Abstract
Tramadol is a synthetic opioid analgesic used for moderate-to-severe pain structurally related to codeine and morphine, where their analgesic mechanism is a result of opioid and non-opioid mechanisms. This study was designed to evaluate the effects of Moringa oleifera leaves extract (MLE) on tramadol-induced testicular toxicity, sperm changes, testicular damage, and oxidative stress in male rats. Forty male albino rats were divided into four groups and treated for 4 weeks (group 1, as control; group 2, MLE; group 3, tramadol; group 4, MLE + tramadol). The relative body weight, relative testes weight, serum total testosterone, luteinizing hormone, follicle-stimulating hormone, sperm counts, vitality, total sperm motility, catalase, and superoxide dismutase activities were significantly decreased in tramadol-treated group when compared with the control group. In contrast, sperm abnormality, immotile sperm percent, testicular injury, and TBARS concentration in testes were significantly increased in the tramadol-treated group. In addition, histopathological examination for the tramadol-treated group has shown incomplete spermatogenesis, moderate degeneration in some seminiferous tubules with a significant decrease in the number of spermatogenic cells and depletion of Leydig cells. The administration of MLE with tramadol ameliorates the testicular toxicity, injury, sperm count, abnormalities, and oxidative stress induced by tramadol.
Keywords: tramadol, Moringa oleifera leaves extract, testicular toxicity, oxidative stress, spermatogenesis
Introduction
Tramadol (Amadol) is prescribed for moderate-to-severe pain and it a synthetic opioid analgesic that structurally related to codeine and morphine, where their analgesic mechanism is a result of opioids and non-opioid mechanisms [1, 2]. It inhibits the neuronal reuptake of norepinephrine and serotonin as do the antidepressant drugs [3]. Also, it has many adverse effects as life-threatening ones such as loss of consciousness, cardiovascular failure, pruritus, nausea, somnolence, headache, dizziness, sweating, constipation, seizures, and serotonin syndrome [4]. Tramadol is well known under the trade names amadol, ultracet, tramacet, altram, tramapap, and acetaminophen [5, 6].
People in all continents have used thousands of indigenous plants for the treatment of various ailments dating back to prehistory. Moringa oleifera is a tree used in traditional medicine worldwide as having value both as a preventative and treatment agent of several health conditions, including the treatment of inflammation, infectious diseases, cardiovascular, gastrointestinal, hematological, hepatic, and renal disorders [7].
Moringa leaves can be a good source of natural antioxidants, and it has strong antiradical activity. Caffeic, p-coumaric and ferulic acids are the dominant phenolic constituents of M. oleifera leave extract [8, 9]. Therefore, the present study was conducted to examine the possible modifying effects of M. oleifera leaves extract (MLE) against testicular toxicity, sperm alternations, oxidative stress, and testicular damage induced by tramadol in male rats.
Materials and Methods
Chemicals and reagents
Tramadol or Amadol tablets
Tramadol, commercially available capsules, each contains 50-mg tramadol hydrochloride (Amadol, manufactured by ADWIA Co. S.A.E., 10th of Ramadan City, Egypt); the dose of tramadol was according to Atici et al. [10].
Moringa oleifera leaves extract
The leaves of M. oleifera were collected from Elgharbya Governorate, Egypt and dried at 35–40°C in van air circulating oven till dryness. The dried leaves were crushed to obtain the powdered form. The derived aqueous extract tested in this study was prepared in our laboratory by mixing 1-g dried and powdered leaves of M. oleifera with 10-ml boiling water for 5 min. The mixture was then filtered twice through a 2- μm pore sterile filter paper into a sterile tube. The aqueous extract stock solution (100 mg/ml) was freshly prepared for each set of experiments and stored at 4°C for up to 5 days according to Pal et al. [11].
Animal groups
The experiments were performed on 40 male albino rats weighing 180 ± 10 g and 12-week old. The rats were kept in the laboratory for 2 weeks before the experimental work and maintained on a standard rodent diet and water available ad libitum. The study protocol was approved (No. 1435-123) by the Scientific Research Ethics Committee of the Institute of Graduate Studies and Research, Alexandria University, Egypt.
