Abstract
Theophylline toxicity results in substantial morbidity and mortality particularly due to its narrow therapeutic index. The development of new treatments for acute theophylline toxicity is a point of research interest. The aim of the present work was to assess the efficacy of L-carnitine (LC) and propranolol in the management of patients with acute theophylline toxicity. The study was conducted on 60 patients with acute theophylline toxicity admitted to the Poison Control Center or Intensive Care Unit at Alexandria Main University Hospital. The studied patients were equally classified into four groups (GPs, 15 patients each): the first group was the control group who received standard treatment protocol for theophylline toxicity. The other three GPs also received standard treatment protocol for theophylline toxicity in addition. The second group (LC group) received LC with a loading dose of 100 mg/kg IV over 30–60 min (maximum 6 g) and the maintenance dose was 50 mg/kg IV every 8 h. The third group (propranolol group) received propranolol, administered slowly intravenous 0.5–1 mg over 1 min; it may be repeated if necessary up to a total maximum dose of 0.1 mg/kg. The fourth group (propranolol and LC) received both IV propranolol and LC in the same doses as previous. Treatment with LC alone or in combination with propranolol resulted in a significant improvement of both clinical and laboratory findings. Although combined therapy yields the best results and outcome, LC can serve as an antidote for acute theophylline toxicity if there is any contraindication to propranolol administration.
Keywords: L-carnitine, β-blocker, propranolol, acute theophylline toxicity, antioxidants
Introduction
Despite the marked decrease in the use of theophylline (a naturally occurring methylxanthine) as a treatment of asthma because of its narrow therapeutic window, yet theophylline intoxication episodes still occur resulting in substantial morbidity and mortality [1]. Theophylline was the second most frequent drug involved in poisoned cases received in the poison control center of Ain Shams University Hospital (PCC, ASUH) through the year 2011 [2]. Theophylline also was the commonest offending drug that caused cardiovascular presentation in the poisoned cases admitted to PCC, ASUH through the year 2013 [3].
Theophylline has a poor therapeutic effect in cases of acute airway spasm, but its continued prescription is mainly for asthma prophylaxis due to its bronchodilator, anti-inflammatory as well as immunomodulatory effects together with its pulmonary vasodilation effect, stimulation of mucociliary clearance and inhibition of mast cell mediator response [4]. It is also used in the treatment of neonatal apnea because of its stimulatory effect on the medullary respiratory center [5].
Minor toxic manifestations include vomiting, tremor or cardiac disturbances without the occurrence of hemodynamic instability, whereas major life-threatening toxic manifestations involved seizures or arrhythmias, resistant to standard therapeutic interventions and combined with hemodynamic compromise [6].
Mechanisms by which xanthine causing toxicities were competitive antagonism of adenosine receptors, inhibition of prostaglandin, enhancing the release of endogenous norepinephrine, and consequent adrenergic receptor stimulation and inhibition of cyclic nucleotide phosphodiesterase (PDE) thus preventing the breakdown of cyclic adenosine monophosphate (cAMP) and guanosine monophosphate (cGMP), translocation of intracellular calcium ions. Moreover, xanthine stimulates β2 adrenergic receptors to increase cAMP with resultant bronchodilatation [4, 7, 8].
It was reported that theophylline-induced tachycardia and seizures were accompanied by alterations in the levels of oxidative stress biochemical markers. This shows that oxidative stress may be involved in theophylline-induced toxicity and that antioxidants may have a role in management [9, 10].
Propranolol is a nonselective beta-blocker. It reverses peripheral vasodilation, hypotension via β2-adrenergic receptors blockage, and reverses tachycardia via β1-adrenergic receptors blockage [11].
L-Carnitine (LC) is an effective antioxidant agent, has an important role in normal mitochondrial oxidation of fatty acids. It can protect human endothelial cells against lipid peroxidation and decreases xanthine oxidase activity [12].
Actually, acute theophylline toxicity constitutes a common toxicity especially in developing countries because of its cheap price and availability of over-the-counter preparations, 10-fold dosing error [13], Intake of sustained-release formulations and absence of specific antidote and presence of drug interactions affecting the drug metabolism and clearance aggravate the problem. Hence, the need developed for recognizing the efficacy of some drugs like LC and propranolol in the management of acute theophylline toxicity, which was the target of the present work.
Subjects and Methods
The study was conducted on 60 patients with acute theophylline toxicity admitted to the Poison Control Center or Intensive Care Unit at Alexandria Main University Hospital.
The patients were enrolled in the study in concordance to the following inclusion criteria: age ranged from 16 to 70 years [4, 14, 15]. Patients admitted within 24 h from exposure to the poison without any prior treatment before admission.
Patients were excluded if they had any of the following conditions: contraindication to propranolol [16], smokers, patients with severe hypoalbuminemia (albumin < 2 g/dl) including cases of chronic malnutrition, hemorrhage, protein-losing nephropathy, enteropathy, hepatic failure [17], patients having malignancy, diabetes mellitus, or cardiac diseases, in addition to cases of coingestion.
The current study was carried out after approval of the Ethics Committee of Faculty of Medicine, Alexandria University (IRB number: 00007555, approval serial number: 0105608). Informed consent was obtained from every patient included in the study or from his family, explaining the aim and the procedure of the research. Complete confidentiality was ensured all through the study procedures.
Diagnosis of acute theophylline toxicity was performed by history-taking or checking the recently used tablets, or by characteristic symptoms and signs of toxicity as well as by serum theophylline concentration.
All patients were subjected to thorough history taking, complete physical examination. Electrocardiography (ECG) was performed and laboratory investigations were measured for every patient both on admission and 24 h after the start of treatment, including serum theophylline level, arterial blood gases, and serum oxidative stress markers: glutathione (GSH) and malondialdehyde (MDA).
The standard treatment protocol for theophylline toxicity was given to all patients. It included maintenance of airway and breathing and stabilization of circulation by giving IV fluids (crystalloids) to treat hypotension and use vasopressors if needed with the management of arrhythmia. Decontamination using activated charcoal was performed if vomiting stopped early. Gastrointestinal tract (GIT) disturbance was managed by antiemetic (ondansetron) and H2 blocker (ranitidine). Treatment of seizures and other complications was done. Close monitoring of serum potassium values with potassium replacement via IV infusion. Elimination can be enhanced by hemodialysis if indicated [11, 18–20].
