IgG’s on ErbB3 phosphorylation induced by 10 ng/ml rhNRG-1. The data are mean±SEM, n=3. Mann Whitney test. B. Effect of anti-NRG-1 antibody (AF377, closed circles) on ErbB3 phosphorylation induced by 10 ng/ml NRG-1 (N), healthy donor plasma (P), combinations of NRG-1 and plasma (N + P), NRG-1, plasma and 1 μg/ml (N + P + Ab1), or 5 μg/ml (N + P + Ab5) of anti-NRG-1 antibody. The data are mean±SEM, n=3. One-way ANOVA, Tukey’s posttest. C. Graphical representation of data showing synergism between recombinant NRG-1 and plasma. Open bar – sum of NRG-1 and plasma added separately to MCF-7 cells; Closed bar – NRG-1 and plasma added to cells simultaneously. Mann Whitney test, n=3. D. Effect of 1 μg/ml anti-NRG-1 antibody and control IgG’s on ErbB3 phosphorylation induced by plasma. Each plasma sample was tested in three independent experiments performed in duplicates. Mann Whitney test. E. Effect of 100 ng/ml recombinant NRG-1 (open bar), 100 ng/ml betacellulin (BTC, grey-shaded) and 100ng/ml heparin-binding epidermal growth factor (FIB-EGF, open) on phosphorylation of ErbB3 in MCF-7 cells. The data are mean ±SEM, n=2.
To confirm the efficiency of neutralizing antibody in human plasma, we tested the effect of two concentrations of neutralizing antibody, 1 μg/ml and 5 μg/ml, on the effect of recombinant NRG-1 in combination with plasma samples obtained from subjects with low concentration of circulating NRG-1 (below 0.5 ng/ml). As shown in Figure 4B, both NRG-1 and plasma are inducing phosphorylation of ErbB3. Neutralizing antibody abolished the effect of recombinant NRG-1 but not plasma on ErbB3 phosphorylation. Surprisingly, we found that recombinant NRG-1 and plasma cooperated in a synergistic manner to induce ErbB3 phosphorylation, suggesting the presence of allosteric non-competitive ErbB3 activators in human plasma (Figure 4C).
Next, we examined the effect of neutralizing antibody in the entire cohort of human plasma samples. We found no difference between the neutralizing and control antibodies, with the exception for one sample. The protein concentration of circulating NRG-1 in this sample was 20 ng/ml. Preincubation with neutralizing antibody resulted in a 25% reduction of plasma-induced ErbB3 phosphorylation in that sample (Figure 4D).
Since our data demonstrated no contribution of circulating NRG-1, we performed an additional analysis to determine the effect of ErbB4 ligands, betacellulin (BTC) and heparin-binding epidermal growth factor (HB-EGF), on activation of ErbB3. Both BTC and HB-EGF are present in the circulation 37–39. As shown in Figure 4E, 100 ng/ml concentration of both BTC and HB-EGF induced phosphorylation of ErbB3. The effect induced by these factors was approximately 20-fold and 50-fold less for BTC and HB-EGF, respectively, compared to the effect of NRG-1. However, these observations demonstrated that plasma factors that do not directly bind the ErbB3 receptor could contribute to its activation.
Circulating pool of full-length NRG-1 is associated with large extracellular vesicles.
To better characterize circulating NRG-1 proteins, we performed western blotting analysis of blood plasma. Our data demonstrated the presence of multiple bands of immunoreactivity with one strong band at approximately 50 kDa, and several weaker bands near~ 70 kDa, 98 kDa and 130 kDa (Figure 5A). No bands, with a molecular weight close to predicted for the extracellular domain of NRG-1 (~ 25 kDa), were detected.
Figure 5. Association of circulating NRG-1 with large extracellular vesicles.