Experimental design
Rats were equally divided into four equal groups (n = 10) and treated for 4 weeks. Group 1, control in which healthy untreated rats; group 2, MLE in which rats receive MLE orally (100 mg/kg BW/day) according to Pal et al. [11]; group 3, Tramadol in which rats receive tramadol orally (40 mg/Kg BW/day); and group 4, treated tramadol plus MLE include rats that receive orally tramadol and MLE.
At the end of the experimental period, animals were anesthetised by intraperitoneal injection with sodium pentobarbital (240 mg/ml) by a 22–25 gauge needle then subjected to a complete necropsy according to Al Suleimani et al. [12]. Blood samples were individually collected from the inferior vena cava of each rat in non-heparinized glass tubes and wait for 30 min at room temperature to clot. Blood serum was separated by centrifugation at 4000 rpm for 10 min. The collected serum was stored at −20°C until analysis.
Sexual hormones
The levels of total testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) were assayed by using the ADVIA Centaur XP system (two-site sandwich immunoassay using direct chemiluminometric technology; Vidas, France).
Sperms morphometric analysis
Testes and epididymis were carefully removed, cleaned from adhering connective tissue in cold saline and weighed. Sampling for the sperm was collected from the caudal epididymis after carefully removed from each testis, put it in 5 ml of Hanks’ balanced salt solution then incubated at room temperature for 15 min. The epididymis was prepared for fertility evaluation to estimate the sperm count, spermatozoa motility parameters, and sperm morphology by computer-assisted semen analysis (CASA System; Germany) with Olympus microscope (Olympus, Tokyo, Japan). A total of 200 spermatozoa from each rat were examined and individually scored normal or abnormal, according to the strict sperm morphology criteria that were used according to the method of Tousson et al. [13] and Eldaim et al. [14].
Tissue preparation
Testes tissues were immediately removed, washed, weighed, and cutting into two different parts—the first part was used for histological examination and the second part was homogenized (10%, w/v)—separately, in ice-cold sucrose buffer (0.25 M) in a Potter–Elvehjem type homogenizer. The homogenate was centrifuged at 10 000 × g for 20 min at 4°C and then uses supernatant for different enzyme assays.
Activities of antioxidant enzymes
Thiobarbituric acid-reactive substances (TBARS) were measured in testes homogenate using the method of Oyouni et al. [15]. The basis of the thiobarbituric acid (TBA) methods is the reaction of Malondialdehyde (MDA) with TBA at low pH and high temperature to form a colored complex, the MDA-TBA complex, with an absorption maximum at 532 nm that can be measured by visible absorption spectrophotometry. Furthermore, the activities of CAT (EC 1.11.1.6) and SOD (EC 1.15.1.1) in the testes homogenates were measured using colorimetric kits (Biodiagnostic, Egypt) and using the specific standard for each parameter according to the manufacturer instructions.
Histopathological examination
Testis was fixed with a 10% buffer neutral formalin solution for 48 h and then processed for paraffin sectioning. Sections of 5 μm were stained with hematoxylin and eosin for histopathological examination according to Tousson [16].
Statistical analysis
Data were expressed as mean values ± SE, and statistical analysis was performed using one-way ANOVA to assess significant differences among treatment groups. The criterion for statistical significance was set at P < 0.05 for the biochemical data. All statistical analyses were performed using SPSS statistical version 21 software package (SPSS® Inc., USA).
Results
Changes in weight
Table 1 showed that relative body weight (RBW) and relative testes weight (RTW) showed a significant (P < 0.05) decrease in the tramadol group (G3) as compared with the control (G1) and MLE (G2) groups, whereas relative Epididymis weights (REW) showed no changes in the tramadol group as compared with the control and MLE groups. On the other hand, RBW and RTW improved after the addition of MLE to the tramadol (G4).
Table 1.