The patients were divided into four equal groups (GPs) 15 patients each) as follows: the first group was the control group who received standard treatment protocol for theophylline toxicity. The other three GPs also received standard treatment protocol for theophylline toxicity in addition. The second group (LC group) received LC with a loading dose of 100 mg/kg IV over 30–60 min (maximum 6 g) and the maintenance dose was 50 mg/kg IV every 8 h [21]. The third group (propranolol group) received beta-blocker (propranolol) administered slowly intravenous 0.5–1 mg over 1 min; it may be repeated if necessary up to a total maximum dose of 0.1 mg/kg [22]. The fourth group (propranolol and LC) received both IV propranolol and LC in the same doses as previous.
The outcome was assessed according to the length of hospitalization and the need for intensive care unit (ICU) admission and categorized into three categories: recurrence of cardiovascular system (CVS) symptoms, improvement/recovery or death.
Statistical analysis of the data
The sample size was calculated using a one-way analysis of variance (ANOVA) test (PASS program version 20).
Data were analyzed using IBM SPSS software package version 20.0. Qualitative data were described using numbers and percentages. Categorical data were evaluated by the chi-square test and Monte Carlo correction. The Kolmogorov–Smirnov test was used to verify the normality of distribution. For normally distributed quantitative variables, a paired t-test was used to compare between two periods, and F-test (ANOVA) to compare between more than two GPs. While for abnormally distributed quantitative variables, Wilcoxon-signed rank test was used to compare between two periods and Kruskal–Wallis test to compare between more than two studied GPs. The significance of the obtained results was examined at the 5% level.
Results
The study enrolled 60 patients; 53 patients were females (88.3%), whereas seven patients were males (11.7%) with a sex ratio of 7.57:1. The age of the patients ranged from 16 to 52 years, with a mean age of 23.20 ± 8.12 years. The majority of cases (80%) took theophylline to commit suicide and 20% of cases were accidental poisoning. No statistically significant differences were detected among the four studied GPs regarding age, sex, marital status, education, and circumstances of poisoning (Table 1).
Table 1.
Comparison between the four studied GPs according to personal history and circumstances of poisoning
| Personal history | GP I (n = 15) | GP II (n = 15) | GP III (n = 15) | GP IV (n = 15) | Total | Test of significance | P * | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Age (years) | ||||||||||||
| ≤30 | 12 | 80.0 | 13 | 86.7 | 14 | 93.3 | 14 | 93.3 | 53 | 88.33 | χ 2 7.596 | MC P = 0.967 |
| >30 | 3 | 20.0 | 2 | 13.3 | 1 | 6.7 | 1 | 6.7 | 7 | 11.67 | ||
| Min.–Max. | 16.0–52.0 | 16.0–40.0 | 16.0–40.0 | 17.0–40.0 | 16.0–52.0 | H = 1.143 | 0.767 | |||||
| Mean ± SD | 24.73 ± 11.37 | 22.53 ± 7.70 | 22.93 ± 6.41 | 22.60 ± 6.67 | 23.20 ± 8.12 | |||||||
| Gender | ||||||||||||
| Male | 1 | 6.7 | 2 | 13.3 | 3 | 20.0 | 1 | 6.7 | 7 | 11.7 | χ 2 = 1.729 | MC P = 0.822 |
| Female | 14 | 93.3 | 13 | 86.7 | 12 | 80.0 | 14 | 93.3 | 53 | 88.3 | ||
| Marital status | ||||||||||||
| Single | 10 | 66.7 | 10 | 66.7 | 10 | 66.7 | 12 | 80.0 | 42 | 70.0 | χ 2 = 2.102 | MC P = 0.972 |
| Married | 4 | 26.7 | 4 | 26.7 | 4 | 26.7 | 3 | 20.0 | 15 | 25.0 | ||
| Divorced | 1 | 6.7 | 1 | 6.7 | 1 | 6.7 | 0 | 0.0 | 3 | 5.0 | ||
| Education | ||||||||||||
| Illiterate | 3 | 20.0 | 4 | 26.7 | 4 | 26.7 | 4 | 26.7 | 15 | 25.0 | χ 2 = 0.909 | MC P = 1.000 |
| Pre-university | 8 | 53.3 | 8 | 53.3 | 7 | 46.7 | 8 | 53.3 | 31 | 51.7 | ||
| University | 4 | 26.7 | 3 | 20.0 | 4 | 26.7 | 3 | 20.0 | 14 | 23.3 | ||
| Circumstances of poisoning | ||||||||||||
| Suicidal | 11 | 73.3 | 12 | 80.0 | 14 | 93.3 | 11 | 73.3 | 48 | 80.0 | χ 2 = 2.648 | MC P = 0.489 |
| Accidental | 4 | 26.7 | 3 | 20.0 | 1 | 6.7 | 4 | 26.7 | 12 | 20.0 | ||
χ 2, chi-square test; MC, Monte Carlo; H, H for Kruskal–Wallis test; GP I, control group; GP III, propranolol group; GP II, LC group; GP IV, propranolol and LC.
* P-value for comparison between the four studied GPs.
There was no statistically significant difference among the four GPs regarding the time of starting treatment after exposure. The serum theophylline level was significantly lower after 24 h of admission than that on admission in all GPs. The highest % change or decrease in serum theophylline level was detected in GPs received LC (GP IV, % change = 86% and GP II, % change 84.9%) (Table 2).
Table 2.