A Western blot analysis of plasma samples obtained from two individuals with the highest level of circulating NRG-1 (Sub1 - 20 ng/ml, and Sub2 - 24 ng/ml). Different concentrations of extracellular domain of recombinant human NRG-1 (rhNRG-1) were used to identify the active form of NRG-1. B. Immunoprecipitation of plasma NRG-1 using mouse IgG2a (IgG, control) or anti-NRG-1 antibody (a-NRG, clone: 7D5) followed by western blot analysis of circulating NRG-1 with goat anti-NRG-1 antibody. C. Representative flow cytometric plots showing NRG-1 positive vesicles (red gate) in plasma samples before (unfiltered) and after (filtered) filtration through filters with pore size of 0.1 μm. Microbeads (5 x 104) with size of 2 μm (blue gate) were used to determine number of NRG-1 positive extracellular vesicles. D. Association between NRG-1 positive vesicles, determined using flow cytometric analysis, and NRG-1 protein levels in plasma samples. The rs and p values are from Spearman correlation (n = 10). E. Levels of NRG-1 protein, determined by ELISA, in plasma samples before (unfiltered) and after (filtered) filtration through 0.1 μm filters. Wilcoxon test. P-value is indicated.
Abundant blood proteins, such as albumin, can contribute to non-specific antibody coupling, making it difficult to analyze specific immunoreactivity in plasma samples. To increase the specificity of NRG-1 immunoblot analysis, we performed immunoprecipitation of plasma NRG-1. Our data clearly demonstrated the presence of only one band of approximately 70 kDa (Figure 5B), the molecular weight predicted for full-length NRG-1.
Since full-length NRG-1 is a transmembrane protein, we tested whether or not circulating NRG-1 is associated with extracellular vesicles generated from cell membrane. We analyzed blood plasma for extracellular vesicles with size between 0.2 μm and 1 μm using flow cytometry. These large extracellular vesicles, also known as microvesicles, bud directly from plasma membranes 40. As shown in Figure 5C, NRG-1-positive extracellular vesicles are present in the human plasma. We found a positive correlation between the number of NRG-1 positive extracellular vesicles and circulating NRG-1 protein determined by ELISA (Figure 5D). Filtration of human plasma with 0.1 μm filters resulted in ~ 30% reduction of NRG-1 protein (Figure 5E), indicating that full-length NRG-1 expressed on >0.1 μm diameter extracellular vesicles represent approximately one-third of the circulating NRG-1 in human blood.
DISCUSSION
In the current study, we demonstrated that human plasma is characterized by high ability to induce ErbB3 activation. However, circulating NRG-1 does not appear to contribute to activation of ErbB3 receptors. This can be explained, at least in part, by the fact that circulating NRG-1 is represented by inactive full-length transmembrane protein associated with large extracellular vesicles.
We used phosphorylation of ErbB3 receptors to determine the activity of blood plasma and circulating NRG-1. It is important to note, however, that activation of ErbB3 receptors cells is not solely limited to the effect of ErbB3 agonists in MCF-7 cells. Our data indicated that in addition to ErbB3, ErbB2 and ErbB4, but not EGFR/ErbB1 are expressed in these cells. The pharmacological analysis further demonstrated the absence of EGFR/ErbB1 expression. Previous data from other studies on the expression of ErbB receptors are consistent with our findings 41–43. Overall, our data indicated that MCF-7 cells can be used to study the functional effects of ErbB3 and ErbB4 receptor ligands.
ErbB3 and ErbB4 receptor ligands, including NRG-1 and 4, BTC and HB-EGF are present in the circulation 17, 44–46, and can contribute to transactivation of ErbB receptors. We found that ErbB4 ligands, BTC and HB-EGF, can minimally induce phosphorylation of ErbB3 receptors in MCF-7 cells. In addition, ErbB receptors can be activated through the activation of G-protein coupled receptor-dependent signaling, a process known as transactivation 47–49. Not surprisingly, our data demonstrated a high level of ErbB3 activating activity in human plasma. We also found that the recombinant extracellular domain of NRG-1 acts in a synergistic manner with blood plasma to activate ErbB3, suggesting that plasma-induced ErbB3 activation is dependent on contributions from allosteric non-competitive mechanisms. An Allosteric mechanism of activation through dimer formation has been previously shown for ErbB receptors50–52. We did not find the expression of ErbB1 in MCF-7 cells. Pharmacological inhibition of ErbB2 also revealed no contribution of this receptor subtype in ErbB3 activation in MCF-7 cells. Since we found the functional activity of ErbB4, it is likely that ErbB3 and ErbB4 interact in an allosteric manner. ErbB3 activity may be regulated by non-receptor tyrosine kinase-dependent mechanisms. The effect of c-Src in synergistic activation of ErbB1 and ErbB2 has been previously described 53–55. In future studies, it would be interesting to test the effect of Src inhibitors alone or in combination with inhibitors of G-protein coupled receptors 56 on plasma-induced ErbB3 or ErbB4 phosphorylation.