Changes in the RBW (g/100 g), RTW (g/100 g BW), and REW (g/100 g BW) in different groups
| Control | MLF | Tramadol | Tramadol + MLF | |
|---|---|---|---|---|
| RBW | 17.9 ± 1.29# | 18.5 ± 0.89# | 14.3 ± 1.05* | 15.5 ± 0.89*# |
| RTW | 1.08 ± 0.09# | 1.09 ± 0.045# | 0.98 ± 0.059* | 1.02 ± 0.06*# |
| REW | 0.330 ± 0.030 | 0.334 ± 0.032 | 0.328 ± 0.025 | 0.330 ± 0.024 |
Data are expressed as mean ± SE of 10 observations. Significant difference from the control group at *P < 0.05. Significant difference from the tramadol group at #P < 0.05. Relative organ weight = organ weight/bodyweight × 100.
Changes in sexual hormones
Figure 1 showed a significant (P < 0.05) decrease in testosterone, LH and FSH in the tramadol group (G3), while there is an improvement in sexual hormones at the administration of MLE with the tramadol (G4) when compared with control (G1) and MLE (G2) groups.
Figure 1.
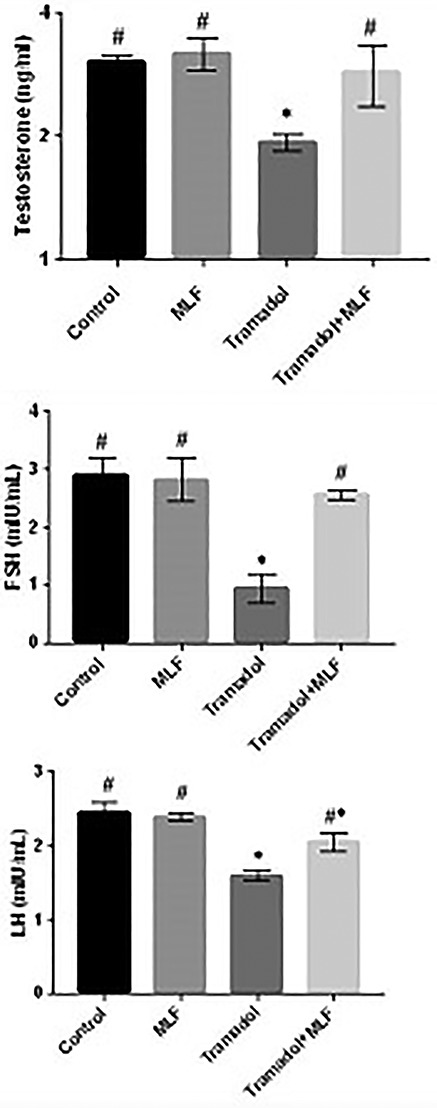
Changes in the testosterone, FSH, and LH levels in different groups under study. Data are expressed as mean ± SE of 10 observations. Significant difference from the control group at *P ˂ 0.05. Significant difference from the Tramadol group at #P ˂ 0.05.
Morphometric alterations of sperms
Table 2 displays that sperm counts and sperm motility significantly (P < 0.05) decreased in the tramadol group (G3), whereas the administration of MLE with the tramadol (G4) showed a significant (P < 0.05) increase when compared with control (G1) and MLE (G2) groups. Sperm abnormality exhibited a significant (P < 0.05) increase in the tramadol group (G3); meanwhile, the administration of MLE with the tramadol (G4) showed an amelioration in the sperm abnormality when compared with control (G1) and MLE (G2) groups.
Table 2.