Comparison between the four studied GPs according to time of starting treatment and serum theophylline level
| GP I (n = 15) | GP II (n = 15) | GP III (n = 15) | GP IV (n = 15) | H | P | |
|---|---|---|---|---|---|---|
| Time of starting treatment after exposure (h) | ||||||
| Min.–Max. | 1.50–14.0 | 2.0–10.0 | 2.50–12.0 | 3.0–14.0 | H = 1.324 | 0.723 |
| Mean ± SD | 5.93 ± 3.76 | 5.53 ± 2.70 | 5.60 ± 2.84 | 6.60 ± 3.29 | ||
| Serum theophylline level (mg/L) | ||||||
| On admission | ||||||
| Min.–Max. | 21.60–88.90 | 26.60–97.0 | 34.40–88.30 | 35.20–90.50 | 3.467 | 0.325 |
| Mean ± SD | 57.19 ± 21.23 | 55.58 ± 24.19 | 62.19 ± 18.24 | 69.05 ± 15.06 | ||
| After 24 h | ||||||
| Min.–Max. | 4.90–29.0 | 0.60–17.0 | 8.20–26.30 | 3.40–21.20 | 16.862* | 0.001* |
| Mean ± SD. | 16.39 ± 8.50 | 8.47 ± 4.86 | 17.09 ± 5.68 | 9.79 ± 4.05 | ||
| Pcontrol | 0.006* | 0.530 | 0.019* | |||
| Significance between GPs | P 1 = 0.001*, P2 = 0.683, and P3 = 0.003* | |||||
| P0 | 0.001* | 0.001* | 0.001* | 0.001* | ||
| % Change | ↓72.0 | ↓84.9 | ↓72.2 | ↓86.0 | ||
Pairwise comparison between each two GPs was carried out using post hoc test (Dunn’s for multiple comparisons test). H, H for Kruskal–Wallis test; χ2, chi-square test; MC, Monte Carlo; P, P-value for comparison between the four studied GPs; P0, comparison between on admission and after 24 h in each GP; Pcontrol, P-value for comparison between GP I and each other GP; P1, P value for comparison between GP II and GP III; P2, P value for comparison between GP II and GP IV; P3, P value for comparison between GP III and GP IV.
*Statistically significant at P ≤ 0.05.
A statistically significant difference was not found among the four GPs regarding the measured serum theophylline level on admission and the difference was developed among them only after 24 h of the start of therapeutic intervention. A statistically significant difference detected only between GP II and either control or GP III and between GP IV and either control or GP III (between any of the two GPs received carnitine and other two GPs; Table 2).
The heart rate (HR) significantly decreased after passing 24 h of therapeutic intervention than that on admission in all GPs. The highest % change or decrease in HR was detected in the GP received combined therapy of propranolol and LC (GP IV; 32.36%) and it was the only GP that had a statistically significant difference with the control GP after 24 h of applying the treatment (Table 3).
Table 3.
Comparison between the four studied GPs according to HR and mean arterial blood pressure
| GP I (n = 15) | GP II (n = 15) | GP III (n = 15) | GP IV (n = 15) | F | P | ||
|---|---|---|---|---|---|---|---|
| HR (beat/min) | On admission | ||||||
| Min.–Max. | 95.0–190.0 | 100.0–170.0 | 125.0–150.0 | 100.0–166.0 | 1.191 | 0.322 | |
| Mean ± SD. | 128.3 ± 26.14 | 130.1 ± 17.27 | 139.0 ± 8.49 | 137.1 ± 17.82 | |||
| On admission | |||||||
| Min.–Max. | 80.0–135.0 | 75.0–125.0 | 75.0–110.0 | 70.0–110.0 | 2.781* | 0.049* | |
| Mean ± SD | 105.0 ± 15.47 | 97.73 ± 14.22 | 94.13 ± 9.48 | 92.33 ± 12.08 | |||
| Pcontrol | 0.427 | 0.113 | 0.048* | ||||
| Significance between GPs | P 1 = 0.873, P2 = 0.669, and P3 = 0.981 | ||||||
| P0 | <0.001* | <0.001* | <0.001* | <0.001* | |||
| % change | ↓17.06 | ↓24.68 | ↓32.26 | ↓32.36 | |||
| Mean BP (mmhg) | On admission | ||||||
| Min.–Max. | 60.0–113.0 | 60.0–97.0 | 40.0–103.0 | 53.0–100.0 | 0.655 | 0.583 | |
| Mean ± SD. | 76.07 ± 14.61 | 69.33 ± 9.41 | 70.80 ± 17.01 | 73.33 ± 14.53 | |||
| After 24 h | |||||||
| Min.–Max. | 70.0–103.0 | 83.0–100.0 | 70.0–108.0 | 80.0–108.0 | 3.777* | 0.015* | |
| Mean ± SD. | 83.67 ± 8.94 | 90.40 ± 5.38 | 88.13 ± 10.91 | 94.07 ± 8.54 | |||
| Pcontrol | 0.157 | 0.498 | 0.009* | ||||
| Significance between GPs | P 1 = 0.890, P2 = 0.656, and P3 = 0.251 | ||||||
| P0 | 0.011* | <0.001* | <0.001* | <0.001* | |||
| % Change | ↑11.9 | ↑32.1 | ↑28.8 | ↑31.6 | |||
Pairwise comparison between each two GPs was carried out using post hoc test (Tukey). F, F for ANOVA test; P, P-value for comparison between the four studied GPs; P0, comparison between on admission and after 24 h in each GP; Pcontrol, P value for comparison between GP I and each other GP; P1, P value for comparison between GP II and GP III; P2, P value for comparison between GP II and GP IV; P3, P value for comparison between GP III and GP IV.
*Statistically significant at P ≤ 0.05.
A statistically significant difference developed among the four GPs regarding ECG only after passing 24 h after the treatment, where 73.3% of GP I patients presented with different types of arrhythmias, whereas the least percentage of arrhythmia was detected in GPs received propranolol; GP III and IV (13.3% each). The highest % change or decrease in sinus arrhythmia occurred with GP IV (73.4%) (Figs 1 and 2).
Figure 1.
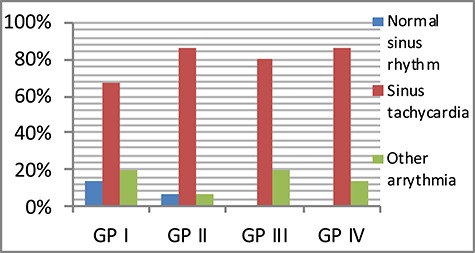
Comparison between the four studied GPs according to heart rhythm on admission (GP I, Control group; GP III, propranolol group; GP II, LC group; GP IV, propranolol and LC).
Figure 2.
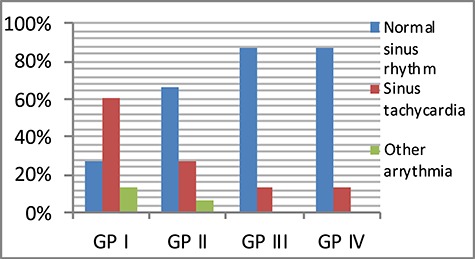
Comparison between the four studied GPs according to heart rhythm after 24 h.
As regards to mean arterial pressure (MAP), it was significantly higher after 24 h of admission than that on admission in all GPs. GPs received LC showed the highest % increase in MAP (32.1% in GP II, 31.6% in GP IV)). A statistically significant difference developed among the four GPs after 24 h. GP IV was the only GP that had a significantly higher MAP than that of the control GP (Table 3).