Our study showed no contribution of circulating NRG-1 to plasma ErbB3 activating activity. That was demonstrated by (1) the absence of association between the level of NRG-1 protein and ErbB3 phosphorylation, and (2) no effect of NRG-1 neutralizing antibody compared to control IgG’s on plasma-induced activation of ErbB3. Thus, an anti-NRG-1 antibody that successfully blocked the effect of the active extracellular domain of NRG-1 did not reduce the phosphorylation of ErbB3 induced by blood plasma. We cannot exclude, however, that full-length, membrane-bound NRG-1 in large extracellular vesicles may contribute to ErbB3 phosphorylation during a longer incubation time during which a plateau corresponding to the phosphorylation of the entire ErbB3 receptor pool is reached. Our current study examined the effect of fifteen minutes of incubation time. Investigation of the effect of purified NRG-1 positive large extracellular vesicles on ErbB3 phosphorylation is warranted.
It has been shown previously that nitration of NRG-1 resulted in loss of its ability to bind and activate ErbB receptors 57, indicating that posttranslational modification could mediate functional inhibition of NRG-1 in blood. In addition, previous analysis of NRG-1 protein expression in cell lines and skeletal muscles revealed the presence of multiple different isoforms, as well as inactive transmembrane and biologically active shed forms of NRG-1 58–62. In the current study, we also demonstrated the presence of multiple bands of NRG-1 immunoreactivity in human plasma. However, immunoprecipitation of plasma NRG-1 using an antibody against the extracellular domain resulted in purification of only one form with a molecular weight close to the predicted weight of full-length NRG-1. The full-length NRG-1 is a transmembrane protein, suggesting that circulating NRG-1 can be associated with extracellular vesicles.
Indeed, our analysis revealed the presence of NRG-1 positive large extracellular vesicles in blood plasma, indicating that matrix metalloproteinase-dependent shedding of extracellular domain of NRG-1 19, 63 is not a unique process contributing to circulating protein pool. Full-length NRG-1 can also be released into the blood through mechanisms related to cell membrane budding. Furthermore, we found that extracellular vesicles-associated NRG-1, accounted for approximately 30% of circulating NRG-1. Interestingly, in our previous study, we demonstrated significant six-fold higher level of NRG-1 protein in blood plasma compared to serum samples obtained from patients with severe coronary artery disease 17. The difference between plasma and serum levels of NRG-1 may indicate that NRG-1 is associated with extracellular vesicles generated from apoptotic cells in diseased myocardium. Apoptotic extracellular vesicles are removed from serum due to their involvement in coagulation and blood clot formation 64–66. However, this should be tested in future studies.
Cytofluorometric approaches to measure NRG-1 positive extracellular vesicles has limitations. The lowest limit of extracellular vesicles detection with this method is 0.2 μm. Because of that, a significant portion of extracellular vesicles with smaller sizes, from 0.04 μm to 0.2 μm, were excluded from the analysis. It is possible that biologically inactive full-length NRG-1 can also be associated with small extracellular vesicles. That may explain the presence of a high molecular weight form of NRG-1, which has not been removed after the filtration through 0.1 μm filters. We cannot exclude, however, that our approach is not sufficient for identification of all forms of circulating NRG-1. The low molecular weight extracellular domain of NRG-1 still may be present in the blood. Biotinylation of the antibody used for immunoprecipitation may result in the reduction of interaction 67 with multiple forms of NRG-1, including the low molecular weight extracellular domain.
In summary, we demonstrate that blood plasma obtained from healthy donor volunteers possesses high ErbB receptor activating activity. Our data indicated that full-length NRG-1, associated with large extracellular vesicles, is present in the circulation. These data expand our understanding of the nature of circulating NRG-1 pool and highlight the importance of the functional assessment of ErbB ligands in biological fluids. Further studies to determine functional significance of ErbB receptor activating activity in cardiovascular disease are warranted.