Changes in sperm counts (number/g epididymis wt.) × 10 [6]), vitality, abnormality, total sperm motility, progressive motility, non-progressive, and immotile sperms percent in the different groups
| Control | MLF | Tramadol | Tramadol + MLF | |
|---|---|---|---|---|
| Sperm counts (million/ml) | 130.9 ± 10.3# | 138.5 ± 11.5# | 83.1 ± 8.3* | 140.0 ± 10.6# |
| Vitality (%) | 66.4 ± 1.50# | 69.1 ± 2.10# | 56.7 ± 1.40* | 66.0 ± 1.50# |
| Sperm abnormality (%) | 32.42 ± 1.39# | 28.55 ± 1.05# | 45.50 ± 1.92* | 37.71 ± 2.06#* |
| Total motility (PR + NP) | 68.12 ± 3.59# | 70.45 ± 4.48# | 60.55 ± 4.08* | 64.30 ± 4.25#* |
| Progressive motility (PR) | 41.60 ± 3.41# | 43.23 ± 4.37# | 32.31 ± 4.75* | 38.05 ± 2.15*# |
| Non-progressive (NP) | 26.40 ± 1.55# | 27.22 ± 1.07# | 24.24 ± 2.33* | 26.25 ± 1.62# |
| Immotile (IM) | 31.88 ± 0.97# | 29.55 ± 1.91# | 42.45 ± 2.27* | 35.70 ± 2.29#* |
Data are expressed as mean ± SE of 10 observations. Significant difference from the control group at *P < 0.05. Significant difference from the Tramadol group at #P < 0.05.
Epididymal progressive and non-progressive motility showed a significant (P < 0.05) decrease in the tramadol group (G3), while the administration of MLE with the tramadol (G4) showed a significant (P < 0.05) increase when compared with control (G1) and MLE (G2) groups. In contrast, the percent of immotile sperm showed a significant (P < 0.05) increase in the tramadol group (G3), while it showed an improvement in the percent after the administration of MLE with the tramadol (G4) when compared with control (G1) and MLE (G2) groups (Table 2).
The percentage of sperm with head defect (no head), sperm abnormalities, and other head defects such as double head, amorphous, banana shape, hookless head, and pinhead sperm was significantly (P < 0.05) increased in the tramadol group (G3); in contrast, the administration of MLE with the tramadol (G4) showed a ameliorate in sperm head abnormalities when compared with control (G1) and MLE (G2) groups (Fig. 2).
Figure 2.
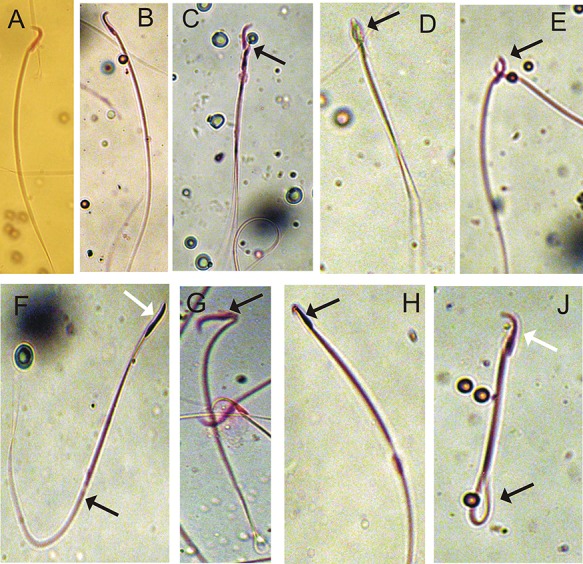
Microphotographs illustrating morphologically normal sperm (A and B) and various sperm defects (C–H) were observed. C, double head; D, amorphous head; E and F, fused heads; G, bent head; H, hook head; and J, coiled tail.
Oxidative stress markers
The results showed a significant (P < 0.05) increase in TBARS concentration in testes of male rats in the tramadol group (G3), while the administration of MLE with the tramadol (G4) showed an ameliorative in TBARS concentration as compared with control (G1) and MLE (G2) groups (Table 3).
Table 3.
Activities of TBARS (nmol/g protein), CAT (U/mg protein), and SOD (U/mg protein) in testes of male rats
| Item | Control | MLF | Tramadol | Tramadol + MLF |
|---|---|---|---|---|
| TBARS | 16.95 ± 1.19# | 17. 55 ± 1.13# | 26.62 ± 1.35* | 21.22 ± 1.00#* |
| Catalase | 61.58 ± 2.99# | 62.70 ± 3.02# | 48.50 ± 3.11* | 51.60 ± 2.43* |
| SOD | 5.48 ± 0.39# | 5.33 ± 0.46# | 2.83 ± 0.51* | 3.06 ± 0.32*# |
Data are expressed as mean ± SE of 10 observations. Significant difference from the control group at *P < 0.05. Significant difference from the Tramadol group at #P < 0.05.