The respiratory rate (RR) on admission was high in all four GPs. RR significantly decreased after 24 h in all GPs except the control GP. The highest % decrease in RR presented in (GP IV; 37%) (Table 4).
Table 4.
Comparison between the four studied GPs according to RR
| RR | GP I (n = 15) | GP II (n = 15) | GP III (n = 15) | GP IV (n = 15) | F | P |
|---|---|---|---|---|---|---|
| On admission | ||||||
| Min.–Max. | 25.0–40.0 | 25.0–40.0 | 25.0–40.0 | 25.0–50.0 | 1.104 | 0.355 |
| Mean ± SD. | 32.13 ± 4.24 | 31.93 ± 5.16 | 30.47 ± 4.34 | 34.0 ± 7.12 | ||
| After 24 h | ||||||
| Min.–Max. | 20.0–45.0 | 19.0–30.0 | 16.0–27.0 | 16.0–28.0 | 11.352* | <0.001* |
| Mean ± SD | 30.0 ± 7.19 | 23.53 ± 3.56 | 21.73 ± 3.17 | 21.07 ± 3.67 | ||
| Pcontrol | 0.002* | <0.001* | <0.001* | |||
| Significance between GPs | P 2 = 0.720, P3 = 0.480, and P4 = 0.980 | |||||
| P0 | 0.247 | <0.001* | <0.001* | <0.001* | ||
| % Change | ↓6.1 | ↓26.0 | ↓27.9 | ↓37.0 | ||
Pairwise comparison between each two GPs was carried out using post hoc test (Tukey). F, F for ANOVA test; P, P-value for comparison between the four studied GPs; P0, comparison between on admission and after 24 h in each GP.
*Statistically significant at P ≤ 0.05.
There were statistical significant differences among the four studied GPs regarding the mean levels of MDA and GSH after 24 h of admission The control and propranolol GPs had a high % of increase MDA levels after 24 h of admission (167.3% and 153.7% respectively), while both GPs received LC showed % decrease in MDA levels (60.5% in GP II, 51.6% in GP IV) (Fig. 3).
Figure 3.
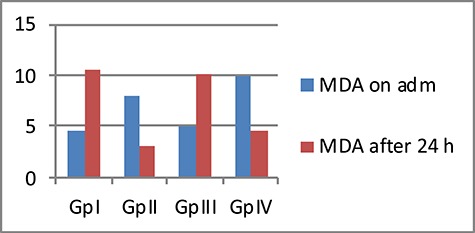
Comparison between the four studied GPs according to mean MDA level (nmol/ml)
On the other hand, all GPs had significantly higher mean GSH levels after 24 h than those on admission. GP II that received LC only had the highest % of increased GSH levels after 24 h of admission (147.7%) (Fig. 4).
Figure 4.
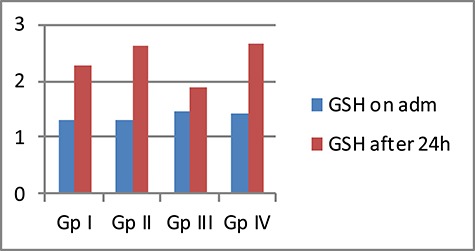
Comparison between the four studied GPs according to mean GSH level (mg/dl)
The mean PH decreased after 24 h of admission in all GPs. The lowest % decrease in PH was in the two GPs received carnitine (GP II; ↓0.33, GP IV; ↓0.03). On the other hand, the mean PaCO2 increased after 24 h of admission in all GPs, with the highest % increase in PaCO2 recorded in the same two GPs received carnitine (GP II; ↑30.67, GP IV; ↑23.47) (Figs 5 and 6).
Figure 5.
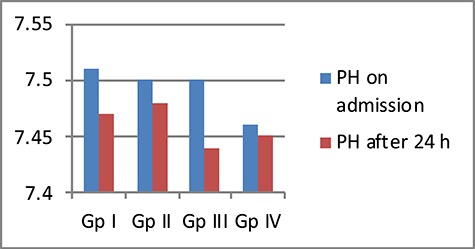
Comparison between the four studied GPs according to mean PH
Figure 6.
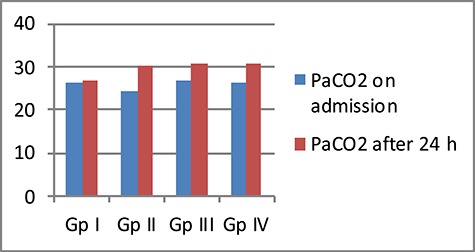
Comparison between the four studied GPs according to mean PaCO2 level (mmHg)
The mean serum HCO3 showed decreased levels in all GPs on time of admission, which improved after 24 h in all GPs with statistically significant differences between its mean levels in the two measuring times in only two GPs that received LC (GP II and GP IV). The highest % increase in HCO3 levels was recorded in the same two GPs received carnitine (GP II; ↑23.28, GP IV; ↑34.72) (Table 5).
Table 5.
Comparison between the four studied GPs according to mean HCO3 levels (mmHg)
| HCO3 | GP I (n = 15) | GP II (n = 15) | GP III (n = 15) | GP IV (n = 15) | F | P |
|---|---|---|---|---|---|---|
| On admission | ||||||
| Min.–Max. | 13.50–23.70 | 8.90–24.60 | 13.50–23.80 | 11.30–27.60 | 1.252 | 0.300 |
| Mean ± SD | 19.82 ± 2.38 | 18.93 ± 3.69 | 19.10 ± 2.89 | 17.44 ± 4.50 | ||
| After 24 h | ||||||
| Min.–Max. | 15.0–22.90 | 19.70–24.60 | 16.70–23.40 | 19.90–25.0 | 9.646* | <0.001* |
| Mean ± SD | 19.63 ± 2.23 | 22.35 ± 1.70 | 19.96 ± 1.81 | 22.34 ± 1.55 | ||
| Pcontrol | 0.001* | 0.962 | 0.001* | |||
| Significance between GPs | P 1 = 0.004*, P2 = 1.000, and P3 = 0.004* | |||||
| P0 | 0.790 | <0.001* | 0.051 | <0.001* | ||
| % Change | ↑0.12 | ↑23.28 | ↑5.70 | ↑34.72 | ||
Pairwise comparison between each two GPs was carried out using post hoc test (Tukey). F, F for ANOVA test; P, P-value for comparison between the four studied GPs; P0, comparison between on admission and after 24 h in each GP; Pcontrol, P value for comparison between GP I and each other GP; P1, P value for comparison between GP II and GP III; P2, P value for comparison between GP II and GP IV; P3, P value for comparison between GP III and GP IV.