ACKNOWLEDGMENTS
We are grateful to Dr. Anne Breggia, Jean S. Mack, Joanne S. Burgess, Susan Bosworth-Farrell, Dana Tripp, and the MMC BioBank, a Core Facility, for subject recruitment and providing logistical support during sample collection.
SOURCES OF FUNDING
This work was supported by the Maine Medical Center Cardiovascular Research Institute 2015 Pilot Project Program, the National Heart, Lung, and Blood Institute of the National Institutes of Health under grants U01 HL100398, R01 HL136560, R01 HL139887 the American Heart Association under grant 17POST33410474. We utilized Maine Medical Center’s Progenitor Cell Analysis Core facility which is supported by NIH/NIGMS grants P30GM106391, COBRE in Stem and Progenitor Cell Biology and Regenerative Medicine and U54GM115516, Northern New England Clinical and Translational Research Network (Translational Technologies Core). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- ACD
anticoagulant citrate dextrose
- AG1478
N-(3-chlorophenyl)-6,7-dimethoxyquinazolin-4-amine hydrochloride
- AST1306
2-Propenamide, N-[4-[[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]amino]-6-quinazolinyl]-, 4-methylbenzenesulfonate (1:1)
- AZD8931
2-(4-(4-(3-chloro-2-fluorophenylamino)-7-methoxyquinazolin-6-yloxy)piperidin-1-yl)-N-methylacetamide
- EC50
half maximal effective concentration
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FITC
fluorescein isothiocyanate
- FSC-H
forward scatter height, flow cytometry
- IC50
half maximal inhibitory concentration
- IgG
immunoglobulin G
- IP
immunoprecipitation
- NRG-1
neuregulin-1β
- OD450
optical density at 450 nm, spectrophotometry
- RT-PCR
real-time polymerase chain reaction
- SSC-H
forward scatter height, flow cytometry
- TAK165
(E)-1-(4-(4-((2-(4-(trifluoromethyl)styryl)oxazol-4-yl)methoxy)phenyl)butyl)-1H-1,2,3-triazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Meyer D and Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–90. [DOI] [PubMed] [Google Scholar]
- 2.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C and Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camenisch TD, Schroeder JA, Bradley J, Klewer SE and McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nature medicine. 2002;8:850–5. [DOI] [PubMed] [Google Scholar]
- 4.Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE and Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galindo CL, Ryzhov S and Sawyer DB. Neuregulin as a heart failure therapy and mediator of reverse remodeling. Current heart failure reports. 2014;11:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odiete O, Hill MF and Sawyer DB. Neuregulin in cardiovascular development and disease. Circulation research. 2012; 111:1376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemmens K, Doggen K and De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–60. [DOI] [PubMed] [Google Scholar]
- 8.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA and Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–9. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM and Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–4. [DOI] [PubMed] [Google Scholar]
- 10.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R and Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. The Journal of biological chemistry. 2004;279:51141–7. [DOI] [PubMed] [Google Scholar]
- 11.Russell KS, Stern DF, Polverini PJ and Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–11. [DOI] [PubMed] [Google Scholar]
- 12.Chun MH. Serum signaling factors and spheroids. Crit Rev Oncol Hematol. 2000;36:89–98. [DOI] [PubMed] [Google Scholar]
- 13.Galindo CL, Ryzhov S and Sawyer DB. Neuregulin as a heart failure therapy and mediator of reverse remodeling. Curr Heart Fail Rep. 2014; 11:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esnafoglu E Levels of peripheral Neuregulin 1 are increased in non-medicated autism spectrum disorder patients. J Clin Neurosci. 2018;57:43–45. [DOI] [PubMed] [Google Scholar]