Antioxidant enzyme activities
As shown in Table 3, catalase (CAT) and superoxide dismutase (SOD) activities were significantly (P < 0.05) decreased in the tramadol group (G3); On the other hand, ameliorate in SOD and CAT activities was observed in the administration of MLE with the tramadol (G4) as compared with control (G1) and MLE (G2) groups.
Effect of MLE on testes histopathology
Testes sections in control (G1) and MLE (G2) groups showed normal seminiferous tubules lining spermatocytes and spermatogenesis (Fig. 3A and B). However, the testis section in the tramadol group (G3) revealed moderate morphological changes as incomplete of spermatogenesis, moderate degeneration in some seminiferous tubules with a significant decrease in the number of spermatogenic cells with a moderate decrease in sperm, and depletion and little numbers of Leydig cells (Fig. 3C). Testesw in rats with the administration of MLE with the tramadol (G4) showed the normal structure of seminiferous tubules with the normal distribution of the spermatogenic cells and an increase in the sperm numbers (Fig. 3D).
Figure 3.
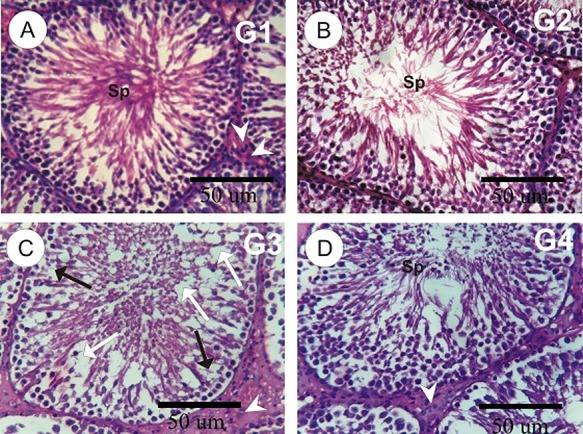
(A–D): photomicrographs of rat testes sections in the different experimental groups. A and B: testes sections in control and MLE rat groups showed normal seminiferous tubules lining spermatocytes and spermatogenesis. C: testis in tramadol group revealed the incomplete of spermatogenesis (black arrows), moderate degeneration (white arrows) in some seminiferous tubules with a decrease in the number of sperms, and depletion and little numbers of Leydig cells (arrowheads). D: testes in tramadol plus MLE showed the normal structure of seminiferous tubules with a normal distribution of the spermatogenic cells.
Discussion
Opiate use is known to decrease the levels of male sex hormones, and this lowered hormonal level is thought to be responsible for the diminished fertility of male opiate users [17]. Amadol or tramadol belongs to the same family of codeine, morphine, and oxycodone [5]. The current results revealed a dramatically drop in the levels of total testosterone, FSH, and LH hormones in the tramadol group compared with the control and MLE groups. However, rat’s administration of MLE with the tramadol showed an amelioration in the levels of total testosterone, FSH, and LH hormones when compared with control and MLE groups. This remarkable reduction of sexual hormones that were found in the current study might be explained by severe damages, which tramadol exerted on Leydig and Sertoli cells by increased generation of free radicals is one of the possible mechanisms involved in opioid-induced Leydig cell degeneration. A moderate decrease of gonadotropin secretion causes atrophy of the testes, as well as a decrease in sperm cell production [18]. Similar results for reduced testosterone have been reported by Abdellatief et al. [19]. Also, our results agree with Tennese and Wevrick [20] who reported the decreased levels of LH and testosterone with increased prolactin hormone after morphine and methadone administration. Also, Inass et al. [21] who observed a decrease of serum levels of FSH, LH, and testosterone and the induction of prolactin hormone (PRL) and E2 secretions after cannabis use.