*Statistically significant at P ≤ 0.05.
There were highly statistically significant differences among the four studied GPs regarding both the total amount of fluid and antiemetic taken within 24 h. The control GP had the highest mean total fluid taken, whereas the lowest mean fluid taken was in GPs received LC (GP II and IV). GP IV had the lowest mean among all studied GPs regarding both lines of treatment (Table 6).
Table 6.
Comparison between the four studied GPs according to management
| Management | GP I (n = 15) | GP II (n = 15) | GP III (n = 15) | GP IV (n = 15) | Test of significance | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |||
| Total IV fluids in 24 h | ||||||||||
| Min.–Max. | 2.0–3.50 | 1.50–3.50 | 1.50–3.50 | 1.50–3.0 | F = 7.026* | <0.001* | ||||
| Mean ± SD | 2.97 ± 0.52 | 2.27 ± 0.53 | 2.70 ± 0.46 | 2.23 ± 0.56 | ||||||
| Pcontrol | 0.003* | 0.498 | 0.002* | |||||||
| Significance between GPs | P 1 = 0.112, P2 = 0.998, and P3 = 0.076 | |||||||||
| Anti-emetic (ondansetron 4 mg) | ||||||||||
| Min.–Max. | 8.0–24.0 | 4.0–12.0 | 4.0–24.0 | 1.50–3.0 | H = 43.662* | <0.001* | ||||
| Mean ± SD | 14.67 ± 4.19 | 8.53 ± 2.56 | 12.8 ± 4.83 | 2.23 ± 0.56 | ||||||
| Pcontrol | 0.003* | 0.390 | <0.001* | |||||||
| Significance between GPs | P 1 = 0.039*, P2 = 0.002*, and P3 < 0.001* | |||||||||
Pairwise comparison between each two GPs was carried out using post hoc test (Tukey). χ2, chi square test; MC, Monte Carlo; H, H for Kruskal–Wallis test; F, F for ANOVA test.
Only two cases in the control GP needed ICU admission and one of them needed MV. The same occurred with GP IV. However, the duration of stay in ICU was longer in the control GP than that in GP IV, reaching 5 days in the control GP versus 2 days maximally in GP IV. There were statistically significant differences among them regarding the days stayed in the poison center. The control GP had the highest mean days of hospital stay and GP IV had the lowest mean days of stay. There were also statistically significant differences among the four GPs regarding the outcome, where recurrence of symptoms occurred in 66.7% of control cases and the highest percentage of improved cases was in GP IV (86.7%) (Table 7).
Table 7.
Comparison between the four studied GPs according to outcome
| Outcome | GP I (n = 15) | GP II (n = 15) | GP III (n = 15) | GP IV (n = 15) | Test of significance | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |||
| Length of hospital stay (days) | ||||||||||
| Days in poison centre | (n = 15) | (n = 15) | (n = 15) | (n = 15) | ||||||
| Min.–Max. | 1.0–4.0 | 1.0–3.0 | 1.0–3.0 | 1.0–3.0 | F = 2.765* | 0.049* | ||||
| Mean ± SD | 2.40 ± 0.74 | 2.13 ± 0.74 | 2.27 ± 0.59 | 1.73 ± 0.59 | ||||||
| Days in ICU | (n = 2) | (n = 0) | (n = 0) | (n = 2) | ||||||
| Min.–Max. | 2.0–5.0 | 1.0–2.0 | # | # | ||||||
| Mean ± SD. | 3.50 ± 2.12 | – | – | 1.50 ± 0.71 | ||||||
| Outcome | ||||||||||
| Recurrence of symptoms | 10 | 66.7 | 5 | 33.3 | 5 | 33.3 | 2 | 13.3 | χ 2 = 14.013* | MC P = 0.023* |
| Improvement/recovery | 5 | 33.3 | 10 | 67.7 | 10 | 67.7 | 13 | 86.7 | ||
| Died | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Pcontrol | 0.068 | 0.068 | 0.003* | |||||||
| Significance between GPs | P 1 = 1.000, FEP2 = 0.390, and FEP3 = 0.390 | |||||||||
Pairwise comparison between each two GPs was carried out using post hoc test (Dunn’s for multiple comparisons test). χ2, Chi square test; MC, Monte Carlo; H, H for Kruskal–Wallis test; P, P value for comparison between the four studied GPs.
*Statistically significant at P ≤ 0.05.
#Excluded from the comparison due to small number of cases.
Discussion
The study enrolled 60 patients with a female to male sex ratio of 7.57:1. The majority of cases (80%) took theophylline to commit suicide.
The age of the patients ranged from 16 to 52 years, as age is one of the important factors affecting theophylline clearance. Theophylline clearance is progressively increased from birth reaching the maximum clearance in young children and stabilizes in the mid-teens then decreases again in the elderly [4, 15]. In the present work, the majority of the intoxicated patients (88.33%) were in the age GP ≤ 30 years as adolescents and young adults are more prone to mood changes and attempted suicide.
No statistically significant difference was found among the four GPs regarding serum theophylline level on admission. The highest % change or decrease in serum theophylline level after 24 h of admission was detected in GPs received LC (GP IV, % change = 86% and GP II, % change = 84.9%). This may indicate the role of LC in theophylline clearance similar to enhancing clearance of valproic acid and so, was used as an antidote for it [23].
The highest % change or decrease in HR after 24 h of admission was detected in the GP received combined therapy (GP IV; 32.36%) and it was the only GP that had a statistically significant difference with the control GP after 24 h of applying the treatment.
β-Blockers have a negative chronotropic effect through blocking β1 receptors [24]. Moreover, a significant negative chronotropic effect was detected by Brooks et al. [25], reporting a reduction of HR from 124 to 86 beats/min after 1 h of intravenous LC injections in their animal studies.
After passing 24 h of the treatment, the least percentage of detected arrhythmia was in GPs received propranolol; GP III and IV (13.3% each). The highest % change or decrease in sinus arrhythmia occurred with GP IV (73.4%). β-Blocker (propranolol) is preferred in the treatment of arrhythmia of theophylline than using Ca channel blocker as the latter can worsen the state of hypotension.