- 15.Geisberg CA, Abdallah WM, da Silva M, Silverstein C, Smith HM, Abramson V, Mayer I, Means-Powell J, Freehardt D, White B, Lenihan D and Sawyer DB. Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J Card Fail. 2013;19:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibuya M, Komi E, Wang R, Kato T, Watanabe Y, Sakai M, Ozaki M, Someya T and Nawa H. Measurement and comparison of serum neuregulin 1 immunoreactivity in control subjects and patients with schizophrenia: an influence of its genetic polymorphism. J Neural Transm (Vienna). 2010;117:887–95. [DOI] [PubMed] [Google Scholar]
- 17.Geisberg CA, Wang G, Safa RN, Smith HM, Anderson B, Peng X- Y, Veerkamp B, Zhao DX, Blakemore D, Yu C and Sawyer DB. Circulating neuregulin-1β levels vary according to the angiographic severity of coronary artery disease and ischemia. Coron Artery Dis. 2011. ;22:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryzhov S, Robich MP, Roberts DJ, Favreau-Lessard AJ, Peterson SM, Jachimowicz E, Rath R, Vary CPH, Quinn R, Kramer RS and Sawyer DB. ErbB2 promotes endothelial phenotype of human left ventricular epicardial highly proliferative cells (eHiPC). J Mol Cell Cardiol. 2018;115:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinowski A, Plowes NJ, Huang Q, Berdejo-lzquierdo C, Russell RR and Russell KS. Metalloproteinase-dependent cleavage of neuregulin and autocrine stimulation of vascular endothelial cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:2567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryzhov S, Matafonov A, Galindo CL, Zhang Q, Tran TL, Lenihan DJ, Lenneman CG, Feoktistov I and Sawyer DB. ERBB signaling attenuates proinflammatory activation of nonclassical monocytes. American journal of physiology Heart and circulatory physiology. 2017;312:H907–H918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannella B, Pitt D, Marchionni M and Raine CS. Neuregulin and erbB receptor expression in normal and diseased human white matter. J Neuroimmunol. 1999;100:233–42. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher MA, Hedl M, Abraham C, Bernard JK, Lozano PR, Hsieh JJ, Almohazey D, Bucar EB, Punit S, Dempsey PJ and Frey MR. ErbB4 signaling stimulates pro-inflammatory macrophage apoptosis and limits colonic inflammation. Cell Death Dis. 2017;8:e2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ky B, Kimmel SE, Safa RN, Putt ME, Sweitzer NK, Fang JC, Sawyer DB and Cappola TP. Neuregulin-1β Is Associated With Disease Severity and Adverse Outcomes in Chronic Heart Failure. 2009;120:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng Z, Gui C, Nong Q, Du F and Zhu L. Serum neuregulin-1β levels are positively correlated with VEGF and Angiopoietin-1 levels in patients with diabetes and unstable angina pectoris. International Journal of Cardiology. 2013;168:3077–3079. [DOI] [PubMed] [Google Scholar]
- 25.Panutsopulos D, Arvanitis DL, Tsatsanis C, Papalambros E, Sigala F and Spandidos DA. Expression of heregulin in human coronary atherosclerotic lesions. J Vase Res. 2005;42:463–74. [DOI] [PubMed] [Google Scholar]
- 26.Nakaoka Y, Nishida K, Narimatsu M, Kamiya A, Minami T, Sawa H, Okawa K, Fujio Y, Koyama T, Maeda M, Sone M, Yamasaki S, Arai Y, Koh GY, Kodama T, Hirota H, Otsu K, Hirano T and Mochizuki N. Gab family proteins are essential for postnatal maintenance of cardiac function via neuregulin-1/ErbB signaling. J Clin Invest. 2007;117:1771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Lengerich B, Agnew C, Puchner EM, Huang B and Jura N. EGF and NRG induce phosphorylation of HER3/ERBB3 by EGFR using distinct oligomeric mechanisms. Proc Natl Acad Sci USA. 2017;114E2836–E2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Macdonald-Obermann J, Westfall C, Piwnica-Worms D and Pike LJ. Quantitation of the effect of ErbB2 on epidermal growth factor receptor binding and dimerization. J Biol Chem. 2012;287:31116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P, Cleveland TEt, Bouyain S, Byrne PO, Longo PA and Leahy DJ. A single ligand is sufficient to activate EGFR dimers. Proc Natl Acad Sci U S A. 2012;109:10861–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen-Plotkin AS, Hu WT, Siderowf A, Weintraub D, Goldmann Gross R, Hurtig HI, Xie SX, Arnold SE, Grossman M, Clark CM, Shaw LM, McCluskey L, Elman L, Van Deerlin VM, Lee VM, Soares H and Trojanowski JQ. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol. 2011;69:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Fan J, Chen M, Li W and Woodley DT. Transforming Growth Factor-Alpha: A Major Human Serum Factor that Promotes Human Keratinocyte Migration. Journal of Investigative Dermatology. 2006;126:2096–2105. [DOI] [PubMed] [Google Scholar]