Antioxidants are molecules that are capable of slowing or preventing the oxidation of other molecules, thereby protecting cells from damages caused by exposure to free radicals, including reactive oxygen species, which are produced during oxidation reactions in biological cells. Antioxidants can be phytochemicals; they range from micromolecules such as glutathione and vitamins to macromolecules such as catalase, glutathione, and peroxidase [22]. Tousson et al. [8] reported that the crude extract of phenolic compounds was obtained from MLE has strong antiradical activity against DPPH radical. Phenolic acids (derivatives of caffeic, p-coumaric, and ferulic acids) are the dominant phenolic constituents of MLE. Oxidative stress and lipid peroxidation are some of the mechanisms that can lead to liver dysfunction. Therefore, lipid peroxidation has been used as an indirect marker of oxidant-induced cell injury. The toxic effects of opioids at the cellular level may be explained by lipid peroxidation. Catalase activity and SOD activity in the tramadol group showed significantly decrease, while TBARS activity showed a significant increase when compared with control and MLE groups. Lipid peroxidation induced by tramadol can eventually result in dysfunction and structural damage of cells [23]. Therefore, decrease in the activity of catalase may result in a number of fatal effects due to the assimilation of superoxide radical and hydrogen peroxide. Our results agree with AKUNNA et al. [24] and Sadek [25] who found that M. oleifera leaf extracts have a chemotherapeutic and ameliorative role against chromium-induced damage in rat testes. Treatment with tramadol and MLE succeeded to modulate these observed abnormalities in testes structure, functions, and sperms resulting from tramadol as indicated by the reduction of enzymes activity and the pronounced improvement of the investigated biochemical, antioxidant, and oxidative stress parameters. Further studies are needed to investigate the impacts of tramadol on human health. In the current study, testicular histopathological results in the tramadol group were supported by sex hormonal dysfunction evident by a highly significant decrease in LH, FSH, and testosterone hormone levels when compared with group control. The present study showed the atrophy of seminiferous tubules with interstitial calcification, focal testicular degeneration with single or multiple layers of vacuolated spermatocytes with a little number of spermatogenesis in tramadol group. This is in agreement with Cajú et al. [26] who detected the decrease of Sertoli and Leydig cells in rats exposed to chronic and acute doses of morphine. Our recommendation, with the increasing use of tramadol for pain control, it is important for physicians to be aware of its potential side effects.
Conclusion
MLE has an ameliorative role against tramadol-induced toxicity, fertility potential, changes in sex hormones, oxidative stress, and testicular injury in male rats. M. oleifera increases the sperm count, reduced the sperm abnormality, and improved the testes’ injury. Hence, these results suggest that MLE can be a reliable therapy for tramadol toxicity and further validate the therapeutic effectiveness of MLE for some diseases.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This study did not receive any grant from any funding agency.
References
- 1. Grond S. and Sablotzki A.. Clinical pharmacology of tramadol. Clin. Pharmacokinet 2004;43:879–23. [DOI] [PubMed] [Google Scholar]
- 2. Gillman P. K. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br. J. Anaesth 2005;95:434–441. [DOI] [PubMed] [Google Scholar]
- 3. Benini F. and Barbi E.. Doing without codeine: why and what are the alternatives? Ital. J. Pediatr 2014;40:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pothiawala S. and Ponampalam R.. Tramadol overdose: a case report. Proc. Singapore Healthc 2011;20:219–223. [Google Scholar]
- 5. Niesters M., Overdyk F., Smith T. et al. Opioid-induced respiratory depression in paediatrics: a review of case reports. Br. J. Anaesth 2012;110:175–182. [DOI] [PubMed] [Google Scholar]