Freeman and Crapo [26] and McCord et al. [27] stated that increased catecholamine stimulates the generation of free radicals via the increase in mitochondrial respiration and auto-oxidation. These free radicals negatively affect myocardial metabolism and function, inducing arrhythmias [28, 29]. This explains the role of an antioxidant in the treatment of arrhythmia.
Colbridge [4] reported that propranolol could treat supraventricular arrhythmia and Imai et al. [30] stated that carnitine significantly decreased ventricular arrhythmias even ventricular fibrillation.
As regards to MAP, GPs received LC showed the highest % increase in MAP (32.1% in GP II, 31.6% in GP IV)). A statistically significant difference developed among the four GPs after 24 h. GP IV was the only one that had a significantly higher MAP than that of the control GP.
The hypotension in theophylline is common and multifactorial and resulted from severe protracted vomiting, β2 stimulated peripheral vasodilatation and inhibition of PDE with increased cAMP causing vascular smooth muscle relaxation. In addition, theophylline stimulates β1 receptors in myocytes producing a positive chronotropic effect, which causes a decrease in time for diastolic filling aggravating the hypotensive state.
β-Blockers can treat hypotension by producing a negative chronotropic effect through blocking β1 receptors, slowing the HR, prolonging the diastolic filling time, thus increases stroke volume and improving cardiac output [24].
Propranolol (nonselective β-blocker) is superior to esmolol (selective β1 blocker; cardioselective) as it has a longer half-life (T1/2) than that of esmolol (only 9 min), and propranolol can reverse metabolic disturbances induced by theophylline as they are β2 mediated [4, 31]. Moreover, propranolol can treat hypotension by counteracting the relaxing effect of stimulated β2 receptors on peripheral vessels as an additional mechanism [31]. However, esmolol is preferred to treat theophylline-induced hypotension in asthmatic patients to avoid bronchospasm induced by β2 blocking effect of propranolol.
Suzuki et al. [32] found that intravenous carnitine caused a 60% increase in coronary blood flow which reached over 100% increase in the study of Brooks et al. [25] with dramatic improvements in left ventricular work, stroke volume, cardiac output, and other parameters of contractility.
Gurlek et al. [33] stated that the addition of LC to the conventional therapy of ischemic cardiomyopathy was more significant than treatment with conventional treatment only as LC produced improvement in left ventricular ejection fraction.
Carnitine maintains energy (ATP) production by facilitation of the transfer of long-chain fatty acid into mitochondria (increases fat oxidation) and regulation of carbohydrate metabolism through increasing glucose oxidation. Cardiomyocytes can use fat for energy by carnitine. Carnitine also enhances lactate extraction from cardiomyocytes, thus improves oxygen consumption and exercise tolerance and many cardiac parameters and delays the onset of fatigue. So, it was indicated in various cardiac disorders. Moreover, it acts as cardioprotective by increasing the resistance of cardiac cell membranes to reactive oxygen species, by decreasing levels of toxic coenzyme A derivatives [34, 35]. Fujiwara et al. [36] reported that LC increased coronary blood flow during exercise.
The RR on admission was high in all four GPs by stimulatory action of theophylline on the respiratory center, causing hyperventilation, and respiratory alkalosis. RR was significantly decreased after 24 h in all GPs except the control GP. The highest % decrease in RR was encountered in GP IV (37%), this confirms the superiority of combined therapy in GP IV than other GPs.
Bernard [37] reported that respiratory alkalosis was the commonest acid–base disturbance encountered in acute theophylline overdose. Greene et al. [20] stated that respiratory alkalosis is common in awake patients, which coincides with the present study where 93.3% of patients were fully conscious (n = 56).
There were statistical significant differences among the four studied GPs regarding the mean levels of MDA and GSH after 24 h of admission The control and propranolol GPs had a high % of increase MDA levels after 24 h of admission (167.3% and 153.7%, respectively), while both GPs received LC showed % decrease in MDA levels (60.5% in GP II, 51.6% in GP IV). On the other hand, all GPs had significantly higher mean GSH levels after 24 h than those on admission. GP II that received LC only had the highest % of increase GSH levels after 24 h of admission (147.7%).
Oxidative stress or imbalance of redox status has been implicated in the toxicity induced by aminophylline including seizures [38]. Gulati et al. [9] reported that seizures, as well as mortality, were declined by antioxidants use (melatonin, N-acetylcysteine) and by nitric oxide (NO) synthase inhibitors. They detected high levels of MDA and NO metabolites in the brain of mice with aminophylline-induced seizures, and these metabolites decreased by antioxidant pretreatment. This was confirmed by reduction of seizures as well as mortality by 10% in case of pretreatment with LC in the study of Roy et al. [39], where the tested efficacy of LC in correction of imbalance of redox status was manifested by the significant suppression of MDA and significantly improved superoxide dismutase activity in treated rats’ brains than control GP.
Carnitine is biosynthesized from the amino acids methionine and lysine. LCs are the biologically active enantiomer having antioxidant property, it protects against oxidative stress induced at the myocardial and endothelial cell level [40]. LC counteracts oxidative stress indirectly by augmenting the activity of cellular scavengers as GSH and vitamin E or by acting as a metal chelator [41] and directly through inhibition of arachidonic acid (AA)-induced nicotinamide adenine dinucleotide phosphate oxidase activation [42].
The mean PH decreased after 24 h of admission in all GPs. The lowest % decrease in PH was in the two GPs received carnitine (GP II; ↓0.33, GP IV; ↓0.03). On the other hand, the mean PaCO2 increased after 24 h of admission in all GPs, with the highest % increase in PaCO2 recorded in the same two GPs received carnitine (GP II; ↑30.67, GP IV; ↑23.47).
The study revealed that the highest % increase in HCO3 levels was recorded in GP IV; ↑34.72). Colbridge [4] reported that propranolol can reverse the β2-mediated metabolic disturbances induced by theophylline such as hypokalemia, hypophosphatemia, hypophosphatemia, hyperglycemia, and acidosis.
The study revealed that GP IV had the lowest mean among all studied GPs regarding the total amount of fluid and antiemetic taken within 24 h. Methylxanthines induce vomiting by multiple mechanisms; through a direct effect on medullary vomiting center, or by increasing the gastric acid and pepsin secretion causing esophagitis and gastritis, they also cause smooth muscle relaxation including relaxation of the lower esophageal sphincter [43].