- 32.Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, Beck S, Marshall G, Davenport S, Callis R, Mills E, Grosios K, Smith P, Barlaam B, Wilkinson RW and Ogilvie D. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB receptor blockade in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1159–69. [DOI] [PubMed] [Google Scholar]
- 33.Xie H, Lin L, Tong L, Jiang Y, Zheng M, Chen Z, Jiang X, Zhang X, Ren X, Qu W, Yang Y, Wan H, Chen Y, Zuo J, Jiang H, Geng M and Ding J. AST1306, a novel irreversible inhibitor of the epidermal growth factor receptor 1 and 2, exhibits antitumor activity both in vitro and in vivo. PLoS One. 2011;6:e21487–e21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sak MM, Breen K, Ronning SB, Pedersen NM, Bertelsen V, Stang E and Madshus IH. The oncoprotein ErbB3 is endocytosed in the absence of added ligand in a clathrin-dependent manner. Carcinogenesis. 2012;33:1031–1039. [DOI] [PubMed] [Google Scholar]
- 35.Fosdahl AM, Dietrich M, Schink KO, Malik MS, Skeie M, Bertelsen V and Stang E. ErbB3 interacts with Hrs and is sorted to lysosomes for degradation. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2017;1864:2241–2252. [DOI] [PubMed] [Google Scholar]
- 36.Levitzki A and Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–8. [DOI] [PubMed] [Google Scholar]
- 37.Anand-Apte B, Ebrahem Q, Cutler A, Farage E, Sugimoto M, Hollyfield J and Folkman J. Betacellulin induces increased retinal vascular permeability in mice. PloS one. 2010;5:e13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson JL, Phelps ED, Doll MA, Schaal S and Ceresa BP. The role of endogenous epidermal growth factor receptor ligands in mediating corneal epithelial homeostasis. Investigative ophthalmology & visual science. 2014;55:2870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto S, Kishida K, Shimomura I, Maeda N, Nagaretani H, Matsuda M, Nishizawa H, Kihara S, Funahashi T, Matsuzawa Y, Yamada A, Yamashita S, Tamura S and Kawata S. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochemical and biophysical research communications. 2002;292:781–6. [DOI] [PubMed] [Google Scholar]
- 40.Raposo G and Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD and Park JW. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63:3154–61. [PubMed] [Google Scholar]
- 42.Soler M, Mancini F, Meca-Cortés Ó, Sánchez-Cid L, Rubio N, López-Fernández S, Lozano JJ, Blanco J, Fernández PL and Thomson TM. HER3 is required for the maintenance of neuregulin-dependent and -independent attributes of malignant progression in prostate cancer cells. 2009;125:2565–2575. [DOI] [PubMed] [Google Scholar]
- 43.Mill CP, Zordan MD, Rothenberg SM, Settleman J, Leary JF and Riese DJ 2nd. ErbB2 Is Necessary for ErbB4 Ligands to Stimulate Oncogenic Activities in Models of Human Breast Cancer. Genes Cancer. 2011;2:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan P, Xu Y, Wan Q, Feng J, Li H, Yang J, Zhong H and Zhang Z. Plasma Neuregulin 4 Levels Are Associated with Metabolic Syndrome in Patients Newly Diagnosed with Type 2 Diabetes Mellitus. Dis Markers. 2018;2018:6974191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Vizcaino E, Vehi C, Camprecios G, Morcillo C, Soley M and Ramirez I. Heparin-binding EGF-like growth factor in human serum. Association with high blood cholesterol and heart hypertrophy. Growth Factors. 2010;28:98–103. [DOI] [PubMed] [Google Scholar]
- 46.Kjaer IM, Olsen DA, Alnor A, Brandslund I, Bechmann T and Madsen JS. EGFR and EGFR ligands in serum in healthy women; reference intervals and age dependency. Clin Chem Lab Med. 2019. [DOI] [PubMed] [Google Scholar]
- 47.Park YJ, Lee H and Lee JH. Macrophage inhibitory cytokine-1 transactivates ErbB family receptors via the activation of Src in SK-BR-3 human breast cancer cells. BMB Rep. 2010;43:91–6. [DOI] [PubMed] [Google Scholar]
- 48.Eguchi S, Frank GD, Mifune M and Inagami T. Metalloprotease-dependent ErbB ligand shedding in mediating EGFR transactivation and vascular remodelling. Biochem Soc Trans. 2003;31:1198–202. [DOI] [PubMed] [Google Scholar]