- 6. Randall C. and Crane J., Tramadol deaths in Northern Ireland: a review of cases from 1996 to 2012. J. Forensic Leg. Med 2014;23:32–36. [DOI] [PubMed] [Google Scholar]
- 7. Ndhlala A., Mulaudzi R., Ncube B. et al. Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules 2014;19:10480–10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tousson E., Hafez E., Massoud A.. et al. Ameliorating effect of propolis and moringa extract against equigan induced neurotoxicity and oxidative stress on rat hippocampus. JBSAR 2016;2:30–37. [Google Scholar]
- 9. Tousson E., Zahran F. and Shalapy M. A., Oral supplementation of Aqueous Moringa and Ginkgo leaf extracts abates oxidative stress and testicular injury associated with boldenone injection in rats. Pharmacol 2016;7:381–389. [Google Scholar]
- 10. Atici S., Cinel L., Cinel I. et al. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Asian J. Plant Sci. Res 2004;114:1001–1011. [DOI] [PubMed] [Google Scholar]
- 11. Pal S. K., Mukherjee P. K. and Saha B. P., Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats. 1995;9:463–465. [Google Scholar]
- 12. Al Suleimani Y. M., Al Mahruqi A. S., Al Za’abi M. et al. Effect of diesel exhaust particles on renal vascular responses in rats with chronic kidney disease. Environ. Toxicol 2017;32:541–549. [DOI] [PubMed] [Google Scholar]
- 13. Tousson E., Bayomy M. F. and Ahmed A. A., Rosemary extract modulates fertility potential, DNA fragmentation, injury, KI67 and P53 alterations induced by etoposide in rat testes. Biomed. Pharmacother 2018;98:769–774. [DOI] [PubMed] [Google Scholar]
- 14. Eldaim A., Tousson E., El Sayed I. E. T. et al. Ameliorative effects of Saussurea lappa root aqueous extract against Ethephon-induced reproductive toxicity in male rats. Environ. Toxicol 2019;34:150–159. [DOI] [PubMed] [Google Scholar]
- 15. Oyouni A. A. A., Saggu S., Tousson E. et al. Mitochondrial nephrotoxicity induced by tacrolimus (FK-506) and modulatory effects of Bacopa monnieri (Farafakh) of Tabuk Region. Pharmacognosy Res 2019;11:20. [Google Scholar]
- 16. Tousson E., Histopathological alterations after a growth promoter boldenone injection in rabbits. Ind. Health 2016;32:299–305. [DOI] [PubMed] [Google Scholar]
- 17. Yassa H. A., Dawood A. E. W. A., Shehata M. M. et al. Subchronic toxicity of cannabis leaves on male albino rats. Exp. Toxicol 2010;29:37–47. [DOI] [PubMed] [Google Scholar]
- 18. Tousson E., El-Moghazy M., Massoud A. et al. Histopathological and immunohistochemical changes in the testes of rabbits after injection with the growth promoter boldenone. Reprod. Sci 2012;19:253–259. [DOI] [PubMed] [Google Scholar]
- 19. Abdellatief R. B., Elgamal D. A. and Mohamed E. E. M., Effects of chronic tramadol administration on testicular tissue in rats: an experimental study. Andrologia 2015;47:674–679. [DOI] [PubMed] [Google Scholar]
- 20. Tennese A. A. and Wevrick R.. Impaired hypothalamic regulation of endocrine function and delayed counterregulatory response to hypoglycemia in Magel2-null mice. Endocrinology 2011;152:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inass E., Hassan M., Fouad G. et al. Toxic effects of paroxetine on sexual and reproductive functions of rats. Egypt. J. Hosp. Med 2005;21:16–32. [Google Scholar]
- 22. Grigorov B., Reactive oxygen species and their relation to carcinogenesis. Trakia J. Sci 2012;10:83–92. [Google Scholar]
- 23. Alvarez J. G. and Storey B. T.. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol. Reprod. Dev 1995;42:334–346. [DOI] [PubMed] [Google Scholar]
- 24. Akunna G. G., Ogunmodede O. S., Saalu C. L. et al. Ameliorative effect of Moringa oleifera (drumstick) leaf extracts on chromium-induced testicular toxicity in rat testes. World J. Life Sci. Med. Res 2012;2:20. [Google Scholar]
- 25. Sadek K. M. Chemotherapeutic efficacy of an ethanolic M oringa oleifera leaf extract against chromium-induced testicular toxicity in rats. Andrologia 2014;46:1047–1054. [DOI] [PubMed] [Google Scholar]
- 26. Cajú F. M., Queiroz G. C. D., Torres S. M. et al. Opioid system manipulation during testicular development: results on sperm production and sertoli cells population. Acta Sci. Biol. Sci 2011;33:219–225. [Google Scholar]