It was found that LC showed a decrease in C-reactive protein, i.e. acts as an anti-inflammatory [44]. Moreover, LC strengthens the gastric mucosal barrier, thus prevents the occurrence of mucosal lesions. It acts by counteracting the effect of lipid peroxidation, hence it prevents the decrease in prostaglandin E2 (PGE2) content of gastric mucosa, which is a potent gastroprotective [45].
The duration of stay in ICU was longer in the control GP than that in GP IV as it reached 5 days in the control GP versus 2 days maximally in GP IV. The control GP had the highest mean days of hospital stay and GP IV had the lowest mean days of stay. There were also statistically significant differences among the four GPs regarding the outcome, where recurrence of symptoms occurred in 66.7% of control cases and the highest percentage of improved cases was in GP IV (86.7%).
Oami et al. [46] reported that nearly one-fourth of patients admitted to ICU had LC deficiency and could benefit from LC supplementation. Carnitine depletion occurred in ICU admission as the patients are usually nourished through parenteral feeding, which lacks sufficient LC content and also LC may be depleted in those patients because of the metabolic load on mitochondrial function in critical illness.
LC can serve as an antidote for theophylline. Firstly, as being an antioxidant, it counteracts the oxidative stress mechanism involved in theophylline toxicity, secondly because of its safety and tolerability. Arsenian [47] recommended LC in his study as the majority of clinical studies with carnitine did not report any side effects or major clinical toxicity.
Conclusion
Early pretreatment with combined therapy; GP IV (propranolol and L-carnitine) proved its superiority over the other studied GPs in the amelioration of both clinical and laboratory manifestations of acute theophylline toxicity. After 24 h of therapeutic intervention, GP IV showed the highest % decrease in serum theophylline level, RR, HR, and sinus arrhythmia with the highest % increase in serum HCO3 level. Moreover, GP IV had the lowest mean value of the total amount of IV fluid intake, antiemetic needed and hospital stay period. Finally, GP IV showed the best outcome (i.e. the highest % of improved or recovered cases and the lowest % of recurrence of symptoms).
Regarding the oxidative stress markers, GP II showed the best results among the four studied GPs followed by GP IV where GP II had the highest % decrease in MDA level together with the highest % increase in GSH level. Efficacy of L-carnitine alone or in combination with propranolol proved the role of oxidative stress mechanism in theophylline toxicity.
Recommendations
The study recommends modification of the existing regimen for the treatment of acute theophylline toxicity by the addition of propranolol and LC. Pretreatment with both drugs is beneficial especially before the development of manifestations. The study also recommends pretreatment with LC alone if there is any contraindication to propranolol administration as showed high efficacy also if used alone.
Funding
There are no funding sources.
Conflict of Interest Statement
There are no conflicts of interest to declare.
References
- 1. Yaman A, Kendirli T, Ödek C, et al. Severe theophylline poisoning treated with continuous venovenous hemodialysis in a child. Turk J Pediatr 2016;58:297–300. [DOI] [PubMed] [Google Scholar]
- 2. El-Masry MK, Tawfik HM. Annual report of the Poison Control Centre of Ain Shams University Hospital, Cairo, Egypt. Ain Shams J Forensic Med Clin Toxicol 2011, 2013;20:10–7. [Google Scholar]
- 3. Tawfik H, El-Helaly H. Toxicological profile of acutely poisoned cases admitted to poison control center, Ain-Shams University Hospitals during year 2013. Ain Shams J Forensic Med Clin Toxicol 2015;24:154–63. [Google Scholar]
- 4. Colbridge M. Theophylline overdose: clinical features and management. Emerg Nurse 2001;8:24–9. [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Feng MX, Qu JM. Low dose theophylline showed an inhibitory effect on the production of IL-6 and IL-8 in primary lung fibroblasts from patients with COPD. Mediators Inflamm 2012;492901. doi: 10.1155/2012/492901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shannon M. Life-threatening events after theophylline overdose: a 10-year prospective analysis. Arch Intern Med 1999;159:989–94. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman RS. Fluid, Electrolyte, and Acid-Base Principles In: Goldfrank LR, Howland MA, Hoffman RS, et al. (eds). Goldfrank’s Toxicologic Emergencies, 7th edn. New York: McGraw-Hill, 2006, 2170. [Google Scholar]
- 8. Boison D. Methylxanthines, seizures, and excitotoxicity. Handb Exp Pharmacol 2011;200:251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gulati K, Ray A, Pal G, Vijayan VK. Possible role of free radicals in theophylline-induced seizures in mice. Pharmacol Biochem Behav 2005;82:241–5. [DOI] [PubMed] [Google Scholar]
- 10. Ray A. Clinical and experimental studies on theophylline toxicity: In search for an antidote. J Clin Toxicol 2014;4:4. [Google Scholar]
- 11. Hoffman RJ. Methylxanthines and selective β2-adrenergic agonists In: Hoffman RS, Nelson LS, Howland MA, et al. (eds). Goldfrank's Toxicologic Emergencies, 10th edn. New York: McGraw Hill, 2015, 2253–79. [Google Scholar]
- 12. Ribas GS, Vargas CR, Wajner M. L-Carnitine supplementation as a potential anti-oxidant therapy for inherited neurometabolic disorders. Gene 2013;533:469–76. [DOI] [PubMed] [Google Scholar]
- 13. Schiff GD, Hegde HK, LaCloche L, Hryhorczuk DO. Inpatient theophylline toxicity: preventable factors. Ann Intern Med 1991;114:748–53. [DOI] [PubMed] [Google Scholar]
- 14. Hendeles L, Jenkins J, Temple R. Revised FDA labeling guidelines for theophylline oral dosage forms. Pharmacotherapy 1995;15:409–27. [PubMed] [Google Scholar]