- 49.Hemi R, Yochananov Y, Barhod E, Kasher-Meron M, Karasik A, Tirosh A and Kanety H. p38 mitogen-activated protein kinase-dependent transactivation of ErbB receptor family: a novel common mechanism for stress-induced IRS-1 serine phosphorylation and insulin resistance. Diabetes. 2011. ;60:1134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Gureasko J, Shen K, Cole PA and Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006; 125:1137–49. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Pickin KA, Bose R, Jura N, Cole PA and Kuriyan J. Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature. 2007;450:741–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aertgeerts K, Skene R, Yano J, Sang BC, Zou H, Snell G, Jennings A, Iwamoto K, Habuka N, Hirokawa A, Ishikawa T, Tanaka T, Miki H, Ohta Y and Sogabe S. Structural analysis of the mechanism of inhibition and allosteric activation of the kinase domain of HER2 protein. The Journal of biological chemistry. 2011;286:18756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcotte R, Zhou L, Kim H, Roskelly CD and Muller WJ. c-Src associates with ErbB2 through an interaction between catalytic domains and confers enhanced transforming potential. Mol Cell Biol. 2009;29:5858–5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu W, Yuan X, Beebe K, Xiang Z and Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biscardi JS, Maa M- C, Tice DA, Cox ME, Leu T-H and Parsons SJ. c-Src-mediated Phosphorylation of the Epidermal Growth Factor Receptor on Tyr845 and Tyr1101 Is Associated with Modulation of Receptor Function. 1999;274:8335–8343. [DOI] [PubMed] [Google Scholar]
- 56.Sriram K and Insel PA. GPCRs as targets for approved drugs: How many targets and how many drugs? 2018:mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nethery DE, Ghosh S, Erzurum SC and Kern JA. Inactivation of neuregulin-1 by nitration. American journal of physiology Lung cellular and molecular physiology. 2007;292:L287–93. [DOI] [PubMed] [Google Scholar]
- 58.Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A and Pandiella A. Differential Shedding of Transmembrane Neuregulin Isoforms by the Tumor Necrosis Factor-α-Converting Enzyme. Molecular and Cellular Neuroscience. 2000;16:631–648. [DOI] [PubMed] [Google Scholar]
- 59.Wang JY, Miller SJ and Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem. 2001;276:2841–51. [DOI] [PubMed] [Google Scholar]
- 60.Aguilar Z and Slamon DJ. The Transmembrane Heregulin Precursor Is Functionally Active. 2001;276:44099–44107. [DOI] [PubMed] [Google Scholar]
- 61.Lebrasseur NK, Cote GM, Miller TA, Fielding RA and Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. American journal of physiology Cell physiology. 2003;284:C1149–55. [DOI] [PubMed] [Google Scholar]
- 62.LeBrasseur NK, Mizer KC, Parkington JD, Sawyer DB and Fielding RA. The expression of neuregulin and erbB receptors in human skeletal muscle: effects of progressive resistance training. European journal of applied physiology. 2005;94:371–5. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z, Cui J, Gao F, Li Y, Zhang G, Liu M, Yan R, Shen Y and Li R. Elevated cleavage of neuregulin-1 by beta-secretase 1 in plasma of schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:161–168. [DOI] [PubMed] [Google Scholar]
- 64.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS and Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. [DOI] [PubMed] [Google Scholar]
- 65.Liu ML, Reilly MP, Casasanto P, McKenzie SE and Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vase Biol. 2007;27:430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zubairova LD, Nabiullina RM, Nagaswami C, Zuev YF, Mustafin IG, Litvinov Rl and Weisel JW. Circulating Microparticles Alter Formation, Structure, and Properties of Fibrin Clots. Sci Rep. 2015;5:17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Høyer-Hansen G, Hamers MJAG, Pedersen AN, Nielsen HJ, Brünner N, Danø K and Stephens RW. Loss of ELISA specificity due to biotinylation of monoclonal antibodies. Journal of Immunological Methods. 2000;235:91–99. [DOI] [PubMed] [Google Scholar]