- 15. Barnes PJ. Theophylline. Pharmaceuticals 2010;3:725–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schroeder JS, Frishman WH, Parker JD, et al. In: Antman EM, Sabatine MS (eds). Cardiovascular Therapeutics: A Companion to Braunwald's Heart Disease, 4th edn. Philadelphia, PA: Elsevier Saunders, 2013. [Google Scholar]
- 17. Throop JL, Kerl ME, Cohn LA. Albumin in health and disease: causes and treatment of hypoalbuminemia. Compendium 2004;26:940–9. [Google Scholar]
- 18. Roberts JR, Carney S, Boyle SM, Lee DC. Ondansetron quells drug-resistant emesis in theophylline poisoning. Am J Emerg Med 1993;11:609–10. [DOI] [PubMed] [Google Scholar]
- 19. Shannon MW. Theophylline and caffeine In: Shannon MW, Borron SW, Burns MJ (eds). Haddad and Winchester's Clinical Management of Poisoning and Drug Overdose, 4th edn. China: Saunders, 2007, 1035–49. [Google Scholar]
- 20. Greene SC, Halmer T, Carey JM, et al. Theophylline toxicity: an old poisoning for a new generation of physicians, Turkish. J Emerg Med 2018;18:37–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perrott J, Murphy N, Zed P. L-Carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother 2010;44:1287–93. [DOI] [PubMed] [Google Scholar]
- 22. Luke Y. Toxicology In: Irwin RS, Lilly CM, Rippe JM (eds). Irwin and Rippe's Manual of Intensive Care Medicine, 6th edn. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2014, 749–836. [Google Scholar]
- 23. Singh O, Juneja D. Extracorporeal therapies: specific poisons In: Singh O, Juneja D (eds). Principles and Practice of Critical Care Toxicology. Chapter 31, 1st edn. India: JP Medical Ltd, 2019, 283. [Google Scholar]
- 24. Kempf J, Rusterholtz T, Ber C, et al. Hemodynamic study as guideline for the use of beta-blockers in acute theophylline poisoning. Intensive Care Med 1996;22:585–7. [DOI] [PubMed] [Google Scholar]
- 25. Brooks H, Goldberg L, Holland R, et al. Carnitine-induced effects on cardiac and peripheral hemodynamics. J Clin Pharm Ther 1977;117:561–8. [DOI] [PubMed] [Google Scholar]
- 26. Freeman BA, Crapo MD. Biology of disease: free radicals and tissue injury. Lab Invest 1984;47:412–26. [PubMed] [Google Scholar]
- 27. McCord JM, Roy RS, Schaffer SW. Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv Myocardiol 1985;5:182–9. [PubMed] [Google Scholar]
- 28. Kim M, Akera T. Oxygen-free radicals: cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol 1987;252:252–7. [DOI] [PubMed] [Google Scholar]
- 29. Goldhaber JL, Ji S, Lamp ST, Weiss JN. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea and reperfusion injury. J Clin Invest 1989;83:1800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imai S, Matsui K, Nakazawa M, et al. Anti-arrhythmic effects of carnitine chloride and its acetyl analogue on canine late ventricular arrhythmia induced by ligation of the coronary artery as related to the improvement of mitochondrial function. Br J Pharmacol 1984;82:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seneff M, Scott J, Friedman B, Smith M. Acute theophylline toxicity and the use of esmolol to reverse cardiovascular instability. Ann Emerg Med 1990;19:671–3. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki Y, Kamikawa T, Yamazaki N. Effect of L-carnitine on cardiac hemodynamics. Jpn Heart J 1980;22:219–25. [DOI] [PubMed] [Google Scholar]
- 33. Gürlek A, Tutar E, Akçil E, et al. The effects of L-carnitine treatment on left ventricular function and erythrocyte superoxide dismutase activity in patients with ischemic cardiomyopathy. Eur J Heart Fail 2000;2:189–93. [DOI] [PubMed] [Google Scholar]
- 34. Pauly DF, Pepine CJ. The role of Carnitine in myocardial dysfunction. Am J Kidney Dis 2003;41:S35–43. [DOI] [PubMed] [Google Scholar]
- 35. Jing L, Zhou LJ, Li WM, et al. Carnitine regulates myocardial metabolism by peroxisome proliferator-activated receptor-alpha (PPARa) in alcoholic cardiomyopathy. Med Sci Monit 2011;17:BR1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fujiwara M, Nakano T, Tamoto S, et al. Effect of L-carnitine in patients with ischemic heart disease. J Cardiol 1991;21:493–504. [PubMed] [Google Scholar]
- 37. Bernard S. Severe lactic acidosis following theophylline overdose. Ann Emerg Med 1991;20:1135–7. [DOI] [PubMed] [Google Scholar]
- 38. Ray A, Gulati K, Anand A, Vijayan VK. Pharmacological studies on mechanisms of aminophylline-induced seizures in rats. Indian J Exp Biol 2005;43:849–53. [PubMed] [Google Scholar]
- 39. Roy UK, Datta S, Pal M, et al. Role of ketamine, levetiracetam, and L-carnitine in aminophylline induced seizure in Wister rat model. Am J Phytomed Clin Therap 2015;3:137–44. [Google Scholar]
- 40. Berni A, Meschini R, Filippi S, et al. L-Carnitine enhances resistance to oxidative stress by reducing DNA damage in ataxia telangiectasia cells. Mut Res/Gen Toxicol Environ Mutagen 2008;650:165–74. [DOI] [PubMed] [Google Scholar]
- 41. Calabrese V, Giuffrida Stella AM, Calvani DA. Butterfield. Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J Nutr Biochem 2006;17:73–88. [DOI] [PubMed] [Google Scholar]
- 42. Pignatelli P, Tellan G, Marandola M, et al. Effect of L-carnitine on oxidative stress and platelet activation after major surgery. Acta Anaesthesiol Scand 2011;55:1022–8. [DOI] [PubMed] [Google Scholar]
- 43. Hoffman RJ. Methylxanthines and selective β2-adrenergic agonists In: Hoffman RS, Nelson LS, Howland MA, et al. (eds). Goldfrank's Manual of Toxicologic Emergencies. New York: McGraw-Hill, 2007, 553–9. [Google Scholar]
- 44. Hakeshzadeh F, Tabibi H, Ahmadinejad M, et al. Effects of L-carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren Fail 2010;32:1109–14. [DOI] [PubMed] [Google Scholar]
- 45. Derin N, Izgut-Uysalvn VN, Agac A, et al. L-Carnitine protects gastric mucosa by decreasing ischemia-reperfusion induced lipid peroxidation. J Physiol Pharmacol 2004;55:595–606. [PubMed] [Google Scholar]
- 46. Oami T, Oshima T, Hattori N, et al. L-Carnitine in critically ill patients—a case series study. Renal Replacement Ther 2018;4:13. [Google Scholar]
- 47. Arsenian MA. Carnitine and its derivatives in cardiovascular disease. Prog Cardiovasc Dis 1997;40:265–86. [DOI] [PubMed] [Google Scholar]


